Abstract
A green-lipped mussel (GLM) preparation was evaluated in a randomized, double-controlled and double-blinded clinical trial. It was hypothesized that the treatment effect would be less than that of the positive control (carprofen) but more than that of the negative control (placebo). Forty-five dogs with chronic pain and a radiographic diagnosis of osteoarthritis that were randomly allocated into one of three groups completed the study. All dogs were fed the test products or placebo for 8 weeks. The dogs were evaluated four times, at 4-week intervals. Six different variables were assessed: veterinary-assessed mobility index, two force plate variables, owner-evaluated chronic pain index and pain as well as locomotion visual analogue scales (VASs). Intake of extra carprofen was also evaluated. A chi-squared and a Mann–Whitney test were used to determine significance between groups. When changed to dichotomous variables, there were more dogs in the GLM than in the placebo group that improved, according to veterinary-assessed mobility, owner-evaluated chronic pain index and pain VAS (P = 0.031, P = 0.025, P = 0.011, respectively). For the same three, the odds ratio and their confidence interval were over one. The extent of improvement was significantly different between the GLM and the control in veterinary-assessed mobility (P = 0.012) and pain VAS (P = 0.004). In conclusion, GLM alleviated chronic orthopedic pain in dogs although it was not as effective as carprofen. As no side-effects were detected, GLM may be beneficial in dogs e.g. when non-steroidal anti-inflammatory drugs cannot be used.
Keywords: Controlled, dog, Lyproflex®, nutraceutical, OA, placebo
Introduction
Over the last few years, there has been a growing interest in new treatment options for osteoarthritis (OA), both for humans and pets, especially dogs. The so-called nutraceuticals have become available, since some patients cannot tolerate or do not want to take the risk of non-steroidal anti-inflammatory drugs (NSAIDs) because of their side-effects (1,2). Nutraceuticals have been described as naturally occurring, biologically effective nutritional supplements that can confer some degree of health benefit and there is a whole new science referred to as ‘bioprospecting’(3) that explores and introduces these new herbs—or animal molecules or products. Currently, several nutraceuticals on the market are claiming to relieve arthritic symptoms. These products generally fall mainly into two distinct product groups, including glucosamine and chondroitin sulfate combinations or polyunsaturated fatty acids (PUFAs), particularly Omega-3 series PUFAs, such as those derived from marine sources. One of the nutraceuticals that may benefit OA is a product based on Perna canaliculus, or the green-lipped mussel (GLM) that has been thoroughly examined by Halpern (4) [see also (5)].
The early GLM-based products were often produced from rejected mussel meat from human food processing, which typically included steam processing as part of the manufacturing process and the early trials using these unstable GLMs, showed poor results. In the 1980s, a method of temperature-controlled cold processing and stabilization of GLM by adding organic acids to prevent oxidation and freeze-drying was patented (6). After 1986, this new stabilized GLM has been in use and is documented to be efficacious in treating experimental arthritis in rats (7,8), clinical arthritis in humans (9,10) and more recently also in dogs (11,12).
The GLM product used in this study, which originates from mussel farms in the Pacific Ocean, has been harvested when the mussels are 12 to 18-months old, and is stabilized, freeze-dried at −40°C and packed immediately thereafter. The GLM product is a rich source of nutrients, including glycosaminoglycans (GAGs), such as chondroitin sulfates, vitamins, minerals and Omega-3 series PUFAs. It is not totally clear how the products function (13) [this is not totally clear for NSAIDs either (14,15)], despite substantial research. Although there has been only a few studies published on the use of stabilized GLM on dogs, it is already available for dogs with OA, as a powder, as capsules or incorporated into pebbles.
The aim of this study was to evaluate a GLM product as an OA nutraceutical for dogs in a randomized, double-controlled and double-blinded trial. We expected the positive control carprofen to significantly reduce pain and locomotion difficulties, and the negative control (placebo) to have no such effect. The effect of the nutraceutical was hypothesized to lie somewhere between those of the positive and negative. Objective, semi-objective and subjective variables were used for assessment. Since the GLM is also used in humans, the treatment outcome is of interest for many people as OA is becoming one of the most prevalent and costly diseases in our aging society. Due to the side-effects associated with NSAIDs, 60–90% of dissatisfied human arthritis patients are reported to seek complementary therapies for their disease (16).
Methods
Dogs
Inclusion criteria were that dogs had clinical signs and a radiographic diagnosis of OA, in either a hip joint or an elbow joint. The owner had to have described at least two of the following signs as being frequent: difficulty in lying down and/or in getting up from a lying position, difficulty in jumping or refusing to jump, difficulty in walking up or down stairs, or definite lameness. Dogs were excluded from the study if they had had prior surgery of the evaluated joint, inadequate clinical symptoms, systemic or infectious disease, neurological deficits, lameness from articular infection, or recent trauma.
