Abstract
The increased use of feed in Egypt's aquaculture and animal industries raises concerns about the possible presence of mycotoxins in feedstuffs. The use of alternative medicine, such as botanicals and nutritional supplements, has become popular with inflammatory cases. The present study aimed to testify the role played by phytic acid (IP6) in enhancing the reproductive and oxidative toxicity induced in aflatoxinB1 (AFB1) treated white male albino rats (Rattus norvegicus) throughout treatment and withdrawal periods. One hundred and twenty white male albino rats were grouped into four groups. Group 1, was injected with 300 μg kg−1 body wt of AFB1 once every 3 days for 15 days and left uninjected for another 15 days to study the withdrawal effect. Group 2, was injected with 300 μg kg−1 body wt of AFB1 once every 3 days for 15 days and treated simultaneously with IP6 daily for another 15 days. Group 3, was treated daily with IP6 (40 mg kg−1 body wt) for 15 days and with no treatment for other 15 days. Group 4, injected with equivalent volume of sterile phosphate buffer saline solution as a control group. Sera were taken at the experimental intervals and assayed for testosterone hormone, follicular-stimulating hormone (FSH) and luteinizing hormone (LH) to determine the toxicological impact of AFB1 and the possibility of amelioration by phytic acid on the reproductive performance of the studied animal. The effects of AFB1 treatment on the absolute and relative weight of testis as well as its histopathologic effect on the testis and the possibility of amelioration by IP6 treatment were evaluated. The activities of enzymatic and non-enzymatic anti-oxidants, in addition to lipid peroxidation were measured in the testis’ homogenate of AFB1-treated rats. A decrease in sex hormone levels, an increase in testicular lipid peroxidation product levels and a significant decrease in testicular glutathione content, catalase and total peroxidase and superoxide dismutase activities were recorded. The histopathologic alterations revealed a degeneration and highly mitotic division within the spermatogenic nuclei, in addition to some karyomegaly and nuclear pyknosis. It is concluded that the reduction in the toxicity of free radicals by phytic acid might be responsible for the protective influence observed.
Keywords: aflatoxicosis, histopathology, hormonal assay, rats
Introduction
Numerous toxigenic fungi and their metabolites have been identified from a variety of substrates. Most of these are produced by genera of Aspergilli, Peniàllia and Fusaria and are most often isolated from cereal grains or corn, Aspergillus fumigatus, A. flavus and A. niger are the most common species that affect humans. Mycotoxins are among the most common food contaminants in animal feed, causing great economic loss in animal mass production and aquaculture (1–4).
Problems associated with mycotoxins tend to be worse in the tropics where high humidity and temperature create optimal conditions for fungal growth. Recently, Luyendy et al. (5), Cupid et al. (6) and Meki et al. (7,8) reported that aflatoxinB1 (AFB1) is a food contaminant fungal toxin that has been implicated as a causative agent in human acute hepatotoxicity, hepatic and extrahepatic carcinogenesis, due to the fact of its formation of AFB1-macromolecular adducts (AFB1-DNA and AFB1-albumin). Furthermore, the oxidative damage has been postulated to play a major role in the mechanisms associated with AFB1-induced cytotoxicity and carcinogenicity in mammalian species and indicates that oxidation of AFB1 generates free radical species via the metabolic pathways and an iron-mediated redox mechanism (9).
Ahmed et al. (10) reviewed the anti-inflammatory and anti-cytotoxicity features of some of the common botanicals and nutritional supplements. They reported that the use of complementary and alternative medicine (CAM) therapies is on the rise and according to reports ∼60–90% of dissatisfied inflammation cases are likely to seek the option of CAM therapy (10).
Phytic acid or Inositol hexaphosphate (IP6) is a sugar molecule attached to six phosphate molecules. It is found in wheat and rice bran, legumes and virtually every kind of mammalian cell. IP6 plays an important role in regulating vital cellular functions, including cell proliferation and differentiation (11–16). Same authors added that phytic acid might be responsible for the anti-oxidant and anti-cancer properties of green tea and grains.
In addition to its other functions, IP6 controls the number and growth of cells. It helps in keeping cells multiplying out of control and overwhelming the immune system. In laboratory and animal experiments, inositol has proved to be beneficial in preventing and slowing the spread of cancer (13,17). IP6 also appears to be a natural anti-oxidant that can reverse the effects of damaging free radicals, fight tumor formation and enhance the body's natural disease resistance (11,12,17).
The present study aimed to investigate the reproductive and oxidative toxicity induced in AFB1-treated white male albino rats (Rattus norvegicus) and to evaluate the role of phytic acid exposure as well as the withdrawal period, in inducing alterations on such toxicological impacts of AFB1. The toxicological impact of AFB1 and the possibility of amelioration by phytic acid and/or the withdrawal period on the reproductive performance of R. norvegicus were determined through detecting the parameters that are sensitive to AFB1 toxicity. These parameters included assaying testosterone hormone, follicular-stimulating hormone (FSH) and luteinizing hormone (LH), the absolute and relative testis weight, the activities of enzymatic and non-enzymatic anti-oxidants and lipid peroxidations, in addition to, investigating the histopathological alterations as sensitive indicators of AFB1 toxicity.
Methods
Rats
One hundred and twenty adult white male albino rats (R. norvegicus) weighing from 150–180 g were kept under observation for about 15 days prior to the experiment, to exclude any intercurrent infection. Rats were housed in metal cages with natural ventilation and illumination at normal atmospheric temperature. Moreover, they were given access of water and supplied daily with commercial pellet diet.
Groups
One hundred and twenty white male albino rats were grouped into four groups. Group 1, was injected intraperitoneally (i.p.) with 300 μg kg−1 body wt of AFB1 once every 3 days for 15 days and left uninjected for another 15 days to study the withdrawal effect. In Group 2, animals were grouped into four groups; the first was injected i.p. with 300 μg kg−1 body wt of the prepared AFB1 once every 3 days for 15 days and left uninjected for another 15 days to study the withdrawal effect. The second group was injected i.p. with 300 μg kg−1 body wt of AFB1 once every 3 days for 15 days and treated simultaneously with phytic acid (IP6) daily for another 15 days. The third group was treated daily with phytic acid (40 mg kg−1 body wt) for 15 days and with no treatment for 15 days. While the fourth group was left as control group and injected by equivalent volume (0.2 ml) of sterile phosphate buffered saline solution (PBSS, pH 7.4).
At different experimental periods (3, 7, 14, 21 and 28 days), six animals from each group were sacrificed under mild diethyl ether anesthesia. Blood samples were taken and centrifuged at 700 g for 30 min. The clear, non-hemolyzed supernatant sera were quickly removed and kept at −20°C for the estimation of testosterone, FSH and LH. Also, testes were homogenized in isotonic solution and kept at −20°C for further investigations to estimate the anti-oxidant parameters.
