Abstract
Soluble egg antigens of the parasitic helminth Schistosoma mansoni (S. mansoni egg antigen [SEA]) induce strong Th2 responses both in vitro and in vivo. However, the specific molecules that prime the development of Th2 responses have not been identified. We report that omega-1, a glycoprotein which is secreted from S. mansoni eggs and present in SEA, is capable of conditioning human monocyte-derived dendritic cells in vitro to drive T helper 2 (Th2) polarization with similar characteristics as whole SEA. Furthermore, using IL-4 dual reporter mice, we show that both natural and recombinant omega-1 alone are sufficient to generate Th2 responses in vivo, even in the absence of IL-4R signaling. Finally, omega-1–depleted SEA displays an impaired capacity for Th2 priming in vitro, but not in vivo, suggesting the existence of additional factors within SEA that can compensate for the omega-1–mediated effects. Collectively, we identify omega-1, a single component of SEA, as a potent inducer of Th2 responses.
Helminth parasites are the most potent natural inducers of T helper 2 (Th2) cell–polarized responses. Infection with Schistosoma mansoni elicits strong Th2 responses in humans and in experimental animal models. The development of this Th2 polarization coincides with the onset of egg production by adult worms (Pearce, 2005). The ability of S. mansoni eggs to induce Th2 differentiation during infection is underscored by the observation that schistosome eggs alone, or soluble S. mansoni egg antigen (SEA), are sufficient to drive Th2 polarization in naive mice even in the absence of infection (Vella and Pearce, 1992; Jankovic et al., 2004).
Various cells of the innate immune system are thought to contribute to the activation of Th2 responses after infection with S. mansoni. Granulocytes like basophils, eosinophils, and mast cells have been shown to represent potential innate sources of Th2-associated cytokines, like IL-4, during infection, which can contribute to the polarization, sustenance, and amplification of Th2 responses (Mitre and Nutman, 2006; Sokol et al., 2008). Although these cells may well support Th2 development, professional APCs, DCs in particular, are thought to play a dominant role in the initiation of these T cell responses (Kapsenberg, 2003). DCs have been shown to efficiently sense, capture, and process antigens derived from S. mansoni eggs (Cervi et al., 2004; van Liempt et al., 2007), resulting in the capacity of these DCs to prime for strong Th2 polarization both in vitro and in vivo (MacDonald et al., 2001; de Jong et al., 2002).
Although the ability of S. mansoni eggs and their soluble antigens to promote potent Th2 responses has been well documented, the specific components responsible for this activity are only beginning to be characterized. In this respect, glycans on proteins from S. mansoni eggs have been shown to contribute to the Th2-polarizing properties of SEA (Okano et al., 1999; Thomas et al., 2003). In addition, IPSE/alpha-1 and peroxiredoxin, which are both glycoproteins secreted by the eggs, have recently been shown to trigger basophils to produce IL-4 (Schramm et al., 2003, 2007) and to induce the development of alternatively activated macrophages (Donnelly et al., 2008), respectively, both of which can contribute to Th2 polarization after exposure to egg antigens. However, the specific molecules responsible for the initiation of Th2 differentiation have remained elusive.
In this paper, we show that omega-1, a glycoprotein present in both SEA (Dunne et al., 1991) and excretory/secretory products (ESPs) from live eggs (Cass et al., 2007), potently instructs human DCs to prime highly Th2-polarized responses from naive human CD4+ T cells in vitro. In addition, we demonstrate that injection of omega-1 alone into IL-4 dual reporter mice is sufficient to prime Th2 responses in vivo, even in the absence of the IL-4Rα chain. Together, these findings demonstrate that omega-1 is a potent initiator, rather than amplifier, of Th2 responses.
RESULTS AND DISCUSSION
ESPs from S. mansoni eggs condition human DCs for Th2 priming
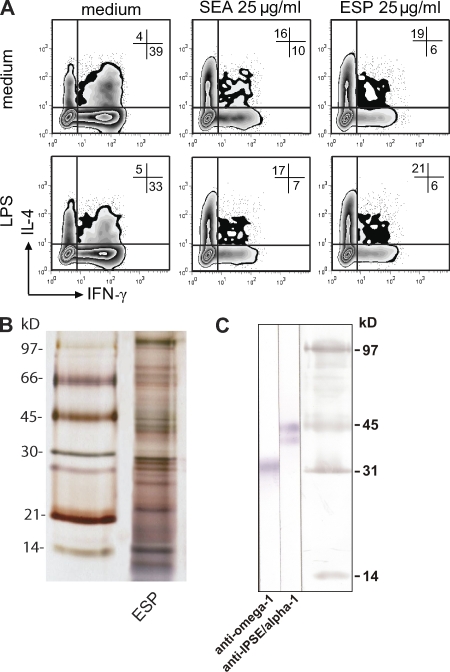
DCs are known to play a pivotal role in the initiation and polarization of T cell responses, and S. mansoni egg preparations have been shown to prime Th2 cells via the functional modulation of DCs (MacDonald et al., 2001; de Jong et al., 2002). To study and identify the components from S. mansoni egg preparations that instruct Th2 development, we used a well established co-culture system of human monocyte-derived DC and naive CD4+ T cells, which is generally thought to mimic in vivo DC-mediated T helper cell polarization (Kapsenberg, 2003). It stands to reason that ESPs from live eggs (Cass et al., 2007) are the first egg-derived molecules to interact with cells of the innate immune system, including DCs. Therefore, we initially tested ESPs for their capacity to condition DCs to prime Th2 development from naive CD4+ T cells. Similar to SEA, exposure of DCs to ESP resulted in a robust Th2 skewing irrespective of the presence or absence of LPS as a neutral maturation factor (Fig. 1 A). In a recent paper, Cass et al. (2007) identified omega-1 and IPSE/alpha-1 as the most abundant proteins within ESP from S. mansoni eggs. Separation of ESP preparations by SDS-PAGE (Fig. 1 B), followed by Western blotting with specific monoclonal antibodies, revealed prominent bands representing IPSE/alpha-1 and omega-1 (Fig. 1 C), which was confirmed by mass spectrometry (not depicted). Both omega-1 and IPSE/alpha-1 are glycoproteins that are specifically expressed in and secreted from S. mansoni eggs. Omega-1 has been demonstrated to display RNase activity and hepatotoxic effects (Dunne et al., 1991; Fitzsimmons et al., 2005), whereas IPSE/alpha-1 has previously been shown to trigger IL-4 production by human and mouse basophils (Schramm et al., 2003, 2007).
