Abstract
Saffron and its constituents have been shown to decrease ischemia-reperfusion (I/R) injury in kidney or brain tissues. In this study, the effects of saffron ethanolic extract and its constituents, crocin and safranal, were evaluated in skeletal muscle during I/R injury. Hind limb ischemia was induced using clamping the common femoral artery and vein. After 2 h ischemia, the clamp of the femoral vessels of animals was taken off and the animal underwent 1h reperfusion. Muscle injuries were evaluated by recording of the electromyographic (EMG) potentials and performing some biochemical analysis including thiobarbituric acid reactive substances (TBARS), total sulfhydryl (SH) groups and antioxidant capacity of muscle (using FRAP assay). The ethanolic extract of saffron (5, 20 and 80 mg kg−1), crocin (50, 200 and 400 mg kg−1), safranal (0.1, 0.25 and 0.5 ml kg−1) and normal saline (10 ml kg−1) were administered intraperitoneally 1 h prior reperfusion. The average peak-to-peak amplitude during I/R was significantly increased in extract, crocin and safranal groups in comparison with control-ischemic group. Following saffron, crocin and safranal administration, the total SH contents and antioxidant capacity were elevated in muscle flap. The MDA level was declined significantly in test groups. It is concluded that saffron extract and its constituents show a protective effect against lower limb I/R in rat.
Keywords: Saffron (Crocus sativus L.), Crocin, Safranal, Oxidative stress, Lower limb ischemia, Reperfusion
Introduction
Skeletal muscle ischemia and reperfusion injury remain an issue of concern because of the morbidity and mortality that follows revascularization of an acutely ischemic limb (1). Many studies have suggested the beneficial effects of nutrients or other agents such as vitamin E (2), C (3), green tea extract (2), melatonin (4,5), carbenoxolone (6) and green propolis (7,8) in reducing or preventing cerebral or muscle injury during ischemia.-reperfusion (I/R).
Crocus sativus L. commonly known as saffron, is a perennial stemless herb of the Iridaceae family and widely cultivated in Iran and other countries such as India and Greece (9). Commercial saffron comprises the dried red stigma with a small portion of the yellowish style attached. Compounds considered pharmacologically active and important are volatile agents (e.g. safranal), bitter principles (e.g. picrocrocin) and dye materials (e.g. crocetin and its glycoside, crocin) (9). In modern pharmacological studies, saffron, or its active constituents, has demonstrated anticonvulsant (10), antidepressant (11), anti-inflammatory (12) and antitumour activities (13,14). Radical scavenger effects as well as learning and memory improving properties (15,16) and promote the diffusivity of oxygen in different tissues were also reported (9). Saffron extract is also chemopreventive and showed protective effects on genotoxins-induced oxidative stress in Swiss albino mice (17–19).
Recently, Assimopoulou et al. (20) showed that the saffron extract, crocin and safranal exhibited significant radical scavenging activity and thus antioxidant activity. There was also a constant decrease in lipoprotein oxidation susceptibility in healthy individuals after administration of 50 mg of saffron twice a day (21). Saffron and its constituents have been shown to decrease I/R injury in kidney (22) or brain tissues (23). Thus, in this study the effect of saffron extract and its constituents was evaluated during I/R on an animal model of I/R injury in another tissue, the rat hind limb. Ischemia damages were evaluated using recording of EMG potentials (to show the viability of nerve) and measuring oxidative markers. Malondialdehyde and SH-groups were measured as parameters for lipid peroxidation and oxidative-stress markers (24). Antioxidant capacity of muscle was evaluated using FRAP assay (24).
Method
Animals
Male Wistar rats, 200–250 g were housed in colony rooms with 12/12 h light/dark cycle at 21 ± 2°C and had free access to food and water. All animal experiments were carried out in accordance with Mashhad University of Medical Sciences, Ethical Committee Acts.
Materials
Safranal and crocin were obtained from Fluka. DTNB (2, 2′-dinitro-5, 5′-dithiodibenzoic acid), TPTZ (2, 4, 6-tri (2-pyridyl)-1, 3, 5-triazine), TBA (2-thiobarbituric acid), n-butanol, Tris, Na2EDTA, sodium acetate, glacial acetic acid, phosphoric acid, potassium chloride, tetramethoxypropane (TMP), ferric chloride (FeCl3.6H2O), ferrous sulfate and hydrochloric acid was obtained from Merck. Xylazine and ketamine were obtained from Loughrea, Co. (Galway, Ireland) and Rotexmedica (GmbH, Germany), respectively. For dilution, saffron and crocin were dissolved in distilled water.
Induction of Ischemia
The rats were anesthetized with intraperitoneal injection of ketamine/xylazine (60 mg kg−1 and 6 mg kg−1, respectively). Additional doses of these agents were used if anesthesia lightened during experiment.
An incision in the inner side of the hind leg from the inguinal ligament to the tendon calcaneus insertion was made.
