Abstract
The effects of lactic acid bacteria-fermented soybean extract (Biofermentics™; BF) on experimental models of hepatic and renal disorders were investigated in vivo and in vitro. In rat, hepatitis induced by feeding of deoxycholic acid (DCA, 0.5 wt/wt, n = 6) or intraperitoneal injection of d-galactosamine (GMN, 500 mg/body wt, n = 6), the increase in serum AST (aspartate aminotransferase) and ALT (alanine aminotransferase) levels were inhibited significantly (P < 0.05) by feeding a diet containing 5% dried BF. Moreover, the BF-administered rat group showed lower concentrations of blood urea nitrogen and a larger amount of urine as compared with values in the control group. Pretreatment of primary cell cultures of rat hepatic and renal cells with BF prior to exposure to dichromate (K2Cr2O7) resulted in a marked decrease of dichromate-induced cytotoxicity as evaluated by the leakage of lactate dehydrogenase The levels of dichromate-induced lipid peroxidation, as monitored by malondialdehyde formation, were also reduced by pretreatment of hepatocytes with BF. These results suggest that BF may play a role in hepatic and renal disorders, and may be useful for maintaining health in humans as well.
Keywords: anti-oxidation, fermentation, hepatitis, lactobacillus, yeasts
Introduction
It is now a world-wide trend to incorporate dietary supplements into comprehensive medicine (1), and evidence-based medicine (EBM) (2,3), which is required for the recognition and acceptance of these supplements. It has become widely recognized that soybeans contain beneficial components for human health, such as soy protein, peptides, oligosaccharides, phospholipids, isoflavones, saponins, minerals and vitamins (4). The fermentation of soybeans results in various compositional and functional changes, and a large variety of peptides and amino acids are produced from soy protein by different kinds of micro-organisms, which include lactic acid bacteria. Aglycones from isoflavone or saponin glucosides are released by β-glucosidase of fungi and lactic acid bacteria (5,6). The aglycon form of isoflavones and saponins has a higher absorptivity than the glycoside form in humans (7).
There are many traditional soybean products fermented by micro-organisms, particularly in the eastern Asian countries, such as soy sauce (lactobacilli and yeasts) (8), ‘natto’ (Bacillus subtilis natto) (9) and tempeh (Rhizopus sp.) (10). These products are fermented mostly by mold, yeast, bacteria or a combination of these micro-organisms. However, there have been few products fermented exclusively by lactic acid bacteria except a few recent soymilk products, fermented mostly by the inoculation of a single pure culture of bifidobacteria (11) or lactic acid bacteria (12) as probiotic food.
The purpose of this study was to evaluate the effects of a new dietary supplement, Biofermentics™ (BF), which consists of soybean fermented by a combination of different species of lactic acid bacteria and yeasts, on in vitro and in vivo hepatic and renal disorder models in rats.
Methods
Preparation of Lactic Acid Bacteria-fermented Soybean Extract (Biofermentics™; BF)
Soymilk was prepared from soybeans of the ‘Tsurunoko’ variety, grown with less agricultural chemicals, in Hokkaido, the northern part of Japan. The soybeans were soaked in water, ground, heated to 98°C for 30 min and expressed through linen. The filtrate was used as soymilk. For fermentation of the soymilk, 12 frozen stock strains of lactic acid bacteria, such as Lactobacillus plantarum, L. casei, L. reuteri and Lactococcus lactis and four strains of the yeast Saccharomyces cerevisiae, were used. Together, the strains were divided into four groups of seed cultures A, B, C and D. Each group consisted of two strains of Lactobacillus, one strain of Lactococcus and one strain of S. cerevisiae. For preparation of the seed cultures, each group of frozen stock was inoculated into the appropriate amount of soymilk and incubated at 37°C for 2 days.
The inoculum sizes were adjusted to ∼1 × 105 ml−1 and 1 × 104 ml−1 for the lactic acid bacteria and yeast, respectively, based on the predetermined cell numbers in the frozen stock cultures. The four seed cultures were then mixed together, inoculated into a large volume of fresh soymilk of approximately 100 times of the inoculum, and incubated at 37°C for 4 days. The large bulk of fermented soymilk was heated to 98°C for 30 min, cooled to room temperature and extracted by the addition of ethyl alcohol at a final concentration of 14% v/v for about 2 weeks. Then, the extract was separated through filter paper. Both the filtrate and precipitate were used for the different products of lactic acid bacteria-fermented soybean extract (Biofermentics; BF). They were usually freeze-dried.