AFB1and IP6 Preparation
The pure crystalline AFB1 toxin (Sigma, 30801) was dissolved in an adequate volume of dimethylsulfoxide (DMSO) as a solvent then was completed to the desired volume by PBSS to perform the final concentration 300 μg kg−1 body wt as determined by Butler and Neal (18) via i.p. route. IP6, (Sigma, P-8810) was dissolved in sterile saline to desire a dose of 40 mg kg−1 body wt and administered daily by gastric intubation (orally) according to Vucenik et al. (16).
Analytical Methods
Hormone Levels by RIA
Hormonal levels were estimated by using radioimmunoassay (RIA) for testosterone (19). RIA kits were purchased from Diagnostic System Laboratories, Inc., Webster, TX, USA. FSH and LH were determined using kits purchased from Diagnostic Automation Inc. according to Uotila et al. (20).
Oxidative Stress
For estimation of the different oxidative stress parameters as well as the anti-oxidant enzymes, a part of testis (0.25 g) was ice-cooled, homogenized in 2.5 ml phosphate buffer pH 7.4, then centrifuged at 30 000g for 15 min at 4°C. The supernatant was collected and preserved at −20°C until used. Estimation of lipid peroxidation was dependent on the malondialdehyde (MDA) formed from the breakdown of polyunsaturated fatty acids. Lipid peroxidation products were quantified by their reaction with the thiobarbituric acid as described by Preuss et al. (21). Briefly, 1.0 ml of the testis supernatant was mixed with 2.0 ml of 7.5% trichloroacetic acid (TCA) then centrifuged at 1000g for 10 min. A 0.2 ml of the formed supernatant was added to 0.7% thiobarbituric acid (TBA) in a boiling water-bath for 15 min. The absorbance of the formed solution was read at 532 nm and a solution of 1, 1, 3, 3-tetramethoxypropane was used as standard.
Thiols in Testes
Total thiol levels in testes homogenate were determined using the method of Koster et al. (22) which based upon the reduction of Ellman's reagent [5,5′-dithiobis-(2-nitrobenzoic acid)] by thiols containing compounds to form 5-thio-2-nitrobenzoic acid and can be measured colorimetrically at 412 nm. Briefly, 0.05 ml of testis homogenate was mixed with 0.75 ml phosphate buffer (0.1 M pH 7.4) and 0.2 ml Ellman's reagent (80 mg of 5,5′-dithiobis-2-nitrobenzoic acid in 100 ml 1% sodium citrate). Mixing was followed by incubation for 5 min at 37°C, distilled water was used instead of sample for blank preparation. Absorbency was measured at 412 nm against blank. Total thiols (μmol l−1) were calculated by following equation: Total thiols = Asample (20 × 106/13 600); whereas 13 600 is the molar extinction coefficient.
Glutathione in Testes
Total glutathione content in testes tissue was estimated according to the method described by Beutler et al. (23). Briefly, 0.2 ml of testis supernatant was added to 1.8 ml of distilled water, then 3.0 ml of precipitating solution (1.67 g of glacial metaphoshoric acid, 0.2 g of EDTA and 30 g NaCl in each 100 ml H2O), was mixed. The mixture was allowed to stand for ∼5 min, and centrifuged, and then 2 ml of the supernatant was added to 8 ml of phosphate solution (0.3 M of NaHPO4) and 1.0 ml of DTNB reagent [40 mg of 5, 5′-dithiobis-(2-nitrobenzoic acid)/100 ml of 1% Sodium Citrate]. Finally, read the absorbance at 412 nm. Standard is used as glutathione reduced form, while the blank is prepared as by adding an 8.0 ml of phosphate solution to 2.0 ml of diluted precipitating solution (3 : 2 parts of distilled water) and 1.0 ml of the DTNB reagent.
Peroxidase Activity
Total peroxidase activity in the supernatant of the testis homogenate was estimated according to the method described by Kar and Mishra (24). Heme peroxidases (myeloperoxidase and eosinophil peroxidase) activity in testis of control and treated rats was measured using a pyrogallol as a substrate in the presence of H2O2 according to the method of Joseph et al. (25). Briefly, 2 ml phosphate buffer solution (pH 6.8), 0.1 ml 1.4% pyrogallol and 0.1 ml 1.1 mM H2O2 were added to 0.025 ml of supernatant. After exactly 5 min, the density of color of formed purpurogallin was measured against the blank by taking absorbance at 420 nm.
SOD Activity
The SOD [EC 1.15.1.1] activity was measured in testis supernatant according to the method described by Zou et al. (26), based on the inhibition of the autoxidation of pyrogallol. Briefly, an aliquot of cell extract was mixed with Tris–cacodylic buffer (50 mM Tris–HCl, 50 mM cacodylic acid, 1 mM diethylenetriamine pentaacetic acid, pH 8.2) and 2 mM pyrogallol. The autoxidation of pyrogallol and the inhibition of this reaction were monitored spectrophotometrically. Under the conditions of this assay, 1 unit of superoxide dismutase (SOD) activity is equivalent to the amount of enzyme that produces a 50% inhibition of the autoxidation of pyrogallol. The rate of pyrogallol's autoxidation was manipulated to be 0.070 O.D. min−1 and 50% depression of autoxidation rate of pyrogallol by SOD was known as 1U.
Catalase Activity
Catalase activity was analyzed according to the method described by Cohen et al. (27) and modified by Sinha (28) by monitoring the enzyme-catalyzed decomposition of hydrogen peroxide using potassium permanganate. Briefly, 0.05 ml sample was added to a test tube then 0.2 ml of 2 M H2O2 was added and the tube was incubated on ice for 3 min. 1 N H2SO4 was used to stop the reaction. Finally, KMnO4 solution was added and absorbance was recorded at 480 nm. In this assay catalase unit is expressed as k, whereas k = log (S0/S2) × (2.3/t), whereas S0 = absorbance of standard−absorbance of blank, S2 = absorbance of standard−absorbance of sample and t = time interval.
Histopathology
Tissue samples of testis were collected and fixed in 10% neutral buffered formalin solution then embedded in paraffin using conventional method. Paraffin sections were prepared and stained with hematoxylin and eosin (29).
Statistical Analysis
The data were analyzed using analysis of variance (ANOVA; 30) followed by LSD analysis to evaluate the variations in-between groups and for multiple comparison between different groups. Results are expressed as mean ± SD. Values of P > 0.05 were considered statistically non-significant, while values of P < 0.05 were considered statistically significant.
