Abstract
Peptidoglycan-derived muramyl dipeptide (MDP) activates innate immunity via the host sensor NOD2. Although MDP is N-acetylated in most bacteria, mycobacteria and related Actinomycetes convert their MDP to an N-glycolylated form through the action of N-acetyl muramic acid hydroxylase (NamH). We used a combination of bacterial genetics and synthetic chemistry to investigate whether N-glycolylation of MDP alters NOD2-mediated immunity. Upon infecting macrophages with 12 bacteria, tumor necrosis factor (TNF) α secretion was NOD2 dependent only with mycobacteria and other Actinomycetes (Nocardia and Rhodococcus). Disruption of namH in Mycobacterium smegmatis obrogated NOD2-mediated TNF secretion, which could be restored upon gene complementation. In mouse macrophages, N-glycolyl MDP was more potent than N-acetyl MDP at activating RIP2, nuclear factor κB, c-Jun N-terminal kinase, and proinflammatory cytokine secretion. In mice challenged intraperitoneally with live or killed mycobacteria, NOD2-dependent immune responses depended on the presence of bacterial namH. Finally, N-glycolyl MDP was more efficacious than N-acetyl MDP at inducing ovalbumin-specific T cell immunity in a model of adjuvancy. Our findings indicate that N-glycolyl MDP has a greater NOD2-stimulating activity than N-acetyl MDP, consistent with the historical observation attributing exceptional immunogenic activity to the mycobacterial cell wall.
In seminal studies, Jules T. Freund reported that the presence of mycobacteria in a water-in-oil emulsion containing protein antigen stimulated a particularly strong delayed hypersensitivity reaction in guinea pigs (Freund et al., 1950; Freund, 1951). Later, it was shown that mycobacterial peptidoglycan (PGN) could replace whole mycobacterial cells in CFA (Lederer et al., 1975) and that synthetic N-acetylmuramyl-l-alanyl-d-isoglutamine PGN moiety, usually referred to as N-acetyl muramyl dipeptide (MDP), presented adjuvant activity (Ellouz et al., 1974; Kotani et al., 1975). However, mycobacterial PGN is unique in that the N-acetyl group at carbon 2 of muramic acid is preferentially hydroxylated to an N-glycolyl group (Adam et al., 1969) via the action of N-acetyl muramic acid hydroxylase (NamH), an enzyme present only in certain aerobic Actinomycetes (Raymond et al., 2005). Notably, these are the very organisms to which Freund attributed increased immunogenic activity (Freund, 1956).
Adjuvants act by stimulating the innate immune response of the host. The response to bacterial PGN is mediated largely by NOD1 and NOD2, two members of the cytosolic-localized Nod-like receptor family, in cooperation with other pattern recognition molecules such as Toll-like receptors (Kufer and Sansonetti, 2007). NOD2 has been implicated in sensing PGN-derived N-acetyl MDP and shown to activate the NF-κB and mitogen-activated protein kinase (MAPK) pathways via polyubiquitination of the RIP2 kinase (Girardin et al., 2003; Yang et al., 2007; Hasegawa et al., 2008). However, the role of NOD2 in recognition of the unique mycobacterial N-glycolyl MDP has not been studied.
Using both bacterial genetics and chemical synthesis, we have determined the importance of the bacterial NamH enzyme for NOD2-mediated sensing and compared the activity of N-acetyl and N-glycolyl MDP in vitro and in vivo. Our findings identify N-glycolyl MDP as more stimulatory than N-acetyl MDP at eliciting NOD2-mediated immune responses in the context of an intact bacterium and as a pure compound. Together, these findings indicate that the NOD2 pathway may be exquisitely tuned to detect mycobacterial infections, and suggest a likely mechanism to explain the remarkable adjuvancy of mycobacterial cell walls.
RESULTS AND DISCUSSION
Macrophage recognition of selected Actinomycetes is NOD2 dependent
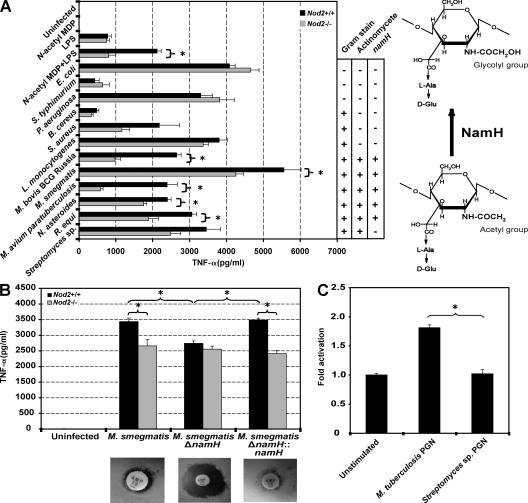
To address the effect of NOD2 on recognition of diverse bacteria, peritoneal macrophages derived from naive WT or Nod2-deficient mice were infected with a panel of live Gram-negative and -positive organisms to measure TNF-α secretion. As shown by others, naive macrophages produced undetectable levels of TNF-α in response to N-acetyl MDP alone, and synergistic NOD2-dependent TNF-α after co-stimulation with MDP and LPS (Fig. 1 A). After infection with Gram-negative Escherichia coli, Salmonella typhimurium, and Pseudomonas aeruginosa, and Gram-positive Bacillus cereus, Staphylococcus aureus, and Listeria monocytogenes, TNF-α levels did not depend on NOD2 (Fig. 1 A). Conversely, as previously described for mycobacterial infection (Ferwerda et al., 2005; Ferwerda et al., 2007; Gandotra et al., 2007; Divangahi et al., 2008; Leber et al., 2008), TNF-α production was significantly reduced in Nod2-deficient cells after infection with different mycobacterial species (Fig. 1 A). NOD2-dependent recognition extended to other Actinomycetales class members (Nocardia asteroides and Rhodococcus equi) but not Streptomyces sp. (Fig. 1 A).