Figure 1.
Immunological and biochemical characterization of ESP from S. mansoni eggs. (A) Monocyte-derived DCs pulsed for 48 h with the different antigen preparations in the absence (top) or presence (bottom) of 100 ng/ml LPS as a maturation factor were co-cultured with allogeneic naive CD4+ T cells for 12 d in the presence of staphylococcal enterotoxin B and IL-2. Intracellular cytokine production was assayed by FACS 6 h after the stimulation of primed T cells with PMA and ionomycin. The frequencies of each population are indicated as percentages in the plot. One representative result from three independent experiments is shown. (B) 5 µg/cm ESP was separated under nonreducing conditions by SDS-PAGE and silver stained. (C) The presence of omega-1 and IPSE/alpha-1 was confirmed on Western blots by staining with specific anti–IPSE/alpha-1 and anti–omega-1 monoclonal antibodies.
Natural omega-1 modulates human DC maturation and cytokine production in vitro
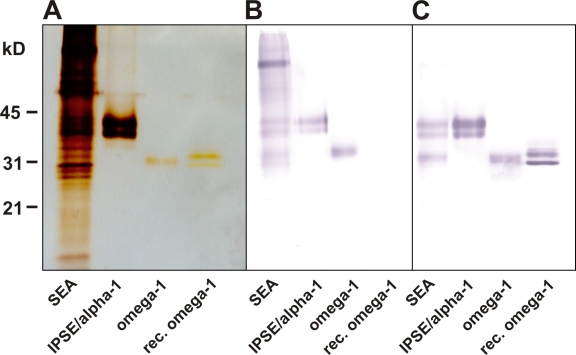
The observation that ESP can instruct human DCs to drive highly polarized Th2 responses prompted the question of whether omega-1 and IPSE/alpha-1 as prominent ESP components are responsible for this activity. Although some immunological properties of IPSE/alpha-1 have been described (Schramm et al., 2003, 2007), the effects of omega-1 and IPSE/alpha-1 on DC-driven T helper cell polarization have not been investigated. To this end, natural omega-1 and IPSE/alpha-1 were purified from SEA (Fig. 2) and used for the conditioning of human DCs in comparison with whole SEA. The concentrations of omega-1 and IPSE/alpha-1 used in these assays were equivalent to those in the unfractionated SEA preparations. As described previously (Kane et al., 2004; van Liempt et al., 2007), stimulation with SEA did not lead to classical maturation of DCs, based on surface marker expression (Fig. S1). Likewise, omega-1 and IPSE/alpha-1 did not induce the expression of these markers on DCs (Fig. S1). Apart from the failure to induce the maturation of DCs, SEA is also known to interfere with TLR-mediated DC activation (Kane et al., 2004; van Liempt et al., 2007). Indeed, when DCs were matured with the TLR4 ligand LPS, a nonpolarizing maturation factor for human DCs, the presence of SEA significantly impaired the LPS-induced up-regulation of CD83 and CD86 surface expression (Fig. 3 A). Strikingly, omega-1 alone was sufficient to suppress the induction of these molecules on LPS-stimulated DCs to a similar extent, whereas IPSE/alpha-1 had no effect (Fig. 3 A).
Figure 2.
SDS-PAGE of SEA, natural omega-1, and natural IPSE/alpha-1 as well as of recombinant omega-1 (silver staining and Western blotting). (A–C) 5 µg/cm SEA, 0.3 µg/cm omega-1, 0.3 µg/cm and IPSE/alpha-1 purified from SEA were separated by SDS-PAGE and silver stained or blotted onto nitrocellulose membrane. Silver staining (A) revealed a weak banding intensity of both natural and recombinant omega-1 compared with IPSE/alpha-1, although the purified proteins were applied to the gel at the same amounts (0.3 µg/cm). The two bands stained by anti-IPSE/alpha-1 represent posttranslational variants of the same protein (Schramm et al., 2003). On Western blots, alkaline phosphatase–labeled A. aurantia agglutinin (B) or a mixture of specific anti–IPSE/alpha-1 and anti–omega-1 monoclonal antibodies followed by alkaline phosphatase–labeled anti–mouse IgG secondary antibody (C) were used for detection. (B) Although A. aurantia agglutinin clearly binds to omega-1 and IPSE/alpha-1 as well as to a variety of other fucosylated components present in SEA, it does not bind to recombinant omega-1, whose glycans are lacking fucose residues (note that the double band of recombinant vs. natural omega-1 is caused by differential glycosylation). (C) In contrast, all purified proteins but no irrelevant SEA components are detected by the mixture of specific monoclonal antibodies.
