Abstract
Vagal withdrawal and sympathetic overactivity accompany various types of stress. Qi training is reported to reduce sympathetic hyper-reactivity in a stressful situation. Turo, which is a type of dance that uses the Meridian Qi System, may reduce the psychological symptoms induced by an imbalance of the autonomic nervous system (ANS). We observed whether Turo training alters psychopathological and psychological symptoms using the Symptom Checklist 90-Revision (SCL-90-R) and examined whether it attenuates the stress response to mental stress in healthy adolescent females using the power spectrum analysis of heart rate variability (HRV). Twenty-one subjects received Turo training and 27 subjects were trained with mimicking movements. The SCL-90-R was measured before and after the 2-month training period. Heart rate (HR), total power (TP) and the LF/HF ratio of HRV were compared between the Turo and control groups during and after mental stress. The somatization and hostility subscales of the SCL-90-R of the Turo group were significantly lower than those of the control group after 2 months. The increases in HR and the LF/HF ratio of HRV induced by the stress test were significantly lower in the Turo group than in the control group. The TP of the Turo group was significantly higher than that of the control group. The psychological symptoms and sympathetic activation induced by the artificial stress were significantly reduced by the Turo training. These findings suggest that Turo training can play a critical role in attenuating psychological symptoms and stress-induced sympathetic activation.
Keywords: autonomic nervous system, heart rate variability, psychological symptoms, Qi-training, stress
Introduction
Some diseases are associated with depressed vagal modulation and enhanced sympathetic function, and various types of stress produce vagal withdrawal and sympathetic overactivity (1). Clinicians suggest that this imbalance of the autonomic nervous system (ANS) may cause not only physical symptoms, but also psychological symptoms such as anxiety, hostility and sleeping disorders that often cause individuals to suffer. Appropriate restoration of ANS regulation would be beneficial to the patient by improving the clinical conditions (2). Heart rate variability (HRV) analysis is a sophisticated non-invasive tool for assessing ANS regulation of the heart. Decreased HRV, indicating disturbed ANS function (3), has been associated with mental stress in laboratory experiments (4). Mental stress induced by the Stroop color word test (CWT) or by mental arithmetic is an established means by which to provoke sympathoexcitatory responses (5, 6).
According to traditional Chinese medicine, the functions of the human body are controlled by a vital force or energy called ‘Qi,’ which circulates among the organs along channels called meridians (7). Meridians are traditionally thought to represent channels through which ‘meridian Qi’ flows (8). The disruption of the meridian channel network is associated with various illnesses.
Turo, which is a sophisticated dance applying the Meridian Qi System, is a type of Qi training that concentrates the consciousness on the meridian pathways to strengthen the existing flow of Qi and simultaneously make use of the muscles related to meridian pathways. Acupuncture, Qi-gong, and Turo training may access and influence this system (9). Acupuncture restores the flow of vital energy in the body by stimulating the organ-specific meridians and enhances cardiac vagal activity and suppresses sympathetic activity in healthy subjects (10). Acupuncture also attenuates sympathoexcitation during mental stress in advanced heart failure patients (11).
A number of studies have been conducted to enhance vagal activity and/or attenuate sympathetic activity using various modalities, including Qi-gong. Qi training increases high frequency (HF) power and decreases the low frequency/high frequency (LF/HF) ratio of HRV, indicating that Qi training increases cardiac vagal tone (12). External Qi therapy reduces the heart rate (HR) and increases the HRV, as indicated by a reduced LF/HF ratio of HRV (13). Turo may have similar effects on restoring ANS imbalance and subsequently on reducing psychological symptoms such as anxiety, hostility and sleep disorders (14). However, there has been no scientific research conducted to examine the potential effects of Turo training on the cardiac autonomic response to mental stress.
Thus, we examined whether Turo training improves psychological symptoms using the Symptom Checklist 90-Revision (SCL-90-R) and whether Turo training attenuates the increase in HR and sympathetic activation that result from mental stress in healthy adolescent females.