Sixty-eight dogs were chosen based on 124 telephone interviews with owners. Of these, 51 dogs will be presented here and the remaining 17 constituted a treatment group for another study (17). Six dogs (two in each group) were excluded from the study at some point for the following reasons: having had a previous operation of the affected hip joint, having a transverse vertebra (n = 2), sustaining a cruciate ligament injury (n = 2) and diagnosed with degenerative myelopathy. There were 15 dogs in each group that finished the study: 12 dogs with canine hip dysplasia (CHD) and three dogs with elbow OA in each group, all confirmed by radiographs. Twenty-five dogs were male and 20 were female. The median age was 6 years (range 1–11) and the median body weight (BW) was 34 kg (range 18–56). There were both uni- and bi-laterally affected dogs. All dogs had either moderate or severe radiological changes in the worst-affected hip or elbow joint (18).
Owners were asked not to give the dogs NSAIDs or corticosteroids for at least 30 days and no Na-pentosan polysulfate (Carthrophen®, Biopharm Pty. Ltd., Australia) for at least 90 days prior to the study. However, this proved not to be always the case, as some owners felt that their dogs were in such pain that they therefore gave them NSAIDs. The use of the additional analgesic pre-trial was, however, recorded in the questionnaire.
Test Products
The product tested in this study was the GLM (Lyproflex® 500 mg; ICENI, OMNI nutraceuticals, Cambridgeshire/Biofarm Oy, Finland). The capsules contain 46–52% protein, 5.9–9.3% fat, 8.0–21.5% carbohydrates, 3.9–5.8% moisture, 14–25% ash, >12 mg eicosatetraenoic acid (ETA)/100 g, > 500 mg eicosapentaenoic acid (EPA)/100 g and >400 mg docosahexaenoic acid (DHA)/100 g. The initial dose was 4 (for dogs ≤ 40 kg BW) or 6 (for dogs > 40 kg BW) capsules/day for 10 days, then continuing with half of the loading dose (i.e. two or three capsules) for the rest of the study. This meant an initial dose of 20–49 mg/kg/day depending on the BW of the dog.
Two control groups were included: the established positive control carprofen (Rimadyl® 50 mg; Pfizer, Helsinki, Finland) at a dose of 2 mg/kg twice a day and a negative control that received all products as placebos. Carprofen was a white pill without the usual stamp and its placebo an identical lactose tablet, GLM was a greenish capsule and its placebo a very similarly colored lactose capsule. In addition, all dogs were administered an ampoule of isotonic sodium chloride solution as a placebo for another study (17). The products were coded and organized by a research assistant who was not involved in the study thereafter.
For ethical reasons, all owners were also given a package of 50 mg carprofen in normal packaging and with the normal stamp on the tablet, at the start of the trial. This could be used as additional pain relief (dose of one tablet for a dog of 20–30 kg BW, two tablets for a dog of 31–40 kg and three tablets for a dog of 41–60 kg) if they felt the dog was in considerable pain. The number of additional carprofen doses used was recorded in the questionnaire.
Study Protocol
The study was designed as a randomized double-controlled, double-blinded clinical trial using the CONSORT guidelines (19). A secretary made the first appointments, and at this first visit (W0), the dogs were assigned into groups, in order of arrival using a computer-generated random list. Only the location of the diseased (hip or elbow OA) was stratified for in the randomization. Initial clinical, orthopedic and neurological examinations were performed and diagnostic criteria included decreased range of motion and pain on stretching the hip or flexing the elbow. Radiographs were taken of the dogs’ hips and/or elbows and other joints if needed. The W0 evaluation and W0 questionnaire was set as baseline, except for pre-trial analgesic medication, where the assessment was made at W−4, as the owners were told not to use them anymore between W−4 and W0. Follow-up visits with questionnaires for reassessment were at 4, 8 and 12 weeks (W4, W8 and W12). The dogs were given the products orally for 8 weeks, from W0 to W8. At W12, the dogs had been off all medication for 4 weeks and were evaluated to determine long-term effects of the different treatments as follow up. All evaluators (veterinarians and owners) and all technical assistants were blinded. Owners of the dogs were required to sign informed consent forms. The study protocol was approved by the Ethics Committee of the University of Helsinki.
Veterinary Evaluation
Two veterinarians subjectively assessed three parameters at W0, W4, W8 and W12: locomotion, jumping and walking stairs using 0–4 descriptive scales. The three scores assigned by the two veterinarians were summed to form a veterinary-assessed mobility index, with a possible minimum score of 0 and a maximum of 24 (2 × 3 × 0–4).
Owner Assessment
Four weeks before the first visit (W−4) and during each following evaluation, owners answered a three-part questionnaire. The first part used a descriptive scale of 0–4 and contained questions about attitude, behavior and locomotion. Of these, 11 questions were combined to form a combined owner-assessed chronic pain index, as described previously (20). The second part contained two 10 cm visual analogue scales (VASs): one for pain and the other for locomotion. The end of the lines to the left represented no pain or no difficulties in locomotion, and to the right, the worst possible pain or the most severe difficulties in locomotion. The third part consisted of questions about possible adverse reactions to treatment, including change in appetite, vomiting, diarrhea and atopic skin reactions. The question about additional analgesics was not a continuous variable but used the following scale: ‘during the last four weeks additional carprofen was given 1 = not at all, 2 = 1–2 times, 3 = about once a week, 4 = about 3–5 times a week, 5 = daily/almost daily’.
