Abstract
We report that like other T cells cultured in the presence of transforming growth factor (TGF) β, Th17 cells also produce interleukin (IL) 9. Th17 cells generated in vitro with IL-6 and TGF-β as well as purified ex vivo Th17 cells both produced IL-9. To determine if IL-9 has functional consequences in Th17-mediated inflammatory disease, we evaluated the role of IL-9 in the development and progression of experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. The data show that IL-9 neutralization and IL-9 receptor deficiency attenuates disease, and this correlates with decreases in Th17 cells and IL-6–producing macrophages in the central nervous system, as well as mast cell numbers in the regional lymph nodes. Collectively, these data implicate IL-9 as a Th17-derived cytokine that can contribute to inflammatory disease.
CD4 T cells have been divided into several subsets as defined by their cytokine products and functions after their activation. These include, but are not limited to, Th1, Th2, and Th17 cells. The initial two T cell subsets described were Th1 cells, which secrete IFN-γ and aid in the clearance of intracellular bacteria and viruses, and Th2 cells, which secrete IL-4 and IL-5 and help control extracellular pathogens. More recently, Th17 cells have been described as a third Th cell type that express the transcription factor RORγt and IL-17A, provide protection against fungi and various other extracellular bacteria, and are pathogenic T cells in the development of autoimmune inflammatory diseases (Zhu and Paul, 2008).
Discovery of the T cell subsets that produce IL-9 has expanded significantly in recent years. IL-9 was primarily studied as a product of Th2 cells, and implicated as an important regulatory cytokine in the lung and the gastrointestinal tract (Faulkner et al., 1997; Townsend et al., 2000; Forbes et al., 2008). Schmitt et al. first reported that IL-9 production is dependent on the initial presence of IL-2, and is greatly increased by the addition of TGF-β in a dose-dependent manner. They also observed that the addition of IL-4 to TGF-β in T cell cultures substantially enhanced T cell IL-9 production (Schmitt et al., 1994). This finding has been recently reexamined by two groups who suggest that the T effector cells produced by TGF-β and IL-4 may represent a unique subset of T cells, as these cells do not express any of the known transcription factors for T cell differentiation, including T-bet, GATA-3, RORγt, and FoxP3 (Dardalhon et al., 2008; Veldhoen et al., 2008). More recent work has shown that T cell–derived IL-9 may mediate immunosuppression. Adaptive T reg cells derived from encephalogenic T cells produce IL-9 (Liu et al., 2006), and our own studies have shown that IL-9 can be colocalized with T reg cells within the tolerant allograft and is functionally important for allograft survival (Lu et al., 2006). Therefore, IL-9 is produced by T cells that play a role in both inflammation and immunosuppression.
In this report, the hierarchy of IL-9 production by defined T cell subsets was compared. To unequivocally address IL-9 production by Th17 cells, IL-17F reporter T cells were used and demonstrated IL-9 production by purified Th17 cells. Given this observation, it was determined whether IL-9 played a functionally significant role in the Th17-mediated disease experimental autoimmune encephalomyelitis (EAE). The data show that IL-9 neutralization and IL-9R deficiency attenuate disease, and this correlates with decreases in Th17 cells and IL-6–producing macrophages in the central nervous system (CNS), as well as mast cell (MC) numbers in the regional lymph nodes. Collectively, these data implicate IL-9 as a Th17-derived cytokine that can contribute to inflammatory disease.
RESULTS AND DISCUSSION
Th17 cells produce IL-9
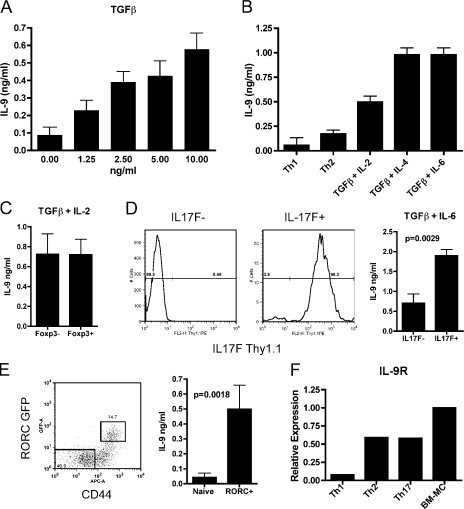
To confirm the observation of Schmitt et al. (1994) that TGF-β can induce IL-9 production, we stimulated T cells with the indicated concentrations of TGF-β and assessed their capacity to produce IL-9 after restimulation. As expected, we saw that TGF-β can induce IL-9 expression in a dose-dependent manner (Fig. 1 A). To further characterize IL-9 production by various T cell subsets, cells were cultured under defined conditions known to induce a spectrum of T cell phenotypes. The data show that Th17 (TGF-β + IL-6) cells and cells stimulated with TGF-β + IL-4 produced similar amounts of IL-9 (Fig. 1 B). As previously reported, it is also shown that conditions that induce the differentiation of adaptive T reg cells (TGF-β + IL-2) generate T cells that produce IL-9. Because the cells differentiated under adaptive T reg cell conditions contain both Foxp3− and Foxp3+ T cells, Foxp3 reporter T cells were used to sort-purify both of these populations to determine their capacity to produce IL-9. The data show that both of these populations produce similar amounts of IL-9 (Fig. 1 C). In general, the hierarchy of IL-9 production by the conditions prescribed in these studies suggests that adaptive T reg cells consistently produced more IL-9 than Th2 cells but less than cell-cultured T cells derived with TGF-β and IL-4 or IL-6 (Th17).
