Abstract
Schistosoma mansoni eggs contain factors that trigger potent Th2 responses in vivo and condition mouse dendritic cells (DCs) to promote Th2 lymphocyte differentiation. Using an in vitro bystander polarization assay as the readout, we purified and identified the major Th2-inducing component from soluble egg extract (SEA) as the secreted T2 ribonuclease, omega-1. The Th2-promoting activity of omega-1 was found to be sensitive to ribonuclease inhibition and did not require MyD88/TRIF signaling in DCs. In common with unfractioned SEA, the purified native protein suppresses lipopolysaccharide-induced DC activation, but unlike SEA, it fails to trigger interleukin 4 production from basophils. Importantly, omega-1–exposed DCs displayed pronounced cytoskeletal changes and exhibited decreased antigen-dependent conjugate formation with CD4+ T cells. Based on this evidence, we hypothesize that S. mansoni omega-1 acts by limiting the interaction of DCs with CD4+ T lymphocytes, thereby lowering the strength of the activation signal delivered.
CD4+ T cells consist of three major subsets (Th1, Th2, and Th17) that display distinct functions in both host defense and autoimmunity (Zhu and Paul, 2008). The delineation of the signals that trigger differentiation of these CD4 subpopulations is important for the development of interventions based on selective induction or ablation of specific Th responses. The presence of polarizing cytokines at the time of initial CD4+ T cell activation is considered to be a primary factor influencing Th phenotype (Zhu and Paul, 2008). Nevertheless, under neutral conditions the strength of TCR stimulation can play a critical role in determining Th1 versus Th2 cell differentiation (Constant et al., 1995; Hosken et al., 1995). A major approach for studying the basis of Th effector choice has been the analysis of pathogen-induced immune responses that polarize toward Th1, Th2, or Th17 phenotypes (Abbas et al., 1996; Ouyang et al., 2008).
Mouse infection with the trematode Schistosoma mansoni is an extensively characterized helminth model for studying Th2 polarization (Pearce et al., 2004). In this system, ova deposited by the parasites in host tissues rather than the worms provide the major Th2 stimulus. Importantly, a water-soluble egg extract (SEA) mimics the key effects of intact ova on Th2 induction. There is growing evidence that the Th2-polarizing activity of SEA is not caused by a direct effect on CD4 T lymphocytes but rather results from prior interaction with DCs. Thus, bone marrow–derived DCs (BMDCs) preincubated with SEA trigger potent egg-specific Th2 recall responses after transfer into naive animals (MacDonald et al., 2001). This function does not require DC secretion of IL-4 but instead depends on the expression of the co-stimulatory molecule CD40 (for review see Perona-Wright et al., 2006). In addition to promoting Th2 responses against itself, SEA can trigger bystander Th2 polarization of unrelated antigen in vivo (Pearce et al., 2004; Perona-Wright et al., 2006) and in vitro with mouse (Jankovic et al., 2004) or human (de Jong et al., 2002) cells, and in each of the latter assays, DCs play a critical role in determining the Th2 phenotype of the CD4+ T cells.
The mechanism by which helminth-exposed DCs trigger Th2 responses is unclear in terms of the recognition events and signaling pathways involved. Indeed, after SEA exposure only minimal gene activation is observed in DCs, in contrast to the potent and extensive induction seen after Toll-like receptor (TLR)–initiated/MyD88-dependent stimulation (Kane et al., 2004). Moreover, SEA has been shown to actually suppress DC responses to TLR ligands (Jankovic et al., 2004; Kane et al., 2004), raising the possibility that its major effects are inhibitory rather than stimulatory.
Our understanding of the mechanism by which S. mansoni eggs promote Th2 responses has been enormously hindered by a lack of information concerning the identity of the parasite molecules that act on DCs to trigger Th2 polarization. SEA is a complex mixture of hundreds of proteins and glycoconjugates that includes structural components of the miracidial embryo as well as its secretory products. Early work implicated LewisX carbohydrate side chains (Thomas et al., 2003) and complex-type N-glycans (Faveeuw et al., 2003) found on multiple schistosome glycoproteins as important Th2 immunomodulatory moieties. In terms of single molecules, IPSE/alpha-1 and peroxiredoxin trigger IgE-dependent IL-4 secretion by basophils (Schramm et al., 2003) and induce alternatively activated macrophages (Donnelly et al., 2008), respectively, but neither are known to directly interact with DCs.
In the present report we performed a classical biochemical purification of the DC-dependent Th2-polarizing activity in SEA and showed that it can be attributed to a single egg-secreted glycoprotein with ribonuclease activity, S. mansoni T2 ribonuclease/omega-1 (Fitzsimmons et al., 2005). We further demonstrate that purified omega-1 directly affects both DC morphology and the ability of these APCs to interact physically with CD4 T lymphocytes. Based on this evidence, we propose that SEA and its omega-1 bioactive component promote Th2 polarization by lowering the strength of the activation signal delivered by DCs.
RESULTS AND DISCUSSION
Ribonuclease T2/omega-1 is the major component in S. mansoni eggs that promotes Th2 polarization in vitro
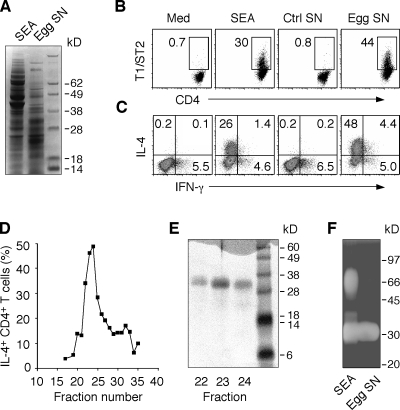
To identify the egg components that are the triggers and not merely the targets of the Th2 response, we used an in vitro bystander Th polarization assay using OVA-specific transgenic (Tg) CD4+ T lymphocytes that reads out the effects of egg products on DCs (Jankovic et al., 2004). In preliminary studies, the activity in SEA that stimulates DC-dependent Th2 polarization of DO.11.10 Tg lymphocytes was shown to be protease sensitive (Fig. S1 A), and upon gel filtration to be confined to the 10–50-kD region of the eluate with the strongest activity correlating with the presence of a band of ∼30 kD in SDS-PAGE (Fig. S1, B and C). Further purification of the active component was hindered by the heterogeneity and abundance of the proteins in this size range within SEA. To circumvent this problem, we used egg culture supernatants that contain the major secretory products of the miracidial embryo and represent a subset of the total proteins in SEA (Fig. 1 A). These supernatants were highly active in the polarization assay triggering the differentiation of CD4+ T lymphocytes expressing T1/ST2, a marker of Th2 cells (Fig. 1 B), as well as IL-4 production (Fig. 1 C). Gel filtration of this material yielded a single sharp peak of activity (Fig. 1 D) containing a 32-kD protein (Fig. 1 E), which when eluted from SDS-PAGE gave a 13–amino acid N-terminal sequence (QNRWDYYVFSVTxP) identical to that of the previously characterized egg secretory protein omega-1, a T2 ribonuclease (Fitzsimmons et al., 2005). In agreement, both SEA and egg supernatants contained a 32-kD molecule with this enzymatic activity (Fig. 1 F).