Then it was divided up and the triceps surae was dissected as a muscle flap, after that insertions to femur was cut. Previously dissected femoral vessels, the artery and vein were clamped with a single clamp of microsurgery. The absence of bleeding was verified in the muscle flap. Then the incision was closed to prevent desiccation. For reperfusion periods, the clamp of the femoral vessels of animals was taken off and then the bleeding of the muscle flap was verified (6). The muscle tissues was homogenized in cold KCl solution (1.5%) to give a 10% homogeny suspension and used for biochemical assays. The results were expressed by n or micromolar tissue (1 ml of homogenate = 0.1 g of tissue). In some references these data are expressed as n or μM/g protein.
Experimental Protocol
Eleven groups of animals were used, each of which contained 8 rats: Group 1 including sham operated animals, group 2 served as ischemic control to which saline (10 ml/kg−1) was injected intraperitoneally (i.p.). Group 3–5 was received the ethanolic extract of saffron (5, 20 and 80 mg kg−1). Crocin was injected to group 6–8 (50, 200 and 400 mg kg−1) and safranal (0.1, 0.25 and 0.5 ml kg−1) was administered to group 9–11. All drugs were administrated intraperitoneally 1 h before reperfusion.
The hind legs of groups 2–11 were exposed to 2 h ischemia and 1 h reperfusion.
Electromyography Data Collection
To determine the muscles and nerve activities during I/R, intramuscular electromyograph (EMG) signals were recorded with PowerLab data acquisition systems. Two pairs of pin electrodes in terminating alligator clips were inserted into the triceps surae (muscle flap) in hind leg, and adductor muscles. The distance between the two electrodes of a pair in each muscle was 5 mm. A grounding electrode was gently attached to the rat tail (25). The EMG signals were collected with sampling frequency of 12 PPM (MacLab/4SP). Duration for each stimulation was 20 ms. The raw EMG signals were low-pass filtered at 50 Hz and EMG signal was expressed as average peak-to-peak amplitude for a 10 min recording periods.
Preparation of Saffron Extract
Crocus sativus L. stigmas were collected from Ghaen (Khorasan province, Northeast of Iran). In the maceration method, 10 g of stigmas were macerated in 500 ml ethanol (80 v/v) for three days. The mixture was subsequently filtered and concentrated under reduced pressure at 40°C. The extract yield was 52% w/w.
Saffron sample was analyzed according to the ISO 3632-2: Bitterness, expressed as direct reading of the absorbance of picrocrocine at about 257 nm, on dry basis was 96.9; safranal, expressed as direct reading of the absorbance at about 330 nm, on dry categories was 41.5 and coloring strength, expressed as direct reading of the absorbance of crocin at about 440 nm, on dry basis was 259.
Biochemical Assays
Thiobarbituric Acid Reactive Species (TBARS) Measurement
Malondialdehyde (MDA) levels, as an index of lipid peroxidation, were measured. MDA reacts with thiobarbituric acid (TBA) as a thiobarbituric acid reactive substance (TBARS) to produce a red colored complex which has peak absorbance at 532 nm (26).
3 ml phosphoric acid (1%) and 1 ml TBA (0.6%) was added to 0.5 ml of muscle homogenate in a centrifuge tube and the mixture was heated for 45 min in a boiling water bath. After cooling, 4 ml of n-butanol was added the mixture and vortex-mixed for 1 min followed by centrifugation at 20000 rpm for 20 min. The organic layer was transferred to a fresh tube and its absorbance was measured at 532 nm. The standard curve of MDA was constructed over the concentration range of 0–40 µM (27).
Ferric Reducing/Antioxidant Power (FRAP) Assay
The FRAP assay measures the change in absorbance at 593 nm owing to the formation of a blue colored FeII-tripyridyltriazine compound from the colorless oxidized FeIII form by the action of electron donating antioxidants (28).
The FRAP reagent consist of 300 mM acetate buffer (3.1 g sodium acetate + 16 ml glacial acetic acid, made up to 1 l with distilled water; pH = 3.6), 10 mM TPTZ in 40 mM HCl and 20 mM FeCl3.6H2O in the ratio of 10:1:1.
Briefly, 50 μl of muscle homogenate was added to 1.5 ml freshly prepared and prewarmed (37°C) FRAP reagent in a test tube and incubated at 37°C for 10 min. The absorbance of the blue colored complex was read against reagent blank (1.5 ml FRAP reagent + 50 μl distilled water) at 593 nm. Standard solutions of FeII in the range of 100 to 1000 mM were prepared from ferrous sulphate (FeSO4.7H2O) in distilled water. The data was expressed as mM ferric ions reduced to ferrous form per liter (FRAP value) (29).
Total Sulfhydryl (SH) Groups Assay
Total SH groups were measured using DTNB (2, 2′-dinitro-5, 5′-dithiodibenzoic acid) as the reagent. This reagent reacts with the SH groups to produce a yellow colored complex which has a peak absorbance at 412 nm (30).
