Abstract
Nuclear factor κB (NF-κB) is one of the main transcription factors involved in regulating apoptosis, inflammation, chronic liver disease, and cancer progression. The IKK complex mediates NF-κB activation and deletion of its regulatory subunit NEMO in hepatocytes (NEMOΔhepa) triggers chronic inflammation and spontaneous hepatocellular carcinoma development. We show that NEMOΔhepa mice were resistant to Fas-mediated apoptosis but hypersensitive to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) as the result of a strong up-regulation of its receptor DR5 on hepatocytes. Additionally, natural killer (NK) cells, the main source of TRAIL, were activated in NEMOΔhepa livers. Interestingly, depletion of the NK1.1+ cells promoted a significant reduction of liver inflammation and an improvement of liver histology in NEMOΔhepa mice. Furthermore, hepatocyte-specific NEMO deletion strongly sensitized the liver to concanavalin A (ConA)–mediated injury. The critical role of the NK cell/TRAIL axis in NEMOΔhepa livers during ConA hepatitis was further confirmed by selective NK cell depletion and adoptive transfer of TRAIL-deficient−/− mononuclear cells. Our results uncover an essential mechanism of NEMO-mediated protection of the liver by preventing NK cell tissue damage via TRAIL/DR5 signaling. As this mechanism is important in human liver diseases, NEMOΔhepa mice are an interesting tool to give insight into liver pathophysiology and to develop future therapeutic strategies.
The transcription factor NF-κB is essential for liver homeostasis, as it is involved in controlling the balance between survival and death signals (Luo et al., 2005). Upon ligand binding, distinct receptors stimulate the IKK complex (IKK1/IKKα, IKK2/IKKβ, and IKKγ/NEMO), which initiates NF-κB activation. The regulatory subunit IKKγ/NEMO is crucial during this process, as NEMO deletion results in complete NF-κB inhibition and embryonic lethality.
Hepatocyte-specific deletion of NEMO and its consequent NF-κB inhibition has detrimental effects on liver homeostasis, as it promotes spontaneous steatohepatitis, fibrosis progression, and development of hepatocellular carcinoma (HCC; Luedde et al., 2007). Additionally, NEMO deletion in hepatocytes promotes severe TNF-mediated liver damage (Beraza et al., 2007). However, the impact of other TNF-related cytokines on acute liver injury and their contribution to the development of the strong parenchymal damage observed in hepatocyte-specific NEMO-deleted (NEMOΔhepa) mice remains unknown.
FasL and TNF-related apoptosis-inducing ligand (TRAIL) belong to the TNF family of cytokines, as they signal through death receptors which contain cytoplasmic death domains (Schutze et al., 2008). Activation of the FasL/Fas system leads to massive apoptotic cell death and fulminant hepatitis (Ogasawara et al., 1993). More recently, liver steatosis has been shown to hypersensitize hepatocytes to Fas-mediated apoptosis, contributing to the progression of steatohepatitis and end-stage liver disease (Zou et al., 2007).
TRAIL, via its receptor DR5, selectively induces apoptosis of transformed and virally infected hepatocytes, and NF-κB–dependent signaling counteracts its detrimental effects (Luo et al., 2004). The cellular responses to TRAIL have been related to the inflammatory status of the liver, and DR5 up-regulation can be mediated by free fatty acids and bile acids, indicating a link between fat metabolism and TRAIL signaling (Higuchi et al., 2004; Malhi et al., 2007). This is supported by findings showing high levels of liver DR5 expression in nonalcoholic steatohepatitis patients (Malhi et al., 2007). Additionally, fatty liver disease activates the innate immune system by triggering NKT cells, promoting an unbalanced Th1/Th2 response in the liver (Guebre-Xabier et al., 2000). This contributes to create a proinflammatory environment where high IFN-γ expression is found. Furthermore, IFN-γ produced by activated NKT cells strongly induces NK cells to express TRAIL (Smyth et al., 2001).
Together, these data suggest that FasL and TRAIL could be involved in mediating the severe liver phenotype observed in NEMOΔhepa mice. In the present study, we aimed to characterize the implication of these cytokines and to uncover the pathophysiological mechanisms contributing to liver inflammation and further disease progression in NEMOΔhepa animals.
We demonstrate that NEMOΔhepa mice are resistant to Fas-mediated apoptosis, whereas they are hypersensitive to TRAIL-mediated cell death. Moreover, we highlight an essential role for NK cells, the main producers of TRAIL, in the spontaneous phenotype observed in NEMOΔhepa mice, as interfering with this cascade significantly improved liver homeostasis and attenuated the impact of T cell–mediated hepatitis in NEMOΔhepa mice.
RESULTS AND DISCUSSION
To better characterize the role of NEMO in hepatocytes, we generated NEMO loxP mice and crossed them with alfp-Cre transgenic animals to create NEMOf/f (WT) and NEMOΔhepa (KO) mice (Fig. S1, A and B). Hepatocyte-specific NEMO deletion led to a complete lack of NF-κB activation after TNF stimulation, confirming our previous results (Fig. S1 C; Luedde et al., 2007). Additionally, in consonance with our previous data (Luedde et al., 2007), NEMOΔhepa animals exhibited spontaneously elevated TNF and TNFR1 levels, although no significant differences were found in TNFR2 expression (Fig. S1 D). Immunohistochemistry (IHC) confirmed higher TNF expression in the nonparenchymal cell compartment in NEMOΔhepa mice (Fig. S1 D). Moreover, 12-wk-old NEMOΔhepa mice displayed high ALT levels (Fig. S1 E), accompanied by signs of nonalcoholic steatohepatitis, activation of progenitor cells, profuse inflammation, and features of chronic liver damage such as hepatocyte dysplasia and anisokaryosis (Fig. S1 F, top). The combination of these events contributed to HCC development in 1-yr-old NEMOΔhepa mice, as indicated by liver tumors and higher liver weight/body weight ratio (Fig. S1, F [bottom] and G).