Figure 1.
S. mansoni egg culture supernatants promote bystander Th2 polarization. (A) Protein composition of SEA and egg supernatants evaluated by Coomassie blue–stained SDS-PAGE. (B and C) Naive DO.11.10 Tg CD4+ lymphocytes were cultured with DC and OVA peptide with or without 40 µg/ml SEA, 30 µg/ml egg supernatant, or control supernatants from mock cultures containing no eggs. After restimulation with PMA/ionomycin, cells were stained for CD4 and T1/ST2 (B) or CD4 and IL-4 plus IFN-γ (C), respectively. The FACS dot plots shown are gated on CD4+ T lymphocytes (percentages are shown). The experiment shown is representative of more than five performed. (D) The frequency of IL-4+ DO.11.10 T cells determined by intracellular cytokine staining (ICS) in response to gel filtration fractions of egg culture supernatants. (E) Coomassie blue–stained SDS-PAGE of fractions 22–24 from the column shown in D. Results are representative of two independent experiments performed with different egg culture supernatant preparations. (F) In situ ribonuclease activity of a 32-kD protein in both SEA and egg supernatants detected by zymogram gel.
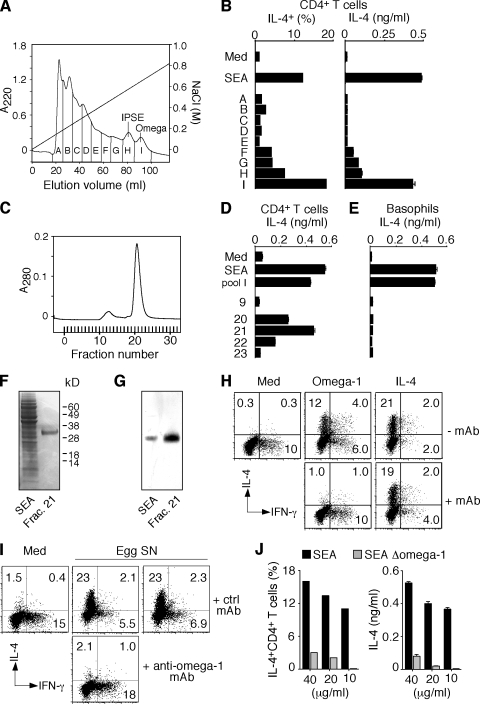
To confirm that omega-1 is also the key molecule in SEA with this activity, we took advantage of the basic charge of omega-1 to separate the protein within this crude extract (Fig. 2 A). We again observed that the major Th2-promoting fraction contained omega-1 (Fig. 2 B). Additional purification by gel filtration yielded a single protein (Fig. 2, C, D, and F), the identity of which was confirmed by its reactivity in Western blotting with a mAb (140-3E11) generated against recombinant omega-1 (Fig. 2 G). Importantly, the same mAb neutralized the in vitro Th2-polarizing activity of purified omega-1 (Fig. 2 H), as well as that of unfractionated egg culture supernatants (Fig. 2 I). Moreover, SEA chemically depleted of omega-1 failed to trigger Th2 polarization (Fig. 2 J) in the same assay. Finally, adoptively transferred DCs (but not B cells) preincubated with the purified protein stimulated Th2 responses comparable to SEA-pretreated DCs (Fig. 3).
Figure 2.
Identification of omega-1 as the major component with Th2-inducing activity in SEA. (A) The elution profile of SEA from an SP-Sepharose column indicating the pools (pools A–I) tested for biological activity. The diagonal line indicates the gradient of NaCl. (B) Th2 response of DO.11.10 Tg cells induced by the different pools in A was measured by ICS and IL-4 secretion. The results from an assay performed at one fixed protein concentration (20 µg/ml) for each pool are shown, along with 40 µg/ml SEA as a positive control. Bars represent the percentage of IL-4+ CD4+ T lymphocytes, and the means ± SD of the IL-4 concentrations. The same pattern of activity was observed when three additional twofold serial dilutions were compared for each pool (not depicted). (C) The elution profile of pool I after separation on a Superdex 75 gel filtration column. (D and E) The indicated fractions were tested in Th2 polarization (D) and basophil (E) assays. Bars represent means ± SD for IL-4 measured by ELISA. (F) Coomassie blue–stained SDS-PAGE of SEA and fraction 21 from the column in C. (G) Western blot of SEA and fraction 21 developed with mAb specific for omega-1. (H and I) Flow cytometry of ICS performed on DO.11.10 Tg T cells stimulated with OVA/DCs in the presence of omega-1 or IL-4 preincubated with or without anti–omega-1 mAb (H) or in the presence of egg supernatants preincubated with control or anti–omega-1 mAb (I). The dot plots are gated on CD4+ T lymphocytes (percentages are shown). (J) Loss of Th2 activity in SEA depleted of omega-1 measured by ICS and IL-4 secretion in a DO.11.10 Tg T cell assay as described. The experiments shown in A–G and H–J are representative of two and three experiments, respectively.
Figure 3.