Figure 1.
NOD2-mediated recognition of selected Actinomycetes by macrophages depends on bacterial NamH. (A, left) Naive peritoneal macrophages from Nod2+/+ and Nod2−/− mice were left unstimulated; stimulated with 10 µg/ml N-acetyl MDP alone, LPS alone, or a combination of N-acetyl MDP and LPS; or were infected with various live Gram-negative and -positive organisms. (right) Schematic representation of the effect of NamH on MDP. (B, top) Naive peritoneal macrophages from Nod2+/+ and Nod2−/− mice were either left uninfected or were infected with WT M. smegmatis, namH-disrupted M. smegmatis (M. smegmatisΔnamH), and namH-disrupted M. smegmatis complemented with namH (M. smegmatisΔnamH::namH). (bottom) Ampicillin zone of inhibition assay on WT M. smegmatis and namH variants. In A and B, the amount of TNF-α released in the supernatant after 16 h was quantified by ELISA. Results represent averaged data from two independent replicates (A) or one representative experiment out of three (B). (C) HEK293 cells were transfected with NOD2 and a NF-κB luciferase reporter in the presence of PGN derived from indicated bacteria. The fold increase in NF-κB activation compared with transfected but unstimulated cells was assessed. Representative data from three independent replicates are shown (means ± SEM). *, P < 0.05.
As a trend, Nod2-deficient cells produced less TNF-α after infection with Gram-positive but not Gram-negative organisms, consistent with the greater quantity of PGN in the cell wall of Gram-positive bacteria (Yang et al., 2001). However, the observation that significant NOD2-dependent recognition was restricted to a subset of aerobic Actinomycetes led us to ask whether these organisms in particular may share a common difference in their PGN.
Bacterial NamH is required for optimal NOD2-dependent recognition
Several differences in PGN amount, location, and structure exist among bacterial species and have in some instances been described to alter immunological activity (Stewart-Tull, 1980). A common feature shared by NOD2-stimulatory Actinomycetes is the elaboration of N-glycolylated PGN via the action of the NamH hydroxylase that converts the UDP-N-acetylmuramic acid PGN precursor moiety to UDP-N-glycolylmuramic acid (UDP-MurNAc + O2 + NADPH + H+ → UDP-MurNGlyc + NADP+ + H2O; Raymond et al., 2005). Because hydroxylation of UDP-N-acetylmuramic acid ultimately affects the structure of MDP (Fig. 1 A, right), we tested the importance of namH for NOD2-dependent bacterial recognition through gene disruption. As previously shown (Raymond et al., 2005), the Mycobacterium smegmatis namH mutant was more susceptible to ampicillin than WT M. smegmatis and the mutant complemented with namH (Fig. 1 B, bottom). After ex vivo infection of WT and Nod2-deficient macrophages, NOD2-dependent TNF-α production was abrogated in the absence of namH and restored with gene complementation (Fig. 1 B).
To verify that NOD2 recognition was sufficient for namH-dependent TNF-α production, we stimulated HEK293 cells transiently expressing NOD2 and a NF-κB luciferase reporter vector with purified PGN derived from the Actinomycetes M. tuberculosis (namH positive) and Streptomyces sp. (namH negative). In this assay, M. tuberculosis–derived PGN, but not Streptomyces sp.–derived PGN, induced NF-κB activation (Fig. 1 C). Therefore, namH is critical for optimal NOD2-mediated recognition of mycobacterial PGN.