Figure 1.
Th17 cells produce IL-9. (A) T cells were stimulated in vitro with the indicated concentration of TGF-β for 4 d. Cells were washed, counted, and restimulated in the presence of αCD3/αCD28 for 24 h before supernatants were collected for ELISA. Results show means ± SD of two independent experiments. (B) Effector T cells were generated in vitro under the indicated conditions for 4 d and were restimulated as in A. Results are representative of at least four independent experiments. (C) Cells from TGF-β + IL-2 cultures were sorted based into FoxP3-GFP–positive and –negative subsets and restimulated as in A. Results are representative of four independent experiments. (D) T cells cultured under Th17 conditions were separated into IL-17F–positive and –negative subsets based on Thy1.1 expression using MACS columns (percentages are shown). Representative purity of both populations is shown. Cells were restimulated as in A for IL-9 production. Results were pooled from two independent experiments, and a Student’s t test was performed to compare the samples. Means ± SD are shown. (E) RORC-GFP mice were immunized with MOG emulsified in CFA, and splenic T cells were harvested 7 d later for sorting into the indicated populations (percentages are shown). Cells were restimulated with PMA/ionomycin for 4 h before supernatant was collected for ELISA. Results show means ± SD of two independent experiments, and a Student’s t test was performed to compare the samples. (F) qRT-PCR from Th1, Th2, and Th17 cells and BM-MCs was performed for IL-9R expression. All samples were standardized to the expression of IL-9R by BM-MCs. Results are representative of three independent samples from each experimental group.
Heterogeneity in T cell differentiation is inherent in the induction of adaptive T reg as well as Th17 cells. As had been executed with the adaptive T reg cell studies, T cells from IL-17F–Thy1.1 reporter mice (Lee et al., 2009) were cultured with TGF-β + IL-6 and Th17 purified by magnetic sorting for Thy1.1. Because none of the existing monocloncal antibodies to mouse IL-9 are suitable for assessing cytoplasmic fluorescence (Veldhoen et al., 2008), an IL-9 ELISA was used to determine IL-9 production. The data show that the IL-17F–positive population (>95% Th17 cells) produced IL-9 after restimulation. Furthermore, the IL-17F–negative population (<1% Th17 cells) produced fourfold less IL-9 than the IL-17F–positive population (Fig. 1 D). We have also performed this experiment an additional time using FACS sorting to obtain purities of >99% for both populations with similar results (unpublished data). To additionally confirm that IL-9 production by Th17 is not solely an in vitro phenomenon, we also used FACS sorting of cells from RORC-GFP mice, which have been previously described to report the expression of RORγt (Ivanov et al., 2006), and IL-17F–Thy1.1 mice. As shown, after RORC-GFP mice receive a CFA immunization, we see a significant amount of IL-9 production from these cells ex vivo as compared with an internal negative control of naive T cells from the same mice (Fig. 1 E). We also performed the same experiment in IL-17F–Thy1.1 reporter mice with similar results. In addition, we further sorted IL-17F–negative CD44hi T cells from these mice and observed that their capacity to produce IL-9 was less than the IL-17F–positive CD44hi T cells (Fig. S1).