Omega-1–pulsed DCs promote Th2-type responses in vivo. BALB/c mice were injected s.c. with BMDCs unstimulated or incubated overnight with 30 µg/ml SEA, 2 µg/ml omega-1, or irradiated T. gondii (RH strain) tachyzoites (1:1). 1.5 × 106 popliteal LN cells/ml from individual animals (n = 3) were pooled by mixing equal numbers of cells from each animal within a group and stimulated with 0.2 µg/ml anti-CD3 mAb, 30 µg/ml SEA, 0.5 µg/ml omega-1, or 10 µg/ml of soluble tachyzoite antigen (STAg). Cytokines were measured by ELISA in 72-h supernatants. Data shown are means ± SD of ELISA values for each pool. ICS was performed after supernatant removal. When restimulated with SEA, the frequency of IL-4+ CD4+ T cells in pooled LN cultures from mice that received SEA- or omega-primed DCs was 14 and 8.8%, respectively, and when the same cultures were restimulated with omega-1 the frequencies were 7.2 and 7.9%. One representative experiment out of three performed is shown. When omega-1–pretreated B cells were injected instead of DCs, no immune response was detected (not depicted).
In addition to omega-1, SEA and egg supernatants contain a similarly charged glycoprotein, IPSE, which triggers IL-4 production from basophils (Schramm et al., 2003) and, therefore, could represent an additional Th2-polarizing factor. We purified IPSE from the same starting material and showed that although highly active in a basophil degranulation assay, the protein failed to promote Th2 differentiation of DO.11.10 Tg cells (Fig. 2 A and Fig. S2). Conversely, purified omega-1 did not trigger IL-4 secretion from basophils (Fig. 2 E). Collectively, these biochemical data revealed that omega-1, a secreted glycoprotein, is the key Th2-inducing egg component acting on DCs.
Inhibition of ribonuclease activity abolishes the Th2-polarizing function of omega-1
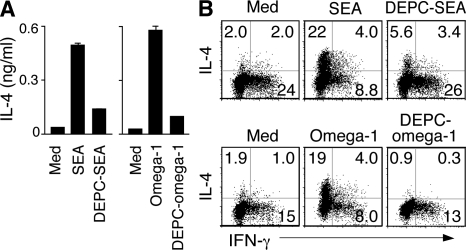
To determine whether the immunological function of omega-1 depends on its enzymatic activity, we treated the purified protein as well as SEA with diethyl pyrocarbonate (DEPC), an irreversible ribonuclease inhibitor that modifies histidine residues essential for the catalytic function of the enzyme. DEPC treatment simultaneously ablated the enzymatic (Fig. S3 A) and Th2-polarizing activities of both materials (Fig. 4) with no detected loss of omega-1 protein as assessed by Western blotting (Fig. S3 B). Although the loss in Th2 polarization seen after DEPC treatment of omega-1 argues that intact ribonuclease activity is required, the possibility that enzymatic inhibition affects other structural domains in omega-1 critical for its immunological function cannot be excluded.
Figure 4.
The Th2-polarizing function of both SEA and omega-1 is sensitive to ribonuclease inhibition. (A and B) Equal amounts of DEPC-treated or -untreated unfractionated SEA (40 µg/ml) or purified omega-1 (0.5 µg/ml) were added to the DC/DO.11.10 Tg CD4 T cell cultures, and IL-4 secretion was measured on day 3 by ELISA (A) and the frequency of IL-4+ cells was assayed by ICS on day 7 after restimulation with anti-CD3 mAb (B). Bars represent means ± SD for IL-4. The dot plots shown are gated on CD4+ T lymphocytes (percentages are shown). When DEPC-treated SEA or DEPC-treated omega-1 was added to the cultures in the presence of exogenous rIL-4, to evaluate possible nonspecific inhibitory effects of carryover DEPC, no decrease in the frequency of IL-4+ DO.11.10+ T cells was observed (not depicted). The data shown are representative of two experiments performed.
Omega-1 inhibits TLR ligand–induced DC activation
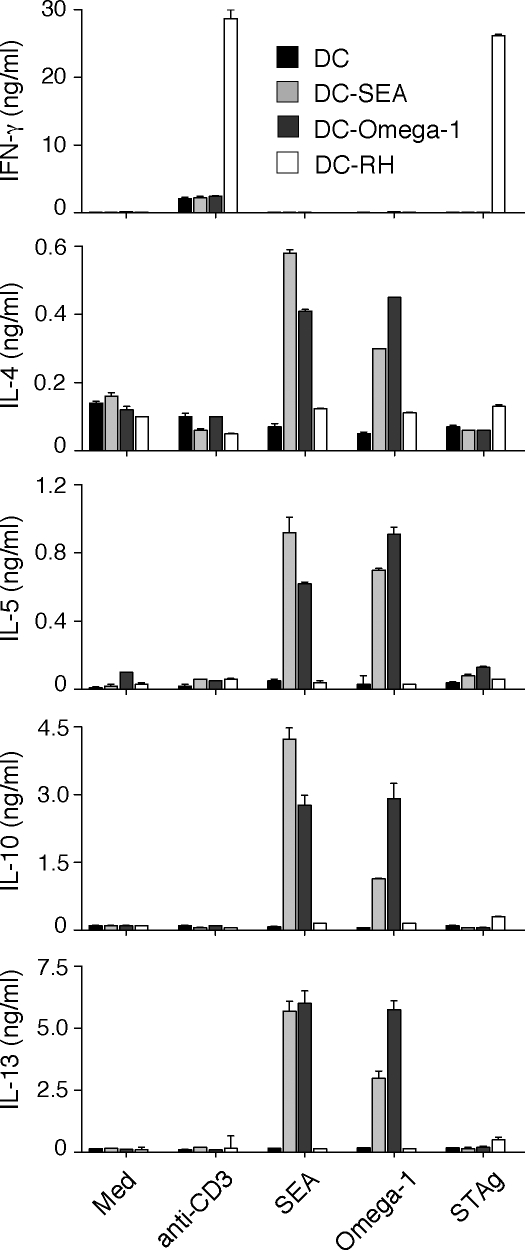
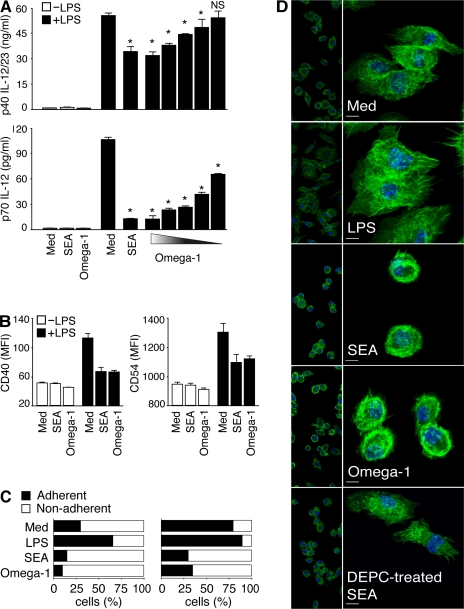
The Th2-polarizing activity of SEA is associated with down-regulation of TLR ligand–induced DC activation (Jankovic et al., 2004; Kane et al., 2004). When tested side-by-side with SEA, purified omega-1 at a 100-fold lower concentration displayed comparable inhibitory activity, suppressing LPS-triggered p40 IL-12/23 and p70 IL-12 secretion (Fig. 5 A), as well as the up-regulated expression of CD40 and CD54 by BMDCs (Fig. 5 B). Nevertheless, although clearly affecting TLR ligand stimulation, TLR signaling does not appear to be involved in the Th2-polarizing function of omega-1, because DCs deficient in both MyD88 and TRIF showed unimpaired omega-1–dependent Th2-inducing activity (Fig. S4 A).