N-glycolyl MDP is sensed by NOD2 and is more potent than N-acetyl MDP at activating RIP2, NF-κB, and c-Jun N-terminal kinase (JNK) but not p38 MAPK
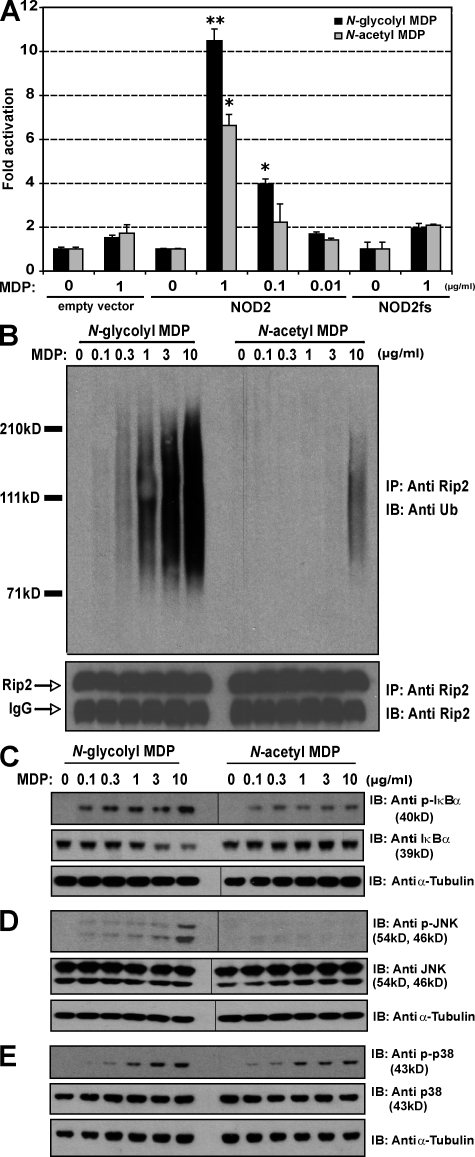
Because mycobacterial PGN contains a mixture of N-glycolylated and N-acetylated muramic acid (Mahapatra et al., 2005), we determined the relative capacity of N-acetyl and N-glycolyl MDP to stimulate NOD2-dependent responses. As shown in Fig. 2 A, both forms of MDP led to activation of NF-κB in HEK293 cells expressing WT NOD2, but N-glycolyl MDP was more stimulatory than N-acetyl MDP at 1 and 0.1 µg/ml. The Crohn's disease (CD)–associated NOD2 variant containing a frame-shift mutation at position 3,020 (NOD2fs) is unresponsive to N-acetyl MDP (Girardin et al., 2003). Likewise, the glycolylated variant was also unable to activate NF-κB via the mutated NOD2 (Fig. 2 A).
Figure 2.
N-glycolyl MDP is more potent than N-acetyl MDP at inducing RIP2 polyubiquitination and at activating NF-κB and JNK. (A) HEK293 cells were transfected with indicated vectors and a NF-κB luciferase reporter in the presence of various concentrations of N-glycolyl or N-acetyl MDP. Fold increase in NF-κB activation compared with transfected but unstimulated cells was assessed. Representative data from three independent replicates are shown (means ± SEM). *, P < 0.05 compared with unstimulated cells; **, P < 0.05 between N-glycolyl versus N-acetyl MDP. (B–E) RAW 264.7 cells were left untreated or treated with various concentrations of N-acetyl or N-glycolyl MDP. (B) Cell lysates were immunoprecipitated (IP) and polyubiquitinated RIP2 proteins were detected by immunoblotting (IB) with the indicated antibody. Total immunoprecipitated RIP2 protein was measured as a control. (C–E) The NF-κB (C), JNK (D), and p38 MAPK (E) activities were measured by immunoblotting with the indicated anti-phospho antibodies. Total IκBα, JNK, p38 MAPK, and α-tubulin protein levels were measured with the indicated antibodies. Representative data from two independent replicates are shown in B–E. Black lines indicate that intervening lanes have been spliced out.
The potency of a compound is a measure of its activity, as expressed by the concentration of compound required to produce a defined response. To determine the relative potency of the two forms of MDP at activating intracellular signaling downstream of NOD2, we stimulated macrophages with increasing concentrations of these compounds ranging from 0.1 to 10 µg/ml. N-glycolyl MDP was ∼30-fold more potent than N-acetyl MDP at inducing polyubiquitination of RIP2 (Fig. 2 B), and 100-fold more potent than N-acetyl MDP at inducing phospho-IκBα (Fig. 2 C). Only N-glycolyl MDP could induce detectable phospho-JNK under current experimental conditions (Fig. 2 D). Both forms of MDP induced similar phosphorylation of p38 MAPK (Fig. 2 E). Collectively, these data indicate that N-glycolyl MDP is more potent than N-acetyl MDP at activating NOD2-mediated RIP2 polyubiquitination and selective downstream pathways.