Rats
Male Wistar rats of 5 weeks of age were purchased from Charles River Co., Tokyo and pre-bred with MF powdery feed (Oriental Yeast Co., Ltd., Tokyo) for a week in a breeding room with a temperature of 23 ± 1°C, humidity of 50 ± 5% and photoperiod cycle of 12 h light/12 h dark.
The care and treatment of rats were done in accordance with the guidelines of institutional animal ethics prescribed by the guidelines of Science Council of Japan for the care and use of laboratory animals.
Oral Administration of BF on Deoxycholic Acid (DCA)-Induced Hepatic and Renal Disorders
At 6 weeks old, the rats were divided into two groups (experimental and control) of 6 rats each, such that the mean and variation of body weights between the two groups were virtually identical. The control group was given MF feed containing exclusively 0.5% DCA (Wako Pure Chemical Industries, Ltd., Osaka) for 6 weeks, while the experimental group was given MF powdery feed containing both 5% BF and 0.5% DCA. Both feed and drinking water were given ad libitum. Blood was collected from the tail vein of each rat at a volume of 0.5 ml at 0, 2, 4 and 6 weeks after the start of the feeding experiment and separated from serum. The concentration of l-asparate aminotransferase (AST), l-alanine aminotransferase (ALT), blood urea nitrogen (BUN), uric acid (UA) and total cholesterol (T-CHL) in the serum were measured using an enzymic test kit (C-test, Wako; Wako pure Chemical Industries, Ltd., Osaka).
During the final week of the experimental period, each rat was housed in a metabolic cage to measure the daily urine amount excreted and concentrations of electrolytes in the urine. The rats were finally anesthetized in a CO2 bag, their blood immediately collected from the abdominal large vein using a syringe and organs weighed. The collected serum was used to determine the concentrations of total protein (TP), alkali phosphatase (ALP), γ-glutamyl transpeptidase (γ-GTP), leucine aminopeptidase (LAP) and glucose (GLU) using multilayer film analytical element, dri-chem colorimetric analyzer 3030 (Fuji Photo Film Co., Ltd., Tokyo), and the concentrations of lipid peroxide (LPO), β-lipoprotein (β-LP) and total bile acid (T-BA) were determined using an enzymic test kit (C-test, Wako; Wako pure Chemical Industries, Ltd., Osaka).
Oral Administration of BF on d-Galactosamine (GMN)-Induced Hepatic Disorders
Male Wistar rats of 6 weeks were divided into two groups of similar mean and variation of body weight. One group used as control (n = 7) was given MF feed only, while the BF group (n = 6) was given MF feed containing 5% BF. Both feed and drinking water were given ad libitum for 3 weeks. At the beginning of the final week of experimental period, all rats were intraperitoneally injected once with a GMN- (Wako Pure Chemical Industries, Ltd., Osaka) solution in a dosage of 500 mg kg−1 body wt. Blood was collected from the tail vein in a volume of 0.5 ml, and serum AST activity was measured on days 1, 2, 3 and 6 following GMN administration.
Estimation of Cytotoxicity
Cytotoxicity caused by exposure to dichromate (K2Cr2O7; Kanto Chemical Co., Tokyo) was estimated by measuring the concentration of lactate dehydrogenase (LDH) leaked from primary cultures of rat hepatocytes and nephrocytes as previously described (13–16). In brief, approximately 2 × 106 collagenase-dispersed (Wako pure Chemical Industries, Ltd., Osaka) rat hepatocytes in medium E were plated onto 60-mm diameter Corning Petri dishes (Iwaki Glass, Ltd., Chiba, Japan) and incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C for 3 h. Following that, BF was added to the primary culture media at 1.3, 2.5, 5.0 or 10 μl ml−1 and incubated further for 20 h. The medium was then discarded and the primary cell sheets were overlaid with salt-glucose medium {SGM: 50 mM 4-(2-hydroxyethyl)-1-piperazinesulfonic acid buffer (pH 7.2) with 100 mM NaCl, 5 mM KCl, 2 mM CaCl and 5 mM glucose} containing dichromate (1 mM) and BF at the above three concentrations and incubated at 37°C for 8 h. The SGM from Petri dishes was centrifuged at 1000 rpm at 4°C for 5 min to remove cell debris, and the supernatant was examined for LDH released from cells by the method described by Mitchell et al. (17).