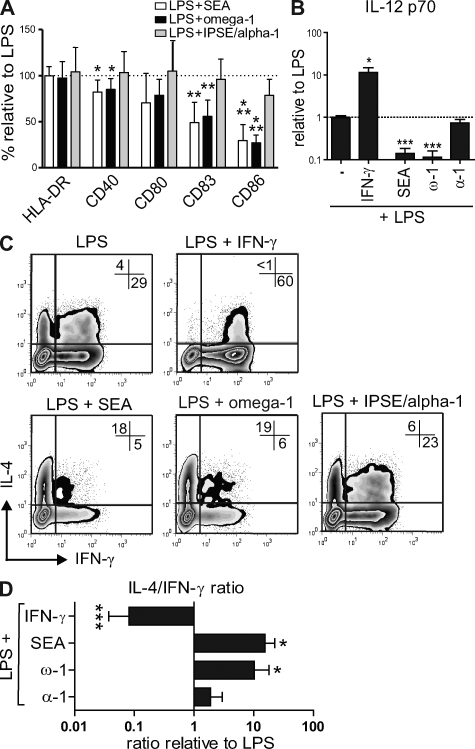
Figure 3.
Omega-1 modulates human DC maturation, cytokine production, and T cell–polarizing capacity with similar characteristics as SEA. (A) DCs were pulsed for 48 h with 25 µg/ml SEA, 500 ng/ml omega-1, or 500 ng/ml IPSE/alpha-1 in combination with 100 ng/ml LPS as a maturation factor, and surface expression of maturation markers was determined by FACS analysis. The expression levels, based on geometric mean fluorescence, of different maturation markers are shown relative to the DCs stimulated with LPS alone, which is set to 100% for each marker (dashed line). (B) DCs were co-cultured for 24 h with a CD40-L–expressing cell line, to mimic the interaction with T cells. IL-12p70 cytokine expression levels are shown relative to the DCs stimulated with LPS alone, which is set to 1 (dashed line). (C and D) T cell–polarizing capacity of the conditioned DCs was evaluated as described in legend for Fig. 1. (C) Representative plots out of at least four independent experiments are shown. (D) Based on intracellular cytokine staining, the ratio of T cells that were single positive for either IL-4 or IFN-γ was calculated relative to the control condition. Error bars in A, B, and D represent the mean ± SD of at least four independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for values significantly different from the LPS control, based on paired analysis (one-sided paired Student's t test). ω-1, omega-1; α-1, IPSE/alpha-1.
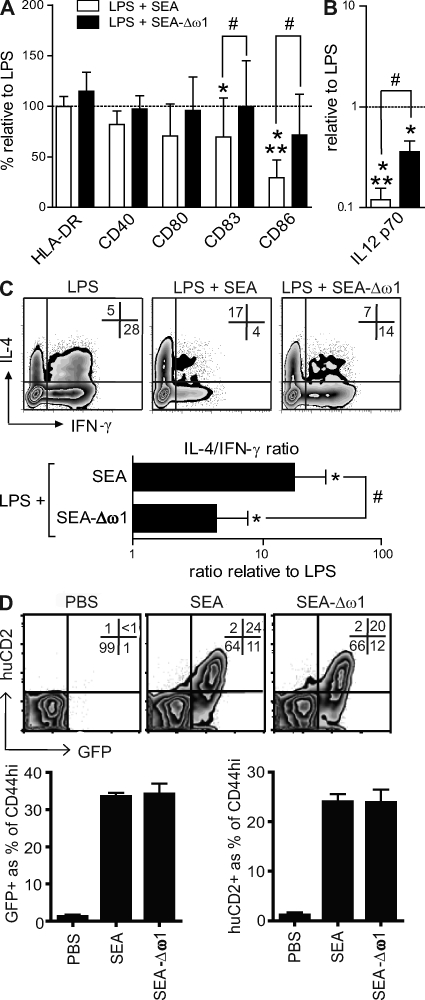
DCs exposed to parasitic helminth-derived antigens, including SEA, are distinguished by their low production of IL-12, which is thought to be a prerequisite for their Th2-inducing capacity (Jankovic et al., 2006). We analyzed the cytokine production of conditioned DCs after restimulation with a CD40-L–expressing cell line, mimicking the interaction with T cells. DCs stimulated with LPS in the presence of SEA displayed a potent reduction in the production of IL-12p70 (Fig. 3 B). Importantly, omega-1 alone was sufficient to inhibit the release of IL-12 (Fig. 3 B). Of note, the impact of 500 ng/ml omega-1 on IL-12 production was equal to that of 25 µg/ml SEA (Fig. 3 B). In contrast, IPSE/alpha-1 did not significantly affect IL-12 production (Fig. 3 B). Collectively, these data demonstrate that omega-1, but not IPSE/alpha-1, down-modulates DC maturation and cytokine production to a similar extent as SEA.
Natural omega-1 primes human DCs to induce a Th2 response in vitro
To evaluate the capacity of DCs exposed to these schistosome egg–derived antigens to direct T helper polarization, human DCs were pulsed for 40 h with the different egg antigens in the presence of LPS as a neutral maturation factor and then co-cultured with naive CD4+ T cells. 2 wk later, cytokine production by the CD4+ T cells was determined by intracellular cytokine staining. In contrast to IFN-γ–stimulated DCs that were used as a Th1-polarizing control, SEA-stimulated DCs potently skewed the response toward a Th2 cytokine profile (Fig. 3, C and D). Omega-1 alone displayed the same Th2-inducing potency as SEA, even at a 50-fold lower protein concentration (500 ng/ml vs. 25 µg/ml; Fig. 3, C and D). Moreover, the robust Th2 priming by omega-1–conditioned DCs was also observed in the absence of LPS as a neutral maturation factor (Fig. S2). In contrast, IPSE/alpha-1–treated DCs did not drive significant Th2 polarization, which is in keeping with the inability of IPSE/alpha-1 to suppress the production of IL-12 by these DCs (Fig. 2, B–D).