Briefly, 1 ml Tris-EDTA buffer (pH = 8.6) was added to 50 μl muscle homogenate in 2 ml cuvettes and sample absorbance was read at 412 nm against Tris-EDTA buffer alone (A1). Then 20 μl DTNB reagent (10 mM in methanol) was added to the mixture and after 15 min (stored in laboratory temperature) the sample absorbance was read again (A2). The absorbance of DTNB reagent was also read as a blank (B). Total thiol concentration (mM) was calculated from the following equation:
Statistical Analysis
Data are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey–Kramer post-hoc test for multiple comparisons. The P-values less than 0.05 were considered to be statistically significant.
Results
Electromyography Data
The average peak-to-peak amplitudes in the control-ischemic group and sham were 1.71 ± 0.03, 1.57 ± 0.068 V and 2.03 ± 0.03, 2.06 ± 0.63 V) respectively during ischemia and reperfusion. The ethanolic extract of saffron, crocin and safranal increased the amplitude compare with ischemia and reperfusion groups (Table 1).
Table 1.
Effects of ethanolic saffron extract, crocin and safranal on average peak-to-peak amplitudes of EMG signals, prior, during and after ischemia-reperfusion in rat skeletal muscle
| Group (n = 8) | Preischemia (v) | Ischemia (v) | Reperfusion (v) |
|---|---|---|---|
| Ischemic-Saline control (10 ml kg−1) | 2.25 ± 0.02 | 1.71 ± 0.03 | 1.57 ± 0.02 |
| Sham-Saline | 2.22 ± 0.03 | 2.03 ± 0.03* | 2.06 ± 0.63*** |
| Ethanolic extract 5 mg kg−1 | 2.21 ± 0.10 | 2.05 ± 0.04** | 2.06 ± 0.04*** |
| Ethanolic extract 20 mg kg−1 | 2.24 ± 0.06 | 2.12 ± 0.08*** | 2.08 ± 0.07*** |
| Ethanolic extract 80 mg kg−1 | 2.26 ± 0.04 | 2.13 ± 0.1** | 2.09 ± 0.06*** |
| Crocin 50 mg kg−1 | 2.26 ± 0.03 | 2.02 ± 0.09* | 2.01 ± 0.03*** |
| Crocin 200 mg kg−1 | 2.27 ± 0.03 | 2.05 ± 0.08* | 2.05 ± 0.04*** |
| Crocin 400 mg kg−1 | 2.26 ± 0.03 | 2.05 ± 0.09* | 2.05 ± 0.04*** |
| Safranal 0.1 ml kg−1 | 2.25 ± 0.03 | 2.04 ± 0.15* | 2.07 ± 0.04*** |
| Safranal 0.25 ml kg−1 | 2.25 ± 0.03 | 2.04 ± 0.06* | 2.03 ± 0.04*** |
| Safranal 0.5 ml kg−1 | 2.26 ± 0.03 | 2.17 ± 0.23*** | 2.17 ± 0.04*** |
Values are mean ± S.E.M. *P <0.05, **P <0.01 and ***P <0.001 when compared with corresponding between groups with Tukey–Kramer test. V = volt.
Thiobarbituric Acid Reactive Species (TBARS) Measurement
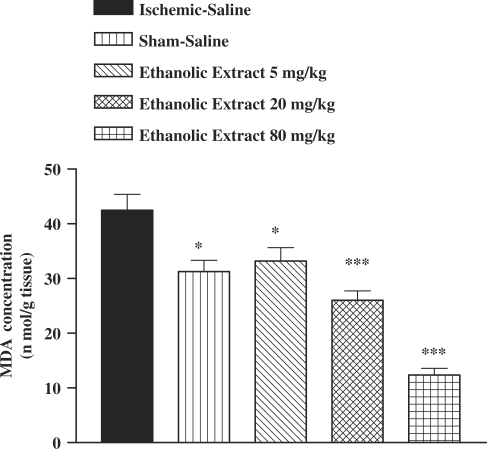
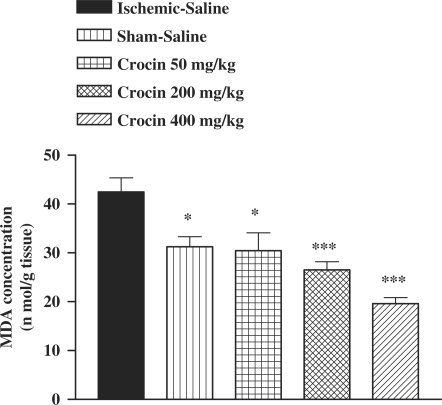
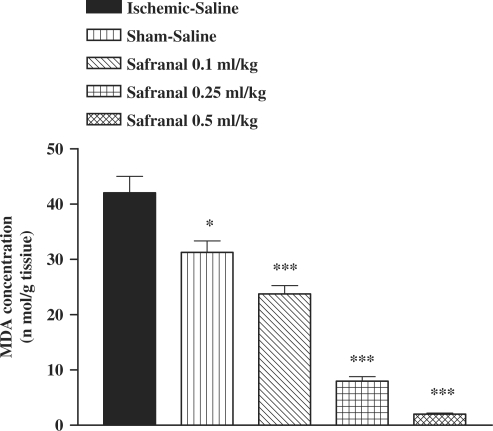
There was an increase in the MDA levels following I/R as compared with sham-operated animals (Fig. 1). The saffron extract, crocin and safranal pretreatment resulted in a significant reduction in the free radical-mediated lipid peroxidation as indicated by a decrease in the MDA levels, at various dose levels (Figs 1, 2 and 3). In safranal-pretreated groups, a reduction in TBARS levels was very prominent in the higher doses.