Methods
Subjects
To avoid the influence of gender and age on HRV (15), 48 healthy female subjects (mean age, 13.3 ± 0.1 years) were recruited from a middle school in Hwa-Sung, Republic of Korea. After the risks and benefits of the study had been explained verbally and in writing to the subjects and their parents, written informed consent was obtained. The study protocol was approved by the Kyung Hee University Institutional Review Board.
Training Groups and Trials
None of the participants had previously practiced Turo. The subjects were randomly divided into a Turo group and a control group using computer-generated randomization. To minimize the placebo effect, both groups were told that they would receive the same physical training. The Turo group comprised 21 subjects who were trained in Turo for 2 months; the control group comprised 27 subjects who were trained in similar movements, but without the concept of meridian Qi flow, for the same period. A certified Turo master taught the procedures of Turo to the subjects on a one-to-one basis. The subjects in the Turo group performed this exercise twice each week for 40 min each session. Each set of Turo comprised 21 postures that potentiate meridian Qi flow (Fig. 1).
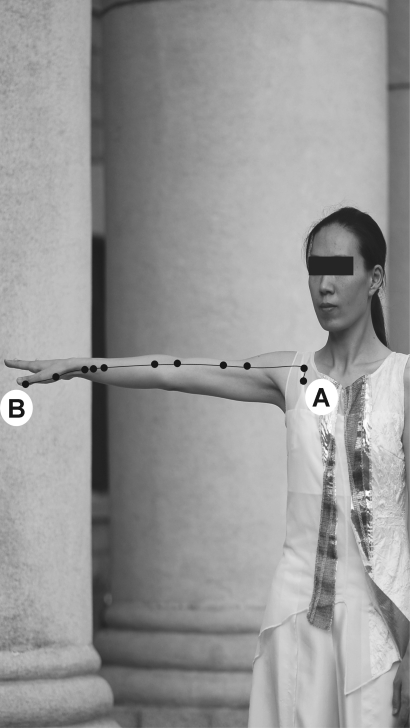
Figure 1.
An example of Turo posture applying Lung meridian. The consciousness of Turo trainee was driven from LU1 (A) to LU11 (B) along the direction of Lung meridian. Turo is a sophisticated dance or Qi-gong applying each meridian Qi system; it is a kind of Qi training that concentrates the consciousness on the meridian pathways to get the Qi flow smooth and activated and that simultaneously makes use of the muscles related to meridian system. Each set of Turo comprises 21 postures which potentiate the Qi flow in the meridian.
The Psychological Symptom Questionnaire
The SCL-90-R is a self-reported checklist composed of 90 items that each describes a physical or psychological symptom. It has been used in a variety of medical populations, including patients with whiplash injuries (16). The checklist provides a profile of somatization (SOM), obsession–compulsion (OC), interpersonal sensitivity (IS), depression (DEP), anxiety (ANX), hostility (HOS), phobic anxiety (PHOB), paranoid ideation (PAR) and psychoticism (PSY). The total SCL-90-R score indicates the general level of distress. The questionnaire was completed by all subjects in their usual environment on a normal weekday and reflected their psychological distress over the past 7 days. The SCL-90-R was compared before and after the 2-month training period.
Experimental Protocol
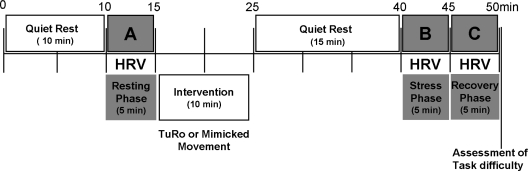
Based on the degree of training, 14 adolescents were selected from the Turo group and 16 volunteers were selected from the control group. The degree of training was quantitatively evaluated by another Turo master after the 2-month training period. Only subjects who reached the appropriate degree of Turo training participated in the HRV measurement. Subjects were asked not to take any medication during the week prior to the measurement. On the test day, subjects abstained from consuming caffeinated beverages or performing heavy exercise. The subjects were restricted from food intake for at least 1 h before the test. For HRV measurement, the subject rested in the supine position for 10 min, and ECG electrodes were positioned over the radial arteries on both wrists. The HRV was recorded at baseline (Resting phase) before the two types of movements (Turo or mimicked movements). After 15 min of rest following the last movement, the HRV was recorded during mental stress (Stress phase) and after mental stress (Recovery phase). Each session lasted 5 min (Fig. 2).