Recombinant omega-1, like natural omega-1, conditions human DCs for Th2 polarization in vitro
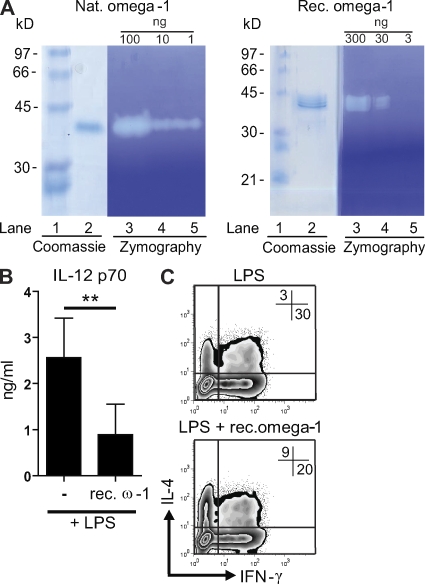
To further establish that omega-1 alone is sufficient to prime Th2 polarization through the functional modulation of DCs, we tested recombinant omega-1 expressed by human embryonic kidney cells (Fig. 2). As described for natural omega-1 (Fitzsimmons et al., 2005), recombinant omega-1 displayed RNase activity (Fig. 4 A), proving its biological activity (Fig. 4 A). In contrast to natural omega-1, the recombinant protein was not bound by the fucose-specific lectin Aleuria aurantia agglutinin, revealing differences in the glycosylation pattern (Fig. 2 B). Importantly, recombinant omega-1 significantly reduced IL-12 production by DCs (Fig. 4 B) and conditioned DCs to prime Th2 responses (Fig. 4 C), albeit with reduced potency compared with natural omega-1 (Fig. 3, B and C).
Figure 4.
Recombinant omega-1 has RNase and Th2-polarizing activity similar to natural omega-1. (A) Recombinant omega-1 is a functional RNase as determined by negative-staining RNase zymography. Samples containing the indicated amount of protein were run under nondenaturing conditions on 11% SDS polyacrylamide gels containing 2 mg/ml of yeast RNA. Protein bands were detected by Coomassie blue staining (lane 2) or SDS was removed and RNase activity was detected by toluidine blue (lanes 3–5). Lane 1 contains molecular mass standards. (B) Monocyte-derived DCs were treated as described in the legend for Fig. 2. IL-12 p70 concentrations were determined by ELISA. Error bars represent the mean ± SD of four independent experiments. (C) T cell–polarizing capacity of the conditioned DCs was evaluated as described in legend for Fig. 1. One representative result from four independent experiments is shown. **, P < 0.01 (one-sided paired Student's t test). ω-1, omega-1.
The data presented so far demonstrate that omega-1 alone, in contrast to IPSE/alpha-1, can initiate Th2 polarization via the modulation of human DCs with similar characteristics as unfractionated SEA.
Omega-1 primes a Th2 response in vivo
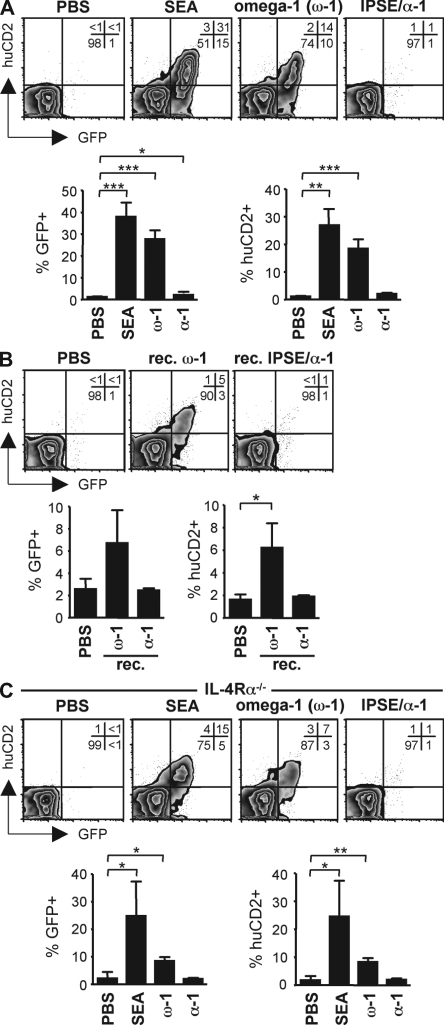
To investigate whether omega-1 has the capacity to prime Th2 responses in vivo, we administered omega-1 to 4get/KN2 IL-4 dual reporter mice (Mohrs et al., 2005). In these mice, IL-4–competent cells are GFP+ and IL-4–producing cells additionally express huCD2, allowing the direct visualization of Th2 differentiation and IL-4 production. After the s.c. injection of SEA, omega-1, or IPSE/alpha-1 into the footpad, the draining popliteal lymph nodes were harvested on day 7 and CD4+CD44high effector T cells were analyzed for the expression of GFP and huCD2 directly ex vivo. Injection of SEA resulted in a significant increase of GFP+ and huCD2+ cells, a result reflecting the induction of Th2 differentiation and acute IL-4 production in vivo (Fig. 5 A). Importantly, omega-1 alone also induced a marked Th2 response and the production of IL-4, whereas IPSE/alpha-1 did not (Fig. 5 A). The Th2-inducing capacity of omega-1 was further substantiated by the observation that immunization with recombinant omega-1 led to the induction of a Th2 response and the production of IL-4 in these mice, although to a lesser degree than natural omega-1 (Fig. 5 B).