Figure 1.
Effect of ethanolic saffron extract on lipid peroxidation following muscle ischemia reperfusion injury. MDA levels were measured in 10% homogenates of muscle samples from rats subjected to 120 min of ischemia and 60 min of reperfusion. All drugs were administrated intraperitoneally 60 min prior to reperfusion. Values are mean ± SEM (n = 8). *P <0.05, ***P <0.001 as compared with vehicle (normal saline) treated animals (One-way ANOVA followed by Tukey–Kramer test).
Figure 2.
Effect of crocin on lipid peroxidation following muscle ischemia reperfusion injury. MDA levels were measured in 10% homogenates of muscle samples from rats subjected to 120 min of ischemia and 60 min of reperfusion. All drugs were administrated intraperitoneally 60 min prior to reperfusion. Values are mean ± SEM (n = 8). *P <0.05, ***P < 0.001 as compared with vehicle (normal saline) treated animals (One-way ANOVA followed by Tukey–Kramer test).
Figure 3.
Effect of safranal on lipid peroxidation following muscle ischemia reperfusion injury. MDA levels were measured in 10% homogenates of muscle samples from rats subjected to 120 min of ischemia and 60 min of reperfusion. All drugs were administrated intraperitoneally 60 min prior to reperfusion. Values are mean ± SEM (n = 8). *P <0.05, ***P <0.001 as compared with vehicle (normal saline) treated animals (One-way ANOVA followed by Tukey–Kramer test).
Modulation of FRAP Value
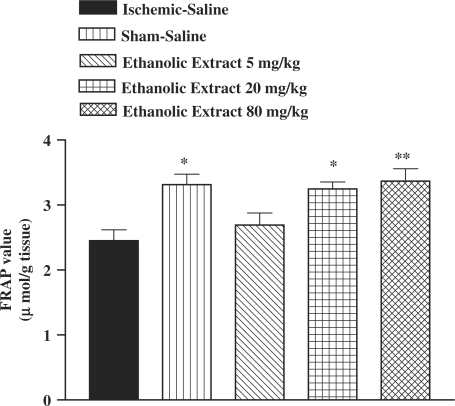
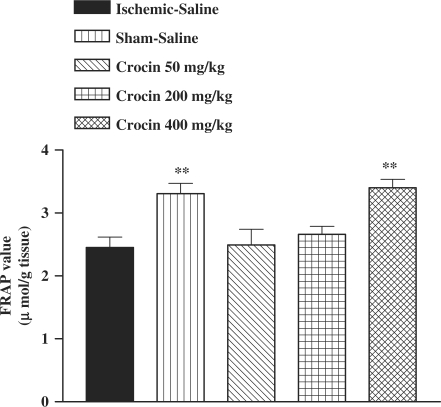
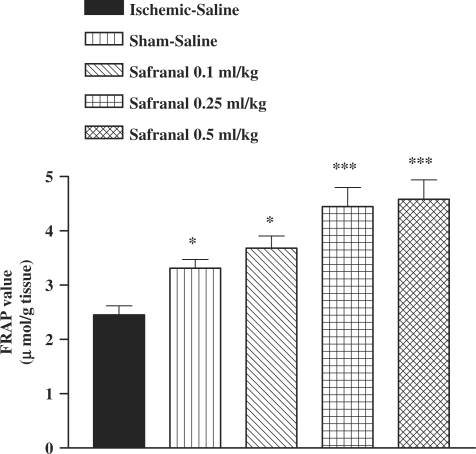
I/R caused a significant reduction in FRAP value of muscle homogenate samples as compared with sham-operated animals (3.31 ± 0.16 versus 2.45 ± 0.17 µmol/g tissue, P < 0.05) (Fig. 4). The ethanolic saffron extract, crocin and safranal pretreatment increased antioxidant power (FRAP value) of muscle homogenate samples (Figs 4, 5 and 6).
Figure 4.