Objective Evaluation of Gait
Gait was analyzed by force plate gait analysis (Kistler forceplate, Type 9286, Kistler Instrumente AG Winterhur, CH-8408, Switzerland), which assesses weight bearing of limbs. The force plate was submerged into the concrete floor so that the plate and floor surfaces were on the same level. The floor was then covered with a 2 mm thick rubber mat that extended from 7 m before to 7 m after the plate, forming a 14 m walkway. A hole was cut in the mat over the force plate and a 3–4 mm gap was left between the force plate mat and the rest of the mat. The signal from the plate was processed and stored using a computer-based software program, and velocities and acceleration were determined by three photoelectric cells placed exactly 1 m apart and a start-interrupt timer system (Aquire 6.0, Sharon Software Inc., DeWitt, MI, USA).
Dogs guided by their owners trotted over the walkway from left to right. The speed was one comfortable for each dog in trot and had to be in the same range (± 0.5 m/s) for the dog each time the test was performed (at W0, W4, W8 and W12). The acceleration was < 0.5 m/s/s and contact had to be made with the plate first by the forelimb and shortly after with the hind limb of the same side for the evaluation to be valid. The test was repeated until sufficient valid results were obtained for both left and right limbs.
Three valid measurements for each side and for each visit were then chosen by a blinded assistant (one not otherwise participating in the study) according to speed, acceleration and with no interferences, such as gait abnormalities or extra body movements. The mean of these three measurements was used for analysis. The ground reaction forces were normalized for each dog's BW and mean peak vertical force (PVF) and mean vertical impulse were used as variables. Only measurements from the most severely affected leg at time W0 were used in the analysis.
Blood Samples
Blood samples were collected from the dogs at each visit. Blood urea nitrogen (BUN), creatinine, serum alanine aminotransferase (ALAT), alkaline phosphatase (AFOS), total protein and albumin were analyzed.
Statistical Analysis
The number of dogs needed in each group was calculated for a two-tailed test (Fisher). The sample size (n = 15/group) was sufficiently large to detect a 47% difference (11) in treatment outcome (effective versus not effective) with a statistical power of 0.8 and allowing for a 5% α-error.
To counteract the effect of the extra NSAID on dogs that at W8 had used extra carprofen more than three times per week, their W8− values were changed into the most negative value measured for any dog at that time. This enabled us to use the whole data in the statistical analyses.
For calculating the percentage of dogs/group that improved between baseline and W8 and the odds ratio, the results of each variable were converted into dichotomous responses of ‘improved’ and ‘not improved’. Dogs that deteriorated and dogs with no change in the evaluated variable were considered ‘not improved’. The difference between the treatment groups and the placebo was calculated using a chi-squared test. The odds ratio was calculated using the common Mantel–Haenszel odds ratio estimate and the confidence interval (CI) was set to 95%. An odds ratio more than 1.0 indicated a beneficial effect of the test treatments.
The changes from baseline to W8 were also calculated for each variable. The difference between the GLM and placebo group was analyzed using the Mann–Whitney test. The changes from W0 to W8 in the force plate variables were proportional in the front and hind legs, although the values were different. Therefore, force plate data of all four legs were analyzed together. The dogs, for which no force plate results could be registered, were considered ‘not improved’ in the dichotomous evaluations and excluded in the median analyses. A correlation test was used to evaluate the association between the assessments of the two veterinarians. Statistical significance was set at P < 0.05. Statistical tests were preformed using SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline Values
Baseline variable median (range) values were: for the veterinary-assessed mobility index: 6 (10–18), PVF: 71.21 (54.7–135.25), vertical impulse: 9.11 (6.02–19.9), owner-evaluated chronic pain index: 16 (4–25), pain VAS: 3.55 (0–8.4) and locomotion VAS: 4.8 (0–8.3). There was no statistical bias between the groups at baseline. The evaluations of the two veterinarians correlated well (R = 0.853, P < 0.01).
Dichotomous Responses
There were four dogs (all from the placebo group) that had used extra carprofen more than three times per week at W8. For three of the variables [veterinary-assessed mobility index (P = 0.031), chronic pain index (P = 0.028) and pain VAS (P = 0.011)] there were significantly more improved dogs in the GLM group compared to the placebo group (Table 1). The odds ratio for the veterinary-assessed mobility index was 5.5 (95% CI 1.14–26.41) indicating that a dog that had received the GLM product was 5.5 times more likely to have a positive response compared to a dog that had received the placebo. The odds ratio for the force plate PVF was 2.50 (95% CI 0.52–11.93), for the force plate impulse 2.40 (95% CI 0.52–10.99), for the owner-assessed chronic pain index was 6.0 (95% CI 1.17–30.72), for the pain VAS 8.0 (95% CI 1.52–42.04) and for the locomotion VAS 4.12 (95% CI 0.88–19.27).
Table 1.