Figure 2.
The experimental protocol is as shown. After a 10-min rest period, HRV was recorded for 5 min during the resting phase (A). The intervention was performed for 10 min. After a 15-min quiet rest, HRV was recorded for 5 min during the recovery phase (C). HRV was recorded during mental stress (Stress phase; B) and after mental stress (Recovery phase; C). Quantified ratings (0.0–10.0) based on a 10-point scale were determined to assess task difficulty on completion of the protocol.
Measurement of HRV
The experiments were performed in an air-conditioned (20 ± 2°C), sound-attenuated room. Electrodes were attached to the subjects while in the supine position. The ECGs were measured using an electrocardiogram amplifier (Heart Rhythm Scanner, Biocom Technologies Inc., USA). The HRV analysis was performed in accordance with the recommendations by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (2). The power spectra were analyzed at two frequency ranges: low frequency (LF: 0.04–0.15 Hz) and high frequency (HF: 0.15–0.40 Hz). The LF component, which corresponds to the 0.15-Hz slow fluctuations of arterial pressure, mainly represents sympathetic modulation of the sinus node, whereas the HF component, which is a measure of respiratory sinus arrhythmias, can be regarded as an index of vagal (parasympathetic) modulation (17). The ratio of the LF and HF components (LF/HF ratio), which is an index of the sympathovagal balance, was also determined. The total power (TP) was evaluated as the sum of all frequency ranges.
Mental Stress Tests
Mental stress testing was performed for 5 min using either the Stroop color word test or mental arithmetic (5, 6). During the Stroop color word test, volunteers were shown a series of names of colors written in a different color of ink from the color specified (e.g. the word ‘red’ written in blue ink). The subject was instructed to identify the color of the ink, not read the word, as quickly as possible. During verbally administered mental arithmetic, the subject was asked to subtract one- or two-digit numbers from two- or three-digit numbers as quickly and accurately as possible. Because sympathetic responses to mental-stress testing are strongly influenced by the perception of task difficulty (18), quantified ratings based on a 10-point scale (0–10) were used to assess the task difficulty on completion of the protocol.
Statistical Analysis
The values are expressed as the mean ± SE. Data were compared between groups using Student's t-test or repeated-measures two-way analysis of variance (ANOVA). Differences were considered statistically significant at P < 0.05.
Results
Effect on Psychological Symptoms
For the SCL-90-R scores before and after Turo training (Table 1), there was a significant time × group interaction in the mean SOM scores [repeated-measures ANOVA; F(1,47) = 6.424, P < 0.05] and in the mean HOS scores [F(1,47) = 5.687, P < 0.05]. However, there was no significant time × group interaction for the other symptoms, the global severity index (GSI), the positive symptom total (PST) or the positive symptom distress index (PSDI).
Table 1.