The control primary cultures, without any exposure to BF or dichromate, were frozen with SGM and thawed. This freeze-thaw procedure was repeated three times. Then, cell sheets were scraped off with a rubber spatula, sonicated with a Handy Sonic model UR-20P (Tomy Seiko Co., Ltd., Tokyo) by pulsing for 1min at an intensity of 8, and then centrifuged at 10 000 r.p.m at 4°C for 10 min. The supernatant was subjected to analysis of total LDH in the control hepatocytes. The percent of LDH released from cells treated with BF and dichromate was calculated against the total LDH from the control hepatocytes. The effect of BF on dichromate-induced cytotoxicity of primary cultures of rat nephrocytes was evaluated in a similar way as hepatocytes. The percentage of LDH leakage from nephrocytes was estimated as the ratio of LDH released in SGM from BF-treated cells to that from non BF-treated cells.
Assay for Lipid Peroxidation
Since dichromate compounds have been shown to facilitate malondialdehyde (MDA) formation through lipid peroxidation in isolated hepatocytes (18), the effect of BF on MDA formation was examined using primary cultures of rat hepatocytes, as previously described (19). In brief, following treatment with dichromate as mentioned in the foregoing section, the cells were washed twice with ice-cold phosphate-buffered saline (PBS) and dislodged from dishes by scraping. The cells were re-suspended in ice-cold PBS and sonicated, and the sonicate was used for the estimation of cellular levels of lipid peroxidation by monitoring MDA formation (20).
Statistical Analysis
The differences between the mean values of the data were evaluated by the Student's t-test for equal variance or Welch's t-test for unequal variance. A P-value of less than 0.05 was considered to be statistically significant. Because of the skewed distribution of AST or ALT in rat experiments, data were normalized by logarithmic transformation for further statistical analysis. However, no transformed values were presented in the ‘results’ section.
Results
Improvement of DCA-induced Hepatic and Renal Disorders
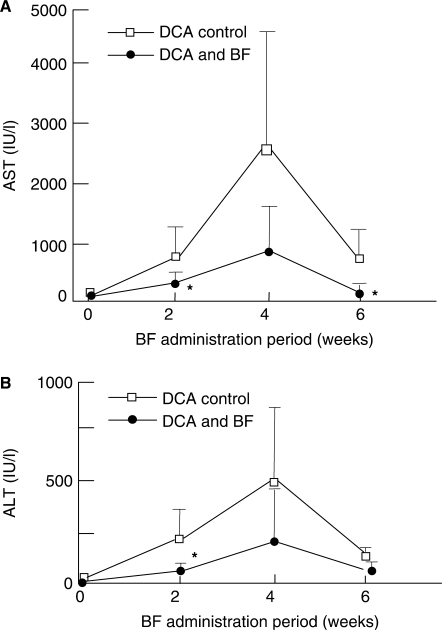
As shown in Fig. 1A, in the DCA-administered control group, serum AST activity rapidly increased to 786 ± 475 IUl at 2 weeks, reached its highest value of 2469 ± 2182 IU/l at 4 weeks, then sharply decreased to 722 ± 502 IU/l at 6 weeks. However, these rapidly increased activities were markedly lowered to 195 ± 105 IU/l, 788 ± 744 IU/l and 147 ± 67 IU/l at 2, 4 and 6 weeks, respectively, in the BF- and DCA- administered group. Thus, the increase of AST activity induced by DCA was clearly reduced by the administration of BF, with reductions significant (P < 0.05) at 2 and 6 weeks. Similarly, in the case of ALT, the increased activity found in controls was also depressed in the BF- administered group (Fig. 1B), with the value at 2 weeks significantly different (P < 0.05) from the DCA- administered control group.
Figure 1.
Effects of oral BF administration on serum AST (A) and ALT (B) activities in rats with hepatic disorders induced by DCA loading. The DCA control group (open square) was given MF powdery feed containing 0.5% DCA only while the DCA and BF group (filled circle) was given MF powdery feed containing both 0.5% DCA and 5% BF. Values indicate mean ± SD (n = 6). *P < 0.05 against the respective control values. BF, Biofermentics™(lactic acid bacteria-fermented soybean extract); AST, l-asparate aminotransferase; ALT, l-alanine aminotransferase; DCA, deoxycholic acid (hepatopathy inducer).