Hepatocyte-specific NEMO deletion promotes hypersensitivity to exogenously administered TNF; however, the animals survived this challenge (Beraza et al., 2007). As NEMO deletion may also contribute to amplifying liver damage triggered by other TNF-related cytokines, we sought to investigate their implication in mediating the liver phenotype of NEMOΔhepa mice.
Fas activation promotes acute hepatic failure (Ogasawara et al., 1993). Hence, stimulation with an anti-Fas mAb (Jo2, 0.5 µg/g) resulted in the death of all NEMOf/f mice within 10 h (Fig. 1 A). Unexpectedly, NEMOΔhepa animals were less sensitive to Jo2, as 90% of the mice survived (Fig. 1 A). Macroscopic view confirmed that NEMOΔhepa livers remained unaffected while NEMOf/f showed significant blood accumulation and a higher liver weight/body weight ratio (Fig. 1 B). Accordingly, NEMOf/f mice showed clear signs of fulminant hepatitis, as high ALT levels, infiltrating PMN and RBC, necrosis, and apoptotic bodies were observed (Fig. 1, C and D). In contrast, we failed to detect any significant effects of Jo2 in NEMOΔhepa mice, as only the preexisting elevated ALT levels and parenchymal damage, caused by the spontaneous phenotype of these mice, was observed (Fig. 1, C and D). TUNEL assay, caspase 8, and caspase 3 activity confirmed resistance to Fas-induced apoptosis in NEMOΔhepa mice (Fig. 1, E and F).
Figure 1.
Hepatocyte-specific NEMO-deleted mice (NEMOΔhepa) are protected against Fas-mediated apoptosis. (A) Survival curve after i.p. injection of 0.5 µg/g Jo2, evidencing the resistance of NEMOΔhepa mice to Fas-mediated death. (B–D) Macroscopic view of livers 3 h after Jo2 (B), liver weight/body weight ratio (B), serum transaminases (C), and H&E staining (D) showed fulminant hepatitis in NEMOf/f mice after Jo2, whereas NEMOΔhepa remained unaffected. (E and F) TUNEL (E) and caspase 8 and 3 (F) activity analysis confirmed Jo2 apoptosis. Activity is represented in times versus untreated NEMOf/f. (G and H) Fas mRNA and IHC analysis in whole liver (G) and isolated primary hepatocytes (H) showed stronger expression in WT compared than in NEMOΔhepa mice. (I) TNF mRNA and cellular location indicated by IHC. (J) Western blotting showed phosphorylation of JNK1 (p45) and JNK2 (p54) in NEMOf/f mice after Jo2. JNK1 and GAPDH act as loading controls. Bars, 50 µm. Data are representative of three independent experiments. n = 4. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (NEMOf/f vs. NEMOΔhepa). §§, P < 0.01; §§§, P < 0.001 (NEMOf/f vs. Jo2/NEMOf/f). Error bars represent SD.
These data may seem contradictory to reports showing that NF-κB inhibition sensitizes hepatocytes to Fas apoptosis (Hatano et al., 2000). However, as we previously described, NF-κB is one of the main factors responsible for activating Fas transcription (Kuhnel et al., 2000). Thus, we hypothesized that NEMOΔhepa mice might have a defective turnover of this receptor, thereby promoting resistance to Jo2 apoptosis. In agreement with this, NEMOΔhepa mice exhibited reduced levels of Fas (25% less) and impaired up-regulation of Fas after Jo2 (Fig. 1 G). IHC confirmed these results, as Fas staining was almost negative on NEMOΔhepa mice after Jo2 (Fig. 1 G). Messenger RNA (mRNA) analysis on isolated primary hepatocytes corroborated the inability of NEMOΔhepa mice to express Fas (Fig. 1 H), which led to impaired NF-κB activation on these cells (not depicted).
Additionally, in accordance with the observation that Jo2/Fas induces TNF expression through NF-κB activation (Lu et al., 2002), we found a strong up-regulation of TNF in NEMOf/f mice after Jo2 (43-fold), whereas it was barely regulated in NEMOΔhepa mice (Fig. 1 I). IHC on liver sections confirmed macrophages as the main source of TNF after Fas activation, which likely explains the modest TNF regulation observed in NEMOΔhepa mice (Fig. 1 I).
Finally, NEMOf/f mice displayed strong c-Jun N-terminal kinase (JNK) phosphorylation, whereas it remained inactive in NEMOΔhepa mice after Jo2 (Fig. 1 J), supporting the absence of cellular stress in our KO animals upon Fas activation. These results suggest that Fas is not an essential mediator of the pathogenesis observed in NEMOΔhepa mice.