Figure 5.
Omega-1 is sufficient to drive Th2 polarization in vivo, independently of IL-4R signaling. (A) 4get/KN2 IL-4 dual reporter mice were injected s.c. with 20 µg SEA, 2 µg omega-1, or 2 µg IPSE/alpha-1 into the footpad. After 7 d, the frequency of GFP+ and huCD2+ within the CD4+CD44high effector T cell population was determined by flow cytometry in the draining popliteal lymph nodes. (B) 4get/KN2 mice were injected with recombinant proteins and analyzed as in A. (C) IL-4Rα−/− 4get/KN2 mice were treated and analyzed as in A. In all panels, representative plots are shown with the frequencies of each population indicated as percentages. Graphs depict the combined data of three to four individual mice per group and show one of at least two independent experiments. Error bars represent the mean ± SD. * P < 0.05; **, P < 0.01; ***, P < 0.001 for values significantly different from the PBS control (two-sided paired Student's t test).
Although IL-4 has been shown to play an important role in both the differentiation and amplification of Th2 responses, there is clear evidence that initial Th2 priming can occur in the absence of IL-4 signaling, as has been shown for SEA (Jankovic et al., 2000, 2006; van Panhuys et al., 2008). To establish whether omega-1 can induce Th2 polarization in the absence of IL-4R signaling, we immunized 4get/KN2 IL-4 dual reporter mice on the IL-4Rα−/− background (Mohrs et al., 1999). Injection of omega-1, as well as SEA, resulted in an increased frequency of GFP+ and huCD2+ cells in IL-4Rα−/− mice (Fig. 5 C), albeit with reduced magnitude, as has been previously reported for SEA (Jankovic et al., 2000). The observation that IL-4R signaling is dispensable for the in vivo priming of Th2 responses by omega-1 further supports that omega-1 itself can provide the initial triggers driving Th2 differentiation rather than simply amplifying the process.
Omega-1 is a major factor in SEA that conditions DCs for Th2 priming but is not the only Th2-inducing component of SEA
Given the potency of omega-1 to condition DCs for Th2 priming in vitro and to drive Th2 polarization in vivo with similar characteristics as SEA, we depleted omega-1 from SEA (Fig. S3) to address the extent to which the Th2-polarizing capacity of SEA can be attributed to omega-1. Depletion of omega-1 almost completely abrogated the inhibitory effect of SEA on LPS-induced in vitro maturation (Fig. 6 A) and IL-12 cytokine production (Fig. 6 B) by DCs. Consistent with this observation, the Th2-polarizing capacity of omega-1–depleted SEA was also significantly reduced compared with whole SEA in the presence (Fig. 6 C) or absence of LPS (Fig. S4). This suggests that omega-1 is a principal factor in SEA mediating the conditioning of DCs for Th2 priming in vitro. In contrast, omega-1–depleted SEA was not impaired in its capacity to prime Th2 responses in vivo (Fig. 6 D). Thus, additional components in SEA are able to compensate for omega-1 with respect to Th2 priming in vivo. An interesting candidate could be the glycoprotein peroxiredoxin present in SEA, as this molecule has recently been shown to induce the development of alternatively activated macrophages (Donnelly et al., 2008), which may render it capable of initiating a Th2 response (Cua and Stohlman, 1997).
Figure 6.
Omega-1 is a major factor in SEA that conditions DCs for Th2 priming but not the only Th2-inducing component present in SEA. (A and B) Monocyte-derived DCs were pulsed for 48 h with 25 µg/ml SEA or 25 µg/ml omega-1–depleted SEA in combination with 100 ng/ml LPS and analyzed for surface expression of maturation markers and IL-12 production as described in the Fig. 2 legend. Error bars represent the mean ± SD of four independent experiments. (C) T cell–polarizing capacity of the conditioned DCs was evaluated as described in the Fig. 1 legend. Depicted are representative plots with percentages indicated. Based on intracellular cytokine staining, the ratio of T cells single positive for either IL-4 or IFN-γ was calculated relative to the control condition. Error bars represent the mean ± SD of five independent experiments. (D) 4get/KN2 mice were injected with recombinant proteins and analyzed as in Fig. 4. Depicted are representative plots with percentages indicated and the combined data of three individual mice per group of two independent experiments. * and #, P < 0.05; **, P < 0.01 for values significantly different from the controls (*) or SEA (#) based on paired analysis (one-sided paired Student's t test). ω-1, omega-1.
In the present study, we identify omega-1, a glycoprotein secreted by S. mansoni eggs, as a strong inducer of Th2 responses in vitro and in vivo. Although omega-1 was known to be secreted by live S. mansoni eggs and to be one of the most abundant molecules present in SEA (Dunne et al., 1988, 1991), its immunological properties have remained elusive. This study shows that omega-1 alone is sufficient to drive Th2 responses both in vitro and in vivo. Using a well established in vitro model to study the T helper polarization of naive human T cell by DCs, we demonstrate that omega-1 can elicit Th2 responses via the conditioning of DCs. Nonetheless, our observations do not exclude the possibility that in vivo omega-1–driven Th2 responses are also supported by cell types other than DCs like, for instance, basophils (Perrigoue et al., 2009; Sokol et al., 2009; Yoshimoto et al., 2009). However, our in vivo studies with IL-4Rα−/− mice demonstrated that IL-4R signaling is dispensable for the in vivo priming of Th2 responses by omega-1. Given that DCs have the unique capacity to initiate Th2 responses independently of IL-4 signaling in vivo (MacDonald and Pearce, 2002), these data support a role for DCs in omega-1–driven Th2 priming. Our findings are corroborated by an independent study by Steinfelder et al. (2009), showing that omega-1–conditioned bone marrow–derived mouse DCs prime Th2 responses in vitro and also upon transfer into naive mice in vivo. Collectively, these studies support a role for omega-1 in driving Th2 responses via the functional modulation of DCs.