The comparison of psychological symptoms between control and Turo groups before and after the 2-month training period using SCL-90-R
| Control (n = 27) | Turo (n = 21) | Time × Group | ||||
|---|---|---|---|---|---|---|
| SCL-90-R | Pre | Post | Pre | Post | F | P |
| SOM | 52.3 ± 2.0 | 50.9 ± 2.0 | 50.8 ± 2.0 | 44.2 ± 1.5 | 6.42 | 0.01 |
| O-C | 50.7 ± 2.5 | 49.5 ± 1.6 | 47.8 ± 2.4 | 42.5 ± 1.7 | 2.52 | 0.11 |
| I-S | 54.0 ± 2.5 | 50.4 ± 2.1 | 46.0 ± 2.4 | 40.4 ± 1.9 | 0.87 | 0.35 |
| DEP | 47.0 ± 1.8 | 47.3 ± 1.6 | 46.1 ± 2.2 | 40.4 ± 1.6 | 3.70 | 0.06 |
| ANX | 48.2 ± 1.5 | 49.5 ± 1.6 | 44.3 ± 1.6 | 40.9 ± 1.0 | 3.55 | 0.06 |
| HOS | 53.9 ± 1.9 | 56.1 ± 2.1 | 48.3 ± 1.8 | 44.9 ± 1.9 | 5.68 | 0.02 |
| PHOB | 51.1 ± 1.6 | 48.9 ± 1.5 | 46.8 ± 1.6 | 44.4 ± 0.8 | 0.00 | 0.93 |
| PAR | 50.7 ± 2.4 | 50.4 ± 2.1 | 47.1 ± 2.0 | 43.8 ± 1.4 | 1.20 | 0.28 |
| PSY | 53.8 ± 1.9 | 52.2 ± 1.8 | 48.2 ± 1.9 | 42.0 ± 0.8 | 3.22 | 0.07 |
| GSI | 51.2 ± 2.1 | 49.9 ± 1.8 | 46.2 ± 2.0 | 43.1 ± 2.2 | 0.35 | 0.55 |
| PSDI | 53.5 ± 2.3 | 53.3 ± 1.9 | 51.2 ± 2.8 | 47.3 ± 2.0 | 1.07 | 0.30 |
| PST | 48.8 ± 1.8 | 48.0 ± 2.1 | 43.6 ± 1.9 | 39.0 ± 2.6 | 1.39 | 0.24 |
SCL-90-R, Symptom Check Lists-90-Revision; SOM, somatization; O-C, obsessive-compulsive; I-S, interpersonal sensitivity; DEP, depression; ANX, anxiety; HOS, hostility; PHOB, phobic anxiety; PAR, paranoid ideation; PSY, psychoticism; GSI, global severity index; PSDI, positive symptom distress index; PST, positive symptom total. Values are shown as mean ± SE.
Autonomic Response to Mental Stress
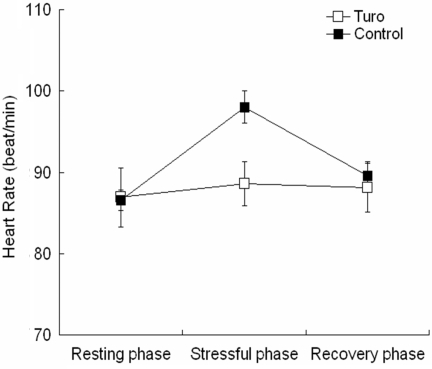
The mean HR of the Turo group was significantly lower than that of the control group during the stress phase (88.6 ± 2.72 versus 98.04 ± 2.02) and similar to that of the control group during the recovery phase (88.11 ± 2.96 versus 89.56 ± 1.77; Fig. 3). There was a significant time × group interaction [repeated-measures ANOVA; F(2,28) = 5.478, P < 0.05] and a significant effect of time [F(2,28) = 3.292, P < 0.05]. However, there was no significant group effect (Fig. 3).
Figure 3.
The effect of Turo on heart rate changes during and after mental stress. Turo significantly attenuated the increase in heart rate during mental stress. Values are means ± SE.
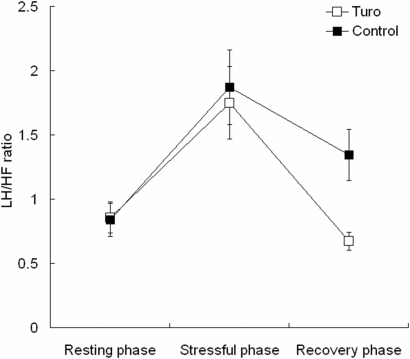
The mean LF/HF ratio of the Turo group was slightly lower than that of the control group during the stress phase (1.75 ± 0.28 versus 1.86 ± 0.29) and markedly lower than that of the control group during the recovery phase (0.67 ± 0.07 versus 1.34 ± 0.20; Fig. 4). There was a significant effect of time [repeated-measures ANOVA; F(2,28) = 13.97, P < 0.05]. The group effect and time × group interaction were not significant.