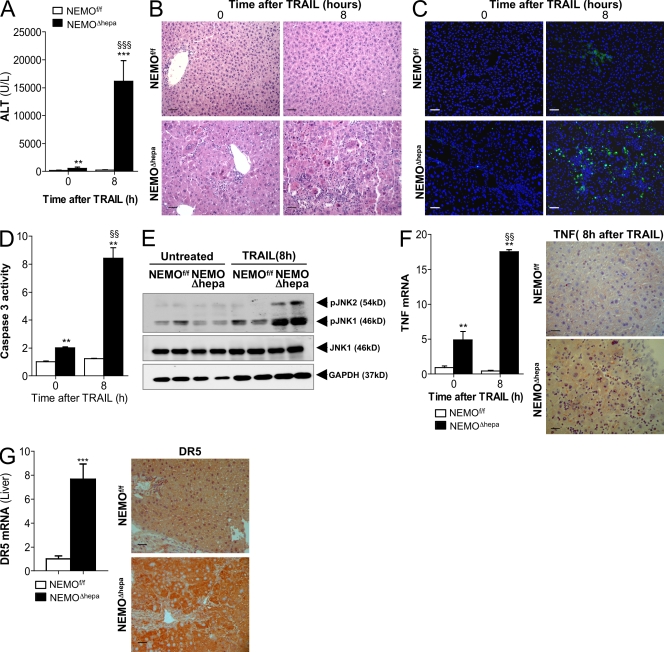
Thus, we next investigated the potential implication of TRAIL in the initiation and progression of chronic liver damage in our NEMO-deficient mice. As expected, low doses of TRAIL had no effect on NEMOf/f mice, as ALT levels and liver architecture remained unaffected (Fig. 2, A and B). In clear contrast, TRAIL had a strong impact on NEMOΔhepa mice, as a significant rise in serum ALT and massive liver damage, indicated by profuse PMN infiltration, necrosis, and apoptosis, were observed (Fig. 2, A and B). TUNEL assay and caspase 3 activity confirmed the severity of TRAIL-mediated apoptosis on NEMOΔhepa mice (Fig. 2, C and D). Previous work indicated that TRAIL induced JNK activation, leading to cellular apoptosis (Herr et al., 1999). Accordingly, we found that TRAIL promoted strong JNK activation in NEMOΔhepa livers, whereas it remained inactive in NEMOf/f mice (Fig. 2 E). Moreover, TRAIL administration promoted a significant induction of TNF in NEMOΔhepa mice that was confirmed by IHC, uncovering the Kupffer cells as its main source (Fig. 2 F). These results suggest that hepatocyte-NEMO deletion promotes hypersensitivity to TRAIL-mediated apoptosis; hence, we next attempted to elucidate the potential mechanisms underlying this effect.
Figure 2.
NEMOΔhepa mice are sensitive to low doses of TRAIL-mediated liver injury. 25 mg/kg of flagged TRAIL was administered i.v. (A) ALT serum levels were significantly elevated in NEMOΔhepa mice after TRAIL. (B–D) H&E staining (B), TUNEL assay (C), and caspase 3 activity (D) showed TRAIL-mediated liver damage and apoptosis in NEMOΔhepa mice. (E) Western blot analysis showed JNK phosphorylation. JNK1 and GAPDH were used as loading controls. (F) RT-PCR analysis of TNF mRNA and IHC showed higher expression in NEMOΔhepa mice. (G) mRNA analysis and IHC evidenced strong DR5 expression in NEMOΔhepa livers compared with NEMOf/f. Bars, 50 µm. All data are representative of three independent experiments. n = 4. **, P < 0.01; ***, P < 0.001 (NEMOf/f vs. NEMOΔhepa). §§, P < 0.01; §§§, P < 0.001 (NEMOΔhepa vs. TRAIL/NEMOΔhepa). Error bars represent SD.
Interestingly, DR5 was highly expressed in livers of untreated NEMOΔhepa mice, where it was mainly present in hepatocytes (Fig. 2 G). This was confirmed in vitro, where NEMOΔhepa hepatocytes showed an eightfold increase in DR5 expression and a complete inability to activate NF-κB in response to TRAIL in contrast to WT cells (Fig. S2, A and B). Accordingly with our in vivo data, WT cells remained unaffected, whereas TRAIL induced JNK activation on hepatocytes lacking NEMO, leading to strong apoptotic cell death which was significantly abrogated by JNK inhibition with SP600125 (Fig. S2, C and D). The protection elicited by JNK inhibition, despite the absence of NF-κB in NEMO-deficient hepatocytes, suggests an essential role of DR5-JNK signaling during TRAIL-induced cellular damage in NEMOΔhepa mice.
NK cells are the main producers of TRAIL in the liver (Smyth et al., 2001; Takeda et al., 2001). Earlier studies demonstrated that genetically or dietary-induced fatty liver disease promotes activation of these cells (Guebre-Xabier et al., 2000; Li et al., 2005). Thus, we hypothesized that similar alterations in the innate immune system might also arise in livers of NEMOΔhepa mice.
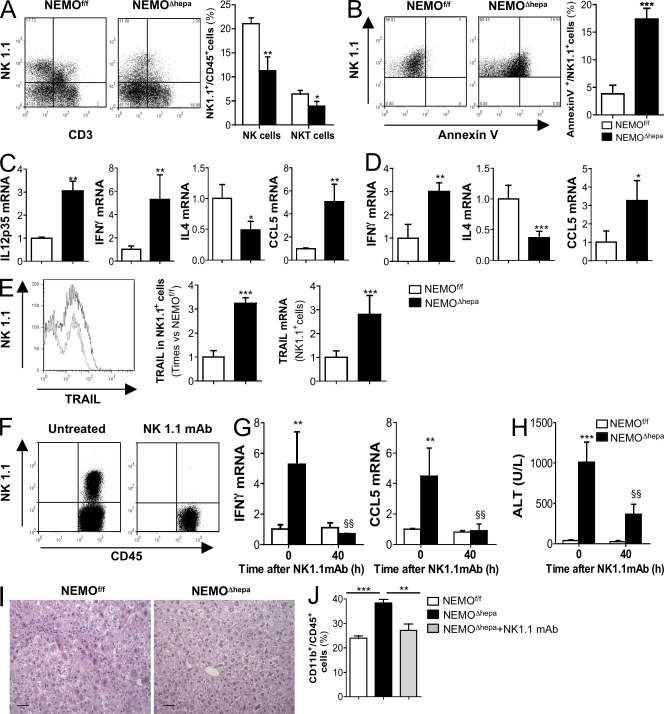
NEMOΔhepa livers had significantly reduced numbers of NK and NKT cells compared with NEMOf/f littermates (Fig. 3 A). As NK cells' activation is associated with their apoptosis, the lower NK1.1+ cell number found in NEMOΔhepa livers could reflect their activation. Hence, NK1.1+ cells from NEMOΔhepa mice exhibited significantly higher levels of annexin V when compared with NEMOf/f mice (Fig. 3 B). Activation of NK cells involves strong production of proinflammatory cytokines, like IFN-γ and IL-12, promoting their cytotoxic activity and apoptosis (Vivier et al., 2008). Accordingly, we found higher IFN-γ and IL-12 (p35) expression associated with lower IL-4 levels in NEMOΔhepa mice compared with WT littermates. Additionally, higher CCL5 (RANTES) levels found in NEMOΔhepa livers (Fig. 3 C) may reflect a compensatory response to restore liver NK cells number (Morris and Ley, 2004). These data were confirmed on isolated NK1.1+ cells from livers of NEMOΔhepa mice showing higher IFN-γ and CCL5 but lower IL4 levels (Fig. 3 D). FACS and mRNA analysis evidenced strong TRAIL expression on liver NK1.1+ cells from NEMOΔhepa compared with WT mice (Fig. 3 E).