Figure 5.
Omega-1 suppresses TLR ligand–mediated DC activation and induces cytoskeletal changes in DCs. (A and B) BMDCs were incubated in medium, 40 µg/ml SEA, or 1 µg/ml omega-1 with or without 40 ng/ml LPS. (A) Levels of p40 IL-12/23 and p70 IL-12 measured by ELISA. When tested in the presence of LPS, twofold dilutions of omega-1 (1–0.06 µg/ml) were assayed. Bars represent mean cytokine concentrations ± SD. *, P < 0.05; and NS refer to IL-12 secretion induced by LPS alone versus in the presence of SEA or omega-1. (B) Surface expression of CD40 and CD54 on DCs in the same cultures. Bars represent geometric mean fluorescences ± SD of two experiments. (C and D) BMDCs were cultured in medium, 40 ng/ml LPS, 40 µg/ml SEA, or 0.5 µg/ml omega-1 for 12 h. (C) The number of viable cells was determined in adherent versus nonadherent populations after culture on nontreated (left) or tissue culture–treated (right) plates. Bars indicate the frequency of cells in the two subpopulations for each culture condition. Results are from one representative experiment out of three performed. (D) CD11c+ BMDCs cultured on multichamber glass slides under the same conditions were stained with phalloidin (green) and DAPI (blue) and analyzed by confocal microscopy. (left) Images demonstrate the homogeneity of cell populations; (right) A zoom-in view, which is a projection of a z-stack. The photomicrographs shown are representative images from five fields examined in one experiment out of three performed. Bars, 5 µm. MFI, mean fluorescence intensity.
Omega-1 alters the adherence properties and morphology of DCs
Previous studies on SEA-treated DCs failed to reveal discrete changes in the expression of known activation markers (Jankovic et al., 2004; Kane et al., 2004), and we observed the same lack of response in omega-1–exposed DCs (Fig. 5 B and not depicted). Some T2 ribonucleases have been shown to alter cell morphology as a result of cytoskeletal changes (Roiz et al., 2006), and this prompted us to examine both SEA- and omega-1–treated DCs for overt changes in appearance and physical behavior. Indeed, SEA- or omega-1–pretreated BMDCs were found to be less adherent to plastic (Fig. 5 C) or glass surfaces (not depicted), as indicated by increased percentages of nonadherent DCs. Because SEA/omega-1 treatment did not significantly alter cell viability as assessed by propidium iodine (PI) staining, the decreases in adherence could not be attributed to nonspecific cytotoxicity (Fig. S4, B and C).
Because of the role of the cytoskeleton in cell adherence, we used phalloidin staining, a procedure that reveals actin-based filaments, to assess SEA- or omega-1–treated DCs for possible changes in cytoskeletal organization. As predicted, cells in untreated cultures showed the spread appearance and membrane-ruffling characteristic of adherent DCs, and this morphology was even more pronounced in LPS-treated cells (Fig. 5 D). In contrast, both SEA- and omega-1–exposed DCs showed no membrane ruffling and displayed a ring of intense actin staining in the cortical region of the cell. Importantly, DEPC treatment, which abolished the Th2-polarizing activity of SEA, also blocked its effects on actin-based DC morphology (Fig. 5 D).
Omega-1–exposed DCs display decreased conjugate formation with CD4+ T cells
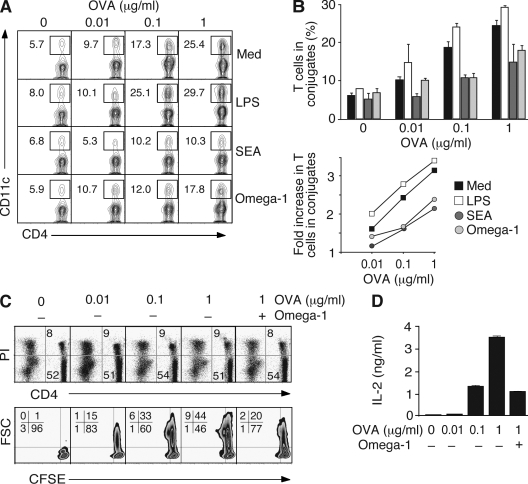
Previous studies demonstrating a role for CD40/CD40L in Th2 induction by SEA (MacDonald et al., 2002) and our experiments in which SEA/omega-1 treatment failed to induce Th2 cell differentiation when DCs were separated from CD4+ T cells in transwells (unpublished data) suggested that Th2 polarization in this system requires direct DC–T cell contact. Because the formation of immunological synapses depends on cytoskeletal polarization at the DC level (Al-Alwan et al., 2003), we hypothesized that modifications in actin distribution induced by omega-1 might result in altered DC–CD4+ T cell interaction. Accordingly, we tested the ability of omega-1–treated DCs to form stable conjugates with CD4+ T lymphocytes in the presence of nominal peptide. In the assay used, increases in peptide concentration lead to increased numbers of Th cells engaged in conjugate formation (Qi et al., 2008), and this interaction is further enhanced by LPS pretreatment of DCs (Fig. 6, A and B). In contrast, SEA- or omega-1–exposed DCs displayed decreased conjugate formation relative to untreated DCs. Importantly, this reduction was particularly evident at higher peptide concentrations, where the frequency of DC–T cell conjugates remained at levels similar to those seen with lower doses of peptide in the absence of any treatment. In agreement with previous findings (Kane et al., 2004), no changes in MHC class II expression were observed in SEA-/omega-1–treated DCs (not depicted), arguing against this explanation for reduced DC–T cell interaction.