In addition to IL-9 secretion, the expression of IL-9R was also evaluated on T cells. Previous work has extensively described IL-9 as influencing MCs and macrophages; however, there are reports that its receptor is also expressed on effector but not naïve T cells (Cosmi et al., 2004; Knoops and Renauld, 2004). As no monoclonal antibodies have been produced to mouse IL-9R, all of these data are based on mRNA expression profiling. To confirm and expand these results, quantitative real-time PCR (qRT-PCR) was performed to quantify IL-9R mRNA in T cells skewed under Th1, Th2, and Th17 conditions. The data show that Th2 and Th17 cells consistently had the highest expression of IL-9R mRNA expression, whereas Th1 cells had minimal expression (Fig. 1 F). Thus, because of the fact that IL-9R is expressed on IL-9–producing T cells, an autocrine impact of IL-9 on T cell differentiation is possible.
IL-9 neutralization ameliorates EAE
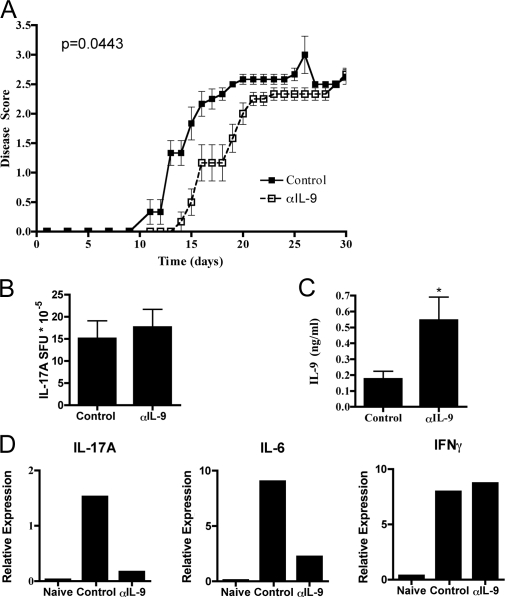
EAE is one disease model in which Th17 cells are the principle mediators of the symptoms observed. To determine if IL-9 contributes to Th17-mediated disease development, chronic EAE was induced in mice with myelin oligodendrocyte peptide (MOG)35-55, CFA, and pertussis toxin while administrating either control Ig antibody or neutralizing αIL-9 antibody beginning the day before immunization. IL-9 blockade consistently delayed the onset of disease, and this difference was statistically significant. However, mice did ultimately succumb with the same disease severity as controls (Fig. 2 A).
Figure 2.
Treatment with neutralizing anti–IL-9 antibody slightly delays EAE. (A) Mice were immunized for EAE and were treated with control Ig (n = 6) or αIL-9 antibody (n = 6) i.p. every other day starting with day −1. Mice were scored for disease severity, and the mean ± SEM for each time point is indicated. Results are representative of three independent experiments. The p-value obtained by a Mann-Whitney U test is indicated. (B and C) CD4 T cells from the peripheral lymph nodes of EAE-immunized mice were taken from each group at day 12 (score = 0 or 1 for all mice used) and stimulated with antigen-presenting cells and MOG for (B) IL-17A spot-forming units (SFU) in ELISPOTs or (C) IL-9 concentration in ELISA. Means ± SD are shown for both graphs. *, P < 0.05 from the control sample by a Student’s t test. Results shown are pooled from three independent experiments. (D) RNA was made from spinal cord samples of EAE-immunized mice from each group at the peak of disease severity in control mice, and the mean expression relative to β-actin is shown. Results are representative of four naive, eight control, and eight αIL-9–treated mice taken from three independent experiments.
Next, to determine if IL-9 blockade qualitatively altered the T cell response to MOG, T cell responses were monitored during disease development. Mice were sacrificed at day 12 after the initial immunization, at which point all mice showed little or no signs of disease. At that time, CD4 T cells were purified from the peripheral lymph nodes. Upon analysis, no significant difference in MOG-specific IL-17A (Fig. 2 B) responsiveness by ELISPOT was observed. However, MOG-specific IL-9 production was significantly enhanced in αIL-9–treated mice (Fig. 2 C), suggesting a possible feedback enhancement of IL-9 production caused by IL-9 blockade.
To assess the immune response in the CNS, qRT-PCR on spinal cord samples from naive mice, EAE mice treated with control Ig, and EAE mice treated with αIL-9 antibody was performed (Fig. 2 D). The data show a consistent decrease in both IL-17A and IL-6 signals in the αIL-9–treated mice but no effect on IFN-γ. These data suggest that IL-9 blockade preferentially attenuates Th17 responses. In addition, no difference in the mRNA expression of Ebi3, IL-10, IL-12a (p35), or IL-12 (p40) was observed (unpublished data).