Results
Changes in Body, Absolute and Relative Organ Weights
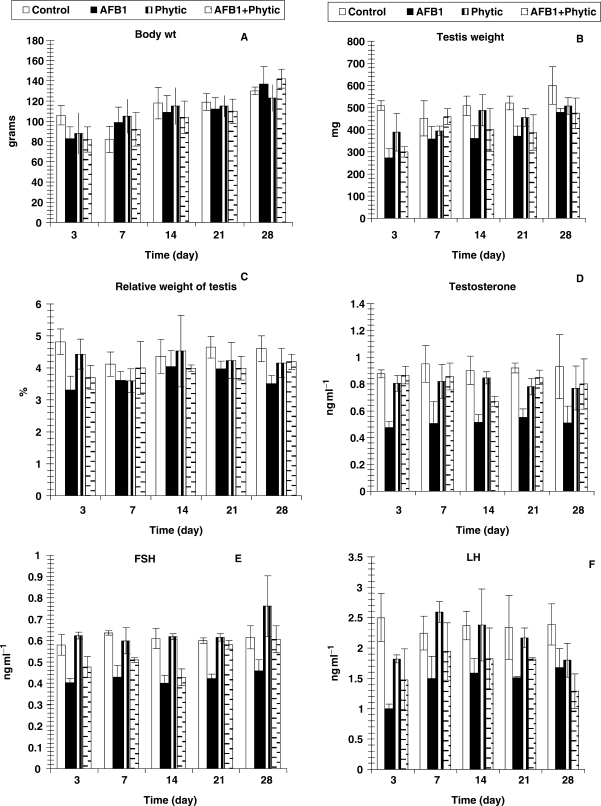
The effects of AFB1, phytic acid and AFB1-phytic acid mixture on the absolute testis weight, relative testis weight and sex hormones level were represented in Table 1 and Fig. 1A and C. Testis absolute weight was significantly decreased as a result of administration of AFB1 and/or phytic acid at all experimental periods (day 3, 7, 14 and 28). The effect was significant at all tested periods except at day 7 regarding AFB1-phytic acid mixture treated group and at day 14 and day 21 for phytic acid treated group. Moreover, the most potent decreased effect was attained at day 3 due to AFB1 and/or phytic acid treatments. Similarly, the relative testis weight was abated to various degrees at the different tested periods. However, while the effect was significant at earlier experimental periods (days 3 and 7), it was not at later ones (days 14, 21 and 28).
Table 1.
Changes in the body weight (g); testis absolute (mg) and relative weights (%) and some sexual hormones in male albino rats treated with aflatoxinB1 and phytic acid
| Treatment period | Recovery period | |||||
|---|---|---|---|---|---|---|
| 3 days | 7 days | 14 days | 21 days | 28 days | ||
| Body weight (g) | Control | 106 ± 9.617 | 82 ± 13.038 | 118 ± 15.652 | 119 ± 8.341 | 130 ± 3.780 |
| AFB1 | 83 ± 11.511 | 99 ± 15.166 | 109 ± 16.335 | 112 ± 11.381 | 137 ± 16.808 | |
| Phytic acid | 88 ± 20.186 | 105 ± 16.583 | 115 ± 18.027 | 115 ± 10.481 | 123 ± 13.038 | |
| AFB1+Phytic | 82 ± 12.549 | 92 ± 16.047 | 104 ± 11.937 | 110 ± 9.350 | 142 ± 16.807 | |
| Testis wt (mg) | Control | 508.4 ± 21.733 a | 451.22 ± 79.550 a | 508.00 ± 43.871 a | 520.96 ± 30.654 a | 599.80 ± 84.715 a |
| AFB1 | 272.44 ± 43.384 c | 358.80 ± 55.693 b | 361.40 ± 57.317 c | 370.45 ± 45.783 b | 479.80 ± 15.465 b | |
| Phytic acid | 388.68 ± 84.767 b | 395.00 ± 21.161 b | 488.80 ± 70.574ab | 455.63 ± 40.841 ab | 507.00 ± 37.195 b | |
| AFB1+Phytic | 299.88 ± 24.033 c | 459.00 ± 35.979 a | 400.20 ± 97.568 bc | 387.12 ± 80.439 b | 474.40 ± 68.449 b | |
| Relative wt (% × 10−3) | Control | 4.822 ± 0.403 a | 4.116 ± 0.388 ab | 4.358 ± 0.533 | 4.650 ± 0.343 | 4.598 ± 0.402 a |
| AFB1 | 3.305 ± 0.439 b | 3.612 ± 0.277 b | 4.038 ± 0.508 | 3.967 ± 0.245 | 3.508 ± 0.257 b | |
| Phytic acid | 4.433 ± 0.469 a | 3.622 ± 0.370 b | 4.526 ± 1.119 | 4.232 ± 0.567 | 4.154 ± 0.449 a | |
| AFB1+Phytic | 3.702 ± 0.378 b | 4.000 ± 0.823 a | 3.991 ± 1.213 | 3.989 ± 0.287 | 4.194 ± 0.826 a | |
| Testosterone (ng ml−1) | Control | 0.876 ± 0.030 a | 0.950 ± 0.137 a | 0.900 ± 0.107 a | 0.920 ± 0.036 a | 0.93 ± 0.239 a |
| AFB1 | 0.476 ± 0.045 b | 0.506 ± 0.165 b | 0.513 ± 0.058 c | 0.550 ± 0.064 c | 0.51 ± 0.126 b | |
| Phytic acid | 0.806 ± 0.059 a | 0.820 ± 0.126 a | 0.846 ± 0.045 a | 0.780 ± 0.062 b | 0.77 ± 0.165 ab | |
| AFB1+Phytic | 0.866 ± 0.065 a | 0.856 ± 0.102 a | 0.670 ± 0.036 b | 0.850 ± 0.055 ab | 0.80 ± 0.118 a | |
| FSH (ng ml−1) | Control | 0.579 ± 0.048 a | 0.636 ± 0.010 a | 0.610 ± 0.0476 a | 0.600 ± 0.0135 a | 0.615 ± 0.0534 ab |
| AFB1 | 0.403 ± 0.018 c | 0.429 ± 0.0537 c | 0.400 ± 0.0367 b | 0.421 ± 0.022 b | 0.458 ± 0.0525 b | |
| Phytic acid | 0.623 ± 0.016 a | 0.599 ± 0.061 a | 0.618 ± 0.0313 a | 0.615 ± 0.018 a | 0.761 ± 0.146 a | |
| AFB1+Phytic | 0.476 ± 0.049 b | 0.509 ± 0.011 b | 0.428 ± 0.0393 b | 0.580 ± 0.020 a | 0.606 ± 0.628 ab | |
| LH (ng ml−1) | Control | 2.499 ± 0.397 a | 2.243 ± 0.278 ab | 2.369 ± 0.235 | 2.339 ± 0.0533 a | 2.384 ± 0.339 a |
| AFB1 | 0.996 ± 0.079 c | 1.493 ± 0.373 c | 1.587 ± 0.244 | 1.510 ± 0.022 c | 1.677 ± 0.315 b | |
| Phytic acid | 1.818 ± 0.065 b | 2.593 ± 0.173 a | 2.379 ± 0.593 | 2.170 ± 0.160 a | 1.797 ± 0.279 b | |
| AFB1+Phytic | 1.475 ± 0.508 bc | 1.944 ± 0.473 bc | 1.823 ± 0.509 | 1.816 ± 0.023 b | 1.289 ± 0.284 b | |
Number of animals = 6; Values are expressed as Means ± SD; AFB1, aflatoxinB1; Means within same parameter with the same letter are not significantly different (P > 0.05).