Figure 6.
Omega-1–exposed DCs are less efficient in forming conjugates with CD4+ T cells. (A and B) BMDCs were cultured on nontreated plates with the same stimuli as in Fig. 5 C, pulsed with increasing amounts of OVA peptide, and incubated with OT-II Tg CD4+ T cells. The frequency of CD4+ T cell–forming conjugates with DC was measured by FACS. (A) A representative set of contour plots gated on CD4+ T lymphocytes (percentages are shown). (B) Quantification of T cells forming conjugates with DCs (means ± SD for duplicates) from one experiment (top). Fold increase in the frequency of OT-II Tg CD4+ T cells in conjugates with DCs was calculated as a function of peptide concentration for each type of DC based on the results from four independent experiments performed, of which two included omega-1 (bottom). (C and D) OT-II Tg T cells were cultured in the presence of DCs and increasing concentrations of OVA peptide for 36 h. Cultures with 1 µg/ml of peptide were tested with and without 0.5 µg/ml omega-1. (C) Viability of CD4+ T cells determined by PI staining of ungated populations (top). The contour plots gated on the CD4+ PI− population shows the percentage of cells that have completed the first cell cycle as determined by CFSE dilution (bottom). Cell size is indicated by forward scatter (FSC) on the y axis. (D) IL-2 in corresponding cultures measured by ELISA. Data shown are the mean concentrations ± SD. The data shown in C and D are representative of two experiments.
The above results suggested that by inhibiting the ability of DCs to form conjugates with T lymphocytes, omega-1 establishes a condition that mimics the effects of low-dose antigen, a well-characterized setting favoring Th2 polarization (Constant et al., 1995; Hosken et al., 1995). Consistent with this hypothesis, omega-1 in the presence of an optimal dose of peptide suppressed T cell activation to the level observed with lower peptide concentrations, as evidenced both by the reduced frequency of CD4+ T cells completing the first cell cycle and by their decreased IL-2 secretion (Fig. 6, C and D).
The data presented in this report establish omega-1 as the principal molecule responsible for the DC-dependent Th2-inducing activity of S. mansoni eggs. The key role of omega-1 in schistosome egg Th2 induction is supported by an accompanying report (see Everts et al. on p. 1673 of this issue) in which the glycoprotein was shown to condition human DCs for Th2 cell differentiation and to trigger IL-4–independent Th2 responses in vivo. Although both studies identify omega-1 as the initial DC trigger, IPSE, a second major egg secretory glycoprotein, is likely to contribute to the Th2 response by providing, through IgE-dependent activation of basophils, an alternative source of IL-4 for sustaining Th2 polarization. A third egg-derived mediator, peroxiredoxin, which lacks a defined signal sequence, may play an additional role by promoting differentiation of alternatively activated macrophages.
Activation of DCs through TLR is required for Th1 as well as Th17 polarization in many microbial systems (Medzhitov, 2007). In contrast, most studies argue for a limited role of TLR signaling in Th2 cell differentiation, and indeed, development of Th2 effectors is commonly associated with suboptimal activation of DCs (Pearce et al., 2004; Perona-Wright et al., 2006). Nevertheless, several S. mansoni egg components (e.g., LewisX) weakly activate DCs through TLR2, 3, or 4 (Thomas et al., 2003; van der Kleij et al., 2002; Aksoy et al., 2005). Regardless, MyD88-deficient mice undergo normal SEA-induced Th2 polarization in vivo (Jankovic et al., 2002) and in vitro (Jankovic et al., 2004). Consistent with the latter findings, omega-1 stimulated Th2 cell differentiation in DC cultures from mice doubly deficient in MyD88/TRIF. One possible explanation for these apparently contradictory observations is that although SEA may contain agonists that signal through TLR, the response to these components is actively inhibited by omega-1 in the same fashion as it suppresses the response to LPS.
The finding that a secreted T2 ribonuclease acts on DCs as a Th2-inducing factor is unexpected, because schistosome eggs contain other molecules such as proteases, which based on prior studies would seem more likely candidates (Donnelly et al., 2006; Sokol et al., 2008). Nevertheless, several plant and fungal allergens (Bufe et al., 1995; Bufe et al., 1996; Arruda et al., 1990) have been identified as ribonucleases. Perhaps more relevant is the recent demonstration by Yang et al. (2008) that the human eosinophil–derived neurotoxin, a ribonuclease A, acts as an endogenous alarmin that enhances Th2 responses by directly affecting DCs. Ribonuclease activity is often associated with cytotoxicity, and indeed, omega-1 was initially characterized as a hepatotoxic agent from S. mansoni (Fitzsimmons et al., 2005). Nevertheless, the Th2-promoting activity of omega-1 in our culture system cannot be explained by cytotoxic effects, because the molecule failed to induce a detectable reduction in DC viability. The precise role that the enzymatic activity of omega-1 plays in its Th2-promoting function is currently under study.