IL-9R deficiency impairs multiple aspects of disease
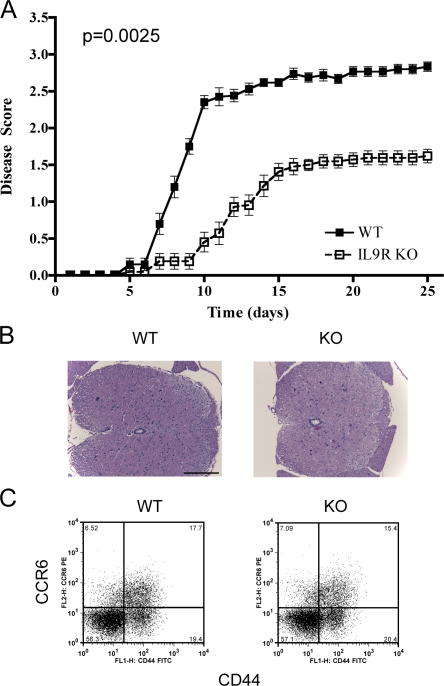
The data show that IL-9 blockade delays the induction of EAE; however, these mice eventually progress to a similar extent as controls. To independently evaluate the role of IL-9, the development of EAE in IL-9R KO mice was evaluated. IL-9R KO mice had delayed onset of disease, similar to what was observed with neutralizing antibody. However, IL-9R–deficient mice consistently had decreased severity over time and this difference was statistically significant. This suggests IL-9 may exert an effect throughout the progression of disease (Fig. 3 A).
Figure 3.
IL-9R deficiency ameliorates the severity of EAE. (A) WT (n = 20) and KO (n = 21) mice were immunized for EAE and scored to generate graphs of mean disease score ± SEM. Results were pooled from three independent experiments, and a Mann-Whitney U test was performed to assess the p-value shown. (B) Transverse spinal cord sections of WT (n = 8) and KO (n = 8) mice from two independent experiments were taken to perform the hematoxylin and eosin staining shown. Bar, 200 µm. (C) On day 7 after EAE immunization, draining lymph nodes of WT and KO mice were taken and CD4 T cells were assessed for CD44 and CCR6 expression (percentages are shown). Results are representative of three independent experiments with four to six mice from each group per experiment.
Because the initial priming of Th17 cells in αIL-9–treated mice appears equivalent to controls in lymph node but decreased in the CNS, we sought to determine if the delay in disease severity in IL-9R–deficient mice may be caused by a decreased ability to traffic into the CNS. Recently, two groups have highlighted the importance of CCR6 expression during the initiation of EAE (Liston et al., 2009; Reboldi et al., 2009); therefore, we examined T cell responses in the lymph node on day 7 after immunization. In this case we consistently found that the initial induction of CCR6 is equivalent between WT and KO mice (Fig. 3 C). In addition, at late time points histochemistry performed on spinal cord samples indicated that lymphocyte infilitrates were also equivalent between these two groups (Fig. 3 B). This suggests that IL-9 responsiveness is not necessary for trafficking of cells to the CNS.
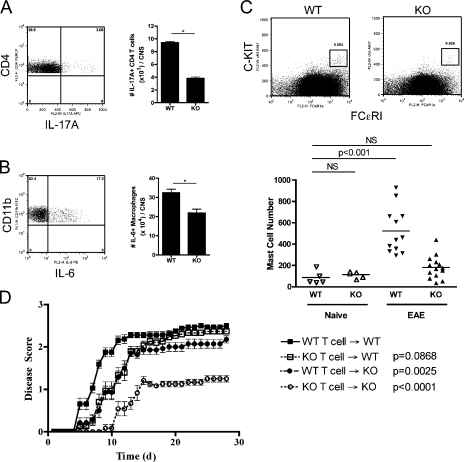
To further characterize the immune response in the CNS as well as to determine the cellular sources of IL-17A and IL-6 observed by qRT-PCR in αIL-9–treated mice, flow cytometry was performed on isolated lymphocytes from the brain and spinal cord of diseased WT and IL-9R KO mice. No significant difference in the total numbers of infiltrating lymphocytes was observed by CNS cell isolation. However, the numbers of IL-17A+ CD4 T cells and IL-6+ macrophages in the CNS were decreased in IL-9R KO mice (Fig. 4, A and B). In addition, IFN-γ responses were not significantly different between groups (unpublished data).
Figure 4.