As seen in Table 1, rats in the BF and DCA group showed significantly (P < 0.01) lower serum BUN values at 4 and 6 weeks, as compared with control groups. No significant effects were observed in UA and T-CHL values following BF administration. As for the biochemical analysis of the serum, no clear change between control and BF groups was observed for TP, ALP, γ-GTP, LAP, GLU, LPO and β-LP (Table 2). However, the concentration of T-BA in the serum of the control group (81 ± 36 nmol ml−1) was considerably different from that of BF group (46 ± 34 nmol ml−1). Thus, the BF-administered group tended to show lower concentrations of total bile acid than the control group. Although the amount of urine was found to be larger in the BF group than in the control group, there were no significant differences in urine electrolyte (Na, K and Cl) concentrations.
Table 1.
Biochemical analysis of the serum from DCA-only and DCA- and BF-administered rats
| Analytical items | Groups | Administration period (weeks) | |||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | ||
| BUN (mg/dl) | DCA-administered | 27.8 ± 2.3 | 28.0 ± 7.9 | 27.1 ± 3.0 | 25.0 ± 2.5 |
| DCA- and BF- administered | 26.2 ± 1.2 | 25.6 ± 4.8 | 21.9 ± 2.2* | 19.8 ± 1.1* | |
| UA (mg/dl) | DCA-administered | 2.2 ± 0.6 | 2.4 ± 0.4 | 2.0 ± 0.5 | 2.3 ± 0.6 |
| DCA- and BF- administered | 2.2 ± 0.5 | 2.4 ± 0.7 | 1.7 ± 0.5 | 2.2 ± 0.5 | |
| T-CHL (mg/dl) | DCA-administered | 69 ± 11 | 121 ± 28 | 162 ± 65 | 118 ± 12 |
| DCA- and BF- administered | 78 ± 11 | 122 ± 17 | 129 ± 12 | 127 ± 16 | |
*P < 0.05 compared to the corresponding value of the DCA administered group.
BF, Biofermentics™ (lactic acid bacteria-fermented soybean extract); BUN, blood urea nitrogen; UA, uric acid; T-CHL, total cholesterol; DCA, deoxycholic acid.
Table 2.
Biochemical analysis of the serum from DCA-only and DCA-and BF-administered rats
| Analytical items | DCA- administered group | DCA-and BF-administered group | |
|---|---|---|---|
| Serum | Total protein (g/dl) | 7.0 ± 0.3 | 7.4 ± 0.3 |
| Alkali phosphatase (IU/l) | 694 ± 143 | 635 ± 127 | |
| γ-Glutamyl transpeptidase (IU/l) | 5.0 ± 1.7 | 5.2 ± 1.8 | |
| Leucine aminopeptidase (U) | 397 ± 199 | 363 ± 156 | |
| Glucose (mg/dl) | 156 ± 55 | 172 ± 25 | |
| Lipid peroxide (nmol/ml) | 2.3 ± 2.4 | 1.7 ± 1.4 | |
| β-Lipoprotein (mg/dl) | 47 ± 32 | 53 ± 34 | |
| Total bile acids (nmol/ml) | 81 ± 36 | 46 ± 34 | |
| Urine | Urine excretion (ml/day) | 21 ± 14 | 30 ± 10 |
| Urine/water intake (%) | 56 ± 7 | 69 ± 18 | |
| Urinary electrolyte (mEq/day): Na | 1.4 ± 0.7 | 2.1 ± 1.1 | |
| K | 2.9 ± 1.3 | 4.6 ± 2.2 | |
| Cl | 1.7 ± 0.8 | 2.7 ± 1.7 |
DCA, deoxycholic acid; BF, Biofermentics™ (lactic acid bacteria-fermented soybean extract).