Our finding that SEA and purified omega-1 both profoundly modify the cytoskeletal organization of DCs implicates a role for altered cell morphology in the Th2-promoting function of these cells. Indeed, the cytoskeletal alterations observed closely correlated with decreased DC–CD4 conjugate formation as well as impaired T cell activation, suggesting that omega-1/SEA exposure creates a setting of weak TCR signal strength analogous to that seen with low-dose antigen. Although we cannot rule out other mechanisms for the immunomodulatory activity of omega-1, this hypothesis provides a framework for resolving a previously noted inconsistency in the signal strength concept as an explanation for helminth-induced Th2 polarization. Thus, helminths release large amounts of parasite products that should generate conditions for high- rather than low-dose antigen effects. In the case of S. mansoni infection, for example, female worms deposit as many as 300 eggs per day, leading to high concentrations of circulating antigen. However, in the presence of omega-1 and possibly other helminth-functional homologues, this high dose of antigen would be offset by inhibition of DC–CD4+ T cell encounter, thus resulting in a decreased signal strength equivalent to that seen with low antigen doses. Further studies on defined helminth Th2-inducing factors will be important in establishing the general validity of this proposed mechanism for parasite-induced Th2 polarization.
MATERIALS AND METHODS
Animals.
C57BL/6 and BALB/c WT mice were obtained from Taconic. BALB/c DO.11.10 TCR Tg animals on the RAG-2−/− background and C57BL/6 OT-II TCR Tg mice were provided by the National Institute of Allergy and Infectious Diseases (NIAID) Animal Supply Contract at Taconic (Jankovic et al., 2004). MyD88−/−TRIF−/− double-deficient animals (Yamamoto et al., 2003) were provided by E. Lien (University of Massachusetts, Worcester, MA) and bred at the NIAID Animal Facility. All experiments were performed under the guidelines and study proposal approved by the NIAID Animal Care and Use Committee. Female 8–12-wk-old mice were used in all experiments.
Parasite preparations.
104 eggs/ml (provided the Biomedical Research Institute, Rockville, MD), isolated from the livers of S. mansoni–infected mice, were cultured in serum-free medium at 37°C (Ashton et al., 2001). After 72 h, eggs were removed by centrifugation and the supernatants were concentrated >10-fold. SEA was prepared according to a standard procedure (Jankovic et al., 2004), whereas omega-1–depleted SEA was obtained after adding back purified IPSE to the remaining SEA fraction after removal of both IPSE and omega-1 by cation exchange chromatography. Irradiated Toxoplasma gondii tachyzoites and soluble tachyzoite antigen were prepared as previously described (Jankovic et al., 2002).
DC immunization.
BALB/c mice were injected s.c. with 3 × 105 BMDCs that were incubated overnight with or without different parasite stimuli. Popliteal LN cells were isolated 7 d later for assay.
Gel filtration of egg supernatants.
Concentrated egg culture supernatant applied to a Superdex 75 10/300 GL column (GE Healthcare) was eluted with 20 mM Tris HCl (pH 8). 0.5-ml fractions were collected for the length of elusion and tested without further treatment in Th2 polarization assays. Active fractions were subsequently concentrated and analyzed by reduced SDS-PAGE. After transfer to a polyvinylidene fluoride (PVDF) membrane, the N-terminal sequence was obtained by Edman degradation.
Purification of omega-1 and IPSE from SEA.
SEA was diluted with 20 mM sodium phosphate (pH 6), applied to an SP-Sepharose column (GE Healthcare), and eluted with a gradient of 0–1 M NaCl in 20 mM sodium phosphate (pH 6). 4-ml fractions were analyzed by SDS-PAGE. Omega-1 was identified with an apparent Mr of 32 kD eluting at ∼0.8 M NaCl, and a protein eluting slightly earlier was identified as IPSE. Material containing IPSE and omega-1 were combined into pools H and I, respectively, whereas fractions eluted previously were combined into seven pools (A–G). All pools were concentrated approximately fourfold, and the buffer was exchanged for 20 mM Tris HCl (pH 8), 0.15 M NaCl. Pools H and I served as a starting material for purification on a Superdex 75 column of omega-1 and IPSE, respectively. As a final verification of identity, the putative omega-1 and IPSE eluted as the major peak were transferred to PVDF and sequenced by Edman degradation.
Ribonuclease activity.
For the zymogram gels, 40 µg SEA and 20 µg of egg supernatants were separated in poly(A)-containing SDS-PAGE, as previously described (Joshi and Dwyer, 2007). Alternatively, samples were tested with the RNase Alert QC System (Applied Biosystems) in which a fluorophore-tagged ribonucleotide is used as a substrate.
Western blotting.
Samples of SEA or purified omega-1 were subjected to reduced SDS-PAGE and transferred to PVDF filters using standard electroblotting methods.
DEPC treatment.
SEA or pool I obtained after ion exchange chromatography of SEA was treated with 12 mM DEPC (Sigma-Aldrich) for 1 h at 37°C. Unreacted DEPC was removed from SEA by ultrafiltration and subsequent additional dilution-and-concentration cycles. After reaction with DEPC, pool I was subjected to gel filtration chromatography on a Superdex 75 column to both remove the unreacted DEPC and to purify omega-1 to homogeneity.
Cell isolation and Th2 polarization assay.
Splenic CD11c+B220−DX5− DCs and DO.11.10 CD4+DX5−CD11c− or OT-II CD4+CD62L+DX5−B220− T cells were isolated by sorting from naive splenocytes, as previously described (Jankovic et al., 2004). 5 × 105 Tg CD4+ lymphocytes/ml were co-cultured with 2.5 × 105 syngeneic splenic DCs/ml and stimulated interchangeably with 1 mM OVA323-339 or 100 µg/ml OVA (Thermo Fisher Scientific). After 72 h, culture supernatants were collected for IL-4 ELISA and cells were expanded in medium containing 10 U/ml rIL-2. Where indicated, 2 µg/ml omega-1 or 50 ng/ml IL-4 were incubated with 30 µg/ml anti–omega-1 mAb (140-3E11) before adding to the DC–CD4+ T cell cultures. Analyses of intracellular cytokine expression were performed as previously described (Jankovic et al., 2004).