N-glycolyl MDP is more active than N-acetyl MDP at inducing synergistic proinflammatory cytokine production
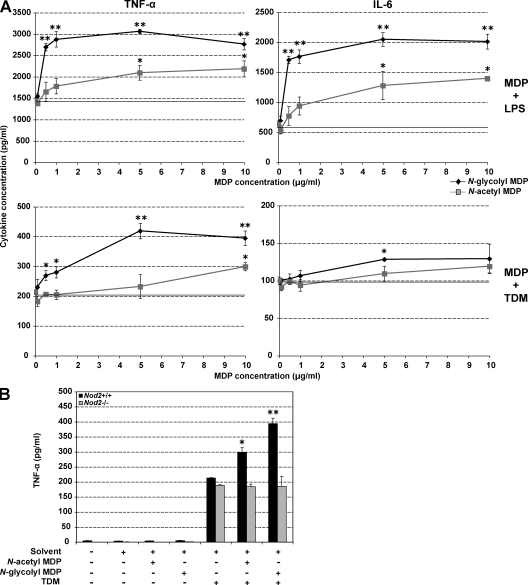
It is known that MDP alone is a potent inducer of TNF-α mRNA, which remains untranslated, and that presence of LPS abrogates this translation block (Wolfert et al., 2002). To compare N-glycolyl and N-acetyl MDP, we stimulated mouse peritoneal macrophages with various concentrations of MDP along with a fixed concentration of LPS, and measured TNF-α and IL-6 production (Fig. 3 A, top). At all concentrations ranging from 0.5 to 10 µg/ml, N-glycolyl MDP was more stimulatory than N-acetyl MDP. In terms of potency, N-glycolyl MDP was active at a 10-fold lower concentration than N-acetyl MDP (0.5 vs. 5 µg/ml). Because Actinomycetes do not produce LPS, we repeated this experiment using M. tuberculosis–derived trehalose 6,6′-dimycolate (TDM), a molecule that has been shown to have synergistic immunological activity in combination with MDP (Masihi et al., 1985). Again, N-glycolyl MDP was more stimulatory than N-acetyl MDP and was active at a 10- to 20-fold lower concentration. (Fig. 3 A, bottom) The synergistic response to MDP plus TDM in Nod2+/+ cells was abrogated in Nod2−/− cells (Fig. 3 B). Thus, N-glycolyl MDP is more stimulatory and more potent than N-acetyl MDP at inducing proinflammatory cytokine production upon co-stimulation with nonmycobacteria- and mycobaceria-derived molecules.
Figure 3.
N-glycolyl MDP is more potent than N-acetyl MDP at inducing proinflammatory cytokine production in macrophages. (A) Naive peritoneal macrophages from Nod2+/+ mice were stimulated for 6 h with LPS (top) or for 12 h with TDM (bottom) in combination with various concentrations of N-glycolyl or N-acetyl MDP. (B) Naive peritoneal macrophages from Nod2+/+ and Nod2−/− mice were either left unstimulated or were stimulated for 12 h with 10 µg/ml N-acetyl MDP alone, 10 µg/ml N-glycolyl MDP alone, TDM alone and a combination of N-acetyl MDP and TDM, or N-glycolyl MDP and TDM. The amount of TNF-α and IL-6 released in the supernatant was quantified by ELISA. Representative data from three independent replicates are shown (means ± SEM). *, P < 0.05 compared with no MDP added; **, P < 0.05 between N-glycolyl versus N-acetyl MDP.
WT M. smegmatis induces increased NOD2-dependent macrophage activation compared with namH-deficient M. smegmatis after i.p. challenge in mice
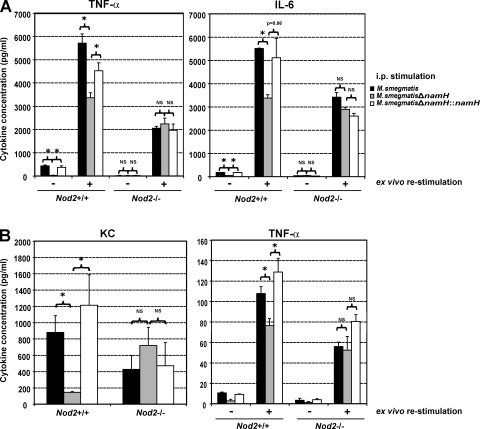
To investigate the effect of N-glycolyl MDP on in vivo innate inflammatory response in the context of a live bacterial infection, we harvested and cultured peritoneal macrophages from Nod2+/+ and Nod2−/− mice after i.p. stimulation with WT M. smegmatis, namH-deficient M. smegmatis, and namH-complemented mutant. 3 d after infection, there was no significant difference in the number of colony-forming units in the spleens of mice infected with the different organisms (unpublished data). Cells harvested from Nod2+/+ mice that had been infected with different strains of M. smegmatis spontaneously released low levels of TNF-α (Fig. 4 A, left). Nonetheless, cells from mice infected with namH-deficient M. smegmatis produced significantly less TNF-α than cells from mice infected by either the WT or the complemented strain. Upon ex vivo restimulation of these cells with live mycobacteria, TNF-α levels were increased, again in a namH-dependent manner (Fig. 4 A, left). The namH dependence for both spontaneous cytokine production and restimulated cells was abrogated in Nod2−/− mice. The same pattern was observed with IL-6 (Fig. 4 A, right).
Figure 4.
Increased NOD2-dependent immunogenicity of WT M. smegmatis compared with M. smegmatisΔnamH during short-term mouse i.p. challenge. (A) Naive Nod2+/+ and Nod2−/− mice (n = 3 per genotype per infection) were injected i.p. with live preparations of either M. smegmatis, M. smegmatisΔnamH, or M. smegmatisΔnamH::namH. After 72 h, peritoneal macrophages were harvested and either left unstimulated or stimulated with M. smegmatisΔnamH. (B) Naive Nod2+/+ and Nod2−/− mice (n = 3 per genotype per stimulation) were injected i.p. with mutanolysin-treated killed preparations of either M. smegmatis, M. smegmatisΔnamH, or M. smegmatisΔnamH::namH. After 2 h, peritoneal lavage was performed, and peritoneal macrophages were harvested and either left unstimulated or stimulated with LPS. The amount of KC in the peritoneal lavage as well as TNF-α and/or IL-6 released in the culture supernatant was quantified by ELISA. Representative data from two independent replicates are shown (means ± SEM). *, P < 0.05.