The molecular basis underlying the immunomodulatory property of omega-1 still remains to be determined. Carbohydrates present in SEA have been found to contribute to the Th2-polarizing properties of this antigen preparation (Okano et al., 1999; Thomas et al., 2003). Because omega-1 is a glycosylated protein, the glycosylation pattern of omega-1 might play a role in its Th2-priming activity. Furthermore, its RNase activity could provide an alternative or additional mechanism through which omega-1 drives Th2 polarization because several RNases have been implicated in Th2 responses (Garcia-Ortega et al., 2005; Yang et al., 2008). Whether the reduced Th2-inducing activity of recombinant compared with natural omega-1 in vitro (Fig. 4 C vs. Fig. 3 C) and in vivo (Fig. 5, B vs. A) is the result of differences in the glycosylation pattern or reduced RNase activity remains to be determined. The specific modification of the glycosylation and/or the RNase activity of omega-1 will define their respective roles and will help to identify the molecular pathways through which omega-1 conditions DCs to initiate Th2 polarization.
Although the immunological processes resulting in Th1 polarization have been extensively characterized, it is still poorly understood exactly how Th2 responses are initiated. SEA has often been used as a model antigen mixture to study the immunological mechanisms underlying the induction of Th2 responses (Jankovic et al., 2004, 2006; Worsley et al., 2008). Now, two groups using different but complementary models have independently identified omega-1 as a single glycoprotein in SEA with potent Th2-polarizing properties. These findings will pave the way for the use of a defined molecule, omega-1, to further delineate the cellular mechanisms and molecular signals that drive Th2 differentiation.
MATERIALS AND METHODS
Preparation and purification of egg antigens.
Freshly isolated S. mansoni eggs from trypsinized livers from infected hamsters were washed in RPMI medium with 300 U/ml penicillin, 300 µg/ml streptomycin, and 500 µg/ml fungizone. To obtain ESP, 3 × 105 eggs/ml were incubated in the same medium for 48 h at 37°C in a humidified incubator. Supernatant containing ESP was harvested and centrifuged to remove residual eggs. SEA was prepared as described previously (de Jong et al., 2002). SEA used for in vivo experiments was supplied by E.J. Pearce (University of Pennsylvania, Philadelphia, PA). Omega-1 and IPSE/alpha-1 were purified from SEA via cation exchange chromatography as previously described (Dunne et al., 1991; Schramm et al., 2003). Omega-1 was then separated from IPSE/alpha-1 by affinity chromatography using specific anti-IPSE/alpha-1 monoclonal antibodies coupled to an NHS-HiTrap Sepharose column according to the manufacturer's instructions (GE Healthcare). Purified components were concentrated and dialyzed. Omega-1–depleted SEA was prepared by adding back purified IPSE/alpha-1 to the remaining SEA fraction left from the cation exchange chromatography. The purity of the preparations was controlled by SDS-PAGE and silver staining. In parallel, Western blotting was performed both with specific anti–omega-1 (140-3E11) and anti–IPSE/alpha-1 (74-1G2) monoclonal antibodies followed by alkaline phosphatase-labeled anti–mouse IgG (Dianova) detection antibody and with alkaline phosphatase–labeled A. aurantia agglutinin, which binds specifically to fucose residues. Protein concentrations were tested using the Bradford or BCA procedure.
Production of recombinant omega-1.
Recombinant omega-1 was purified from human 293 human embryonic kidney cells transfected with the expression vector pSecTag2-omega-1. The pSecTag2 plasmid was obtained from Invitrogen. Secreted recombinant omega-1 was sequentially purified from the culture medium by immobilized metal affinity chromatography and size exclusion chromatography.
Zymography.
Ribonuclease activity was determined as described previously (Fitzsimmons et al., 2005).
Human DC culture, stimulation, and analysis.
Monocytes were isolated from venous blood of healthy volunteers using Institutional Review Board–approved protocols by density centrifugation on ficoll followed by a Percoll gradient, as previously described (de Jong et al., 2002), and were cultured in RPMI medium supplemented with 10% FCS, 500 U/ml of human rGM-CSF (gift from Schering-Plough) and 250 U/ml of human rIL-4 (R&D Systems). On day 3, culture medium including the supplements was replaced, and on day 6 immature DCs were stimulated with the indicated reagents in the presence of 100 ng/ml of ultrapure LPS (Escherichia coli 0111 B4 strain; InvivoGen). As a Th1 control, DCs were also pulsed with 1,000 U/ml IFN-γ. After 48 h, DCs were harvested for co-culture with naive T cells. In addition, 104 matured DCs were co-cultured with 104 CD40L-expressing J558 cells for 24 h to determine cytokine production by the DCs after activation by CD40L. IL-12p70 concentrations were determined by ELISA using mouse anti–human IL-12 (clone 20C2) as capture antibody and biotinylated mouse anti–human IL-12 (clone C8.6) as detection antibody (both obtained from BD). The expression of maturation markers on the pulsed DCs was determined by FACS (FACSCalibur; BD) through staining with CD83-PE (Beckman Coulter), HLA-DR-PerCP, CD40-APC, CD80-FITC, and CD86-PE (all BD).
Human T cell culture and determination of T cell polarization.