Figure 1.
Changes in body wt (g) absolute testis and relative weights and some sexual hormones of male albino rats treated with AFBI and phytic acid. Number of animals is six and values are expressed as means ± SD.
Alterations in Sex Hormones
Sex hormones were lowered to various extents at different experimental periods. The effect of AFB1 administration was significant at all tested periods. With the exception of day 21, the treatment of male albino rats with phytic acid (IP6) produced a non-significant change of testosterone level. The administration of phytic acid to AFB1-intoxicated rats induced marked (P < 0.05) amelioration of altered testosterone concentration. In contrast, the FSH and LH levels were not significantly improved as a result of administration of phytic acid-AFB1 mixture as compared to AFB1 alone (Table 1 and Fig. 1D–F).
Changes in the Oxidative Stress and Anti-oxidant Parameters
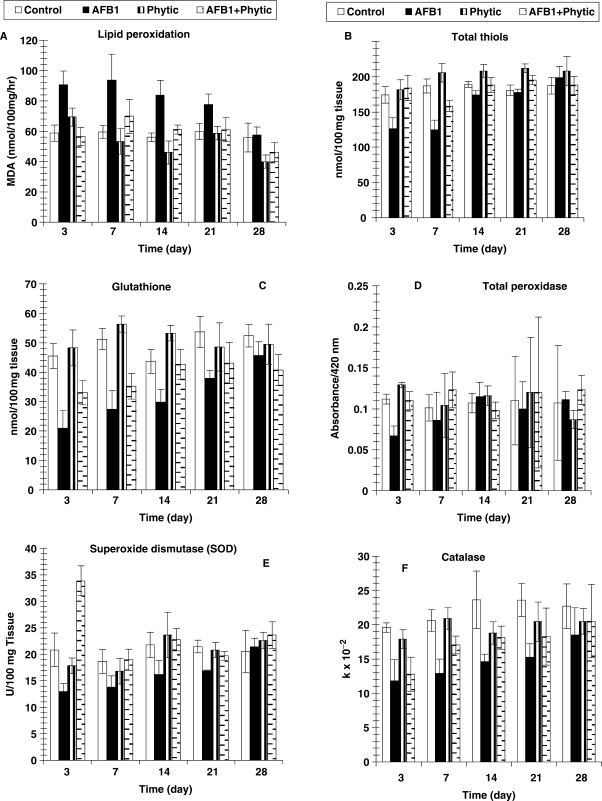
The testis malondialdehyde level was significantly increased in AFB1-intoxicated animals. The administration of phytic acid to AFB1-intoxicated group induced great amelioration of this oxidative stress marker (Table 2 and Fig. 2A and F).
Table 2.
Changes in the different parameters indicating oxidative stress status in rats treated with aflatoxinB1 and phytic acid
| Treatment period | Recovery period | |||||
|---|---|---|---|---|---|---|
| 3 days | 7 days | 14 days | 21 days | 28 days | ||
| Testis lipid peroxidation (nmol MDA/100 mg/h) | Control | 58.82 ± 5.32b | 59.53 ± 4.47b | 56.13 ± 2.82bc | 59.78 ± 5.32b | 55.89 ± 9.45a |
| AFB1 | 90.63 ± 9.23b | 93.67 ± 16.97a | 83.84 ± ± 9.57a | 77.65 ± 6.87a | 57.54 ± 5.10a | |
| Phytic acid | 69.32 ± ± 5.92b | 53.32 ± 8.34b | 46.06 ± 7.54c | 58.74 ± 4.45b | 39.90 ± 4.35b | |
| AFB1+Phytic | 56.55 ± 6.0b | 69.93 ± 11.28b | 61.11 ± 2.94b | 60.98 ± 7.98b | 46.21 ± 6.20ab | |
| Total thiols (nmol/100 mg tissue) | Control | 174.29 ± 11.64a | 186.75 ± 10.10a | 189.11 ± 4.42b | 180.634 ± 7.43c | 187.28 ± 11.62 |
| AFB1 | 126.49 ± 15.62b | 124.77 ± 13.58c | 174.17 ± 5.73c | 177.543 ± 4.87c | 198.77 ± 16.11 | |
| Phytic acid | 181.59 ± 14.10a | 205.45 ± 13.06a | 208.33 ± 9.22a | 211.897 ± 6.43a | 207.99 ± 20.70 | |
| AFB1+Phytic | 183.81 ± 17.75a | 158.25 ± 7.96b | 188.12 ± 10.44b | 195.312 ± 6.23b | 187.99 ± 12.86 | |
| Testis glutathione (nmol/100 mg tissue) | Control | 45.471 ± 4.150 a | 51.239 ± 3.604 a | 43.687 ± 4.012 b | 53.707 ± 5.292 | 52.402 ± 3.775 |
| AFB1 | 20.931 ± ± 5.975 c | 27.386 ± 6.409 b | 29.818 ± 4.308 c | 38.020 ± 2.537 | 45.706 ± 4.644 | |
| Phytic acid | 48.268 ± 6.123 a | 56.374 ± 2.800a | 53.254 ± 2.635 a | 48.570 ± 8.150 | 49.507 ± 6.863 | |
| AFB1+Phytic | 33.048 ± 4.135 b | 35.255 ± 4.362 b | 42.684 ± 5.097 b | 43.059 ± 6.963 | 40.795 ± 5.247 | |
| Total peroxidase (absorbance) | Control | 0.112 ± 0.006a | 0.101 ± 0.016 | 0.107 ± 0.012 | 0.110 ± 0.054b | 0.107 ± 0.070 |
| AFB1 | 0.067 ± 0.112b | 0.086 ± 0.034 | 0.115 ± 0.017 | 0.100 ± 0.033b | 0.111 ± 0.010 | |
| Phytic acid | 0.129 ± 0.003a | 0.104 ± 0.039 | 0.116 ± 0.012 | 0.120 ± 0.067a | 0.087 ± 0.011 | |
| AFB1+Phytic | 0.110 ± 0.011a | 0.123 ± 0.022 | 0.098 ± 0.010 | 0.120 ± 0.092a | 0.123 ± 0.018 | |
| Superoxide dismutase (U/100 mg tissue) | Control | 20.83 ± 3.175ab | 18.67 ± 2.254 | 21.77 ± 2.411 | 21.47 ± 1.1985a | 20.50 ± 3.970 |
| AFB1 | 13.00 ± 1.513c | 13.81 ± 2.108 | 16.20 ± 2.654 | 16.85 ± 0.1729b | 21.43 ± 1.795 | |
| Phytic acid | 17.87 ± 1.436b | 16.80 ± 2.402 | 23.63 ± 4.290 | 20.80 ± 1.417a | 22.60 ± 1.566 | |
| AFB1+Phytic | 33.88 ± 2.804a | 19.00 ± 2.000 | 22.76 ± 2.112 | 19.70 ± 0.821ab | 23.63 ± 2.500 | |
| Testis catalase (k ×10−2) | Control | 19.593 ± 0.697 a | 20.627 ± 1.585 a | 23.634 ± 4.216 a | 23.559 ± 2.405 a | 22.723 ± 3.252 |
| AFB1 | 11.799 ± 3.090 b | 12.876 ± 2.142 c | 14.631 ± 1.065 b | 15.247 ± 2.002b | 18.492 ± 3.983 | |
| Phytic acid | 17.927 ± 1.364 a | 20.920 ± 1.563 a | 18.803 ± 1.634 ab | 20.464 ± 2.856 ab | 20.476 ± 1.841 | |
| AFB1+Phytic | 12.785 ± 2.502 b | 17.075 ± 1.293 b | 18.120 ± 1.678 b | 18.267 ± 4.153 ab | 20.519 ± 5.353 | |
Number of animals = 6; Values are expressed as Means ±SD; AFB1, aflatoxinB1; Means within a row with the same letter are not significantly different (P > 0.05).