IL-9R expression by both T cells and other cell types contributes to adoptively transferred EAE. (A and B) Lymphocytes from the brains and spinal cords of WT (n = 4) and KO (n = 4) mice were isolated, counted, and stained for (A) CD4 T cells and IL-17A and (B) macrophages and IL-6 after restimulation. The frequency of cytokine-producing cells by FACS was used to calculate the absolute numbers of cells shown. *, P < 0.01 by the Student’s t test. Results show means ± SD and are representative of three independent experiments. (C) Peripheral lymph nodes of mice were pooled and enriched for FCϵRI expression and stained for c-kit to identify MCs. Representative FACS plots are shown of WT and KO mice. Absolute numbers of MCs in lymph nodes were determined for WT naive (n = 5), KO naive (n = 5), WT EAE (n = 12), and KO EAE (n = 14) mice pooled from four independent experiments. A Student’s t test between the WT naive group and each other group was performed to assess statistical significance. Horizontal bars represent means. (D) T cells from WT and KO mice with EAE were transferred to either WT or KO mice to cause disease. Mice were scored and the mean disease scores ± SEM are shown. A Mann-Whitney U test was done to compare the WT T cell → WT group with each of the indicated groups to obtain the p-values shown. Results were pooled from two independent experiments, and the numbers of mice used are as follows: WT T cell → WT, n = 16; KO T cell → WT, n = 15; WT T cell → KO, n = 14; and KO T cell → KO, n = 12.
It has been reported that MC deficiency can decrease the severity of EAE (Secor et al., 2000), and that MC accumulation in the regional lymph nodes accompanies disease development (Tanzola et al., 2003). Given the fact that IL-9 is a growth and differentiation factor of MCs (Zhou et al., 2001), MC accumulation in immunized WT and IL-9R KO mice was determined during the development of EAE. First, there are no defects in MC numbers in naive IL-9R KO mice (Steenwinckel et al., 2007). Second, WT EAE-immunized mice had elevated MC numbers (Brenner et al., 1994). Third, no MC accumulation was observed in immunized IL-9R KO mice (Fig. 4 C). Hence, it appears that IL-9 is critical for the inflammation-induced accumulation of MCs during disease development.
A functional role for IL-9 in encephalitogenic T cells was sought because differentiated Th17 cells express IL-9R mRNA. As such, adoptive transfer studies were performed using WT or IL-9R KO MOG-primed T cells. Furthermore, adoptive transfer of WT T cells into WT or IL-9R KO hosts was performed to address whether host expression of IL-9R was important to the development of disease (Fig. 4). The transfer of IL-9R KO T cells → WT and WT T cells → IL-9R KO hosts caused a slight delay in disease onset and reduced severity at early time points, but the mice eventually succumbed to the same extent as WT T cells → WT controls. However, the transfer of IL-9R KO T cells → IL-9R KO hosts displayed both delayed onset and reduced severity of disease. Overall, these results suggest that IL-9R expression on both the encephalitogenic T cells and other host cell types contributes to the effect seen in IL-9R KO mice.
The findings presented establish (a) the hierarchy of IL-9 production by differentiated T cell subsets and purified subsets derived from these cells, (b) that purified Foxp3+ adaptive T reg and Th17 cells produce IL-9, (c) that IL-9 contributes to the development of EAE, (d) that IL-9 influences the expression of IL-17A and IL-6 in the CNS, and (e) that IL-9 mediates the accumulation of MCs in the regional lymph nodes during the development of EAE. Collectively, the findings implicate IL-9 as a mediator of Th17-driven inflammatory diseases.
The data show that both Th17 and TGF-β + IL-4 T cells produce high levels of IL-9 upon restimulation. Furthermore, Foxp3+ and Foxp3− T cells from in vitro generation of adaptive T reg cells also produce IL-9. To definitively show that Th17 cells produce IL-9, reporter Th17 cells were isolated and IL-9 production was confirmed. These results add to the growing list of IL-9–producing T cells, which includes natural T reg, adaptive T reg, and Th2 cells (Gessner et al., 1993; Schmitt et al., 1994; Hauber et al., 2004; Liu et al., 2006; Lu et al., 2006). In contrast, Veldhoen et al. (2008) reported that neither Th2, adaptive T reg, natural T reg, nor Th17 cells produce IL-9. The differences between that study and the findings in this paper, as well as others (Gessner et al., 1993; Schmitt et al., 1994; Hauber et al., 2004; Liu et al., 2006; Lu et al., 2006), has yet to be resolved.