Percentage of improved dogs and median (range) of improvement for evaluated variables, per group from W0 to W8
| Carprofen (n = 15) | GLM (n = 15) | Placebo (n = 15) | ||||
|---|---|---|---|---|---|---|
| Improved P= | Improvement Median (range), P= | Improved P= | Improvement Median (range), P= | Improved | Improvement Median (range) | |
| Veterinary mobility index | 66.7% | 3 (0–8) | 66.7% | 1 (−3 to 7) | 26.7% | −3 (−14 to 3) |
| 0.031 | 0.001 | 0.031 | 0.012 | |||
| Force plate PVF | 66.7% | 3.2 (−8.2 to 11.8) | 46.7% | 0.17 (−5.6 to 12) | 26.7% | −0.9 (−33.6 to 10) |
| 0.031 | 0.079 | 0.264 | 0.201 n = 14 | n = 13 | ||
| Force plate Impulse | 80.0% | 0.4 (−0.5 to 1.3) | 53.3% | 0.20 (−1 to 1.54) | 33.3% | −0.0 (−3.3 to 0.8) |
| 0.011 | 0.009 | 0.277 | 0.123 n = 14 | n = 13 | ||
| Chronic pain index | 80.0% | 9 (−9 to 19) | 80.0% | 2 (−2 to 6) | 40.0% | −3 (−25 to 8) |
| 0.028 | <0.001 | 0.028 | 0.102 | |||
| Pain VAS | 85.7% | 1.4 (−6 to 8.4) | 66.7% | 0.6 (−3.3 to 3.3) | 20.0% | −1.7 (−7 to 3.2) |
| 0.001 | <0.001 | 0.011 | 0.004 | |||
| Locomotion VAS | 85.7% | 3.1(−1.9 to 6.2) | 60.0% | 0.2 (−3.8 to 3.5) | 26.7% | −1 (−6.6 to 5) |
| 0.002 | 0.001 | 0.070 | 0.057 | |||
For each treatment group: First column: Percentage of dogs in the group that improved. Below: P = Difference in percentage of improved between treatment groups and placebo. Second column: Median (with range) of change from W0 to W8 [(+), improvement; (−), deterioration] in evaluated variables for the carprofen-, GLM- and placebo-groups. P = Difference in improvement between treatment groups and placebo (the force plate values do not include three dogs for whom no results were obtained). n, number of patients per group; GLM, green-lipped mussel; PVF, peak vertical force; VAS, visual analogue scale.
Medians of the Change from W0 to W8
All variables showed a similar trend of improvement, with carprofen being the most efficient, placebo the least and GLM being between these two (Table 1). There was a significant difference between the GLM and the placebo in two variables [veterinary-assessed mobility index (P = 0.012) and pain VAS (P = 0.004)] and a third variable was close to significant [locomotion VAS (P = 0.057)].
Extra Carprofen
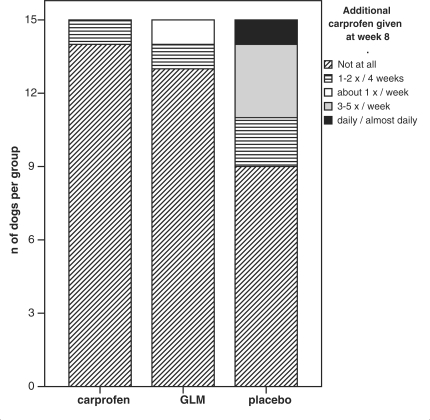
At W−4, before the owners were requested to stop all medication, 14% of the carprofen group, 13% of the GLM group and 8% of the placebo group were given NSAIDs once a week or more. At W8, (Fig. 1) 0, 7 and 27% of the respective groups were given additional carprofen once a week or more. At follow-up (W12), the respective numbers were 33, 14 and 29%. The differences between both GLM and carprofen compared to the placebo group at time W8 were significant (P = 0.021 and P = 0.008, respectively).
Figure 1.
At the end of the treatment period (W8), there was 4/15 dogs in the placebo group given extra carprofen 3–7 days/week (n = number of dogs).
Complications and Side-effects
Three dogs (one in the GLM group, two in the placebo group) were so lame during the visits that no usable data were obtained from the force plate. Two of these dogs (one in the GLM group, one in the placebo group) were euthanized between W8 and W12 due to severe pain. In populations, neither our findings of clinical side-effects nor clinical chemistry in any of the blood parameters were severe or related to any particular group. Palatability was never a concern.
Discussion
In our study, dogs showed a beneficial clinical response to treating OA-induced pain and locomotion difficulties with GLM. More dogs improved in the GLM group compared to the placebo group and the extent of treatment effects was between that of our two control groups, as can be seen from the median values in Table 1. The carprofen had in previous studies shown 56–80% of improvement in dogs with OA (graded by veterinarians and owners) whereas the placebo in the same studies showed improvement in only 23–38% of the cases (21, 22). As these numbers are close to the results we obtained in our study for the two control groups (Table 1), they indicate that our cohort reflects reality well and that we can trust the results of our treatment group. The fact that extra carprofen was used significantly more often in the placebo group at W8 is also a positive result for the tested product.