To determine T cell polarization, 5 × 103 48-h-pulsed DCs were co-cultured with 2 × 104 naive T cells that were purified using a human CD4+/CD45RO− column kit (R&D Systems) in the presence of 100 pg/ml staphylococcal enterotoxin B (Sigma-Aldrich) in 96-well flat-bottom plates (Costar). On day 5, 10 U/ml rhuIL-2 (Novartis) was added and the cultures were expanded for another 7 d. For intracellular cytokine production, the primed CD4+ T cells were restimulated with 50 ng/ml PMA plus 2 µg/ml ionomycin for 6 h (Sigma-Aldrich). 10 µg/ml brefeldin A was added during the last 2 h (Sigma-Aldrich). The cells were stained with a combination of IL-4–PE and IFN-γ–FITC antibodies (BD).
In vivo experiments.
WT (Mohrs et al., 2005) and IL-4Rα−/− (Mohrs et al., 1999) 4get/KN2 mice were bred and housed in the animal facility of the Trudeau Institute and used at 8–12 wk of age. All experimental procedures were approved by the Institutional Animal Care and Use Committee. Mice were immunized s.c. into one hind footpad with 20 µg SEA, 2 µg omega-1, or 2 µg IPSE in a volume of 30 µl and the draining popliteal lymph nodes were analyzed 1 wk later.
Statistical analysis.
Data were analyzed for statistical significance using a one-sided paired Student's t test. All p-values <0.05 were considered significant.
Online supplemental material.
Fig. S1 shows the expression of maturation markers on human DC after stimulation with of LPS, SEA, omega-1, or IPSE/alpha-1. Fig. S2 shows the T cell–polarizing capacity of omega-1–conditioned human DC in the absence of LPS. Fig. S3 shows the efficacy of depletion of omega-1 from SEA. Fig. S4 shows the T cell–polarizing capacity of DC conditioned with SEA or omega-1–depleted SEA in the absence of LPS. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082460/DC1.
Acknowledgments
The authors thank Edward J. Pearce for generously providing us with critical reagents and advice and Achim Gronow for expert technical assistance with purifying omega-1.
This work was supported by the Dutch Organization for Scientific Research (NWO grant No ZONMW 912-03-048, ZONMW-VENI 016.066.093), the European commission (contract number EEG LSHB-CT-2006-018996 GABRIEL to B. Everts), the National Institutes of Health (grant No AI073462 to M. Mohrs and AI072296 to M. Mohrs), and Deutsche Forschungsgemeinschaft (SFB/TR22-TP A12).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: ESP, excretory/secretory product; SEA, S. mansoni egg antigen; Th2, T helper 2.
References
- Cass C.L., Johnson J.R., Califf L.L., Xu T., Hernandez H.J., Stadecker M.J., Yates J.R., III, Williams D.L. 2007. Proteomic analysis of Schistosoma mansoni egg secretions.Mol. Biochem. Parasitol. 155:84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervi L., MacDonald A.S., Kane C., Dzierszinski F., Pearce E.J. 2004. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses.J. Immunol. 172:2016–2020 [DOI] [PubMed] [Google Scholar]
- Cua D.J., Stohlman S.A. 1997. In vivo effects of T helper cell type 2 cytokines on macrophage antigen-presenting cell induction of T helper subsets.J. Immunol. 159:5834–5840 [PubMed] [Google Scholar]
- de Jong E.C., Vieira P.L., Kalinski P., Schuitemaker J.H., Tanaka Y., Wierenga E.A., Yazdanbakhsh M., Kapsenberg M.L. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals.J. Immunol. 168:1704–1709 [DOI] [PubMed] [Google Scholar]
- Donnelly S., Stack C.M., O'Neill S.M., Sayed A.A., Williams D.L., Dalton J.P. 2008. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages.FASEB J. 22:4022–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne D.W., Hillyer G.V., Vazquez G. 1988. Schistosoma mansoni cationic egg antigens (CEF6): immunoserology with oxamniquine-treated patients and involvement of CEF6 in the circumoval precipitin reaction.Am. J. Trop. Med. Hyg. 38:508–514 [DOI] [PubMed] [Google Scholar]
- Dunne D.W., Jones F.M., Doenhoff M.J. 1991. The purification, characterization, serological activity and hepatotoxic properties of two cationic glycoproteins (alpha 1 and omega 1) from Schistosoma mansoni eggs.Parasitology. 103:225–236 [DOI] [PubMed] [Google Scholar]
- Fitzsimmons C.M., Schramm G., Jones F.M., Chalmers I.W., Hoffmann K.F., Grevelding C.G., Wuhrer M., Hokke C.H., Haas H., Doenhoff M.J., Dunne D.W. 2005. Molecular characterization of omega-1: a hepatotoxic ribonuclease from Schistosoma mansoni eggs.Mol. Biochem. Parasitol. 144:123–127 [DOI] [PubMed] [Google Scholar]
- Garcia-Ortega L., Lacadena J., Villalba M., Rodriguez R., Crespo J.F., Rodriguez J., Pascual C., Olmo N., Onaderra M., del Pozo A.M., Gavilanes J.G. 2005. Production and characterization of a noncytotoxic deletion variant of the Aspergillus fumigatus allergen Aspf1 displaying reduced IgE binding.FEBS J. 272:2536–2544 [DOI] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Noben-Trauth N., Caspar P., Paul W.E., Sher A. 2000. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile.J. Immunol. 164:3047–3055 [DOI] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Caspar P., Sher A. 2004. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling.J. Immunol. 173:2419–2427 [DOI] [PubMed] [Google Scholar]