In addition, the data show that IL-9 blockade by antibody or by IL-9R deficiency can ameliorate EAE. However, it must be noted that the phenotype of the IL-9R KO mice is much less robust than reported in IL-6 KO (Korn et al., 2007), IL-23 (p19 and p40) KO (Becher et al., 2002; Cua et al., 2003), and RORγt KO mice (Ivanov et al., 2006), which are completely protected from disease. Functionally, the data show that disease in IL-9R KO mice correlates with a reduction of IL-17A+ CD4 T cells and IL-6+ macrophages in the CNS of mice, as well as a decrease in MC numbers in the lymph nodes of mice. The later finding is not surprising, as IL-9 is known as a growth and activation factor for MCs (Faulkner et al., 1997; Townsend et al., 2000; Forbes et al., 2008). Extensive work performed by Melissa Brown’s group has also shown that MC-deficient W/Wv mice display suboptimal EAE (Secor et al., 2000; Tanzola et al., 2003; Gregory et al., 2005), and we have observed a similar phenotype in MC-deficient Wsh mice (unpublished data).
The findings presented, in the context of the emerging literature, establish that IL-9 cannot be readily assigned as being either a pro- or antiinflammatory cytokine. Rather, its function may be as an autocrine differentiation factor for inflammatory T cells and/or T reg cells, or as a paracrine factor regulating the activities of macrophages and/or MCs to mediate inflammation or suppression.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Charles River Laboratories. IL-9R–deficient mice (Steenwinckel et al., 2007) were provided by J.-C. Renauld (Ludwig Institute, Brussels, Belgium) and were bred in-house. FoxP3-GFP reporter mice were bred in-house after being provided by A. Rudensky (University of Washington, Seattle, WA). Spleen cells from IL-17F–Thy1.1 reporter mice were provided by C.T. Weaver (University of Alabama at Birmingham, Birmingham, AL) and have been previously described (Lee et al., 2009). RORC-GFP mice (Ivanov et al., 2006) were purchased from the Jackson Laboratory. All experiments using mice were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Dartmouth College.
EAE induction and clinical evaluation.
Age-matched female C57BL/6 and IL-9R KO mice that were 6–10 wk old were immunized subcutaneously with 200 µg of MOG35-55 peptide (Peptides International) emulsified in CFA (Sigma-Aldrich) on day 0 and an i.p. injection of 400 ng pertussis toxin (Sigma-Aldrich) on days 0 and 2. Mice were scored daily as previously described (Becher et al., 2002). In some experiments, mice were also treated with 200 µg of control Ig or αIL-9 (MM9C1) antibody (MedImmune) i.p. every other day starting the day before immunization. For adoptively transferred EAE, T cells from WT and KO mice on day 14 after EAE induction were isolated and further cultured for 3 d in vitro to promote Th17 cell differentiation, as previously described (Ogura et al., 2008). Each mouse received 1.5–2 × 106 cells intravenously and 200 ng pertussis toxin on the day of the cell transfer and 7 d after the initial immunization.
Histochemistry.
Spinal cords from perfused mice with EAE were isolated. Transverse sections of spinal cords (5-µm) were cut and stained with hematoxylin and eosin. Photographs of the sections were taken at 20× magnification using a microscope (BX41; Olympus).
In vitro T cell differentiation.
CD4+ CD25− FoxP3GFP− cells were cultured as previously described in RPMI 1640 to obtain Th1 and Th2 cells (Lu et al., 2006). Th17 cells were generated with 2.5 ng/ml TGF-β, 20 ng/ml IL-6, 10 µg/ml αIFN-γ, and 10 µg/ml αIL-4 using 5 µg/ml of plate-bound CD3 (145-2C11; BioXCell) and CD28 (PV-1; BioXCell), respectively. For the differentiation of cells in the presence of TGF-β and either IL-2 or IL-4, all samples received 5 ng/ml TGF-β with either 100 U/ml IL-2 or 10 ng/ml IL-4.
qRT-PCR.
RNA from various CD4 T cell subsets was isolated according to the manufacturer’s directions using RNAeasy (QIAGEN). RNA was extracted from spinal cords, further treated with DNase using RNAqueous (Applied Biosystems), and transcribed to cDNA using a cDNA synthesis kit (iScript; Bio-Rad Laboratories). PCR was performed using iQ SYBR Green Supermix (Bio-Rad Laboratories) on an iCycler (Bio-Rad Laboratories), as previously described (Becher et al., 2002). All samples were normalized to expression of GAPDH or β-actin.
ELISAs and ELISPOTs.