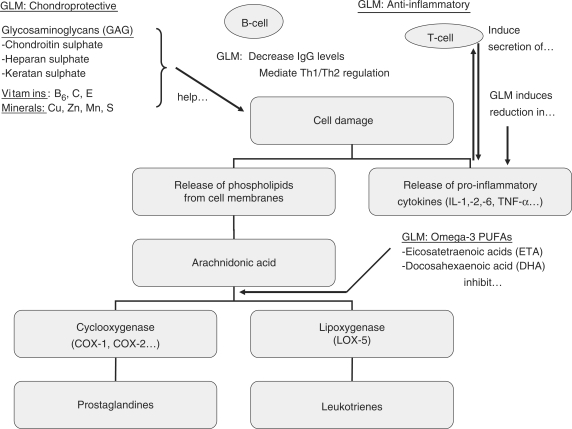
This positive outcome opens a discussion about possible working mechanisms of the GLM (Fig. 2). In the early GLM clinical trials on human patients, the outcomes were not good and often contradictory (23,24). Twenty years later, possibly after having stabilized the product by freeze-drying and lyophilizing, the results of clinical trials for GLM have been significantly promising (9–12). The lyophilizing process might have been the more important as in fact, the difference in lipid, sterol or fatty acid composition of frozen and freeze-dried GLM has been shown to be non-existent; the only major difference was between total lipid composition on a dry weight basis because of the removal of water in the deep-frozen product (25). The potent anti-inflammatory activity of GLM powder was confirmed in vivo using the established rat paw oedema model; rats fed mussel lipids perorally developed neither adjuvant-induced polyarthritis nor collagen-induced auto-allergic arthritis (8). However, these lipids showed only marginal inhibition of carrageenan-induced paw edema in rats (acute irritation assay, which is the standard test for NSAIDs), indicating that they do not mimic rapid-acting NSAIDs (8,13). Macrides and others (7) found that the ETAs of GLM had considerable anti-inflammatory activity. In vitro, the extracted lipids have been shown to possess significant cyclo-oxygenase (COX) and lipoxygenase (LOX-5) inhibitory activity; hence, the GLM seems to be working on the same mechanisms as newer NSAIDs (8).
Figure 2.
Main active constituents of the green lipped mussel and their effect on the inflammation pathways of osteoarthritis. The main active constituents, according to how we understand their working mechanisms now: The Omega-3 PUFAs (especially the ETAs) have anti-inflammatory activity; they possess significant cyclo-oxygenase (COX 1 and 2) and lipoxygenase (LOX-5) inhibitory activity. Due to their glycosaminoglycan content (especially chondroitin sulphate with its high glucosamine content), the GLM may have chondroprotective properties. The vitamins and minerals are needed in cartilage anabolism. GLM, green-lipped mussel; Th, T helper cell; IL, interleukin; TNF, tumor necrosis factor.
This dual inhibition of both LOX- and COX metabolic pathways may offer an explanation for the reported clinical efficacy and favorable gastrointestinal tolerability of GLM. Platelet aggregation remains unaltered and the lipid fraction be non-gastrotoxic in fasted disease-stressed arthritic rats at a dose of 300 mg/kg (treatment dose 20 mg/kg) (8,13). This shows that the GLM does not have the negative side-effects of the NSAIDs. Recently, new GLM extracts were tested (26) and a Tween-20 extract (that draws out membrane-bound proteins by a cationic detergent) effectively inhibits both COX-1 and COX-2 activity. It also induced a significant reduction in TNF-α, IL-1, IL-2 and IL-6 and decreased IgG levels, indicating that GLM may regulate the immune system and promote humoral and cellular activity (26).
The active components possess a molecular weight above 100 kDa and when a proteolytic enzyme was added to the extract, it eliminated the component effective against inflammatory cytokines, suggesting that at least part of the active substance resides in the protein moiety associated with the glycogen, probably as a glycoprotein (26), as already had been suggested earlier (27). However, the component effective against COX enzymes was resistant to this induced proteolysis, indicating that there are different types of active components (26). It was suggested that GLM mediates T-(lymphocytes) helper cells Th1/Th2 regulation as it relates to inflammation and therefore plays an immunomodulatory role (26). The chondroitin sulfate and the other GAGs of the GLM further work as building blocks in cartilage anabolism; glucosamine is one of their main constituents. They help the joint capsule to hold water and to adapt to changes in pressure, thereby absorbing shock induced by abnormal join stress (28). The role of minerals and vitamins has not been studied, but it is possible that they also contribute to the positive effects of the GLM. As seen earlier, the GLM probably acts through several different working mechanisms.
Three studies exist on stabilized GLM as a treatment for canine OA and our findings are consistent with two of them. Bierer and Bui (11) conducted three 6-week, randomized, double-blinded trials, in which they compared three different GLM dog feeds with control feeds. They used a total arthritic score by summing eight variables. As in our study, all individual variables showed no significant improvement, although a significant change was observed in the total arthritis score in favor of all three GLM test groups. In our study, a different set of variables was used. The veterinarians evaluated only mobility and not range of motion, crepitus, etc., as we had noted that owner compliance was much higher if provocations that hurt the dogs were not used.
Two force plate measurements were chosen as objective variables. Force plate has been used in similar studies to evaluate treatments of hip (22,29–31) and elbow (22,31,32) joints. The best variables for these conditions were considered to be PVF and vertical impulse, both of which were included here. The change from baseline to end of treatment in vertical impulse and PVF in our study was in the same range as in previous studies (30) but the range was larger. Furthermore, we used three owner-assessed variables: two VAS scores, one of them widely used in studies assessing human pain and a chronic pain index that has been shown to correlate well with chronic pain due to hip OA in dogs (20). In our study two of these three owner-assessed variables showed significance between the GLM group and the placebo. Thus, while our variables were different from the eight variables of Bierer and Bui (11), also only three of our six variables showed a significant improvement between W0 and W8 in the GLM group, compared to the placebo group.