Figure 4.
The effect of Turo on LF/HF ratio changes during and after mental stress. Turo significantly attenuated the increase in LF/HF ratio after mental stress. Values are means ± SE.
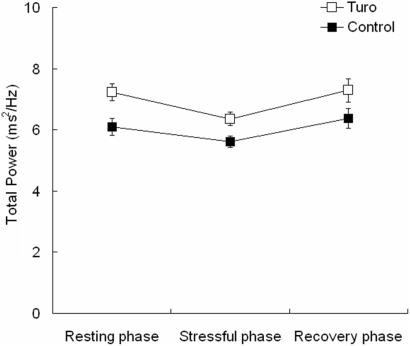
The mean TP before, during and after mental stress was 7.23 ± 0.27, 6.36 ± 0.22 and 7.29 ± 0.37 for the Turo group and 6.09 ± 0.28, 5.61 ± 0.19 and 6.38 ± 0.33 for the control group, respectively (Fig. 5). There was a significant group effect [repeated-measures ANOVA; F(1,28) = 10.63, P < 0.05]. However, the time effect and time × group interaction were not significant.
Figure 5.
The effect of Turo on TP changes during and after mental stress. Turo significantly attenuated the increase in TP after mental stress. Values are means ± SE.
The Perceived Difficulty During Mental Stress
The perceived difficulty induced by mental stress was similar between the two groups (5.20 ± 0.58 versus 4.4 ± 0.60; P > 0.05).
Discussion
Turo training ameliorated the SOM and HOS symptoms in the SCL-90-R test. In addition, the enhanced HR and LF/HF ratio induced by mental stress were significantly attenuated in the Turo group compared to the control group. The TP of the Turo group was significantly enhanced, regardless of mental stress. Thus, Turo training might have therapeutic effects on psychological and stress-related disorders.
Our results agree with the previous reports that Qi-gong training is effective for maintaining mental fitness (19). The Turo group showed significant improvement in negative psychological symptoms such as SOM and HOS. There were statistically significant effects of time to both the Turo and control groups in five subscales of psychological symptoms, including OC, IS, PHOB, HOS and PSY. In training, the control group copied the movements of the Turo group, but received no guidance on the circulation of consciousness and breathing methods. Even though the concept of consciousness and breathing were absent, physiological changes in the control group subjects were not blocked completely; the mental fitness of the control group subjects could have been affected because Turo comprises movements that use muscles related to the meridian pathways. Therefore, our control group may not have been a perfect control, possibly resulting in meaningful differences between the two groups in only two psychological subscales, SOM and HOS.
A large body of evidence suggests that Qi training is effective in treating psychological and stress-related disorders (8, 19). In addition, stress reduction with Qi training results in the decrease of several cardiovascular abnormalities, including hypertension, elevated cholesterol levels and chronic stress (20, 21). Several types of Qi-gong have been reported to modulate autonomic functions using the power spectrum of HRV (12, 13). However, the effects of Turo training on the cardiac autonomic response to mental stress have received little attention. During the stressful phase, we found no statistically significant differences between the groups in the LF/HF ratio of HRV, whereas during the recovery phase, the LF/HF ratio of the Turo group showed a notably prompt recovery to the baseline condition, indicating that the effect of Turo on autonomic imbalance is more likely to emerge in the stress recovery phase.
The value of HR as a reliable estimator of autonomic activity has been questioned because HR is subject to many control mechanisms and pathological phenomena (22). However, the mean HR of the control group significantly increased with the same pattern as the LF/HF ratio of HRV during the mental-stress phase compared to the resting phase, indicating that the sympathovagal balance became higher. Therefore, it is highly likely that the alteration of HR is a result of the reciprocal coordination of a decrease in parasympathetic nerve activity and an increase in sympathetic nerve activity, indicating that HRV might not be suitable for detecting the subtle effect of Turo on ANS function during the stress phase.