CD4 T cells from the draining lymph nodes of EAE-immunized mice were isolated using a CD4+ selection kit (StemCell Technologies Inc.). Cells were cultured in the presence of irradiated antigen-presenting cells and MOG peptide for 24 h. An IL-17A (eBioscience) ELISPOT was performed with antibodies from the indicated manufacturer. CD4 T cells from in vitro cultures were restimulated for the time indicated in the figures before collection of supernatants. Splenic T cells from RORC-GFP mice immunized with MOG emulsified in CFA were isolated 7 d later, sorted on a FACSAria (BD), and recalled in vitro with PMA/ionomycin for 4 h. An IL-9 ELISA (PeproTech) was performed according to the manufacturer’s recommendations using duplicates or triplicates of each sample and a standard curve of the recombinant cytokine.
Antibodies and reagents.
Antibodies against the following were used for staining and, unless indicated otherwise, were obtained from BioLegend: CCR6-PE, CD11b-FITC, CD11c-allophycocyanin (APC), CD4-PerCP, CD44-FITC and -APC, CD45-A700, c-kit–A647, FCϵRI-PE (eBioscience), Thy1.1-PE (eBioscience), IL-6–PE, IL-17A–FITC and –APC, and IFN-γ–PE and –APC. Anti-PE beads and LS columns were purchased from Miltenyi Biotec and used according to the manufacturer’s directions to enrich for Thy1.1-PE+ T cells.
CNS lymphocyte isolation.
Brains and spinal cords of perfused mice were isolated as previously described (Becher et al., 2002). In brief, tissue was digested with DNase (Roche) and liberase (Roche), homogenized, and separated over a Percoll gradient before use.
MC enrichment.
Lymph node cells were stained with c-kit–A647 and FCϵRI-PE. Cells were enriched using anti-PE beads (StemCell Technologies Inc.) according to the manufacturer’s directions before being used for FACS analysis. MC numbers were calculated as follows: MC numbers total LN = (MC number in enriched sample) × (total number of cells in LN)/(number of LN cells used at the start of enrichment).
Intracellular staining.
For cytokine staining, cells were restimulated in vitro for 5 h in the presence of 50 ng/ml PMA (EMD), 500 ng/ml ionomycin (EMD), and 1 µl/ml monensin (BioLegend), followed by surface and intracellular cytokine staining according to the manufacturer’s directions (BD).
Graphs and statistics.
All graphs were created and the indicated statistical analysis was performed using Prism (GraphPad Software, Inc.).
Online supplemental material.
Fig. S1 demonstrates ex vivo IL-9 production by in vivo–generated Th17 cells from IL-17F reporter mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090246/DC1.
Acknowledgments
We would like to thank J.-C. Renauld for providing us with IL-9R KO mice.
This work was supported by National Institutes of Health research grant AI048667. E.C. Nowak was supported by grant T32 AI07363 (awarded to William R. Green, PhD).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: CNS, central nervous system; EAE, experimental autoimmune encephalomyelitis; MC, mast cell; MOG, myelin oligodendrocyte peptide; qRT-PCR, quantitative real-time PCR.
References
- Becher B., Durell B.G., Noelle R.J. 2002. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12.J. Clin. Invest. 110:493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner T., Soffer D., Shalit M., Levi-Schaffer F. 1994. Mast cells in experimental allergic encephalomyelitis: characterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides.J. Neurol. Sci. 122:210–213 [DOI] [PubMed] [Google Scholar]
- Cosmi L., Liotta F., Angeli R., Mazzinghi B., Santarlasci V., Manetti R., Lasagni L., Vanini V., Romagnani P., Maggi E., et al. 2004. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines.Blood. 103:3117–3121 [DOI] [PubMed] [Google Scholar]
- Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain.Nature. 421:744–748 [DOI] [PubMed] [Google Scholar]
- Dardalhon V., Awasthi A., Kwon H., Galileos G., Gao W., Sobel R.A., Mitsdoerffer M., Strom T.B., Elyaman W., Ho I.C., et al. 2008. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3− effector T cells.Nat. Immunol. 9:1347–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner H., Humphreys N., Renauld J.C., Van Snick J., Grencis R. 1997. Interleukin-9 is involved in host protective immunity to intestinal nematode infection.Eur. J. Immunol. 27:2536–2540 [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Groschwitz K., Abonia J.P., Brandt E.B., Cohen E., Blanchard C., Ahrens R., Seidu L., Mckenzie A., Strait R., et al. 2008. IL-9– and mast cell–mediated intestinal permeability predisposes to oral antigen hypersensitivity.J. Exp. Med. 205:897–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner A., Blum H., Rollinghoff M. 1993. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice.Immunobiology. 189:419–435 [DOI] [PubMed] [Google Scholar]
