Abstract
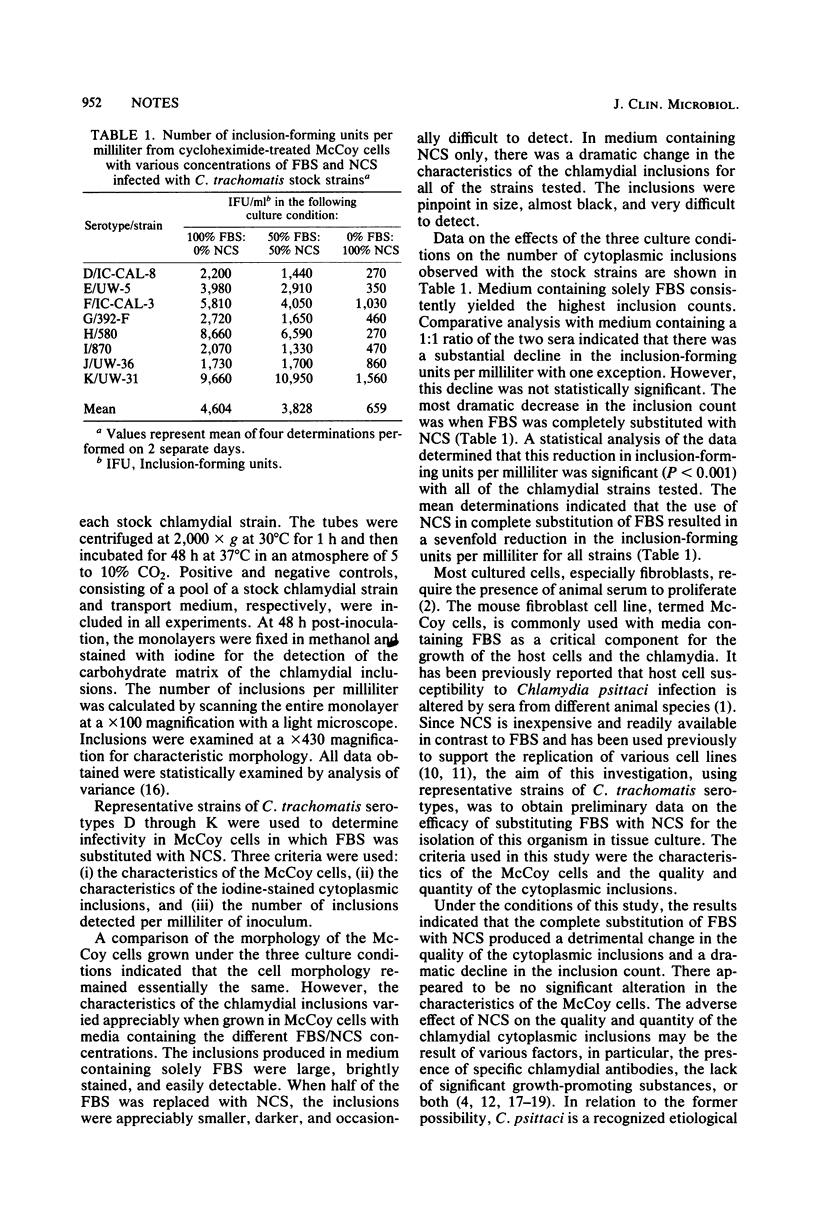
By using representative strains of Chlamydia trachomatis serotypes, the efficacy of substituting fetal bovine serum with newborn calf serum for the isolation of this organism in tissue culture was examined. The criteria used were the quality and quantity of the iodine-stained cytoplasmic inclusions and the characteristics of the McCoy cells. Complete substitution of fetal bovine serum with newborn calf serum produced a detrimental change in the quality of the cytoplasmic inclusions and a dramatic decline in the inclusion count (P less than 0.001) with all of the chlamydial strains tested. There appeared to be no significant alteration in the characteristics of the McCoy cells. It is recommended from this preliminary investigation that newborn calf serum should not be used for the isolation of C. trachomatis in tissue culture.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beem M. O., Saxon E. M. Respiratory-tract colonization and a distinctive pneumonia syndrome in infants infected with Chlamydia trachomatis. N Engl J Med. 1977 Feb 10;296(6):306–310. doi: 10.1056/NEJM197702102960604. [DOI] [PubMed] [Google Scholar]
- Boulanger P., Bannister G. L. Abortion Produced Experimentally In Cattle With An Agent Of The Psittacosis-Lymphogranuloma-Venereum Group of Viruses. Can J Comp Med Vet Sci. 1959 Aug;23(8):259–265. [PMC free article] [PubMed] [Google Scholar]
- Evans R. T. Suppression of Chlamydia trachomatis inclusion formation by fetal calf serum in cycloheximide-treated McCoy cells. J Clin Microbiol. 1980 Apr;11(4):424–425. doi: 10.1128/jcm.11.4.424-425.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommell G. T., Rothenberg R., Wang S., McIntosh K. Chlamydial infection of mothers and their infants. J Pediatr. 1979 Jul;95(1):28–32. doi: 10.1016/s0022-3476(79)80077-3. [DOI] [PubMed] [Google Scholar]
- Harrison H. R., English M. G., Lee C. K., Alexander E. R. Chlamydia trachomatis infant pneumonitis: comparison with matched controls and other infant pneumonitis. N Engl J Med. 1978 Mar 30;298(13):702–708. doi: 10.1056/NEJM197803302981303. [DOI] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Keddell J. E. Efficacy of various cell culture procedures for detection of Chlamydia trachomatis and applicability to diagnosis of pediatric infections. J Clin Microbiol. 1981 Apr;13(4):705–708. doi: 10.1128/jcm.13.4.705-708.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malewicz B., Jenkin H. M. Development of dengue virus plaques under serum-free overlay medium. J Clin Microbiol. 1979 May;9(5):609–614. doi: 10.1128/jcm.9.5.609-614.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. H., Brunstedt J., Andersson A., Frimodt-Møller C. Preservation of beta cell function in adult human pancreatic islets for several months in vitro. Diabetologia. 1979 Feb;16(2):97–100. doi: 10.1007/BF01225457. [DOI] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Schachter J., Grossman M., Holt J., Sweet R., Goodner E., Mills J. Prospective study of chlamydial infection in neonates. Lancet. 1979 Aug 25;2(8139):377–380. doi: 10.1016/s0140-6736(79)90400-8. [DOI] [PubMed] [Google Scholar]
- Schachter J., Lum L., Gooding C. A., Ostler B. Pneumonitis following inclusion blennorrhea. J Pediatr. 1975 Nov;87(5):779–780. doi: 10.1016/s0022-3476(75)80309-x. [DOI] [PubMed] [Google Scholar]
- Tipple M. A., Beem M. O., Saxon E. M. Clinical characteristics of the afebrile pneumonia associated with Chlamydia trachomatis infection in infants less than 6 months of age. Pediatrics. 1979 Feb;63(2):192–197. [PubMed] [Google Scholar]