During the resting phase, there were no statistically significant differences between the two groups in either HR or the LF/HF ratio of HRV. The TP of HRV did not show a time effect, but did show a group effect. The TP of the Turo group was much higher than that of the control group during all phases, including the resting phase, indicating low levels of stress as a result of sympathetic inhibition. Healthy people with higher levels of psychological stress have significantly lower HRV parameters (23). Therefore, it appears that long-term Turo training has a cumulative effect on the TP of the HRV and might exert a relieving effect on psychological stress.
Short-term HRV is regulated by cardiac ANS activity. The responses of HRV during emotional tasks have been considered a marker of autonomic regulation. Previous research on fitness, exercise and HRV demonstrated that the greater parasympathetic response at rest and the reduction of parasympathetic activity during and after mental challenge is influenced by aerobic training (24). In addition, aerobic fitness reduces sympathetic responses to laboratory stressors in young women with parental hypertension (25). Similarly, Turo training lowered sympathetic activation induced by mental stress compared to that in a general exercise group. These findings can be explained by the untested assumption that Turo training initiates and strengthens the Qi flow through the meridian system.
This pilot study had several limitations such as a small sample size, a possibly affected control treatment and non-persistent follow-up. These concerns make it difficult to draw clear conclusions on the efficacy of Turo training. Rigorous trials with large sample sizes and adequate experimental design are needed to define the role of Turo in mitigating psychological symptoms. However, our results clearly demonstrate that Turo training produces not only subjective improvement of psychological distress, but also objective recovery of ANS disturbance induced by artificial stress, using HRV analysis. We think that these preliminary data provide evidence of the potential benefits of Turo training in psychological fitness.
Conclusion
A Turo exercise program has the potential to play a critical role in attenuating the increase in heart rate and sympathetic activation induced by mental stress. Turo might be useful as an alternative modality in treating psychological and stress-related disorders.
Acknowledgements
This study was supported by the SRC program of KOSEF (Korea Science and Engineering Foundation, R11-2005-014), Republic of Korea.
References
- 1.Sloan RP, Korten JB, Myers MM. Components of heart rate reactivity during mental arithmetic with and without speaking. Physiol Behav. 1991;50:1039–45. doi: 10.1016/0031-9384(91)90434-p. [DOI] [PubMed] [Google Scholar]
- 2.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 3.Horsten M, Ericson M, Perski A, Wamala SP, Schenck-Gustafsson K, Orth-Gomer K. Psychosocial factors and heart rate variability in healthy women. Psychosom Med. 1999;61:49–57. doi: 10.1097/00006842-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Sloan RP, Shapiro PA, Bagiella E, Boni SM, Paik M, Bigger JT, Jr, et al. Effect of mental stress throughout the day on cardiac autonomic control. Bio Psychol. 1994;37:89–99. doi: 10.1016/0301-0511(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 5.Hjemdahl P, Freyschuss U, Juhlin-Dannfelt A, Linde B. Differentiated sympathetic activation during mental stress evoked by the Stroop test. Acta Physiol Scand Suppl. 1984;5:25–9. [PubMed] [Google Scholar]
- 6.Middlekauff HR, Nguyen AH, Negrao CE, Nitzsche EU, Hoh CK, Natterson BA, et al. Impact of acute mental stress on sympathetic nerve activity and regional blood flow in advanced heart failure: implications for ‘triggering’ adverse cardiac events. Circulation. 1997;96:1835–42. doi: 10.1161/01.cir.96.6.1835. [DOI] [PubMed] [Google Scholar]