Because the M. smegmatis namH mutant is more sensitive to lysozyme than WT M. smegmatis (Raymond et al., 2005), it is possible that infection with the former organism leads to increased PGN shedding and macrophage exhaustion rather than decreased stimulation. To control for this possibility, we digested the PGN from heat-killed M. smegmatis and namH variants, and performed a short-term i.p. stimulation of WT and Nod2-deficient mice. After 2 h, KC levels were significantly increased in the peritoneum of WT mice stimulated with N-glycolyl MDP–containing organisms (Fig. 4 B, left). This increase was abrogated in Nod2-deficient mice. Furthermore, akin to what was observed after 72 h, macrophages recruited to the peritoneum of WT mice after a 2-h challenge with M. smegmatis and the complemented namH mutant were more responsive to Toll-like receptor 4 stimulation than macrophages from namH-deficient M. smegmatis–treated mice (Fig. 4 B, right). This effect was again dependent on the presence of NOD2 because it was abrogated in Nod2-deficient mice (Fig. 4 B, right). Thus, optimal NOD2-dependent macrophage activation by mycobacteria in vivo requires expression of the bacterial NamH.
N-glycolyl MDP confers increased immunological activity to live mycobacteria and is a critical active component of CFA
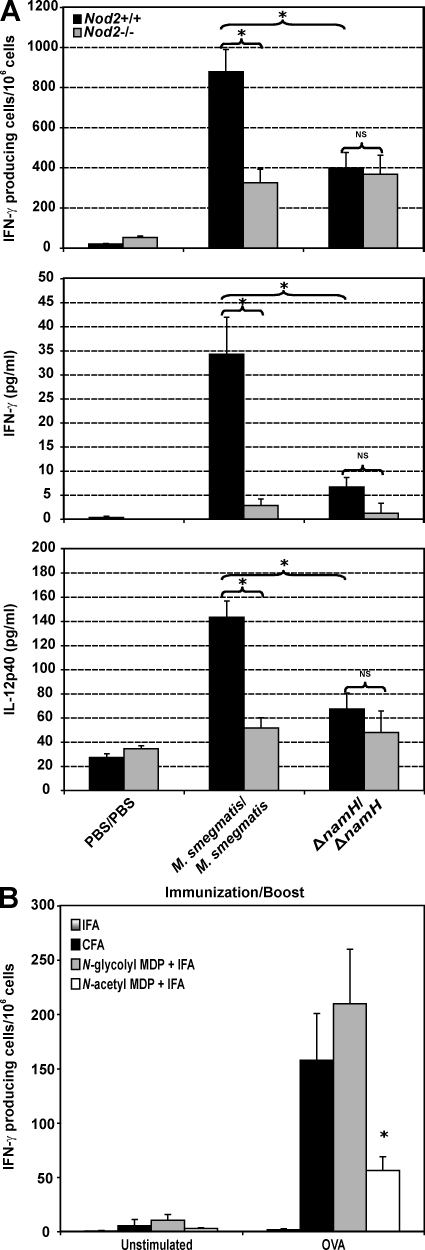
Our group recently showed that activation of the IFN-γ–IL-12 axis during mycobacterial infection is impaired in Nod2-deficient mice (Divangahi et al., 2008). To investigate the NOD2-dependent contribution of mycobacterial NamH to T cell activation, we performed a short-term immunization experiment using live WT M. smegmatis and namH-deficient M. smegmatis. 14 d after immunization, no bacteria could be detected in the mouse spleens (unpublished data). As shown in Fig. 5 A, the number of IFN-γ–producing splenocytes (top) and the level of IFN-γ production by these cells (middle) were significantly increased in Nod2+/+ compared with Nod2−/− cells after immunization with WT M. smegmatis. This NOD2 dependence was lost after immunization with namH-deficient M. smegmatis. Similar results were obtained when measuring IL-12p40 production by splenic antigen-presenting cells (Fig. 5, bottom).
Figure 5.
N-glycolyl MDP is a critical active constituent of the adjuvant-active mycobacterial cell wall. (A) Naive Nod2+/+ and Nod2−/− mice (n = 4 per genotype per immunization) were immunized i.p. with either M. smegmatis or M. smegmatisΔnamH and rechallenged 14 d later with the same organism. Saline-injected mice were used as a control. The frequency of IFN-γ–producing splenocytes (top) as well as total IFN-γ (middle) and IL-12p40 (bottom) production by these cells was quantified using ELISPOT and ELISA, respectively. (B) C57BL/6 mice (n = 3–4 per immunization) were immunized s.c. with the indicated emulsified preparations for 7 d. The frequency of OVA-specific IFN-γ–producing T cells in the draining lymph nodes was analyzed by ELISPOT of unstimulated and OVA-stimulated cells. Representative data from two independent replicates are shown (means ± SEM). *, P < 0.05.