- Jankovic D., Steinfelder S., Kullberg M.C., Sher A. 2006. Mechanisms underlying helminth- induced Th2 polarization: default, negative or positive pathways? Chem. Immunol. Allergy. 90:65–81 [DOI] [PubMed] [Google Scholar]
- Kane C.M., Cervi L., Sun J., McKee A.S., Masek K.S., Shapira S., Hunter C.A., Pearce E.J. 2004. Helminth antigens modulate TLR-initiated dendritic cell activation.J. Immunol. 173:7454–7461 [DOI] [PubMed] [Google Scholar]
- Kapsenberg M.L. 2003. Dendritic-cell control of pathogen-driven T-cell polarization.Nat. Rev. Immunol. 3:984–993 [DOI] [PubMed] [Google Scholar]
- MacDonald A.S., Pearce E.J. 2002. Cutting edge: polarized Th cell response induction by transferred antigen-pulsed dendritic cells is dependent on IL-4 or IL-12 production by recipient cells.J. Immunol. 168:3127–3130 [DOI] [PubMed] [Google Scholar]
- MacDonald A.S., Straw A.D., Bauman B., Pearce E.J. 2001. CD8- dendritic cell activation status plays an integral role in influencing Th2 response development.J. Immunol. 167:1982–1988 [DOI] [PubMed] [Google Scholar]
- Mitre E., Nutman T.B. 2006. Basophils, basophilia and helminth infections.Chem. Immunol. Allergy. 90:141–156 [DOI] [PubMed] [Google Scholar]
- Mohrs M., Ledermann B., Kohler G., Dorfmuller A., Gessner A., Brombacher F. 1999. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling.J. Immunol. 162:7302–7308 [PubMed] [Google Scholar]
- Mohrs K., Wakil A.E., Killeen N., Locksley R.M., Mohrs M. 2005. A two-step process for cytokine production revealed by IL-4 dual-reporter mice.Immunity. 23:419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M., Satoskar A.R., Nishizaki K., Abe M., Harn D.A., Jr 1999. Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens.J. Immunol. 163:6712–6717 [PubMed] [Google Scholar]
- Pearce E.J. 2005. Priming of the immune response by schistosome eggs.Parasite Immunol. 27:265–270 [DOI] [PubMed] [Google Scholar]
- Perrigoue J.G., Saenz S.A., Siracusa M.C., Allenspach E.J., Taylor B.C., Giacomin P.R., Nair M.G., Du Y., Zaph C., van Rooijen N., et al. 2009. MHC class II-dependent basophil-CD4(+) T cell interactions promote T(H)2 cytokine-dependent immunity.Nat. Immunol. 10:697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm G., Falcone F.H., Gronow A., Haisch K., Mamat U., Doenhoff M.J., Oliveira G., Galle J., Dahinden C.A., Haas H. 2003. Molecular characterization of an interleukin-4-inducing factor from Schistosoma mansoni eggs.J. Biol. Chem. 278:18384–18392 [DOI] [PubMed] [Google Scholar]
- Schramm G., Mohrs K., Wodrich M., Doenhoff M.J., Pearce E.J., Haas H., Mohrs M. 2007. Cutting edge: IPSE/alpha-1, a glycoprotein from Schistosoma mansoni eggs, induces IgE-dependent, antigen-independent IL-4 production by murine basophils in vivo.J. Immunol. 178:6023–6027 [DOI] [PubMed] [Google Scholar]
- Sokol C.L., Barton G.M., Farr A.G., Medzhitov R. 2008. A mechanism for the initiation of allergen-induced T helper type 2 responses.Nat. Immunol. 9:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol C.L., Chu N.Q., Yu S., Nish S.A., Laufer T.M., Medzhitov R. 2009. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response.Nat. Immunol. 10:713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfelder S., Andersen J., Cannons J.L., Feng C.G., Joshi M., Dwyer D., Caspar P., Schwartzberg P.L., Sher A., Jankovic D. 2009. Ribonuclease T2 (omega 1) is the major Th2 polarizing component in schistosome eggs and acts on dendritic cells by limiting T cell conjugate formation.J. Exp. Med. 206:1681–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P.G., Carter M.R., Atochina O., Da'Dara A.A., Piskorska D., McGuire E., Harn D.A. 2003. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism.J. Immunol. 171:5837–5841 [DOI] [PubMed] [Google Scholar]
- van Liempt E., van Vliet S.J., Engering A., Garcia Vallejo J.J., Bank C.M., Sanchez-Hernandez M., van Kooyk Y., van Die I. 2007. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation.Mol. Immunol. 44:2605–2615 [DOI] [PubMed] [Google Scholar]
- van Panhuys N., Tang S.C., Prout M., Camberis M., Scarlett D., Roberts J., Hu-Li J., Paul W.E., Le Gros G. 2008. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation.Proc. Natl. Acad. Sci. USA. 105:12423–12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella A.T., Pearce E.J. 1992. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0-like stage.J. Immunol. 148:2283–2290 [PubMed] [Google Scholar]
- Worsley A.G., Leibundgut-Landmann S., Slack E., Phng L.K., Gerhardt H., Sousa C.R., MacDonald A.S. 2008. Dendritic cell expression of the Notch ligand jagged2 is not essential for Th2 response induction in vivo.Eur. J. Immunol. 38:1043–1049 [DOI] [PubMed] [Google Scholar]
- Yang D., Chen Q., Su S.B., Zhang P., Kurosaka K., Caspi R.R., Michalek S.M., Rosenberg H.F., Zhang N., Oppenheim J.J. 2008. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2–MyD88 signal pathway in dendritic cells and enhances Th2 immune responses.J. Exp. Med. 205:79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Yasuda K., Tanaka H., Nakahira M., Imai Y., Fujimori Y., Nakanishi K. 2009. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4(+) T cells.Nat. Immunol. 10:706–712 [DOI] [PubMed] [Google Scholar]