Figure 2.
Changes in oxidative stress and some anti-oxidant enzymes in testis tissue homogenate of albino rats treated with AFBI and phytic acid. Number of animals is six and values are expressed as means ± SD.
The testis total thiols and glutathione contents were deleteriously decreased as a result of AFB1-intoxication. The co-administration of phytic acid exerted marked increase of these deteriorated variables. Moreover, testis total peroxidase activity of AFB1-intoxicated rats was significantly decreased only at day 3. Also, SOD activity was significantly affected at day 7 and day 21 due to AFB1-intoxication, while the testis catalase activity was significantly lowered at all tested periods except at day 28. The administration of phytic acid to the AFB1-intoxicated animals induced marked improvement of these altered anti-oxidant enzymes activities at all tested periods (Table 2 and Fig. 2C and F).
Histopathological Alterations
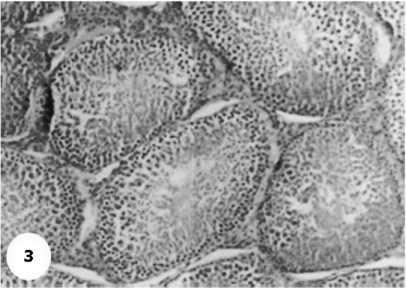
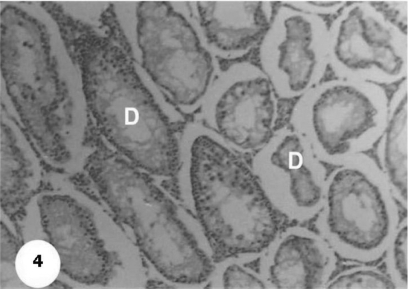
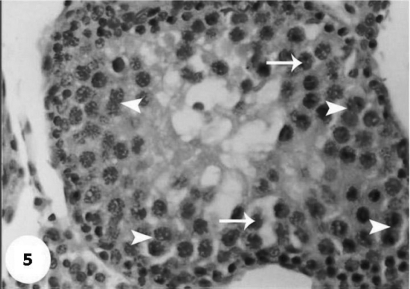
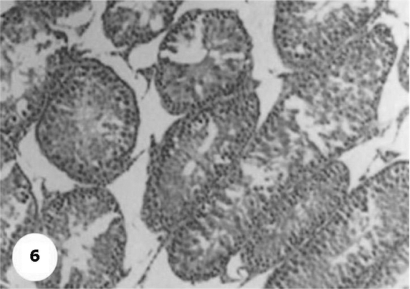
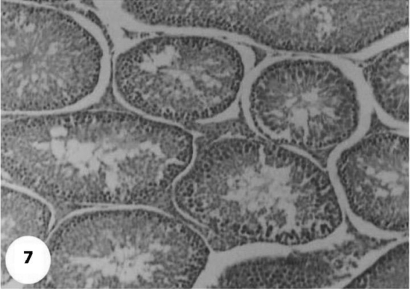
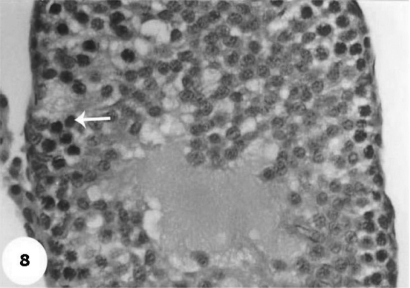
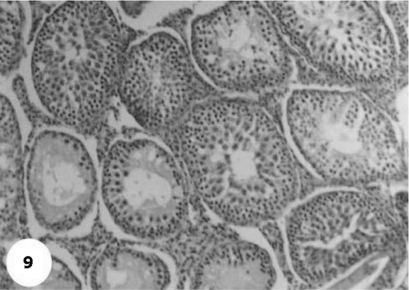
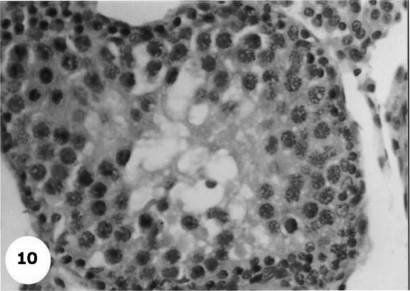
Control sections of testis revealed that the seminiferous tubules consist of several layers of epithelial cells (Fig. 3). These spermatogonia cells are cuboidal cells, having clear cytoplasm and rounded nuclei, which may show division. Sertoli cells are large epithelial cells having vesicular nucleus with large nucleolus. Next to the spermatogonia cells is a zone of spermatocytes, the nuclei of which are usually in mitotic division. A large number of small cells, the spermatids, are seen external to spermatocytes. The spermatids become developed further into spermatozoa. The latter usually lie in groups with their heads projecting between the deeper cells, and are connected with one of the sertoli cells of the lining epithelium. By contrast, sections treated with AFB1 showed degenerative and highly mitotic division after 3 days of injection. Also, spermatozoa were seemed to disappear in association with karyomegaly in some nuclei of the spermatogonic cells, while others show pyknotic. Meanwhile, at 3rd day post-AFB1 injection, H and E sections of testis show degeneration in the seminiferous tubules with complete absence of spermatozoa, in addition, some spermatogonic nuclei show karyomegaly and high incidence of mitotic divisions (Figs 4 and 5). On the other hand, treatment with phytic acid for 1 week shows a marked regenerative effect upon the histopathologic features of the seminiferous tubules (Fig. 6). A reduction in spermatids number within the lumen of seminiferous tubules, a marked mitotic divisions and pyknosis of some spermatogonic nuclei were observed in the section of testis of AFB1-injected group after 2 weeks of injection (Figs 7 and 8). Testis sections of rats treated with AFB1–phytic acid mixture show regenerative features after 2 weeks of treatments, in spite of the absence of spermatids (Figs 9 and 10).