To specifically test the relative adjuvant activity of the two forms of MDP present in CFA, we immunized mice s.c. with preparations of OVA emulsified in (a) IFA alone, (b) CFA, (c) N-glycolyl MDP with IFA, or (d) N-acetyl MDP with IFA. After 7 d, OVA-specific IFN-γ–producing cells from the draining lymph nodes were enumerated by ELISPOT. IFA alone was not sufficient to generate antigen-specific immunity, whereas CFA induced a high frequency of OVA-specific IFN-γ–producing T cells (Fig. 5 B). Strikingly, N-glycolyl MDP plus IFA induced a comparable response to CFA, which was significantly greater than N-acetyl MDP plus IFA. As a control, there was no response in nondraining lymph nodes (unpublished data). These results point to a key role of N-glycolylated MDP in the adjuvant activity of CFA.
Concluding remarks
The major findings of this work are that the disruption of namH leads to impaired NOD2-mediated recognition of mycobacterial PGN, and that N-glycolyl MDP is more potent at modulating host response compared with N-acetyl MDP. Thus, it appears that the mammalian NOD2 pathway is exquisitely sensitive to PGN from mycobacteria and related organisms, and hence, that NOD2 serves as a major receptor for CFA. Efforts are currently underway to address the role of NamH in relationship to NOD2 using more virulent bacterial organisms. Determining whether increased potency of N-glycolyl MDP is a result of altered affinity, stability, or cytosolic delivery is expected to lead to a better understanding of the mechanism of action of CFA.
NOD2 is a susceptibility gene for CD, a polygenic systemic inflammatory disease featuring recurring lesions in the gastrointestinal tract (Hugot et al., 2001). Cells from humans with CD-associated NOD2 polymorphisms manifest a reduced response to MDP (Inohara et al., 2003), which could increase susceptibility to intracellular bacterial infection. Because only a small minority of all individuals with CD-associated NOD2 polymorphisms will develop CD in their lifetime, it is possible that decreased resistance to specific bacteria will play an important role in disease pathogenesis. Given the importance of NOD2 as a key modulator of the host response to N-glycolyl MDP–containing bacteria, it will be a priority to investigate the role of these organisms in the pathogenesis of CD.
MATERIALS AND METHODS
Mice.
Nod2+/− males backcrossed six generations onto a C57BL/6 background were obtained from the Congenics Facility at Yale University. They were bred with C57BL/6 mice purchased from Harlan Laboratories to establish a Nod2−/− breeding colony at the McGill University Health Centre. A WT breeding colony was established from the Nod2+/+ littermates from this breeding, and these animals were used for experiments in Figs. 1, 3, 4, and 5 A. For Fig. 5 B, C57BL/6 mice were purchased from the Jackson Laboratory. All study mice were 8–12 wk old, and experiments were conducted in accordance with the guidelines of animal research ethics boards of McGill and Harvard Universities.
Bacterial strains, growth conditions, and bacteria-derived reagents.
M. bovis BCG Russia, M. smegmatis mc2155, and M. avium ssp. paratuberculosis (MAP) K10 were grown as previously described (Divangahi et al., 2008). For MAP K10, 1 µg/ml mycobactin J (Allied Monitor Inc.) was added to the culture medium. S. aureus (American Type Culture Collection), E. coli (American Type Culture Collection), S. typhimurium (American Type Culture Collection), P. aeruginosa (American Type Culture Collection), and recent clinical isolates of L. monocytogenes, B. cereus, R. equi, N. asteroides, and Streptomyces sp., as well as E. coli DH5-α used for cloning purposes, were cultured at 37°C in Luria broth (Difco) at 250 rpm. Kanamycin (50 µg/ml for E. coli and mycobacteria) and hygromycin (100 µg/ml for E. coli and 50 µg/ml for mycobacteria) were used when needed.
Pure LPS from E. coli 055:B5, N-acetyl MDP (98% purity), Streptomyces sp.–derived PGN, TDM from M. tuberculosis (99% purity), CFA, and IFA were purchased from Sigma-Aldrich. M. tuberculosis–derived PGN was a gift from P. Brennan and J. Spencer (Colorado State University, Fort Collins, CO). N-glycolyl MDP was custom synthesized (Carbohydrate Synthesis; Kobayashi et al., 1980) and shown to be >95% pure by nuclear magnetic resonance spectrometry. N-acetyl and N-glycolyl MDP were free of endotoxin contamination, as confirmed by the Limulus amebocyte lysate assay (Pyrotell; Associates of Cape Cod, Inc.).
Construction and complementation of the M. smegmatis namH mutant.
The hygromycin resistance cassette from PSC301 was digested by XmnI and inserted into XbaI-digested and T4 polymerase-treated pUC19 to generate the pUC19::Hyg suicide vector. Subsequently, a 1,070-bp fragment derived from the M. smegmatis namH gene (amplified using KONamHF [5′-CCGCATATGTCGCCTCGTGGTTC-3′] and KONamHR [5′-AGCGGATCCTCTCGTCGGGGATC-3′]) was inserted into pUC19::Hyg using NdeI and BamHI. This plasmid was electroporated in M. smegmatis, and the hygromycin-resistant clones were confirmed by Southern blotting to have the plasmid inserted in namH. To complement the ΔnamH mutant, the full-length M. smegmatis namH gene was amplified along with its putative promoter using MsNamHF (5′-CGAGCTAGCTGGTGTGGTTGATCG-3′) and MsNamHR (5′-GATAAGCTTCGATGTGCCCGACG-3′). This fragment was inserted in pSUM37 using NheI and HindIII to generate the pSUMnamH complementation vector.