In the second dog trial, Dobenecker (33) used a smaller dose of GLM [25% of what was used by Bierer and Bui (11) and of our initial dose] and found no statistical improvement in the GLM dogs, compared to the placebo group. The third canine trial showed a significantly decreasing pain scale throughout the study (12) but obtained only close to significant differences compared to the placebo group (that in our opinion was quite unsuitable as a placebo, including both brewers’ yeast and dried fin-fish, which probably both would work positively in OA).
As far as side-effects from these products, they were neither severe nor related to any group. Carprofen and other NSAIDs can potentially have severe side-effects such as hepatic disease (especially in Labradors), renal toxicosis and irritation of the gastrointestinal tract (1,2), whereas GLM is reported to have none (9,10,34). In fact, research suggests that GLM may have chondroprotective properties due to its GAG, especially chondroitin sulfate, content (35–37). In addition, GLM has a slower onset (34,38–41) but a longer effect (23). The preliminary human study by Gibson et al. (23) indicated that the beneficial effects of GLM treatment, could last for 2–3 weeks after cessation of therapy, if given at least for 2 months. Our follow-up evaluation was at W12, 4 weeks after discontinuation of the trial, and the beneficial effects were still evident as could be seen e.g. by a smaller intake of extra carprofen in the GLM group compared to the two other groups.
Carprofen, by contrast, rapidly triggers the clinical response, but this vanishes quickly upon discontinuing the drug, which also was documented in our study where a third of owners in the carprofen group were using extra carprofen at follow up, compared to none at the end of the treatment period. The placebo group seemed to react in accordance with the seasonal disease pattern of our geographical region, although the means changed only slightly and the CI were large, making an exact interpretation difficult: they started showing more signs of pain when the weather changed (42,43) to a humid, raw cold (our placebo group became worse between W0 to W8) (Fig. 1) and later in the spring when the weather turned warm and dry (W8 to W12) the dogs were better again.
In future studies, to obtain an optimal effect from the GLM product, should reconsider some aspects of the treatment regime. As OA often is a clinically variable disease, not having homogeneous groups is a major drawback, and this likely influenced the results. However, although the cohort of dogs was non-homogeneous, observed as a wide range even at the start of the trial, the documented trend of improvement was clear and similar for all variables and may even have been more evident, if we had more dogs. The positive effects of the GLM could eventually have been underestimated, rather than overestimated: if a non-articular concurrent pain such as spondylosis or secondary muscle pain, etc. was present, there would have been a positive analgesic effect for these also in the NSAID carprofen group, while the GLM might have helped primarily in arthritic disorders (9–12). The choice of patients and the treatment time might also have an impact on the results.
Radiographically, all dogs in our study had moderate or severe OA. Although the radiological data does not correlate well with the true clinical picture, at least not in dog hip joint (20), these dogs might have been too seriously affected to benefit optimally from the product. In one of the older human studies, severity of the disease was shown to have an impact on treatment outcome; mild and moderate knee OA responded very well to GLM treatment, whereas patients with severe knee OA did not benefit from treatment (34). Also, our 2-month study period might have been too short, as some earlier human studies have been unable to show a significant improvement compared to controls until patients had ingested fatty acid products for 3–6 months (34,38–41).
In conclusion, our results suggest that the modern stabilized and freeze-dried GLM is more effective than the placebo in treating chronic pain due to moderate to severe OA and that it has no side-effects. For dogs that can not use NSAIDs or corticosteroids and for patients who need analgesic support over extended periods of time, oral GLM may be an acceptable alternative for treating chronic arthritis pain, although it does not alleviate pain as well as carprofen. As dogs are used as models for human OA, we hope these promising results will stimulate new human research in this area.
Acknowledgements
Funding was provided by Finnish Foundation of Veterinary Research, Helvi Knuuttila Foundation. The authors are grateful for grants received from the Finnish Kennel Club, Pfizer Finland, Boeringer Ingelheim Germany, Vetcare Finland and Heel Germany, which financed the acquisition of a force plate that made objective analyses possible. Further, we would like to thank Pfizer Finland and ICENI England for contributing the products and their placebos used in the different treatment groups in this study, without cost. We also wish to thank our two anonymous reviewers, Ms Patty Willis and the Editor for their valuable comments.