Amelioration of GMN-induced Hepatic Disorders
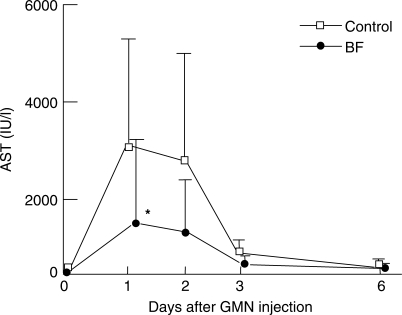
As shown in Fig. 2, serum AST activity in the control group increased sharply to 3172 ± 2379 IU/l on day 1 and 2811 ± 2210 IU/l on day 2, then decreased sharply to 372 ± 323 IU/l on day 3 after injection of GMN. In the BF group, AST was significantly (P < 0.05) lowered to 1423 ± 1857, 1009 ± 1395 and 142 ± 161 IU/l on those days, respectively, that is, to ∼45% the level of values in the control group.
Figure 2.
Effects of oral BF administration on serum AST activity in rats with d-galactosamine (GMN)-induced hepatic disorders. The control group (open square) was given MF powdery feed only for 3 weeks, while the BF group (filled circle) was given MF powdery feed containing 5% BF. On the first day of the fourth week of feeding period, the rats of both groups were injected intraperitoneally with GMN solution (500 mg/kg body wt). Serum AST was measured on days 1, 2, 3 and 6 after GMN injection. Values indicate mean ± SD (n = 7). *P < 0.05 against the respective control values. BF, Biofermentics™ (lactic acid bacteria-fermented soybean extract).
Cytotoxicity
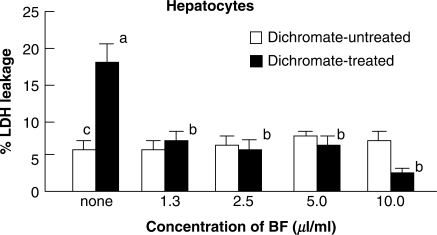
As shown in Fig. 3, when the hepatocytes were treated with 1 mM dichromate, they were damaged and leaked LDH at a level of 18% of total LDH in normal cells. However, when they were treated with dichromate and BF at the same time, the LDH leakage was significantly inhibited to a level of 7.0% of untreated control cells, indicating that BF treatment had a protective effect against cytotoxicity caused by dichromate treatment. No adverse effects were observed with BF treatment in the range of concentrations employed.
Figure 3.
Effects of BF treatment on dichromate-induced cytotoxicity in primary cultures of rat hepatocytes. Hepatocytes were treated with BF alone (open square) or with a combination of BF and dichromate (1 mM) (filled square) for 8 h at 37°C in SGM. The effect of BF was evaluated as a percent of decreased LDH leakage against the total LDH from control hepatocytes without any treatment. Bars indicate mean ± SD (n = 4). P < 0.05 of superscript a as compared with values of superscript b and c. BF, Biofermentics™ (lactic acid bacteria-fermented soybean extract). LDH, lactate dehydrogenase.
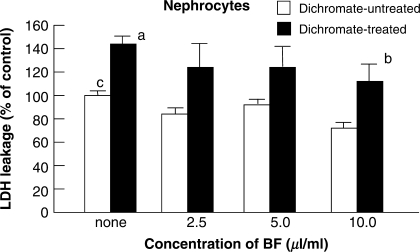
Figure 4 shows that the treatment of nephrocytes with BF at a concentration of more than 2.5 μl ml−1 for 16 h also revealed the tendency to suppress (P < 0.07) cytotoxicity caused by dichromate.
Figure 4.
Effects of BF treatment on dichromate-induced cytotoxicity in primary cultures of rat nephrocytes. Nephrocytes were treated with BF alone (open square) or with a combination of BF and dichromate (1 mM) (filled square) for 8 h at 37°C in SGM. The effect of BF was evaluated by the percent decrease of LDH leakage in the nephrocytes treated by BF and dichromate. Bars indicate mean ± SD (n = 4). P < 0.05 of superscript a as compared with the value of superscript c. P < 0.07 of superscript b as compared with the value of superscript a. BF, Biofermentics™ (lactic acid bacteria-fermented soybean extract).