Figure 3.
Hepatocyte-specific deletion of NEMO promotes spontaneous activation of liver NK/NKT cells, and administration of NK1.1-depleting mAb attenuates the damaging phenotype in NEMOΔhepa mice. (A) FACS analysis revealed a lower number of NK1.1+CD3− and NK1.1+CD3+ cells in NEMOΔhepa mice. The graph represents the percentage of NK1.1+ cells related to the percentage of CD45+ cells in the liver (B) FACS analysis of NK1.1+/annexin V+ related to % of CD45+ cells revealed strong apoptosis of NK1.1+ cells in livers from NEMOΔhepa mice. (C) RT-PCR showed strong expression of IL-12 (p35), IFN-γ, and CCL5 but lower IL-4 mRNA in livers. Data are presented as times versus NEMOf/f untreated. (D) mRNA analysis of isolated NK1.1+ cells confirmed cell activation and increased cytokine expression. (E) FACS analysis showed stronger TRAIL expression on NK1.1+ cells from livers from NEMOΔhepa mice. mRNA analysis on isolated NK1.1+ cells confirmed this. (F and G) FACS analysis proved effective depletion of NK1.1+ cells (F) 40 h after NK1.1 mAb administration that reduced IFN-γ and CCL5 mRNA levels (G). (H–J) Serum ALT (H), H&E staining (I), and FACS analysis (J; CD11b+ cells) were used as markers of liver damage and inflammation. Bars, 50 µm. All data are representative of three independent experiments. n = 4. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (NEMOf/f vs. NEMOΔhepa). §§, P < 0.01 (NEMOΔhepa vs. Nk1.1mAb/NEMOΔhepa). Error bars represent SD.
These data suggest that hepatocyte-NEMO deletion correlates with an altered NK/NKT cell response. Moreover, the proclivity of TRAIL-producing NK cells to promote cytotoxicity against self-hepatocytes (Ochi et al., 2004) appears to be enhanced in NEMOΔhepa mice, suggesting that this may represent a potential mechanism involved in the chronic inflammation and parenchymal damage observed in NEMOΔhepa mice.
To test this hypothesis, we investigated the potential beneficial effects of NK/NKT cell inactivation in NEMOΔhepa mice by using a NK1.1 mAb (PK-136). FACS and mRNA analysis confirmed efficient cell depletion after NK1.1 mAb administration (Fig. 3 F) that attenuated IFN-γ and CCL5 expression, correlating with strong amelioration of liver damage in NEMOΔhepa mice (Fig. 3, H and I). FACS analysis confirmed the attenuation of the proinflammatory environment in NK1.1 mAb-treated NEMOΔhepa mice, as a significant reduction of CD11b+ cells was detected (Fig. 3 J).
Based on our present data, which identify the critical detrimental role of NK1.1+ cells in the pathogenesis of liver injury in NEMOΔhepa mice (Fig. 3), we further characterized the contribution of these cells during concanavalin A (ConA) hepatitis, where NKT cells activation have a major implication (Toyabe et al., 1997; Takeda et al., 2000).
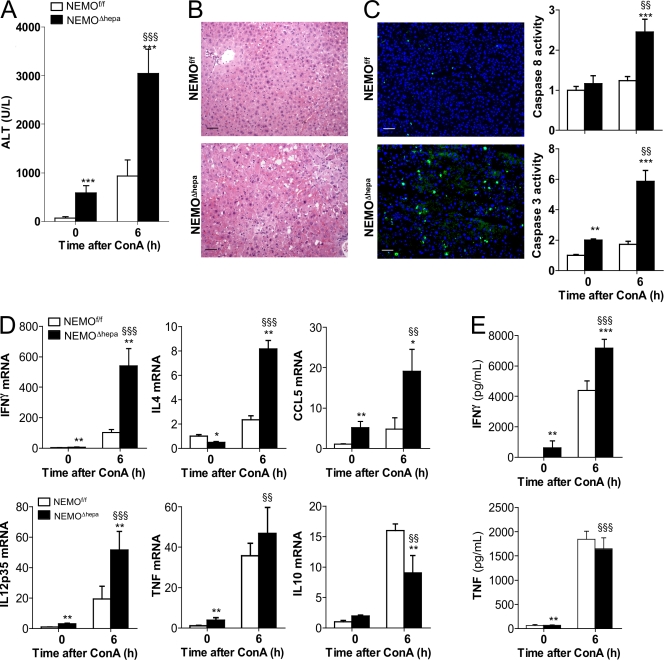
Administration of 15 mg/kg ConA caused 100% mortality of NEMOΔhepa mice within 16 h, whereas all NEMOf/f mice survived (Fig. S3). To better define the molecular mechanism leading to the mortality of NEMOΔhepa mice, we next administered 25 mg/kg ConA and sacrificed the animals 6 h after injection. ConA triggered a more severe hepatitis in NEMOΔhepa mice compared to NEMOf/f littermates. Massive destruction of liver parenchyma in NEMOΔhepa mice were indicated by high ALT, strong inflammation, and necrotic and apoptotic cell death (Fig. 4, A–C). Moreover, ConA induced significantly higher levels of IFN-γ, IL-4, and CCL5 in NEMOΔhepa compared with NEMOf/f livers (Fig. 4 D). ELISA confirmed systemic IFN-γ elevation (Fig. 4 E).