Ex vivo macrophage culture, infection, and stimulation.
Peritoneal macrophages were harvested from naive Nod2+/+ and Nod2−/− mice without prestimulation. 105 cells per well were purified by adherence and cultured in 96-well plates with or without live bacterial infection (2 CFU/cell) or stimulation with 10 ng/ml LPS, 10 µg/ml TDM, and N-acetyl and N-glycolyl MDP. TDM was dissolved in petroleum ether, coated to the wells of a 96-well plate, and allowed to evaporate before macrophage addition with and without MDP. The culture supernatants were collected at designated intervals and stored at −20°C until cytokine measurements. Cytokine production by macrophages was assayed using ELISA (R&D Systems) to measure TNF-α and IL-6 in culture supernatants.
Antibiotic sensitivity assay.
107 cells from log-phase M. smegmatis strains were resuspended in 200 µl 7H9 and spread onto 25 ml 7H10 in standard 15-cm-diameter Petri dishes. 10-µg ampicillin disks (Oxoid Ltd.) were placed on inoculated plates. After a 48-h incubation at 37°C, the diameter of the zone of inhibition was measured.
HEK293 cell transfection and stimulation.
Plasmids containing cDNA for the WT human NOD2 or the CD-associated human NOD2 3020insC (NOD2fs) were gifts from S. Girardin (University of Toronto, Toronto, Canada). 3xFLAG-tagged NOD2wt and NOD2fs were PCR amplified and subcloned into the pIRES-puro3 expression vector (Clontech Laboratories, Inc.). For NF-κB activation assays, 105 HEK293 cells per well were seeded into 24-well plates and transfected overnight using FuGene6 (Roche) with 1 ng of NOD2, NOD2fs, or empty vector, 75 ng pTAL-NF-κB-luc (Clontech Laboratories, Inc.), and 7.5 ng pGL4-RL (Promega), along with defined concentrations of PGN or MDP. After 16 h, a dual-luciferase reporter assay (Promega) was performed on cell lysates.
Immunoprecipitation and Western blot analysis.
Immunoprecipitation and immunoblotting were performed as previously described (Yang et al., 2007). Experiments were performed using the RAW 264.7 macrophage cell line stimulated with MDP for 1 h.
i.p. M. smegmatis challenges.
Nod2+/+ and Nod2−/− mice were injected i.p. with 0.5 ml PBS containing ∼107 live or heat-killed, mutanolysin-treated M. smegmatis (Sigma-Aldrich) or modified strains for 72 and 2 h, respectively. Peritoneal lavage was performed at 2 h with 3 ml PBS, and peritoneal macrophages from infected mice were harvested and cultured as described with or without stimulation. KC production in the peritoneal lavage as well as TNF-α and IL-6 production in culture supernatants was assayed using ELISA.
Immunizations and analysis of T cell response.
Nod2+/+ and Nod2−/− mice were immunized i.p. with 0.5 ml PBS alone or containing ∼106 live M. smegmatis or M. smegmatisΔnamH. After 14 d, each mouse was given a 5-d boost with the same preparation used for immunization. The number of IFN-γ–producing splenocytes (0.5 × 106/well) as well as total IL-12p40 and IFN-γ production by unstimulated cells was measured using ELISPOT (R&D Systems) and ELISA, respectively.
C57BL/6 mice received s.c. injections (two injection sites, 100 µl per site) of 200 µg OVA (Sigma-Aldrich) mixed with IFA, CFA, 30 µg N-glycolyl MDP plus IFA, or 30 µg N-acetyl MDP plus IFA. 7 d after immunization, a single-cell suspension was prepared from draining lymph nodes (inguinal) and nondraining lymph nodes (axillary). Cells were incubated for 24 h with medium alone or containing 100 µg OVA, and then subjected to IFN-γ ELISPOT.
Acknowledgments
This work was funded by an operating grant (MOP-86536) from the Canadian Institutes for Health Research (CIHR) to M.A. Behr. F. Coulombe is supported by a studentship award from the Fonds de la Recherche en Santé du Québec (FRSQ), M.B. Reed is a New Investigator of the CIHR, and M.A. Behr is a Chercheur-Boursier Senior of the FRSQ and a William Dawson Scholar of McGill University.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: CD, Crohn's disease; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MDP, muramyl dipeptide; NamH, N-acetyl muramic acid hydroxylase; PGN, peptidoglycan; TDM, trehalose 6,6′-dimycolate.