Figure 3.
A photomicrograph section of testis of control group shows the normal histology of the somniferous tubules and their normal layer distributions. H & E × 10.
Figure 4.
Section shows degeneration in the somniferous tubules (D) with absence of spermatozoa after 3 days of AFB1 injection. H & E × 10.
Figure 5.
A magnified photograph for testis section at third day post-AFB1 injection show karyomegaly of some spermatogonic nuclei (arrow) and mitotic divisions in other nuclei (head of arrow). H & E × 40.
Figure 6.
A photomicrograph shows a marked regenerative effect after 1 week post-treatment with phytic acid. H & E × 10.
Figure 7.
Section for testis of AFB1-injected group shows absence of spermatids in lumen and a marked regenerative effect after 2 weeks of injection. H & E × 10.
Figure 8.
A magnified photomicrograph for section of testis of AFB1-injected group shows absence of spermatids in lumen and a marked mitotic divisions and pyknotic of some spermatogonic nuclei (arrow), in addition to absence of the spermatozoa within the lumen after 2 weeks of injection. H & E × 10.
Figure 9.
A photomicrograph shows a marked regenerative effect after 2 week post-treatment with phytic acid. H & E × 10.
Figure 10.
A magnified photograph for section of testis of AFB1-treated with phytic acid after 2 weeks show somewhat regenerative features although absence of spermatides. H & E × 40.
Discussion
Hensarling et al. (31) and Ehrlich and Ciegler (32,33) indicated that phytate depresses aflatoxin production by rendering zinc, a necessary co-factor for aflatoxin biosynthesis, unavailable to the mold. On the other hand, IP6 ubiquitous in plants and animals is not only a natural anti-oxidant, but also a precursor/storage of intracellular inositol phosphates, that is important for various cellular functions (34).
The reduction in the testosterone, FSH and LH hormones level recorded in the present study after AFB1-injection was in agreement with those observed in Japanese quail (35); leghorn chickens (1); mice (34,36) and rabbits (37). The observed effect of AFB1 may be due to a depressed Leydig cell function (36,38) and/or due to the aflatoxin's documented impairment of lipid metabolism (39).
The present study revealed the amerolative effect of IP6 upon the pathological and hormonal alterations induced by the AFB1-treatment and this could be explained by the fact that IP6 plays an important role in regulating vital cellular functions, including cell proliferation and differentiation (11–16). They added that IP6 might be responsible for the anti-oxidant and anti-cancer properties of green tea and grains, in addition to its vital role in enhancing the functional capabilities of nervous system and reproductive functions (14–16).
In view of oxidative stress, the present results indicated that AFB1 induced marked increase in testis lipid peroxidation and caused a significant decrease in testis total thiols glutathione, peroxidase, SOD and catalase activities, which are in agreement with the findings of Verma and Nair (38); Abdel-Wahhab and Aly (40); Abdel-Moneim (41), Kandeil and Abu El-Saad, (42) and Lee et al. (17). Strelic et al. (43) mentioned that AFB1 administration led to long-term increase in glutathion-S-transferase (GST) activity in the plasma and liver. Such oxidative damage has been postulated to play a major role in AFB1-induced cytotoxicity and carcinogenicity in mammalian species (9). In contrast, Lee et al. (17) revealed that the number and the area of the pre-neoplastic lesions in the liver were negatively correlated with the GST activity with supplementing IP6 and/or inositol in the drinking water.
The induced increase in lipid peroxidation after AFB1-injection may be due to the fact that onset of lipid peroxidation in susceptible sperm leads to the progressive accumulation of lipid hydroperoxides in sperm plasma membranes, which then decomposes to form MDA under stress and toxic conditions (41,42,44).
The restoration of glutathione and LPO levels was observed. This may point out the role of phytic acid in promoting stability of cellular, nuclear and organelle membranes (13,15,16,45). Such observation was supported by the findings of Karimi et al. (46), who suggested that some natural product, including phytic acid, can inhibit lipid peroxidation by scavenging free radicals and increasing intracellular concentration of glutathione.
The present study clarified that AFB1 induces histopathological alterations in the seminiferous tubules and whole nuclei of treated-testes. These findings are parallel with other studies of Japanese quail (35), Leghorn chickens (1), rats (47,48) and roosters (49). They reported degeneration and desquamation in the epithelium decrease in the size and thickness of the germinative layer of seminiferous tubules and lowered plasma testosterone levels. These findings may be due to the direct effects of AFB1 on seminiferous tubules, responsible for sperm production or due to its effect on the duct system responsible for sperm maturation (47). It could be also due to reduction in rates of oxygen consumption by adenosine diphosphate (ADP) utilization in gonadal mitochondria (50). Similarly, Agnes and Akbarsha (51) recorded disruption of spermatogenic and androgenic compartments of the testes by AFB1 injection. This reflects an alteration of epididymal functions towards the post-testicular sperm maturation process by AFB1.
In partial agreement with the present investigations, Agnes and Akbarsha (51) reported reduced fertility of similarly treated animals, as sperm concentration in the epididymis and sperm motility was decreased, whereas sperm abnormalities were increased. In agreement with the present findings, Koksal et al. (44) confirmed that the severe pathological changes in testicular tissue are associated with a high level of lipid peroxidation, findings that suggest overproduction of reactive oxygen substances (ROS) playing a role in the mechanism of testicular degeneration associated with infertility. The recorded amerolative effect of IP6 upon pathological alterations induced by AFB1 treatment could be explained by its role in regulating vital cellular functions including cell proliferation and differentiation as well as its anti-oxidant and anti-cancer properties. The role of IP6 in enhancing reproductive functions was proved in the present study and agreed with findings of other authors (13,15,16).