Figure 4.
ConA promotes stronger liver damage in NEMOΔhepa mice than in NEMOf/f animals. (A–C) ALT serum levels (A), H&E staining and TUNEL analysis (B), and quantification of caspase 8 and 3 activity (C) measured 6 h after 25 mg/kg ConA showed strong liver damage in NEMOΔhepa mice. (D) mRNA levels of IFN-γ, IL-4, CCL5, IL-12p35, TNF, and IL-10. All RT-PCR data are presented as times versus NEMOf/f untreated. (E) ELISA of IFN-γ and TNF in serum before and 6 h after 25 mg/kg ConA. Bars, 50 µm. All data are representative of three independent experiments. n = 4. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (NEMOf/f vs. NEMOΔhepa). §§, P < 0.01; §§§, P < 0.001 (NEMOΔhepa vs. ConA/NEMOΔhepa). Error bars represent SD.
Activated macrophages are the main producers of IL-12, which promotes NKT cell activation and further IFN-γ production (Trinchieri, 2003). This phenomenon may also occur in NEMOΔhepa mice, as IL12-p35 was significantly upregulated before ConA and was further increased following injury (Fig. 4 D).
Expression of the antiinflammatory cytokine IL-10, which negatively regulates TNF, IFN-γ, and IL-12 after ConA (Louis et al., 1997), was significantly lower in NEMOΔhepa, indicating their inability to orchestrate a proper antiinflammatory response to ConA hepatitis (Fig. 4 D). As expected, up-regulation of TNF was observed in WT and NEMOΔhepa mice after ConA but no significant differences were observed between genotypes (Fig. 4, D and E).
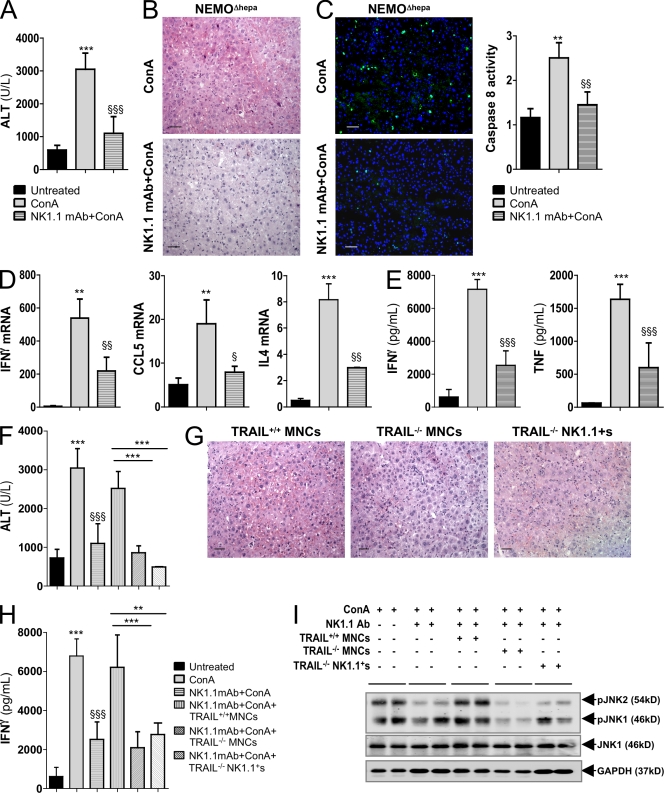
NK/NKT cell depletion lead to a remarkable improvement of liver parenchyma, indicated by lower ALT, necrosis, inflammation, and infiltration of PMNs (Fig. 5, A and B). Attenuation of ConA induced liver apoptosis after NK1.1 mAb treatment was confirmed by TUNEL assay and caspase 8 activity (Fig. 5 C). Accordingly, IFN-γ, CCL5, and IL-4 levels were lower after ConA in NK1.1-depleted NEMOΔhepa mice (Fig. 5 D), and systemic IFN-γ and TNF serum levels were significantly reduced (Fig. 5 E). Remnant cytokine expression despite NK1.1 mAb is likely explained by the fact that ConA promotes activation of other cell compartments like macrophages.
Figure 5.
NK1.1 cell depletion and adoptive transfer of TRAIL−/− MNCs protects NEMOΔhepa mice against ConA-mediated fulminant hepatitis. (A–C) ALT serum levels (A), H&E staining (B), TUNEL analysis, and quantification of caspase 8 (C), 6 h after 25 mg/kg ConA revealed strong attenuation of ConA hepatitis in NEMOΔhepa mice when NK1.1 mAb was administered 40 h before insult. (D and E) mRNA levels of IFN-γ, CCL5, and IL-4 (D) and ELISA of IFN-γ and TNF (E) on serum confirmed attenuation of ConA damage in NEMOΔhepa mice after NK1.1 mAb. (F–H) Adoptive transfer of TRAIL−/− MNCs and liver TRAIL−/− NK1.1+ cells after NK1.1 mAb protected while TRAIL+/+ MNC transfer restored ConA injury in NEMOΔhepa mice as shown by serum ALT (F), H&E staining (G), and ELISA (H) of IFN-γ serum levels. (I) ConA-mediated JNK activation was attenuated by NK1.1 mAb and restored by TRAIL+/+ MNCs adoptive transfer. TRAIL−/− cells maintained JNK inactivated. JNK1 and GAPDH act as loading control. Bars, 50 µm. All data are representative of three independent experiments. n = 4. **, P < 0.01; ***, P < 0.001 (NEMOΔhepa vs. ConA/NEMOΔhepa). §, P < 0.05; §§, P < 0.01; §§§, P < 0.001 (ConA/NEMOΔhepa vs. NK1.1mAb/ConA/NEMOΔhepa).