- Gregory G.D., Robbie-Ryan M., Secor V.H., Sabatino J.J., Jr., Brown M.A. 2005. Mast cells are required for optimal autoreactive T cell responses in a murine model of multiple sclerosis.Eur. J. Immunol. 35:3478–3486 [DOI] [PubMed] [Google Scholar]
- Hauber H.P., Bergeron C., Hamid Q. 2004. IL-9 in allergic inflammation.Int. Arch. Allergy Immunol. 134:79–87 [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells.Cell. 126:1121–1133 [DOI] [PubMed] [Google Scholar]
- Knoops L., Renauld J.C. 2004. IL-9 and its receptor: from signal transduction to tumorigenesis.Growth Factors. 22:207–215 [DOI] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Gao W., Awasthi A., Jager A., Strom T.B., Oukka M., Kuchroo V.K. 2007. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells.Nature. 448:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.K., Turner H., Maynard C.L., Oliver J.R., Chen D., Elson C.O., Weaver C.T. 2009. Late developmental plasticity in the T helper 17 lineage.Immunity. 30:92–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A., Kohler R.E., Townley S., Haylock-Jacobs S., Comerford I., Caon A.C., Webster J., Harrison J.M., Swann J., Clark-Lewis I., et al. 2009. Inhibition of CCR6 function reduces the severity of experimental autoimmune encephalomyelitis via effects on the priming phase of the immune response.J. Immunol. 182:3121–3130 [DOI] [PubMed] [Google Scholar]
- Liu Y., Teige I., Birnir B., Issazadeh-Navikas S. 2006. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE.Nat. Med. 12:518–525 [DOI] [PubMed] [Google Scholar]
- Lu L.F., Lind E.F., Gondek D.C., Bennett K.A., Gleeson M.W., Pino-Lagos K., Scott Z.A., Coyle A.J., Reed J.L., Van Snick J., et al. 2006. Mast cells are essential intermediaries in regulatory T-cell tolerance.Nature. 442:997–1002 [DOI] [PubMed] [Google Scholar]
- Ogura H., Murakami M., Okuyama Y., Tsuruoka M., Kitabayashi C., Kanamoto M., Nishihara M., Iwakura Y., Hirano T. 2008. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction.Immunity. 29:628–636 [DOI] [PubMed] [Google Scholar]
- Reboldi A., Coisne C., Baumjohann D., Benvenuto F., Bottinelli D., Lira S., Uccelli A., Lanzavecchia A., Engelhardt B., Sallusto F. 2009. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE.Nat. Immunol. 10:514–523 [DOI] [PubMed] [Google Scholar]
- Schmitt E., Germann T., Goedert S., Hoehn P., Huels C., Koelsch S., Kuhn R., Muller W., Palm N., Rude E. 1994. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma.J. Immunol. 153:3989–3996 [PubMed] [Google Scholar]
- Secor V.H., Secor W.E., Gutekunst C.A., Brown M.A. 2000. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis.J. Exp. Med. 191:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenwinckel V., Louahed J., Orabona C., Huaux F., Warnier G., Mckenzie A., Lison D., Levitt R., Renauld J.C. 2007. IL-13 mediates in vivo IL-9 activities on lung epithelial cells but not on hematopoietic cells.J. Immunol. 178:3244–3251 [DOI] [PubMed] [Google Scholar]
- Tanzola M.B., Robbie-Ryan M., Gutekunst C.A., Brown M.A. 2003. Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course.J. Immunol. 171:4385–4391 [DOI] [PubMed] [Google Scholar]
- Townsend J.M., Fallon G.P., Matthews J.D., Smith P., Jolin E.H., Mckenzie N.A. 2000. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development.Immunity. 13:573–583 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Uyttenhove C., Van Snick J., Helmby H., Westendorf A., Buer J., Martin B., Wilhelm C., Stockinger B. 2008. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset.Nat. Immunol. 9:1341–1346 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Mclane M., Levitt R.C. 2001. Th2 cytokines and asthma. Interleukin-9 as a therapeutic target for asthma.Respir. Res. 2:80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Paul W.E. 2008. CD4 T cells: fates, functions, and faults.Blood. 112:1557–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]