- 7.Flowers J. What is qi? Evid Based Complement Alternat Med. 2006;3:551–2. doi: 10.1093/ecam/nel074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin P. Efficacy of Tai Chi, brisk walking, meditation, and reading in reducing mental and emotional Stress. J Psychosom Res. 1992;36:361–70. doi: 10.1016/0022-3999(92)90072-a. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi H, Ishii M. Mind-Body, Ki (Qi) and the skin: commentary on Irwin's ‘Shingles immunity and health functioning in the elderly: Tai Chi Chih as a behavioral treatment'. Evid Based Complement Alternat Med. 2005;2:113–6. doi: 10.1093/ecam/neh071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JD, Kuo TB, Yang CC. An alternative method to enhance vagal activities and suppress sympathetic activities in humans. Auton Neurosci. 2002;100:90–5. doi: 10.1016/s1566-0702(02)00150-9. [DOI] [PubMed] [Google Scholar]
- 11.Middlekauff HR, Hui K, Yu JL, Hamilton MA, Fonarow GC, Moriguchi J, et al. Acupuncture inhibits sympathetic activation during mental stress in advanced heart failure patients. J Card Fai. 2002;8:399–406. doi: 10.1054/jcaf.2002.129656. [DOI] [PubMed] [Google Scholar]
- 12.Lee MS, Huh HJ, Kim BG, Ryu H, Lee HS, Kim JM, et al. Effects of Qi-training on heart rate variability. Am J Chin Med. 2002;30:463–70. doi: 10.1142/S0192415X02000491. [DOI] [PubMed] [Google Scholar]
- 13.Lee MS, Rim YH, Jeong DM, Kim MK, Joo MC, Shin SH. Nonlinear analysis of heart rate variability during Qi therapy (external Qigong) Am J Chin Med. 2005;33:579–88. doi: 10.1142/S0192415X05003181. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Chae YB, Hahm DH, An KE, Park HJ, Lee HJ. The effect of TuRo (Qi dance therapy) on the psychological health in adolescent female students. Kor J Meridian Acupoint. 2006;23:69–78. [Google Scholar]
- 15.Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability–the ARIC study. Atherosclerosis risk in communities. Am J Cardiol. 1995;76:906–12. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- 16.Derogatis LR, Rickels K, Rock A. The SCL-90 and the MMPI: A step in the validation of a new self-report scale. Br J Psychiatry. 1976;128:280–89. doi: 10.1192/bjp.128.3.280. [DOI] [PubMed] [Google Scholar]
- 17.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 18.Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol. 1992;454:373–87. doi: 10.1113/jphysiol.1992.sp019269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MS, Jeong SM, Oh SW, Ryu H, Chung HT. Effects of chundosunbup Qi-training on psychological adjustments: a cross-sectional study. Am J Chin Med. 1998;26:223–30. doi: 10.1142/S0192415X98000270. [DOI] [PubMed] [Google Scholar]
- 20.Alexander CN, Schneider RH, Staggers F, Sheppard W, Clayborne BM, Rainforth M, et al. Trial of stress reduction for hypertension in older African Americans. II. Sex and risk subgroup analysis. Hypertension. 1996;28:228–37. doi: 10.1161/01.hyp.28.2.228. [DOI] [PubMed] [Google Scholar]
- 21.Barnes VA, Treiber FA, Turner JR, Davis H, Strong WB. Acute effects of transcendental meditation on hemodynamic functioning in middle-aged adults. Psychosom Med. 1999;61:525–31. doi: 10.1097/00006842-199907000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik M, Camm AJ. Components of heart rate variability–what they really mean and what we really measure. Am J Cardio. 1993;72:821–2. doi: 10.1016/0002-9149(93)91070-x. [DOI] [PubMed] [Google Scholar]
- 23.Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Int J Psychophysiol. 2000;37:121–33. doi: 10.1016/s0167-8760(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 24.Boutcher SH, Nugent FW, McLaren PF, Weltman AL. Heart period variability of trained and untrained men at rest and during mental challenge. Psychophysiol. 1998;35:16–22. [PubMed] [Google Scholar]
- 25.Buckworth J, Dishman RK, Cureton KJ. Autonomic responses of women with parental hypertension. Effects of physical activity and fitness. Hypertension. 1994;24:576–84. doi: 10.1161/01.hyp.24.5.576. [DOI] [PubMed] [Google Scholar]