Recent work showing that TRAIL−/− mice are protected from ConA-induced liver injury highlighted the important role of TRAIL in this model (Zheng et al., 2004). Thus, we examined if this cytokine may be an essential mediator of ConA liver damage in our NEMOΔhepa mice. We performed intrahepatic adoptive transfer of splenic mononuclear cells (MNCs) from TRAIL−/− or TRAIL+/+ mice before ConA administration in NK1.1+-depleted mice. Liver damage was strongly attenuated in NEMOΔhepa mice receiving TRAIL−/− MNCs in contrast to TRAIL+/+ MNC transferred mice, where injury was restored (Fig. 5, F and G). Additionally, adoptive transfer of TRAIL+/+ MNCs into NK1.1-depleted NEMOΔhepa mice restored both liver and systemic IFN-γ expression after ConA, whereas it remained low in NEMOΔhepa mice receiving TRAIL−/− MNCs (Fig. 5 H). The essential implication of NK cell–derived TRAIL in mediating ConA hepatitis in NEMOΔhepa mice was further confirmed by adoptive transfer of isolated NK1.1+ cells from TRAIL−/− mice, which preserved liver protection against ConA injury elicited by NK1.1 mAb (Fig. 5, F–H).
During ConA-mediated hepatitis, JNK activation is related to the degree of apoptosis and the severity of the cellular stress (Trautwein et al., 1998). Additionally, we showed the detrimental effects of the TRAIL-JNK cascade in NEMOΔhepa mice both in vivo and in vitro (Fig. 2 and Fig. S2). Accordingly, strong JNK activation was observed in NEMOΔhepa mice after ConA, whereas it was clearly blunted after NK1.1 mAb despite ConA administration (Fig. 5 I). JNK activation was restored after adoptive transfer of TRAIL+/+ MNCs in NEMOΔhepa livers after ConA despite NK1.1 mAb treatment. On the contrary, transfer of both TRAIL−/− MNCs and TRAIL−/− NK1.1+ cells maintained the resistance of NK1.1-depleted NEMOΔhepa mice to ConA-mediated JNK activation (Fig. 5 I).
Previous studies using a NK1.1 mAb described that NKT cells are an essential cell subset responsible for ConA-mediated liver injury as selective depletion of NK cells with an anti-asialo GM1 antibody had no impact on ConA hepatitis (Toyabe et al., 1997). However, our present data strongly support the hypothesis that NK cell–derived TRAIL plays a key role in the progression of ConA-induced liver damage in NEMOΔhepa mice. Accordingly, we observed that NK cell depletion with asialo-GM1 mAb significantly reduced ALT levels (2.17-fold; Fig. S4 A), a comparable effect to that observed with NK1.1 mAb (Fig. 5 A; 2.56-fold reduction). Accordingly, hematoxylin and eosin (H&E) and TUNEL assay revealed significant protection against ConA after NK cell depletion (Fig. S4, B and C), accompanied by reduced CCL5 in asialo-GM1–treated NEMOΔhepa mice. Interestingly, despite the clear protection elicited by NK cell depletion, we found high IL-4 mRNA expression in asialo-GM1 mAb–treated KOs (Fig. S4 D) suggesting remnant activation of NKT cells. Moreover, downregulation of serum IFN-γ levels supports the implication of this cytokine in the activation and production of TRAIL from NK cells in an autocrine fashion (Smyth et al., 2001; Takeda et al., 2001). This data strongly supports the essential role of TRAIL-producing NK cells in mediating the dramatic impact of ConA on NEMOΔhepa mice.
Our present study uncovers a new mechanism by which hepatocyte-specific NEMO deletion contributes to spontaneous liver inflammation and injury. We show that NEMO deletion sensitizes the liver to TRAIL-mediated apoptosis and this contributes to disease progression. We propose a dual mechanism consisting of up-regulation of DR5 on hepatocytes and the activation of TRAIL-producing NK cells to cause the detrimental effects of NEMO deletion in the liver. The first mechanism is supported by studies showing that steatosis and free fatty acids promote up-regulation of DR5 mRNA expression (Malhi et al., 2007). Additionally, the profuse bile acid accumulation found in livers of NEMOΔhepa mice (unpublished data) may further promote DR5 expression (Higuchi et al., 2004). In addition to increased DR5 expression, fatty liver disease also triggers NKT cell activation, leading to a proinflammatory cytokine response (Li et al., 2005), which we also observed in our NEMOΔhepa mice.
Additionally, it has been shown that NKT cells promote NK cell activation and TRAIL production through IFN-γ expression (Smyth et al., 2001). At present, the relevance of intestinal bacterial products transported into the liver through the blood stream and its role in NK and NKT cell activation is unclear in this model. However, this mechanism could also be relevant, as LPS induces macrophages to produce IL-12, triggering IFN-γ production by NKT cells in the absence of IL-4 (Dobashi et al., 1999; Guebre-Xabier et al., 2000). This scenario closely resembles the liver environment found in untreated NEMOΔhepa mice.
Our present work strengthens the essential role of hepatocyte NEMO/NF-κB to maintain liver homeostasis because absence of NEMO in these cells activates the innate immune system, thus promoting liver damage. The cascade of molecular events leading from steatohepatitis to fibrosis and tumor development observed in NEMOΔhepa mice reflects the pathogenesis of liver cirrhosis and HCC in humans. Further work in this model could yield novel therapeutic strategies targeting the NK cell/TRAIL axis to modulate chronic liver disease progression.
MATERIALS AND METHODS
Generation of hepatocyte-specific conditional NEMO KO mice.
We generated mice carrying the loxP site–flanked (floxed [f]) NEMO gene (NEMOf/f) as described in detail in the supplemental figures. We generated NEMOΔhepa mice by crossing NEMOf/f with alfp-cre transgenic animals. For the experiments described, we used 8-wk-old male (Jo2 and TRAIL [provided by S. Bronk, Mayo Clinic College of Medicine, Rochester, MN) and female (NK1.1 mAb and ConA) mice. Animals were treated according to the guidelines of the National Academy of Sciences (National Institutes of Health publication 86-23, revised 1985). Animal husbandry and procedures were approved by the authority for environment conservation and consumer protection of the state North Rhine–Westfalia (LANUV) and the University Hospital Aachen Animal Care Facility's guidelines.