Effect of ethanolic saffron extract on antioxidant power of muscle homogenate samples following muscle ischemia reperfusion injury. FRAP values were measured in 10% homogenate samples from rats subjected to 120 min of ischemia and 60 min of reperfusion. All drugs were administrated intraperitoneally 60 min prior to reperfusion. Values are mean ± SEM (n = 8). *P <0.05, **P <0.01 as compared with vehicle (normal saline) treated animals (One-way ANOVA followed by Tukey–Kramer test).
Figure 5.
Effect of crocin on antioxidant power of muscle homogenate samples following muscle ischemia reperfusion injury. FRAP values were measured in 10% homogenate samples from rats subjected to 120 min of ischemia and 60 min of reperfusion. All drugs were administrated intraperitoneally 60 min prior to reperfusion. Values are mean ± SEM (n = 8). **P <0.01 as compared with vehicle (normal saline) treated animals (One-way ANOVA followed by Tukey–Kramer test).
Figure 6.
Effect of safranal on antioxidant power of muscle homogenate samples following muscle ischemia reperfusion injury. FRAP values were measured in 10% homogenate samples from rats subjected to 120 min of ischemia and 60 min of reperfusion. All drugs were administrated intraperitoneally 60 min prior to reperfusion. Values are mean ± SEM (n = 8). *P <0.05, ***P <0.001 as compared with vehicle (normal saline) treated animals (One-way ANOVA followed by Tukey–Kramer test).
Total Thiol Concentration
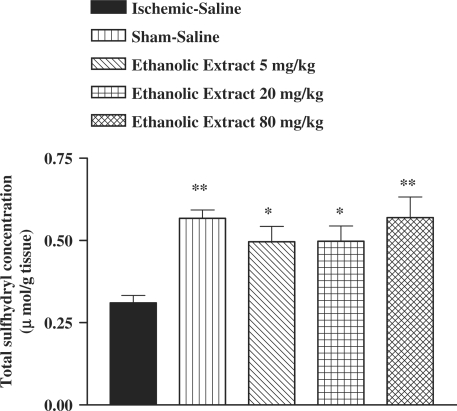
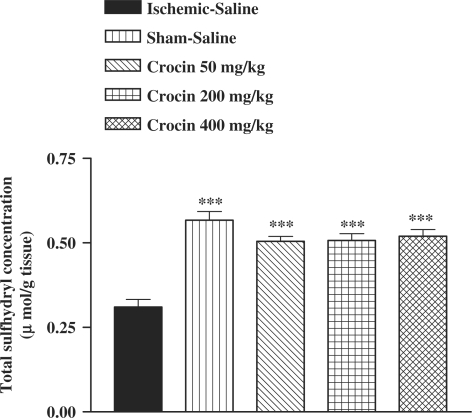
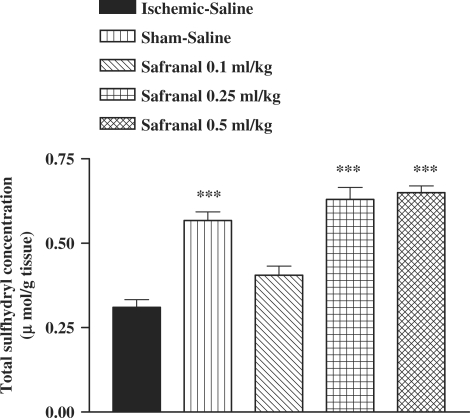
Following I/R injury, a significant reduction in total SH groups (0.57 ± 0.03 versus 0.31 ± 0.02 mM, P < 0.01) in muscle homogenate samples was observed (Fig. 7). The ethanolic saffron extract, crocin and safranal pretreatment caused a significant elevation in total thiol concentration, as compared with control-ischemic group (Figs 7, 8 and 9).
Figure 7.
Effect of ethanolic saffron extract on total thiol concentrations following muscle ischemia reperfusion injury. Total sulfhydryl (SH) groups were measured in 10% muscle homogenate samples from rats subjected to 120 min of ischemia and 60 min of reperfusion. All drugs were administrated intraperitoneally 60 min prior to reperfusion. Values are mean ± SEM (n = 8). *P <0.05, **P <0.01 as compared with vehicle (normal saline) treated animals (One-way ANOVA followed by Tukey–Kramer test).
Figure 8.
Effect of crocin on total thiol concentrations following muscle ischemia reperfusion injury. Total sulfhydryl (SH) groups were measured in 10% muscle homogenate samples from rats subjected to 120 min of ischemia and 60 min of reperfusion. All drugs were administrated intraperitoneally 60 min prior to reperfusion. Values are mean ± SEM (n = 8). ***P <0.01 as compared with vehicle (normal saline) treated animals (One-way ANOVA followed by Tukey–Kramer test).
Figure 9.
Effect of safranal on total thiol concentrations following muscle ischemia reperfusion injury. Total sulfhydryl (SH) groups were measured in 10% muscle homogenate samples from rats subjected to 120 min of ischemia and 60 min of reperfusion. All drugs were administrated intraperitoneally 60 min prior to reperfusion. Values are mean ± SEM (n = 8). ***P <0.001 as compared with vehicle (normal saline) treated animals (One-way ANOVA followed by Tukey–Kramer test).