In conclusion, the present study suggests that severe pathological changes in testicular tissue are associated with a high level of lipid peroxidation and drastic inhibition in the enzymatic and non-enzymatic anti-oxidant activities. Moreover the overproduction of ROS may play a role in the mechanism of testicular degeneration associated with infertility in case of aflatoxicosis. According our findings, IP6 is advised to be used in case of aflatoxicosis because it is abundantly present in regular diet, efficiently absorbed from the gastrointestinal tract and safe.
References
- 1.Sharlin J, Howarth B, Thompson F, Wyatt R. Decreased reproductive potential and reduced feed consumption in mature white leghorn males fed aflatoxin. Poultry Sci. 1981;60:2701–8. doi: 10.3382/ps.0602701. [DOI] [PubMed] [Google Scholar]
- 2.Hafez A, Megalla S, Abdel-Fattah HM, Kamel YY. Aflatoxin and aflatoxicosis. II. Effects of aflatoxin on ovaries and testicles in mature domestic fowls. Mycopathologia. 1982;77:137–9. doi: 10.1007/BF00518797. [DOI] [PubMed] [Google Scholar]
- 3.Jantrarotai W, Lovell R. Subchronic toxicity of dietary aflatoxin B1 to channel catfish. J. Aquatic Animal Health. 1990;2:248–54. [Google Scholar]
- 4.Abou El, Magd M. Zagazig, Egypt: Pharmacology, Forensic Medicine and Toxicology Dept. Fac. Vet. Med. Zagazig Univer; 1996. Immunotoxicological studies on the effect of aflatoxin in O. niloticus. PhD Thesis. [Google Scholar]
- 5.Luyendy K, Copple B, Barton C, Ganey P, Roth R. Augmentation of aflatoxinB1 hepatotoxicity by endotoxin: involvement of endothelium and the coagulation system. Tox Sci. 2003;72:171–81. doi: 10.1093/toxsci/kfg007. [DOI] [PubMed] [Google Scholar]
- 6.Cupid B, Lightfoot T, Russell D, Gant S, Turner P, Dingley K, et al. The formation of AFB1-macromolecular adducts in rats and humans at dietary levels of exposure. Food and Chemi Toxicol. 2004;42:559–69. doi: 10.1016/j.fct.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Meki A, Abdel-Ghaffar S, El-Gibaly I. Aflatoxin B1 induces apoptosis in rat liver: protective effect of melatonin. Neuroendocrinol Lett. 2001;22:417–26. [PubMed] [Google Scholar]
- 8.Meki A, Esmail E, Hussein A, Hassaneine H. Caspase-3 and heat shock protein-70 in rat liver treated with aflatoxin B1: effect of melatonin. Toxicon. 2004;43:93–100. doi: 10.1016/j.toxicon.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Towner R, Mason R, Reinke L. In vivo detection of aflatoxin-induced lipid free radicals in rat bile. Biochim Biophys Act. 2002;1573:556–2. doi: 10.1016/s0304-4165(02)00326-4. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed S, Anuntiyo J, Malemud CJ, Haqqi TM. Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. Evid based Compl Alter Med. 2005;2:301–08. doi: 10.1093/ecam/neh117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C. Inositol hexaphosphate inhibits cell transformation and activator protein 1 activation by targeting phosphatidylinositol-3′ kinase-1. Cancer Res. 1997;57:2873–8. [PubMed] [Google Scholar]
- 12.Saied I, Shamsuddin A. Up-regulation of the tumor suppressor gene p53 and WAF1 gene expression by IP6 in HT-29 human colon carcinoma cell line. Anticancer Res. 1998;273:1479–84. [PubMed] [Google Scholar]
- 13.Shamsuddin A, Yang G, Vucenik I. Novel anti-cancer functions of IP6: growth inhibition and differentiation of human mammary cancer cell lines in vitro. Anticancer Res. 1996;273:3287–92. [PubMed] [Google Scholar]
- 14.Vucenik I, Yang G, Shamsuddin A. Comparison of pure inositol hexaphosphate and high-bran diet in the prevention of DMBA-induced rat mammary carcinogenesis. Nutr Cancer. 1997;57:7–13. doi: 10.1080/01635589709514546. [DOI] [PubMed] [Google Scholar]
- 15.Vucenik I, Kalebic T, Tantivejkul K, Shamsuddin A. Novel anticancer function of inositol hexaphosphate: inhibition of human rhabdomyosarcoma in vitro and in vivo. Anticancer Res. 1998a;275:1377–84. [PubMed] [Google Scholar]
- 16.Vucenik I, Tantivejkul K, Zhang Z, Cole K, Saied I, Shamsuddin A. IP6 in treatment of liver cancer. I. IP6 inhibits growth and reverses transformed phenotype in HepG2 human liver cancer cell line. Anticancer Res. 1998b;18:4083–90. [PubMed] [Google Scholar]
- 17.Lee H-J, Lee S, Choi H. Dietary Administration of Inositol and/or Inositol-6-phosphate prevents chemically-induced rat hepatocarcinogenesis. Asian Pacific J Cancer Prev. 2005;6:41–7. [PubMed] [Google Scholar]
- 18.Butler W, Neal G. The effect of aflatoxin B1 on the hepatic structure and RNA synthesis in rats fed a diet marginally deficient in choline. Cancer Res. 1973;33:2878–85. [PubMed] [Google Scholar]
- 19.Carlstrom K, Gershagen S, Rannevik G. Free testosterone and testosterone/SHBG index in hirsute women: a comparison of diagnostic accuracy. Gynecol Obstet Invest. 1987;24:256–61. doi: 10.1159/000298811. [DOI] [PubMed] [Google Scholar]
- 20.Uotila M, Ruoslahti E, Engvall E. Two-site sandwich enzyme immunoassay with monoclonal antibodies to human alpha-fetoprotein. J Immunol Methods. 1981;42:11. doi: 10.1016/0022-1759(81)90219-2. [DOI] [PubMed] [Google Scholar]
- 21.Preuss HG, Jarrel ST, Scheckenobach R, Lieberman S, Anderson RA. Comparative effects of chromium vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr. 1998;17:116–23. doi: 10.1080/07315724.1998.10718736. [DOI] [PubMed] [Google Scholar]
- 22.Koster JF, Bieermond P, Swaak AJ. Intracellular and extracellular sulfhydryl levels in rheumatoid arthritis. Ann Rheu Dis. 1986;45:44–6. doi: 10.1136/ard.45.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beutler E, Duron O, Mikus-Kelly B. Improved method for determination of blood glutathione. J Lab Clin Med. 1963;61:882. [PubMed] [Google Scholar]