Models of liver injury.
Jo2 (0.5 µg/g of body weight; BD) was injected i.p. and mice were sacrificed 3 h later. ConA (Sigma-Aldrich) was administered i.v. for either 6 h (15 mg/kg of body weight) or 24 h (25 mg/kg). Flagged TRAIL (Higuchi et al., 2002) was administered i.v. at a concentration of 25 mg/kg.
Analysis of gene and serum protein expression.
RNA was isolated with PeqGold-RNA pure kit (PEQLAB) from livers. Quantitative real-time PCR was performed using SYBR green reagent (Invitrogen) in a 7300 Real-Time PCR system (Applied Biosystems). GAPDH was used to normalize gene expression, which is represented as times versus NEMOf/f basal expression. Liver and serum TNF and serum IFN-γ levels were quantified by ELISA (R&D systems and BD, respectively).
Western blotting.
Immunoblotting on liver protein extracts were performed with IKKγ/NEMO, p65, and JNK1 (Santa Cruz Biotechnology Inc.), phospho-JNK1/JNK2 (Tyr183/185; Invitrogen), and GAPDH antibody (Biogenesis).
Histological evaluation and apoptosis determination.
H&E staining was performed on paraffin-embedded liver sections. Apoptosis was evaluated by TUNEL assay performed on frozen liver sections using the in situ cell death detection kit (Roche) according to manufacturer's instructions. Caspase 8 and 3 activity were quantified on liver protein extracts as previously described (Luedde et al., 2007). DR5 detection on liver sections was performed by IHC using a DR5 antibody (ProScience), followed by Cy-3–labeled secondary antibody (Jackson ImmunoResearch Laboratories) or by the polyclonal Enhanced Vision reaction (Dako). TNF IHC was performed on frozen sections using rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc.).
NK1.1+ cell depletion.
NK1.1 antibody (250 µg/mouse i.p.) was purified from hybridoma PK-136 (American Type Culture Collection) culture supernatants. Depletion efficiency was determined by FACS analysis 40 h after administration. NK cell–specific depletion was obtained by i.p. administration of 250 µg Asialo-GM1 mAb (Wako Chemicals USA, Inc.) per mouse.
MNC isolation, adoptive transfer, and flow cytometric analysis.
Liver and splenic MNCs were obtained as previously described (Zheng et al., 2004) with small modifications. In brief, MNCs were isolated by collagenase digestion and erythrocytes were removed using PharmLyse lysing buffer (BD). MNCs were purified by density gradient centrifugation using Lymphocyte (LSM 1077; PAA). 2 × 107 splenic MNCs were injected in the lateral left liver lobe of NEMOΔhepa mice using a 29-gauge needle 1 h before i.v. administration of 25 mg/kg ConA.
Flow cytometry analysis and cell sorting.
Cells were stained with CD45-APC-Cy7, NK1.1-PE-Cy7, CD11b-PE, annexin V–FITC (BD), and CD3-APC (eBioscience). All samples were analyzed by flow cytometry (FACS Canto II; BD). For NK cell isolation, livers cells were isolated, stained, and purified by high speed sorting using a FACSAria (BD). CD45+NK1.1+ cells were further analyzed or transferred into animals while CD45+CD19+ cells were discarded. Purity of isolated NK1.1+ cells was >98%.
Statistical analysis.
Data are expressed as mean ± standard deviation of the mean. Statistical significance was determined by two-way analysis of variance followed by a Student's t test.
Online supplemental material.
Fig. S1 shows the gene-targeting strategy for the generation of hepatocyte-specific NEMO KO mice. Fig. S2 demonstrates the effect of TRAIL on primary hepatocytes. Fig. S3 shows the survival curve after ConA treatment. Fig. S4 shows the effects of asialo-GM1 mAb administration during ConA hepatitis. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082152/DC1.
Acknowledgments
We want to thank Steve Bronk for providing us with the TRAIL protein. We also want to thank Sara Dutton Sackett for editing the manuscript.
This work was supported by the SFB542, C15.
The authors declare that they have no competing financial interests.
Footnotes
Abbreviations used: ConA, concanavalin A; HCC, hepatocellular carcinoma; IHC, immunohistochemistry; JNK, c-Jun N-terminal kinase; MNC, mononuclear cell; mRNA, messenger RNA; TRAIL, TNF-related apoptosis-inducing ligand.