Measurement of cell division.
OT-II cells were labeled with 5- and 6-CFSE (Invitrogen) before stimulation. CFSE dilution was determined by flow cytometry 36 h later.
Cytokine measurements.
IL-4 and IL-2 were assayed using commercial ELISA kits (R&D Systems), whereas IFN-γ, IL-5, IL-13, and IL-10 were measured using laboratory-based ELISAs (Jankovic et al., 2002).
Basophil assay.
The basophil-enriched non–B/non–T cell population was isolated from spleens from 10-d Nippostrongylus brasiliensis–infected BALB/c mice provided by J. Urban (United States Department of Agriculture, Beltsville, MD) and J. Pesce (NIAID, Bethesda, MD), as previously described (Jankovic et al., 1997). Cells were cultured at 106 cells/ml in the presence of the stimuli indicated in the figures for 24 h, when culture supernatants were collected and assayed for IL-4 by ELISA.
BMDC cultures.
For all experimentss BMDCs were harvested on day 7 from bone marrow cultures generated as previously described (MacDonald et al. 2001). After 12 h of culture, nonadherent and adherent cells, recovered after a 5-min incubation with 5 mM EDTA, were collected, counted separately, and pooled for further analyses.
Phalloidin staining and confocal microscopy.
5 × 105 FACS-sorted CD11c+ BMDCs/ml were cultured on chambered coverglass (Thermo Fisher Scientific) with the stimuli indicated in the figures. After 18 h, cells were washed, fixed with 3.7% paraformaldehyde, and stained with FITC-labeled phalloidin (Invitrogen) and DAPI. Images were acquired with a microscope (model SP5; Leica) and analyzed with IMARIS software (Bitplane).
DC–T cell conjugation assay.
5 × 105 OT-II T cells per well were incubated for 45 min at 37°C in 96-well U-bottom plates with 1.5 × 106 BMDCs that had been pulsed with peptide. Conjugates were enumerated by flow cytometry after the cell mixture was stained at 4°C for CD4 and CD11c and repeatedly washed (Qi et al., 2008).
Statistics.
The statistical significance of differences between data means was evaluated using the Student's two-tailed t test. P < 0.05 was considered statistically significant.
Online supplemental material.
Fig. S1 depicts a partial biochemical characterization of Th2-polarizing activity in SEA. In Fig. S2, we show that IPSE triggers IL-4 secretion from basophils but does not promote DC-dependent Th2 polarization. Fig. S3 demonstrates ribonuclease activity of omega-1 before and after DEPC treatment. Fig. S4 shows that omega-1 does not require MyD88/TRIF signaling in DCs for Th2 polarization and does not affect DC viability. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082462/DC1.
Acknowledgments
We thank C. Eigsti for FACS sorting; L. Koo, S. Becker, and J. Kabat for assistance with the confocal microscopy; M. Garfield for Edman degradation analysis; and Dr. F. Lewis and his colleagues for supplying schistosome eggs and SEA. We also thank Drs. J. Urban and J. Pesce for providing spleens from N. brasiliensis–infected mice, and Drs. R. Goldszmid and E. Lien for GM-CSF 3T3 cells and MyD88/TRIF double-deficient mice, respectively. Finally, we are grateful to Dr. D. Burnette for advice on actin staining, Drs. G. Schramm and H. Haas for the gifts of mAb 140-E311 and omega-1–depleted SEA, and Drs. T. Nutman and T. Wynn for critical reading of the manuscript.
This work was supported by the Intramural Research Program of the NIAID, National Institutes of Health, as well as the German Academic Exchange Service and the Karl-Enigk Foundation (S. Steinfelder).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: BMDC, bone marrow–derived DC; DEPC, diethyl pyrocarbonate; ICS, intracellular cytokine staining; PI, propidium iodine; SEA, soluble egg extract; SN, supernatant; Tg, transgenic; TLR, Toll-like receptor.
References
- Abbas A.K., Murphy K.M., Sher A. 1996. Functional diversity of helper T lymphocytes.Nature. 383:787–793 [DOI] [PubMed] [Google Scholar]
- Aksoy E., Zouain C.S., Vanhoutte F., Fontaine J., Pavelka N., Thieblemont N., Willems F., Ricciardi-Castagnoli P., Goldman M., Capron M., et al. 2005. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells.J. Biol. Chem. 280:277–283 [DOI] [PubMed] [Google Scholar]
- Al-Alwan M.M., Liwski R.S., Haeryfar S.M., Baldridge W.H., Hoskin D.W., Rowden G., West K.A. 2003. Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent.J. Immunol. 171:4479–4483 [DOI] [PubMed] [Google Scholar]
- Arruda L.K., Platts-Mills T.A., Fox J.W., Chapman M.D. 1990. Aspergillus fumigatus allergen I, a major IgE-binding protein, is a member of the mitogillin family of cytotoxins.J. Exp. Med. 172:1529–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton P.D., Harrop R., Shah B., Wilson R.A. 2001. The schistosome egg: development and secretions.Parasitology. 122:329–338 [DOI] [PubMed] [Google Scholar]
- Bufe A., Schramm G., Keown M.B., Schlaak M., Becker W.M. 1995. Major allergen Phl p Vb in timothy grass is a novel pollen RNase.FEBS Lett. 363:6–12 [DOI] [PubMed] [Google Scholar]
- Bufe A., Spangfort M.D., Kahlert H., Schlaak M., Becker W.M. 1996. The major birch pollen allergen, Bet v 1, shows ribonuclease activity.Planta. 199:413–415 [DOI] [PubMed] [Google Scholar]
- Constant S., Pfeiffer C., Woodard A., Pasqualini T., Bottomly K. 1995. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells.J. Exp. Med. 182:1591–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong E.C., Vieira P.L., Kalinski P., Schuitemaker J.H., Tanaka Y., Wierenga E.A., Yazdanbakhsh M., Kapsenberg M.L. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals.J. Immunol. 168:1704–1709 [DOI] [PubMed] [Google Scholar]