Discussion
The results obtained in the present investigation suggest that the saffron extract and its active constituents, crocin and safranal, have an overall protective effect against muscle I/R injury in a rat model.
A number of processes have been implicated in the pathogenesis of oxygen deprivation-induced cell injury. These include the disturbances of cell calcium homeostasis, depletion of adenine nucleotides, activation of enzymes like phospholipases with production of toxic lipid metabolites, proteases and endonucleases and generation of free radicals (ROS) that can cause oxidative damage to cellular macromolecules (31,32). Antioxidant therapy has been well documented to help in the improvement of organ functions (33).
We assessed the effect of the saffron extract, crocin and safranal by studying their effects on lipid peroxidation. MDA levels increased significantly following muscle I/R. The saffron extract and its bioactive constituents reversed the increase of MDA levels to a considerable extent, thereby confirming its antioxidant role in I/R.
Under acute and chronic pathologic conditions such as ischemia, the balance between oxidant and antioxidant systems has been interrupted (34,35). Therefore we evaluate the antioxidant or reducing potential of muscle homogenate samples following muscle I/R, using FRAP assay. As expected following this, a significant reduction in antioxidant power, as indicated by FRAP value, was observed. The extract, crocin and safranal increased the antioxidant power of muscle homogenate samples.
Sulfhydryl (SH) groups are highly-reactive constituents of protein molecules, and they participate in important biochemical and metabolic process such as cell division, blood coagulation, maintenance of protein systems and enzymatic activation including antioxidant enzymes (catalase, superoxide dismutase, etc.) (36). There are also important scavengers of oxygen-derived free radicals (37). SH groups known to be sensitive to oxidative damage and depleted following ischemic insult (38), therefore we studied the effect of these agents on total thiol concentration during muscle I/R. Similarly, in our studies, total sulfhydryl groups were decreased following I/R injury. These agents exhibited higher SH contents in muscle tissues than their respective controls, indicating that helped in replenishing the total thiol pool.
As we said crocin and crocetin are main constituents that have antioxidant activity, these means that these components were studied for these effects. There are a lot other ingredients in saffron extract which should evaluate for this effect. In our study the saffron extract was more potent than crocin. This may be attributed to a synergistic action of many constituents such as crocins, crocetin, dimethyl crocetin, safranal and flovonoids which quench free radicals and have antioxidant effects and may have role in protective effect of saffron on muscle I/R. In addition saffron contains proteins, sugars, vitamins (especially riboflavin), amino acids, minerals and gums. Also it seems crocin from Fluka is not completely pure.
Among the constituents of saffron stigmas, crocins and crocetin are the most abundant agents with established antioxidant and antitumor effects (9, 13 and 18). These carotenoids scavenge free radicals, especially superoxide anions and thereby may protect cells from oxidative stress (39). The aqueous saffron extract and its active constituent, crocin also prevent renal I/R-induced oxidative injury in rats (22). In our study the saffron extract was more potent than crocin. This may be attributed to a synergistic action of many constituents such as crocins, crocetin, dimethyl crocetin, safranal and flovonoids which quench free radicals and have antioxidant effects and may have role in protective effect of saffron on muscle I/R. In addition saffron contains proteins, sugars, vitamins (especially riboflavin), amino acids, minerals and gums (9). It is also possible that crocin may be impure.
Safranal also showed anti-ischemia activity in this study. This monoterpene aldehyde, which is the major constituent of the essential oil of saffron (20) showed good antioxidant activity. Our recent studies showed that safranal also attenuated cerebral ischemia induced oxidative damage in rat hippocampus (23). As safranal showed suitable activity in almost all doses, it may be concluded that safranal is major antioxidant constituent or played a major role in protecting I/R-induced oxidative injury. But it should be mentioned that safranal is usually present in less than 1% of saffron extract (40).
It seems that I/R injury reduced the conductivity and viability of muscles and nerves. In this study, the saffron extract and its constituents maintained the nerve conductivity. Nerve conduction is decreased during I/R (41,42). Mild muscle necrotic changes occur after 2–3 h ischemia (43,44). Oxidative stress and the production of oxygen free radicals during I/R is one mechanism of ischemic fiber degeneration, causing a breakdown of the blood-nerve barrier, endoneurial edema and lipid peroxidation (45). Saffron and its constituents prevented lipid peroxidation and showed antioxidant activity in this study. These effects and other activities such as anti-inflammatory effect (12) may preserve viability of nerve conductivity. A reduction in the EMG potential was more severe in reperfusion phase than the ischemia stage as shown in another study (46).
In conclusion, the present study showed that saffron extract and its constituents, crocin and safranal, have protective effect on I/R injury-induced oxidative stress in rats muscle that at least partly is due to antioxidant properties of saffron and its constituents.
Acknowledgements
The authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences for financial support.