References
- 1.FDA. US ADE (Adverse Drug Experience) reports summary 1998. Animal Pharm. 1999;435:9. [Google Scholar]
- 2.MacPhail CM, Lappin MR, Meyer DJ, Smith SG, Webster CRL, Armstrong PJ. Hepatocellular toxicosis associated with administration of carprofen in 21 dogs. J Am Vet Med Assoc. 1998;212:1895–901. [PubMed] [Google Scholar]
- 3.Cooper EL. Bioprospecting: a CAM frontier. Evid Based Complement Alternat Med. 2005;2:1–3. doi: 10.1093/ecam/neh062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halpern GM. The Inflammation Revolution: A Natural Solution for Arthritis, Asthma & Other Inflammatory Disorders. NY: SquareOne Publishers; 2005. [Google Scholar]
- 5.Cooper EL. The amazing science behind nature's ‘miracle from the sea’. Evid Based Complement Alternat Med. 2005;2:569–70. [Google Scholar]
- 6.Broadbent JM, Kosuge Y. 1985. Stabilized mussel extract. New Zealand patent 211928 (April 29, 1985); Australian patent PG 4775/84 (May 1, 1984) [Google Scholar]
- 7.Macrides TA, Treschow AP, Kalafatis N, Wright PFA. The anti-inflammatory effects of Omega-3 tetraenoic fatty acids isolated from a lipid extract from the New Zealand green-lipped mussel. In: Proceedings of the 88th American Oil Chemists Society Annual Meeting: May 1997, Seattle. 1997 [Google Scholar]
- 8.Whitehouse MW, Macrides TA, Kalafatis N, Betts WH, Haynes DR, Broadbent J. The anti-inflammatory activity of a lipid fraction from the New Zealand green lipped mussel. Inflammopharmacology. 1997;5:237–46. doi: 10.1007/s10787-997-0002-0. [DOI] [PubMed] [Google Scholar]
- 9.Gibson SLM, Gibson RG. The treatment of arthritis with a lipid extract of Perna canaliculus: a randomized trial. Complement Ther Med. 1998;6:122–6. [Google Scholar]
- 10.Cho SH, Jung YB, Seong SC, Park HB, Byun KY, Lee DC, et al. Clinical efficacy and safety of Lyprinol, a patented extract from New Zealand green-lipped mussel (Perna canaliculus) in patients with osteoarthritis of the hip and knee: a multicenter 2-month clinical trial. Allergy Immunol. 2003;6:212–6. [PubMed] [Google Scholar]
- 11.Bierer TL, Bui LM. Improvement of arthritic signs in dogs fed green-lipped mussel (Perna canaliculus) J Nutr. 2002;132(Suppl):1634–6. doi: 10.1093/jn/132.6.1634S. [DOI] [PubMed] [Google Scholar]
- 12.Pollard B, Guilford WG, Ankenbauer-Perkins KL, Hedderley D. Clinical efficacy and tolerance of an extract of green-lipped mussel (Perna canaliculus) in dogs presumptively diagnosed with degenerative joint disease. New Zel Vet J. 2006;54:114–8. doi: 10.1080/00480169.2006.36622. [DOI] [PubMed] [Google Scholar]
- 13.Rainsford KD, Whitehouse MW. Gastroprotective and anti-inflammatory properties of green lipped mussel (Perna canaliculus) preparation. Arzn Forsch. 1980;30:2128–32. [PubMed] [Google Scholar]
- 14.Lees P, May SA, White D. Pharmacokinetics and dosage regimens of anti-inflammatory drugs. Ann Rech Vet. 1990;21(Suppl):73–8. [PubMed] [Google Scholar]
- 15.Fox SM, Johnston SA. Use of carprofen for the treatment of pain and inflammation in dogs. J Am Vet Med Assoc. 1997;210:1493–8. [PubMed] [Google Scholar]
- 16.Ahmed S, Anuntiyo J, Malemud CJ, Haqqi TM. Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. Evid Based Complement Alternat Med. 2005;2:301–8. doi: 10.1093/ecam/neh117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hielm-Björkman A, Tulamo R-M, Salonen H, Raekallio M. Evaluating a complementary therapy for moderate to severe canine osteoarthritis. Part II: a homeopathic combination preparation (Zeel®) eCAM. doi: 10.1093/ecam/nem143. doi:10.1093/ecam/nem143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federation Canine International. 1991. FCI Hip Dysplasia and Elbow classification. workshop in Dortmund, 14.6 1991 (brochure) [Google Scholar]
- 19.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Lancet. 2001;357:1191–4. [PubMed] [Google Scholar]
- 20.Hielm-Björkman AK, Kuusela E, Liman A, Markkola A, Saarto E, Huttunen P, et al. Evaluation of methods for assessment of pain associated with chronic osteoarthritis in dogs. J Am Vet Med Assoc. 2003;222:1552–8. doi: 10.2460/javma.2003.222.1552. [DOI] [PubMed] [Google Scholar]
- 21.Holtsinger RH, Parker RB, Beale BS, Friedman RL. The therapeutic efficacy of carprofen (Rimadyl-V™) in 209 clinical cases of canone degenerative joint disease. Vet Comp Orthop Traumatol. 1992;5:140–4. [Google Scholar]