- 24.Kar M, Mishra D. Catalase, peroxidase and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976;57:315–9. doi: 10.1104/pp.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph P, Murthy KR, Nelson JL, Kulkrani AP. Peroxidase: a novel pathway for chemical oxidation in human term placenta. Placenta. 1992;13:545–54. doi: 10.1016/0143-4004(92)90019-p. [DOI] [PubMed] [Google Scholar]
- 26.Zou GL, Gui XF, Zhong XL, Zhu YF. Improvements in pyrogallol autoxidation method for the determination of SOD activity. Progress Biochem Biophys. 1986;71:73. [Google Scholar]
- 27.Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extract. Anal Biochem. 1970;34:30–8. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 28.Sinha A. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–95. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 29.Carleton H, Drury R, Willington E, Conergon H. Cited from Carleton, Histological Techniques. 4th. Toronto: Oxford University Press No 4; 1967. [Google Scholar]
- 30.1985. PC-STAT One way analysis of variance. Version 1A(C) copyright. The University of Georgia. Programs coded by Roa M, Blane K, Zonneberg M. University of Georgia, USA. [Google Scholar]
- 31.Hensarling T, Jacks T, Lee L, Ciegler A. Production of aflatoxins on soybean and cottonseed meals. Mycopathologia. 1983;83:125–7. doi: 10.1007/BF00436896. [DOI] [PubMed] [Google Scholar]
- 32.Ehrlich K, Ciegler A. Effect of phytate on aflatoxin formation by Aspergillus parasiticus and Aspergillus flavus in synthetic media. Mycopathologia. 1984;30;87:99–103. doi: 10.1007/BF00436636. [DOI] [PubMed] [Google Scholar]
- 33.Ehrlich K, Ciegler A. Effect of phytate on aflatoxin formation by Aspergillus parasiticus grown on different grains. Mycopathologia. 1985;92:3–6. doi: 10.1007/BF00442651. [DOI] [PubMed] [Google Scholar]
- 34.Yang G, Shamsuddin A. IP6-induced growth inhibition and differentiation of HT-29 human colon cancer cells: involvement of intracellular inositol phosphates. Anticancer Res. 1995;15:2479–87. ISSN: 0250-7005. [PubMed] [Google Scholar]
- 35.Ottinger M, Doerr J. The early influence of aflatoxin upon sexual maturation in the male Japanese quail. Poultry Sci. 1980;59:1750–4. doi: 10.3382/ps.0591750. [DOI] [PubMed] [Google Scholar]
- 36.Verma R, Nair A. Effect of aflatoxins on testicular steroidogenesis and amelioration by vitamin E. Food Chem Toxicol. 2002;40:669–72. doi: 10.1016/s0278-6915(01)00131-4. [DOI] [PubMed] [Google Scholar]
- 37.Salem M, Kamel K, Yousef M, Hassan G, EL-Nouty F. Protective role of ascorbic acid to enhance semen quality of rabbits treated with sublethal doses of aflatoxinB1. Toxicology. 2001;162:209–18. doi: 10.1016/s0300-483x(01)00366-3. [DOI] [PubMed] [Google Scholar]
- 38.Verma R, Nair A. Ameliorative effect of vitamin E on aflatoxin-induced lipid peroxidation in the testis of mice. Asian J Androl. 2001;3:217–21. [PubMed] [Google Scholar]
- 39.Kirubagaran R, Joy K. Toxic effects of mercury on testicular activity in the fresh water teleost, Clarias batrachus. J Fish Biol. 1992;41:305–15. [Google Scholar]
- 40.Abdel-Wahab M, Aly S. Antioxidant and radical scavenging properties of vegetable extracts in rats fed aflatoxin-contaminated diet. J Agric Food Chem. 2003;51:2409–14. doi: 10.1021/jf0209185. [DOI] [PubMed] [Google Scholar]
- 41.Abdel-Moniem A. Effect of Nigella sativa on aflatoxin B1 induced oxidative stress in male albino rats. Egypt Germ Soc Zool J Comp Physiol. 2004;44:301–22. [Google Scholar]
- 42.Kandeil M, Abu El-Saad A. Biochemical effects of ascorbic acid on oxidative stress induced by aflatoxinB1 in male albino rats. Fourth International Science Conference Fac Vet Med Mansoura Univ. 2005:265–82. [Google Scholar]
- 43.Strelic N, Saicic Z, Magic Z, Spasic M, Trutic N, Krtolica K. AflatoxinB1- induced changes of glutathione-S-transferase activity in the plasma and liver of the rat. Vojnosanit Pregl. 2003;60:415–20. doi: 10.2298/vsp0304415s. [DOI] [PubMed] [Google Scholar]
- 44.Koksal I, Usta M, Orhan I, Abbasoglu S, Kadioglu A. Potential role of reactive oxygen species on testicular pathology associated with infertility. Asian J Androl. 2003;5:95–9. [PubMed] [Google Scholar]
- 45.Kumar M, Samarth R, Kumar M, Selvan SR, Saharan B, Kumar A. Protective effect of Adhatoda vascia Nees against radiation-induced damage at cellular, biochemical and chromosomal levels in Swiss Albino mice. Evid based Compl Alter Med (eCAM) 2006;3:1–8. doi: 10.1093/ecam/nel098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karimi G, Ramezani M, Tahoonian Z. Cisplatin nephrotoxicity and protection by milk thistle extract in rats. Evid based Compl Alter Med (eCAM) 2005;2:383–6. doi: 10.1093/ecam/neh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egbunike G, Emerole G, Aire T, Ikegwuonu FI. Sperm production rates, sperm physiology and fertility in rats chronically treated with sublethal doses of aflatoxin B1. Andrologia. 1980;12:467–75. doi: 10.1111/j.1439-0272.1980.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 48.Egbunike G. Steroidogenic and spermatogenic potentials of the male rat after acute treatment with Aflatoxin B1. Andrologia. 1982;14:440–6. doi: 10.1111/j.1439-0272.1982.tb02291.x. [DOI] [PubMed] [Google Scholar]
- 49.Ortatatli M, Ciftci M, Tuzcu M, Kaya A. The effects of aflatoxin on the reproductive system of roosters. Res Vet Sci. 2002;72:29–36. doi: 10.1053/rvsc.2001.0516. [DOI] [PubMed] [Google Scholar]
- 50.Davis P, Bergg J, Keough K. Respiration of mitochondria from the gonads of the sea urchin Strongylocentrotus droebachiensis: the effects of petroleum fractions on oxygen consumption. Comp Biochem Physiol. 1985;80C:155–60. doi: 10.1016/0742-8413(85)90148-3. [DOI] [PubMed] [Google Scholar]
- 51.Agnes V, Akbarsha M. Spermatotoxic effect of aflatoxin B1 in the albino mouse. Food Chem Toxicol. 2003;41:119–30. doi: 10.1016/s0278-6915(02)00171-0. [DOI] [PubMed] [Google Scholar]