References
- 1.Ascher E, Hanson JN, Cheng W, Hingorani A, Scheinman M. Glycine preserves function and decreases necrosis in skeletal muscle undergoing ischemia and reperfusion injury. Surgery. 2001;129:231–5. doi: 10.1067/msy.2001.112594. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda K, Negishi H, Yamori Y. Antioxidant nutrients and hypoxia/ischemia brain injury in rodents. Toxicology. 2003;189:55–61. doi: 10.1016/s0300-483x(03)00152-5. [DOI] [PubMed] [Google Scholar]
- 3.Kearns SR, Daly AF, Sheehan K, Murray P, Kelly C, Bouchier-Hayes D. Oral vitamin C reduces the injury to skeletal muscle caused by compartment syndrome. J Bone Joint Surg Br. 2004;86:906–11. doi: 10.1302/0301-620x.86b6.14177. [DOI] [PubMed] [Google Scholar]
- 4.Erkanli K, Kayalar N, Erkanli G, Ercan F, Sener G, Kirali K. Melatonin protects against ischemia/reperfusion injury in skeletal muscle. J Pineal Res. 2005;39:238–42. doi: 10.1111/j.1600-079X.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang WZ, Fang XH, Stephenson LL, Khiabani KT, Zamboni WA. Melatonin reduces ischemia/reperfusion-induced superoxide generation in arterial wall and cell death in skeletal muscle. J Pineal Res. 2006;41:255–60. doi: 10.1111/j.1600-079X.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 6.Hosseinzadeh H, Nassiri Asl M, Parvardeh S. The effects of carbenoxolone, a semisynthetic derivative of glycyrrhizinic acid, on peripheral and central ischemia-reperfusion injuries in the skeletal muscle and hippocampus of rats. Phytomedicine. 2005;12:632–7. doi: 10.1016/j.phymed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Inokuchi Y, Shimazawa M, Nakajima Y, Suemori S, Mishima S, Hara H. Brazilian green propolis protects against retinal damage in vitro and in vivo. Evid. Based Complement Alternat Med. 2006;3:71–7. doi: 10.1093/ecam/nek005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimazawa M, Chikamatsu S, Morimoto N, Mishima S, Nagai H, Hara H. Neuroprotection by Brazilian Green Propolis against In vitro and In vivo Ischemic Neuronal Damage. Evid Based Complement Alternat Med. 2005;2:201–7. doi: 10.1093/ecam/neh078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ríos JL, Recio MC, Giner RM, Máňez S. An update review of saffron and its active constituents. Phytother Res. 1996;10:189–93. [Google Scholar]
- 10.Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigmas in mice. Arch Irn Med. 2002;5:44–7. [Google Scholar]
- 11.Hosseinzadeh H, Karimi Gh, Niapoor M. Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocin and safranal, in mice. Acta Hort. 2004;650:435–45. [Google Scholar]
- 12.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:1–8. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullaev FJ. Biological effects of saffron. Biofactors. 1993;4:83–6. [PubMed] [Google Scholar]
- 14.Escribano J, Alonso GL, Coca-Prados M, Fernandez JA. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996;100:23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhang YX, Sugiura M, Saito H, Shoyama Y. Acute effects of Crocus sativus L. on passive avoidance performance in mice. Biol Pharmacol Bull. 1994;17:217–21. doi: 10.1248/bpb.17.217. [DOI] [PubMed] [Google Scholar]
- 16.Abe K, Sugiura M, Ymaguchi S, Shoyama Y, Saito H. Saffron extract prevents acetaldehyde-induced inhibition of long-term potentiation in the rat dentate gyrus in vivo. Brain Res. 1999;851:287–9. doi: 10.1016/s0006-8993(99)02174-5. [DOI] [PubMed] [Google Scholar]
- 17.Abdullaev J, Caballero-Ortega H, Riveron-Nigrete L, Pereda-miranda R, Rivera-Luna R, Manuel Hernandez J, Perez-Lopez I, Espinosa-Aguirre JJ. In vitro evaluation of chemopreventive potential of saffron. Rev Inves Clin. 2002;54:430–6. [PubMed] [Google Scholar]
- 18.Nair SC, Kurumboor SK, Hasegawa JH. Saffron chemoprevention in biology and medicine: a review. Cancer Biother. 1995;10:257–64. doi: 10.1089/cbr.1995.10.257. [DOI] [PubMed] [Google Scholar]
- 19.Premkumar K, Abraham SK, Santhiya ST, Ramesh A. Protective effects of saffron (Crocus sativus L.) on genotoxins-induced oxidative stress in Swiss albino mice. Phytother Res. 2003;17:614–17. doi: 10.1002/ptr.1209. [DOI] [PubMed] [Google Scholar]
- 20.Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 21.Verma SK, Bordia A. Antioxidant property of saffron in man. Indian J Med Sci. 1998;52:205–7. [PubMed] [Google Scholar]