References
- Beraza N., Luedde T., Assmus U., Roskams T., Vander Borght S., Trautwein C. 2007. Hepatocyte-specific IKK gamma/NEMO expression determines the degree of liver injury.Gastroenterology 132:2504–2517 [DOI] [PubMed] [Google Scholar]
- Dobashi H., Seki S., Habu Y., Ohkawa T., Takeshita S., Hiraide H., Sekine I. 1999. Activation of mouse liver natural killer cells and NK1.1(+) T cells by bacterial superantigen-primed Kupffer cells.Hepatology. 30:430–436 [DOI] [PubMed] [Google Scholar]
- Guebre-Xabier M., Yang S., Lin H.Z., Schwenk R., Krzych U., Diehl A.M. 2000. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage.Hepatology. 31:633–640 [DOI] [PubMed] [Google Scholar]
- Hatano E., Bradham C.A., Stark A., Iimuro Y., Lemasters J.J., Brenner D.A. 2000. The mitochondrial permeability transition augments Fas-induced apoptosis in mouse hepatocytes.J. Biol. Chem. 275:11814–11823 [DOI] [PubMed] [Google Scholar]
- Herr I., Wilhelm D., Meyer E., Jeremias I., Angel P., Debatin K.M. 1999. JNK/SAPK activity contributes to TRAIL-induced apoptosis.Cell Death Differ. 6:130–135 [DOI] [PubMed] [Google Scholar]
- Higuchi H., Bronk S.F., Taniai M., Canbay A., Gores G.J. 2002. Cholestasis increases tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-R2/DR5 expression and sensitizes the liver to TRAIL-mediated cytotoxicity.J. Pharmacol. Exp. Ther. 303:461–467 [DOI] [PubMed] [Google Scholar]
- Higuchi H., Grambihler A., Canbay A., Bronk S.F., Gores G.J. 2004. Bile acids up-regulate death receptor 5/TRAIL-receptor 2 expression via a c-Jun N-terminal kinase-dependent pathway involving Sp1.J. Biol. Chem. 279:51–60 [DOI] [PubMed] [Google Scholar]
- Kellendonk C., Opherk C., Anlag K., Schutz G., Tronche F. 2000. Hepatocyte-specific expression of Cre recombinase.Genesis. 26:151–153 [DOI] [PubMed] [Google Scholar]
- Kuhnel F., Zender L., Paul Y., Tietze M.K., Trautwein C., Manns M., Kubicka S. 2000. NFkappaB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis.J. Biol. Chem. 275:6421–6427 [DOI] [PubMed] [Google Scholar]
- Li Z., Soloski M.J., Diehl A.M. 2005. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease.Hepatology. 42:880–885 [DOI] [PubMed] [Google Scholar]
- Louis H., Le Moine O., Peny M.O., Quertinmont E., Fokan D., Goldman M., Deviere J. 1997. Production and role of interleukin-10 in concanavalin A-induced hepatitis in mice.Hepatology. 25:1382–1389 [DOI] [PubMed] [Google Scholar]
- Lu B., Wang L., Medan D., Toledo D., Huang C., Chen F., Shi X., Rojanasakul Y. 2002. Regulation of Fas (CD95)-induced apoptosis by nuclear factor-kappaB and tumor necrosis factor-alpha in macrophages.Am. J. Physiol. Cell Physiol. 283:C831–C838 [DOI] [PubMed] [Google Scholar]
- Luedde T., Beraza N., Kotsikoris V., Van Loo G., Nenci A., De Vos R., Roskams T., Trautwein C., Pasparakis M. 2007. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma.Cancer Cell. 11:119–132 [DOI] [PubMed] [Google Scholar]
- Luo J.L., Maeda S., Hsu L.C., Yagita H., Karin M. 2004. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression.Cancer Cell. 6:297–305 [DOI] [PubMed] [Google Scholar]
- Luo J.L., Kamata H., Karin M. 2005. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy.J. Clin. Invest. 115:2625–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H., Barreyro F.J., Isomoto H., Bronk S.F., Gores G.J. 2007. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity.Gut. 56:1124–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M.A., Ley K. 2004. Trafficking of natural killer cells.Curr. Mol. Med. 4:431–438 [DOI] [PubMed] [Google Scholar]
- Ochi M., Ohdan H., Mitsuta H., Onoe T., Tokita D., Hara H., Ishiyama K., Zhou W., Tanaka Y., Asahara T. 2004. Liver NK cells expressing TRAIL are toxic against self hepatocytes in mice.Hepatology. 39:1321–1331 [DOI] [PubMed] [Google Scholar]
- Ogasawara J., Watanabe-Fukunaga R., Adachi M., Matsuzawa A., Kasugai T., Kitamura Y., Itoh N., Suda T., Nagata S. 1993. Lethal effect of the anti-Fas antibody in mice.Nature. 364:806–809 [DOI] [PubMed] [Google Scholar]
- Rodriguez C.I., Buchholz F., Galloway J., Sequerra R., Kasper J., Ayala R., Stewart A.F., Dymecki S.M. 2000. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP.Nat. Genet. 25:139–140 [DOI] [PubMed] [Google Scholar]
- Schutze S., Tchikov V., Schneider-Brachert W. 2008. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization.Nat. Rev. Mol. Cell Biol. 9:655–662 [DOI] [PubMed] [Google Scholar]
- Smyth M.J., Cretney E., Takeda K., Wiltrout R.H., Sedger L.M., Kayagaki N., Yagita H., Okumura K. 2001. Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) contributes to interferon γ–dependent natural killer cell protection from tumor metastasis.J. Exp. Med. 193:661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Hayakawa Y., Van Kaer L., Matsuda H., Yagita H., Okumura K. 2000. Critical contribution of liver natural killer T cells to a murine model of hepatitis.Proc. Natl. Acad. Sci. USA. 97:5498–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Hayakawa Y., Smyth M.J., Kayagaki N., Yamaguchi N., Kakuta S., Iwakura Y., Yagita H., Okumura K. 2001. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells.Nat. Med. 7:94–100 [DOI] [PubMed] [Google Scholar]
- Toyabe S., Seki S., Iiai T., Takeda K., Shirai K., Watanabe H., Hiraide H., Uchiyama M., Abo T. 1997. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice.J. Immunol. 159:1537–1542 [PubMed] [Google Scholar]
- Trautwein C., Rakemann T., Brenner D.A., Streetz K., Licato L., Manns M.P., Tiegs G. 1998. Concanavalin A-induced liver cell damage: activation of intracellular pathways triggered by tumor necrosis factor in mice.Gastroenterology. 114:1035–1045 [DOI] [PubMed] [Google Scholar]
- Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity.Nat. Rev. Immunol. 3:133–146 [DOI] [PubMed] [Google Scholar]
- Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. 2008. Functions of natural killer cells.Nat. Immunol. 9:503–510 [DOI] [PubMed] [Google Scholar]
- Zheng S.J., Wang P., Tsabary G., Chen Y.H. 2004. Critical roles of TRAIL in hepatic cell death and hepatic inflammation.J. Clin. Invest. 113:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C., Ma J., Wang X., Guo L., Zhu Z., Stoops J., Eaker A.E., Johnson C.J., Strom S., Michalopoulos G.K., et al. 2007. Lack of Fas antagonism by Met in human fatty liver disease.Nat. Med. 13:1078–1085 [DOI] [PubMed] [Google Scholar]