Lipid Peroxidation
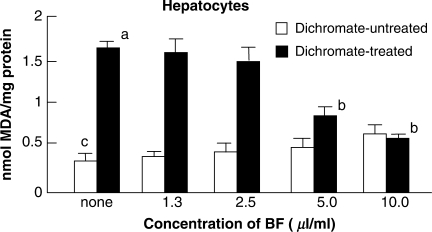
As shown in Fig. 5, the treatment of hepatocytes with 1 mM dichromate alone for 8 h revealed a significant increase (P < 0.05) in MDA formation of 1.7 nmol mg−1 protein, when compared with MDA formation of 0.3 nmol mg−1 protein for the control cells without any treatment. However, when the cells were treated with BF at concentrations of 5 or 10 μl/ml combined with dichromate, significant (50% or more) inhibitions (P < 0.05) of MDA formation was observed, indicating that BF treatment exhibited a protective effect against cytotoxity caused by dichromate. No increase in MDA formation occurred among cells treated with BF alone in the test range of 1.3–10 μl ml−1.
Figure 5.
Effects of BF treatment on dichromate-induced lipid peroxidation. Hepatocytes were treated with BF alone (open square) or with dichromate (1 mM) and BF(filled square) for 8 h at 37°C in salt-glucose medium. The effect of BF treatment was estimated by monitoring decreased malondialdehyde (MDA) formation through lipid peroxidation. Values indicate mean ± SD (n = 4). P < 0.05 of superscript a as compared with the value of superscript c. P < 0.05 of superscript b as compared with the value of superscript a. BF, Biofermentics™ (lactic acid bacteria-fermented soybean extract).
Discussion
DCA, a secondary bile acid, is highly toxic and causes cholestatic hepatopathy in experimental animals (21). When the concentration of bile acids exceeds the binding capacity of binding proteins located in the cytosol of the hepatocytes, bile acids induce apoptosis and necrosis by damaging mitochondria (22). Hydrophobic bile acids induce alterations in membrane fluidity associated with impairment of mitochondrial respiration and depolarization. In this study, it was considered that the suppression of bile acid concentration in hepatocytes was one of the mechanisms for BF to reduce the damage to hepatocytes. We found that the BF administered group tended to show lower values of total bile acid concentration in serum than control groups (Table 2). As a reason why the concentration of bile acid in hepatocytes was suppressed by BF, it is suspected that the absorption of DCA by intestinal mucosa was decreased by inhibiting the conjugation of bile acids with amino acids and by lowering the active transportation activity of bile acids in intestinal mucosa, though this supposition is unclear and requires further study.
In a model of human viral hepatitis, GMN inhibits the protein and the nucleic acid synthesis of hepatocytes (23,24). It has been reported that various kinds of dietary amino acids, such as l-serine, l-aspargine, l-histidine, l-lysine, l-tyrosine, l-glycine and l-glutamine, were effective in protecting rats from GMN-induced injury (25). Moreover, the effects of oligosaccharides and dietary fiber have been shown to prevent GMN-induced hepatic injury (26). Therefore, we believe that amino acids and oligosaccharides, ingredients of BF originating from soymilk, are partly attributable to protection against GMN-induced hepatic disorders.
Hexavalent chromium is reduced to stable form of trivalent chromium in hepatic and renal cells (15). It has been considered that the active oxygen (hydroxyl radical •OH) produced in these cells causes damage to the cell membrane and DNA through lipid peroxidation, which leads to leakage of LDH in the cell. It has also been reported that aglycones from saponin or isofravone glycosides, both ingredients of soymilk, are released by β-glucosidase of lactic acid bacteria (5,6). As such, we suspect that the anti-oxidative powers of BF were conserved in the process of fermentation by the mixed culture of the lactic acid bacteria and yeast in this study. Therefore, the anti-oxidative effect of BF prevented lipid peroxidation by chromium, and we believe that the power of BF as an anti-oxidant is similar to that of a lot of other anti-oxidation substances such as catechin, chlorogenic acid, vitamin E and melatonin, as previously tested (14,15,19). There have been many reports about the therapeutic benefits of plant herbs and fruits, notably on their anti-oxidant properties (27–29). Accordingly, taking fermented soybean extract, such as the BF used in this article, as an anti-oxidant every day may contribute to better overall health of humans
There have been reports about the functionality of the soybean as fermented by different micro-organisms (30,31). Moreover, it has been postulated that products of fermentation with two or more bacteria, are considerably more complicated than those with a single kind of bacterium (32,33). Although we did not examine the fermentation products of each bacterium used for the production of BF in this experiment, further study is needed for both single and co-cultivation to isolate the specific fermentation products responsible for the anti-oxidative effects of BF.
In conclusion, this study suggests that BF may be applied to an improvement of hepatic and renal disorders, BF may also possess other health benefits in humans.