- Donnelly S., Dalton J.P., Loukas A. 2006. Proteases in helminth- and allergen-induced inflammatory responses.Chem. Immunol. Allergy. 90:45–64 [DOI] [PubMed] [Google Scholar]
- Donnelly S., Stack C.M., O'Neill S.M., Sayed A.A., Williams D.L., Dalton J.P. 2008. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages.FASEB J. 22:4022–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts B., Perona-Wright G., Smits H.H., Hokke C.H., van der Ham A.J., Fitzsimmons C.M., Doenhoff M.J., van der Bosch J., Mohrs K., Haas H., et al. 2009. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses.J. Exp. Med. 206:1673–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faveeuw C., Mallevaey K., Paschinger K., Wilson I.B., Fontaine J., Mollicone R., Oriol R., Altmann F., Lerouge P., Capron M., Trottein F. 2003. Schistosome N-glycans containing core α3-fucose and core β2-xylose epitopes are strong inducers of Th2 responses in mice.Eur. J. Immunol. 33:1271–1281 [DOI] [PubMed] [Google Scholar]
- Fitzsimmons C.M., Schramm G., Jones F.M., Chalmers I.W., Hoffmann K.F., Grevelding C.G., Wuhrer M., Hokke C.H., Haas H., Doenhoff M.J., Dunne D.W. 2005. Molecular characterization of omega-1: a hepatotoxic ribonuclease from Schistosoma mansoni eggs.Mol. Biochem. Parasitol. 144:123–127 [DOI] [PubMed] [Google Scholar]
- Hosken N.A., Shibuya K., Heath A.W., Murphy K.M., O'Garra A. 1995. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor–αβ–transgenic model.J. Exp. Med. 182:1579–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Dombrowicz D., Barbieri S., Caspar P., Wynn T.W., Paul W.E., Cheever A.W., Kinet J.P., Sher A. 1997. FcϵRI-deficient mice infected with Schistosoma mansoni mount normal Th2-type responses while displaying enhanced liver pathology.J. Immunol. 159:1868–1875 [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Hieny S., Caspar P., Collazo C.M., Sher A. 2002. In the absence of IL-12, CD4+ T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10−/− setting.Immunity. 16:429–439 [DOI] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Caspar P., Sher A. 2004. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling.J. Immunol. 173:2419–2427 [DOI] [PubMed] [Google Scholar]
- Joshi M.B., Dwyer D.M. 2007. Molecular and functional analyses of a novel class I secretory nuclease from the human pathogen, Leishmania donovani.J. Biol. Chem. 282:10079–10095 [DOI] [PubMed] [Google Scholar]
- Kane C.M., Cervi L., Sun J., McKee A.S., Masek K.S., Shapira S., Hunter C.A., Pearce E.J. 2004. Helfminth antigens modulate TLR-initiated dendritic cell activation.J. Immunol. 173:7454–7461 [DOI] [PubMed] [Google Scholar]
- MacDonald A.S., Straw A.D., Bauman B., Pearce E.J. 2001. CD8− dendritic cell activation status plays an integral role in influencing Th2 response development.J. Immunol. 167:1982–1988 [DOI] [PubMed] [Google Scholar]
- MacDonald A.S., Straw A.D., Dalton N.M., Pearce E.J. 2002. Cutting edge: Th2 response induction by dendritic cells: a role for CD40.J. Immunol. 168:537–540 [DOI] [PubMed] [Google Scholar]
- Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response.Nature. 449:819–826 [DOI] [PubMed] [Google Scholar]
- Ouyang W., Kolls J.K., Zheng Y. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation.Immunity. 28:454–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.J., Kane C.M., Sun J., Taylor J.J., McKee A.S., Cervi L. 2004. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni.Immunol. Rev. 201:117–126 [DOI] [PubMed] [Google Scholar]
- Perona-Wright G., Jenkins S.J., MacDonald A.S. 2006. Dendritic cell activation and function in response to Schistosoma mansoni.Int. J. Parasitol. 36:711–721 [DOI] [PubMed] [Google Scholar]
- Qi H., Cannons J.L., Klauschen F., Schwartzberg P.L., Germain R.N. 2008. SAP-controlled T–B cell interactions underlie germinal center formation.Nature. 455:764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiz L., Smirnoff P., Bar-Eli M., Schwartz B., Shoseyov O. 2006. ACTIBIND, an actin-binding fungal T2-RNase with antiangiogenic and anticarcinogenic characteristics.Cancer. 106:2295–2308 [DOI] [PubMed] [Google Scholar]
- Schramm G., Falcone F.H., Gronow A., Haisch K., Mamat U., Doenhoff M.J., Oliveira G., Galle J., Dahinden C.A., Haas H. 2003. Molecular characterization of an Interleukin-4-inducing factor from Schistosoma mansoni eggs.J. Biol. Chem. 278:18384–18392 [DOI] [PubMed] [Google Scholar]
- Sokol C.L., Barton G.M., Farr A.G., Medzhitov R. 2008. A mechanism for the initiation of allergen-induced T helper type 2 responses.Nat. Immunol. 9:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P.G., Carter M.R., Atochina O., Da'Dara A.A., Piskorska D., McGuire E., Harn D.A. 2003. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism.J. Immunol. 171:5837–5841 [DOI] [PubMed] [Google Scholar]
- van der Kleij D., Latz E., Brouwers J.F., Kruize Y.C., Schmitz M., Kurt-Jones E.A., Espevik T., de Jong E.C., Kapsenberg M.L., Golenbock D.T., et al. 2002. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization.J. Biol. Chem. 277:48122–48129 [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K., Akira S. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway.Science. 301:640–643 [DOI] [PubMed] [Google Scholar]
- Yang D., Chen Q., Su S.B., Zhang P., Kurosaka K., Caspi R.R., Michalek S.M., Rosenberg H.F., Zhang N., Oppenheim J.J. 2008. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2–MyD88 signal pathway in dendritic cells and enhances Th2 immune responses.J. Exp. Med. 205:79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Paul W.E. 2008. CD4 T cells: fates, functions, and faults.Blood. 112:1557–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]