- 22.Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm Pharmacol Sci. 2005;8:387–93. [PubMed] [Google Scholar]
- 23.Hosseinzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron) attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharmacol Sci. 2005;8:394–9. [PubMed] [Google Scholar]
- 24.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–56. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 25.Ossowska K, Lorence-Koci E, Schulze G, Wolfarth S. The influence of dizolcipine (MK-801) on the reserpine-enhanced electromyographic stretch reflex in rats. Neurosci Lett. 1996;203:73–6. doi: 10.1016/0304-3940(95)12264-8. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez J, Perez-Alvarez JA, Fernandez-lopez JA. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997;99:345–53. [Google Scholar]
- 27.Uchiama M, Miahara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:279–86. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 28.Benzie IFF, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 29.Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 30.Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 31.Rhodena E, Teloken C, Lucas M, Rhoden C, Mauri M, Zettler C, Bello-Kleind A, Barros E. Protective effect of allopurinol in the renal ischemia–reperfusion in uninephrectomized rats. General Pharmacol. 2002;35:189–93. doi: 10.1016/s0306-3623(01)00105-7. [DOI] [PubMed] [Google Scholar]
- 32.Montagna G, Hofer CG, Torres AM. Impairment of cellular redox status and membrane protein activities in kidneys from rats with ischemic acute renal failure. Biochem Biophys Acta. 1998;1407:99–108. doi: 10.1016/s0925-4439(98)00029-5. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Lott JA, Kauffman EM, Sharma HM. Effect of herbal mixture MAK-4 on organ functions in WHHL rabbits. Biochem Arch. 1997;13:285–96. [Google Scholar]
- 34.Abdollahi M, Ranjbar R, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit. 2004;10:141–7. [PubMed] [Google Scholar]
- 35.Parihar MS, Hemnani T. Phenolic antioxidants attenuate hippocampal neuronal cell damage against kainic acid induced excitotoxicity. J Biosci. 2003;28:121–8. doi: 10.1007/BF02970142. [DOI] [PubMed] [Google Scholar]
- 36.Jansen EV. Sulfhydryl-disulfide interchanges. Science. 1959;130:1319–23. doi: 10.1126/science.130.3385.1319. [DOI] [PubMed] [Google Scholar]
- 37.Dormandy TL. An approach to free radicals in medicine and biology. Acta Physiol Scand. 1980;492:153–68. [PubMed] [Google Scholar]
- 38.Soszynski M, Bartosz G. Decrease in accessible thiols as an index of oxidative damage to membrane proteins. Free Rad Biol Med. 1980;23:463–9. doi: 10.1016/s0891-5849(97)00117-2. [DOI] [PubMed] [Google Scholar]
- 39.Bors W, Saran M, Michel C. Radical intermediates involved in the bleaching of the carotenoid crocin. Hydroxyl radicals, superoxide anions and hydrated electrons. Int J Radiat Biol Relat Stud Phys Chem Med. 1982;41:493–501. doi: 10.1080/09553008214550571. [DOI] [PubMed] [Google Scholar]
- 40.Caballero-Ortega H, Pereda-Miranda R, Abdullaev FI. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem. 2007;100:1126–31. [Google Scholar]
- 41.Oguzhanoglu A, Kurt T, Sahiner T. Nerve conduction parameters during ischemia-reperfusion in the rat sciatic nerve. Electromyogr Clin Neurophysiol. 2000;40:487–90. [PubMed] [Google Scholar]
- 42.Schmelzer JD, Zochodne DW, Low PA. Ischemic and reperfusion injury of rat peripheral nerve. Proc Natl Acad Sci USA. 1989;86:1639–42. doi: 10.1073/pnas.86.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korthals JK, Maki T, Gieron MA. Nerve and muscle vulnerability to ischemia. J Neurol Sci. 1985;71:283–90. doi: 10.1016/0022-510x(85)90066-8. [DOI] [PubMed] [Google Scholar]
- 44.Iida H, Schmelzer JD, Schmeichel AM, Wang Y, Low PA. Peripheral nerve ischemia: reperfusion injury and fiber regeneration. Exp Neurol. 2003;184:997–1002. doi: 10.1016/S0014-4886(03)00385-6. [DOI] [PubMed] [Google Scholar]
- 45.Nagamatsu M, Schmelzer JD, Zollman PJ, Smithson IL, Nickander KK, Low PA. Ischemic reperfusion causes lipid peroxidation and fiber degeneration. Muscle Nerve. 1996;19:37–47. doi: 10.1002/mus.880190103. [DOI] [PubMed] [Google Scholar]
- 46.Mitsui Y, Schmelzer JD, Zollman PJ, Mitsui M, Tritschler HJ, Low PA. Alpha-lipoic acid provides neuroprotection from ischemia-reperfusion injury of peripheral nerve. J Neurol Sci. 1999;163:11–6. doi: 10.1016/s0022-510x(99)00017-9. [DOI] [PubMed] [Google Scholar]