References
- Adam A., Petit J.F., Wietzerbin-Falszpan J., Sinay P., Thomas D.W., Lederer E. 1969. FEBS Lett. 4:87–92 [DOI] [PubMed] [Google Scholar]
- Divangahi M., Mostowy S., Coulombe F., Kozak R., Guillot L., Veyrier F., Kobayashi K.S., Flavell R.A., Gros P., Behr M.A. 2008. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity.J. Immunol. 181:7157–7165 [DOI] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. 1974. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives.Biochem. Biophys. Res. Commun. 59:1317–1325 [DOI] [PubMed] [Google Scholar]
- Ferwerda G., Girardin S.E., Kullberg B.J., Le Bourhis L., De Jong D.J., Langenberg D.M., Van Crevel R., Adema G.J., Ottenhoff T.H., Van Der Meer J.W., Netea M.G. 2005. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis.PLoS Pathog. 1:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda G., Kullberg B.J., De Jong D.J., Girardin S.E., Langenberg D.M., Van Crevel R., Ottenhoff T.H., Van Der Meer J.W., Netea M.G. 2007. Mycobacterium paratuberculosis is recognized by Toll-like receptors and NOD2.J. Leukoc. Biol. 82:1011–1018 [DOI] [PubMed] [Google Scholar]
- Freund J. 1951. The effect of paraffin oil and mycobacteria on antibody formation and sensitization; a review.Am. J. Clin. Pathol. 21:645–656 [DOI] [PubMed] [Google Scholar]
- Freund J. 1956. The mode of action of immunologic adjuvants.Bibl. Tuberc. 1956:130–148 [PubMed] [Google Scholar]
- Freund J., Lipton M.M., Morrison L.R. 1950. Demyelination in the guinea pig in chronic allergic encephalomyelitis produced by injecting guinea pig brain in oil emulsion containing a variant of Mycobacterium butyricum.Arch. Pathol. (Chic). 50:108–121 [PubMed] [Google Scholar]
- Gandotra S., Jang S., Murray P.J., Salgame P., Ehrt S. 2007. Nucleotide-binding oligomerization domain protein 2-deficient mice control infection with Mycobacterium tuberculosis.Infect. Immun. 75:5127–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin S.E., Boneca I.G., Viala J., Chamaillard M., Labigne A., Thomas G., Philpott D.J., Sansonetti P.J. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection.J. Biol. Chem. 278:8869–8872 [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Fujimoto Y., Lucas P.C., Nakano H., Fukase K., Nunez G., Inohara N. 2008. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation.EMBO J. 27:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot J.P., Chamaillard M., Zouali H., Lesage S., Cezard J.P., Belaiche J., Almer S., Tysk C., O'Morain C.A., Gassull M., et al. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease.Nature. 411:599–603 [DOI] [PubMed] [Google Scholar]
- Inohara N., Ogura Y., Fontalba A., Gutierrez O., Pons F., Crespo J., Fukase K., Inamura S., Kusumoto S., Hashimoto M., et al. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease.J. Biol. Chem. 278:5509–5512 [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Fukuda T., Yukimasa H., Fujino M., Azuma I., Yamamura Y. 1980. Synthesis of muramyl dipeptide analogs with enhanced adjuvant activity.Bull. Chem. Soc. Jpn. 53:2570–2577 [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Shimono T., Morisaki I. 1975. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids.Biken J. 18:105–111 [PubMed] [Google Scholar]
- Kufer T.A., Sansonetti P.J. 2007. Sensing of bacteria: NOD a lonely job.Curr. Opin. Microbiol. 10:62–69 [DOI] [PubMed] [Google Scholar]
- Leber J.H., Crimmins G.T., Raghavan S., Meyer-Morse N.P., Cox J.S., Portnoy D.A. 2008. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen.PLoS Pathog. 4:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer E., Adam A., Ciorbaru R., Petit J.F., Wietzerbin J. 1975. Cell walls of Mycobacteria and related organisms; chemistry and immunostimulant properties.Mol. Cell. Biochem. 7:87–104 [DOI] [PubMed] [Google Scholar]
- Mahapatra S., Scherman H., Brennan P.J., Crick D.C. 2005. N glycolylation of the nucleotide precursors of peptidoglycan biosynthesis of Mycobacterium spp. is altered by drug treatment.J. Bacteriol. 187:2341–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masihi K.N., Brehmer W., Lange W., Werner H., Ribi E. 1985. Trehalose dimycolate from various mycobacterial species induces differing anti-infectious activities in combination with muramyl dipeptide.Infect. Immun. 50:938–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J.B., Mahapatra S., Crick D.C., Pavelka M.S., Jr 2005. Identification of the namH gene, encoding the hydroxylase responsible for the N-glycolylation of the mycobacterial peptidoglycan.J. Biol. Chem. 280:326–333 [DOI] [PubMed] [Google Scholar]
- Stewart-Tull D.E. 1980. The immunological activities of bacterial peptidoglycans.Annu. Rev. Microbiol. 34:311–340 [DOI] [PubMed] [Google Scholar]
- Wolfert M.A., Murray T.F., Boons G.J., Moore J.N. 2002. The origin of the synergistic effect of muramyl dipeptide with endotoxin and peptidoglycan.J. Biol. Chem. 277:39179–39186 [DOI] [PubMed] [Google Scholar]
- Yang S., Tamai R., Akashi S., Takeuchi O., Akira S., Sugawara S., Takada H. 2001. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture.Infect. Immun. 69:2045–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Yin C., Pandey A., Abbott D., Sassetti C., Kelliher M.A. 2007. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2.J. Biol. Chem. 282:36223–36229 [DOI] [PubMed] [Google Scholar]