Acknowledgement
We are grateful for the review of this article from the clinical standpoint by Dr Morie Sekiguchi (M.D., Ph. D., F.A.C.C.).
References
- 1.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, et al. Trends in alternative medicine use in the United States, 1990-1997: Results of a follow-up national survey. JAMA. 1998;280:1569–75. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 2.Kaminogawa S, Nanno M. Modulation of immune functions by foods. Evid Based Complement Alternat Med. 2004;1:241–50. doi: 10.1093/ecam/neh042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naylor CD. Gray zones of clinical practice: some limits to evidence-based medicine. Lancet. 1995;345:840–2. doi: 10.1016/s0140-6736(95)92969-x. [DOI] [PubMed] [Google Scholar]
- 4.Csáky I, Fekete S. Soybean: feed quality and safety. Part 1: biologically active components. Acta Veterinaria Hungarica. 2004;52:299–313. doi: 10.1556/AVet.52.2004.3.6. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda S, Miyazaki T, Matsumoto Y, Ohba R, Teramoto Y, Ohta N, et al. Hydrolysis of isoflavones in soybean cooked syrup by Lactobacillus casei subsp. rhamnosus IFO 3425. J Ferment Bioeng. 1992;74:301–4. [Google Scholar]
- 6.Coulon S, Chemardin P, Gueguen Y, Arnaud A, Galzy P. Purification and characterization of an intracellular β-glucosidase from Lactobacillus casei ATCC 393. App Biochem Biotech. 1998;74:105–14. [Google Scholar]
- 7.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–9. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 8.Kataoka S. Functional effects of Japanese style fermented soy sauce (Shoyu) and its components. J Biosci Bioeng. 2005;100:227–34. doi: 10.1263/jbb.100.227. [DOI] [PubMed] [Google Scholar]
- 9.Katsuyama H, Ideguchi S, Fukunaga M, Fukunaga T, Saijoh K, Sunami S. Promotion of bone formation by fermented soybean (Natto) intake in premenopausal women. J Nutr Sci Vitaminol. 2004;50:114–20. doi: 10.3177/jnsv.50.114. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima N, Nozaki N, Ishihara K, Ishikawa A, Tsuji H. Analysis of isoflavone content in tempeh, a fermented soybean, and preparation of new isoflavone-enriched tempeh. J Biosci Bioeng. 2005;100:685–7. doi: 10.1263/jbb.100.685. [DOI] [PubMed] [Google Scholar]
- 11.Shimakawa Y, Matsubara S, Yuki N, Ikeda M, Ishikawa F. Evaluation of bifidobacterium breve strain Yakult-fermented soymilk as a probiotic food. Int J Food Microbiol. 2003;81:131–6. doi: 10.1016/s0168-1605(02)00224-6. [DOI] [PubMed] [Google Scholar]
- 12.Beasley S, Tuorila H, Saris PEJ. Fermented soymilk with a monoculture of Lactococcus lactis. Int J Food Microbiol. 2003;81:159–62. doi: 10.1016/s0168-1605(02)00196-4. [DOI] [PubMed] [Google Scholar]
- 13.Susa N, Ueno S, Furukawa Y. Comparative test for cytotoxicity of hexavalent and trivalent chromium in primary cultures of hepatocytes. Kitasato Arch Exp Med. 1989;62:53–7. [PubMed] [Google Scholar]
- 14.Susa N, Ueno S, Furukawa Y. Protective effects of thiol compounds on chromate-induced toxicity in vitro and in vivo. Environ Health Perspect. 1994;102:247–50. doi: 10.1289/ehp.94102s3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Susa N, Ueno S, Furukawa Y, Sugiyama M. Protective effect of vitamin E on chromium (VI)-induced cytotoxity and lipid peroxidation in primary cultures of rat hapatocytes. Arch Toxicol. 1996;71:20–4. doi: 10.1007/s002040050353. [DOI] [PubMed] [Google Scholar]
- 16.Susa N, Ueno S, Furukawa Y, Sugiyama M. Protective effect of deferoxamine on chromium (VI)-induced DNA single-strand breaks, cytotoxity and lipid peroxidation in primary cultures of rat hapatocytes. Arch Toxicol. 1997;71:345–50. doi: 10.1007/s002040050397. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell DB, Santone KS, Acosta D. Evaluation of cytotoxity in cultured cells by enzyme leakage. J Tissue Cult Method. 1980;6:113–6. [Google Scholar]