- 22.Vasseur PB, Johnson AL, Budsberg SC, Lincoln JD, Toombs JP, Whitehair JG, et al. Randomized, controlled trial of the efficacy of carprofen, a nonsteroidal anti-inflammatory drug, in the treatment of osteoarthritis in dogs. J Am Vet Med Assoc. 1995;206:807–11. [PubMed] [Google Scholar]
- 23.Gibson RG, Gibson SLM, Conway V, Chappell D. Perna canaliculus in the treatment of arthritis. Practice. 1980;224:955–60. [PubMed] [Google Scholar]
- 24.Huskisson EC, Scott J, Bryans R. Seatone is ineffective in rheumatoid arthritis. Br Med J. 1981;282:1358–9. doi: 10.1136/bmj.282.6273.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy KJ, Mann NJ, Sinclair AJ. Fatty acid and sterol composition of frozen and freeze-dried New Zealand green lipped mussel (Perna canaliculus–) from three sites in New Zealand. Asia Pac J Clin Nutr. 2003;12:50–60. [PubMed] [Google Scholar]
- 26.Mani S, Lawson JW. In vitro modulation of inflammatory cytokine and IgG levels by extracts of Perna canaliculus. BMC Complement Alternat Med. 2006;6:1. doi: 10.1186/1472-6882-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller TE, Dodd J, Ormrod DJ, Geddes R. Anti-inflammatory activity of the glycogen extracted from Perna canaliculus (NZ green-lipped mussel) Agents Actions. 1993;38:C139–42. doi: 10.1007/BF01991164. [DOI] [PubMed] [Google Scholar]
- 28.Bauer JE. Evaluation of nutraceuticals, dietary supplements, and functional food ingredients for companion animals. J Am Vet Med Assoc. 1999;218:1755–60. doi: 10.2460/javma.2001.218.1755. [DOI] [PubMed] [Google Scholar]
- 29.Budsberg SC, Chambers JN, Van Lue SL, Foutz TL, Reece L. Prospective evaluation of ground reaction forces in dogs undergoing unilateral total hip replacement. Am J Vet Res. 1996;57:1781–5. [PubMed] [Google Scholar]
- 30.Budsberg SC, Johnston SA, Schwarz PD, De Camp CE, Claxton R. Efficacy of etodolac for the treatment of osteoarthritis of the hip joints in dogs. J Am Vet Med Assoc. 1999;214:206–10. [PubMed] [Google Scholar]
- 31.Moreau M, Dupuis J, Bonneau M, Desnoyers M. Clinical evaluation of a neutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet Rec. 2003;152:323–9. doi: 10.1136/vr.152.11.323. [DOI] [PubMed] [Google Scholar]
- 32.Theyse LFH, Hazewinkel HA, Van Den Brom WE. Force plate analyses before and after surgical treatment of unilateral fragmented coronoid process. Vet Comp Orthop Traumatol. 2000;13:135–40. [Google Scholar]
- 33.Dobenecker B, Beetz, Kienzle E. A placebo-controlled double-blind study on the effect of nutraceuticals (chondroitin sulfate and mussel extract) in dogs with joint diseases as perceived by their owners. J Nutr. 2002;132(Suppl):1690–1. doi: 10.1093/jn/132.6.1690S. [DOI] [PubMed] [Google Scholar]
- 34.Audeval B, Bouchacourt P. Etude controlée en double aveugle contre placebo, de l’extrait de moule Perna canaliculus (moule aux orles vertes) dans la gonoarthhrose. Gaz Medicale [in French] 1986;93:111–6. [Google Scholar]
- 35.Bassleer C, Henrotin Y, Franchimont P. In vitro evaluation of drugs proposed as chondroprotective agents. Int J Tissue React. 1992;14:231–41. [PubMed] [Google Scholar]
- 36.Korthauer W, Torre J. Treatment of deforming arthropathy in working dogs with ‘canosan’, a new glycosaminoglycan preparation. Kleintierprax. 1992;37:467–78. [Google Scholar]
- 37.Bucci LR. Chondroprotective agents: glucosamine salts and chondroitin sulfates. Townsend Lett Dr. 1994;1:52–4. [Google Scholar]
- 38.Cleland LG, French J, Betts HW, Murphy G, Elliot M. Clinical and biochemical effects of dietary fish oil supplements in rheumatoid arthritis. J Rheumatol. 1988;15:1471–5. [PubMed] [Google Scholar]
- 39.Kremer JM. Clinical studies on Omega-3 fatty acid supplementation in patients who have rheumatoid arthritis. Rheum Dis Clin North Am. 1991;17:391–402. [PubMed] [Google Scholar]
- 40.Volker D, Garg M. Dietary N-3 fatty acid supplementation in rheumatoid arthritis- mechanisms, clinical outcomes, controversies, and future directions. J Clin Biochem Nutr. 1996;20:83–97. [Google Scholar]
- 41.Cobb CS, Ernst E. Systematic review of a marine nutriceutical supplement in clinical trials for arthritis: the effectiveness of the New Zealand green-lipped mussel Perna canaliculus. Clin Rheumatol. 2005;25:275–84. doi: 10.1007/s10067-005-0001-8. [DOI] [PubMed] [Google Scholar]
- 42.Aikman H. The association between arthritis and the weather. Int J Biometeorol. 1997;40:192–9. doi: 10.1007/s004840050041. [DOI] [PubMed] [Google Scholar]
- 43.Strusberg I, Mendelberg RC, Serra HA, Strusberg AM. Influence of weather conditions on rheumatic pain. J Rheumatol. 2002;29:335–8. [PubMed] [Google Scholar]