- 18.Ueno S, Susa N, Furukawa Y, Akikawa K, Itagaki I, Komiyama T, et al. The relationship between the development of toxicity and lipid peroxidation induced by chromium compounds in rats. Kitasato Arch Exp Med. 1988;61:137–47. [PubMed] [Google Scholar]
- 19.Susa N, Ueno S, Furukawa Y, Ueda J, Sugiyama M. Potent protective effect of melatonin on chromium (VI)-induced DNA single-strand breaks, cytotoxity, and lipid peroxidation in primary cultures of rat hapatocytes. Toxicol App Pharmacol. 1997;144:377–84. doi: 10.1006/taap.1997.8151. [DOI] [PubMed] [Google Scholar]
- 20.Buege JA, Aust S. Microsomal lipid peroxidation. Methods Enzymol. 1987;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 21.Shiina T, Shin R, Ishihara K, Isoda M. Effects of heat treated cells of intestinal lactic acid bacteria in rats fed a deoxycholic acid diet. Jikken Dobutsu. 1990;39:325–35. doi: 10.1538/expanim1978.39.3_325. (in Japanese with English abstract) [DOI] [PubMed] [Google Scholar]
- 22.Palmeria CM, Rolo AP. Mitochondrially-mediated toxicity of bile acids. Toxycol. 2004;203:1–15. doi: 10.1016/j.tox.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Keppler DOR, Rudigier JFM, Bischoff E, Decker KFA. The trapping of uridine phosphates by D-galactosamine, D-glucosamine, and 2-deoxy-D-galactose. A study on the mechanism of galactosamine hepatitis. Eur J Biochem. 1970;17:246–53. doi: 10.1111/j.1432-1033.1970.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 24.Decker K, Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;71:78–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Ishihara M, Egashira Y, Ohta T, Sanada H. Effects of various kind of dietary amino acids on the hepatotoxic action of D-galactosamine in rats. Biosci Biotechnol Biochem. 1999;63:319–22. doi: 10.1271/bbb.63.319. [DOI] [PubMed] [Google Scholar]
- 26.Daizo A, Egashira Y, Sanada H. Suppressive effect of corn bran hemicellulose on liver injury induced by D-galactosamine in rats. Nutrition. 2005;21:1044–51. doi: 10.1016/j.nut.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Gupta V, Gupta A, Saggu S, Divekar HM, Grover SK, Kumar R. Anti-stress and adaptogenic activity of L-arginine supplementation. Evid Based Complement Alternat Med. 2005;2:93–7. doi: 10.1093/ecam/neh054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azaizeh H, Ljubuncic P, Portnaya I, Said O, Cogan U, Bomzon A. Fertilization-induced changes in growth parameters and antioxidant activity of medicinal plants used in traditional Arab medicine. Evid Based Complement Alternat Med. 2005;2:549–56. doi: 10.1093/ecam/neh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljubuncic P, Dakwar S, Portnaya I, Cogan U, Azaizeh H, Bomzon A. Aqueous extracts of Teucrium polium possess remarkable antioxidant activity in vitro. Evid Based Complement Alternat Med. 2006;3:329–38. doi: 10.1093/ecam/nel028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng IC, Shang HF, Lin TF, Wang TH, Lin HS, Lin SH. Effect of fermented soy milk on the intestinal bacterial ecosystem. World J Gastroenterol. 2005;11:1225–7. doi: 10.3748/wjg.v11.i8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YC, Yu RC, Chou CC. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006;23:128–35. doi: 10.1016/j.fm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Lin FM, Chiu CH, Pan TM. Fermentation of a milk-soymilk and Lyceum chinense Miller mixture using a new isolate of lactobacillus paracasei subsp. paracasei NTU101 and Bifidobacterium longum. J Ind Microbiol Biotechnol. 2004;31:559–64. doi: 10.1007/s10295-004-0184-z. [DOI] [PubMed] [Google Scholar]
- 33.Chien HL, Huang HY, Chou CC. Transformation of isoflavone phytoestrogens during the fermentation of soymilk with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006;23:772–8. doi: 10.1016/j.fm.2006.01.002. [DOI] [PubMed] [Google Scholar]