Abstract
Angiomyolipomata (AML) belong to a family of tumors known as perivascular epithelioid cell tumors (PEComas) that share a common immunophenotypic profile of muscle and melanocytic differentiation. These tumors are clonal in nature and have a strong association with tuberous sclerosis. Genetic analyses have reported allelic imbalance at the TSC2 locus on 16p13. In the context of non-TSC, non-LAM associated AMLs and non-renal PEComas, the functional status of the TSC2 signaling pathway has not been reported. Studies over the last several years have uncovered a critical role of the TSC1/2 genes in negatively regulating the Rheb/mTOR/p70S6K cascade. Here, we examined the activity of this pathway in sporadic AMLs and PEComas using immunohistochemical and biochemical analyses. We found increased levels of phospho-p70S6K, a marker of mTOR activity, in 15 of 15 non-TSC AMLs. This was accompanied by reduced phospho-AKT expression, a pattern that is consistent with the disruption of TSC1/2 function. Western blot analysis confirmed mTOR activation concurrent with the loss of TSC2 and not TSC1 in sporadic AMLs. Similarly, elevated phospho-p70S6K and reduced phospho-AKT expression was detected in 14/15 cases of extra-renal PEComas. These observations provide the first functional evidence that mTOR activation is common to sporadic, non-TSC-related AMLs and PEComas. This suggests the possibility that mTOR inhibitors such as rapamycin may be therapeutic for this class of disease.
Keywords: AML, PEComa, immunohistochemistry, tuberous sclerosis, TSC2, p70S6K
Introduction
The World Health Organization recently recognized as a distinct entity a heterogeneous family of neoplasms with perivascular epithelioid cell differentiation known as PEComa 1. These lesions are defined as “mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells”. While the origin of these cells is unknown, PEComas include angiomyolipoma (AML), lymphangio-leiomyomatosis (LAM), clear cell ‘sugar’ tumor of the lung, clear cell myomelanocytic tumor of the falciform ligament, and other “unusual clear cell tumors” designated as PEComas not otherwise specified (NOS) 2. Such tumors may be conceptualized as monotypic epithelioid or spindled non-renal AML, akin to the monotypic tumors known to occur in TSC-associated renal AML. The clinical course of these tumors is highly variable ranging from indolent benign lesions, to lesions with an aggressive clinical course including distant metastases. Due to their relative scarcity, little is known about the underlying molecular mechanism. Currently, there is no effective medical therapy.
As suggested by its designation, perivascular epithelioid cells (PEC) are characterized histologically by their epithelioid appearance and their physical relationship to blood vessels1. They display a distinct immunophenotype that includes the expression of melanocytic and smooth muscle markers (e.g., gp100 and smooth muscle actin) but not epithelial antigens. Since the PECs have no normal anatomic counterpart, the origin of these tumors remains elusive.
AML and LAM represent a subset of PEComas that are strongly associated with tuberous sclerosis complex (TSC) 3. The latter is an autosomal dominant disease characterized by the multi-system development of benign tumors stemming from underlying mutations of either the TSC1 or TSC2 tumor suppressor gene4. AML of the kidney develops in >50% of TSC patients and is a major source of morbidity secondary to hemorrhage and destruction of renal parenchyma. Recent studies suggest that up to one-third of adult TSC females also have manifestations of LAM based on radiologic characteristics of their lungs 5, 6. LAM occurs almost exclusively in females, although one case of LAM in a male patient has been reported7. The coexistence of AML and LAM in TSC suggests a common pathogenetic mechanism stemming from the disruption of the TSC1/2 pathway. However, it is not known if sporadic AMLs and other less common forms of PEComas suffer from the functional consequences of the loss of TSC1/2 activity.
A small number of studies that have examined the cytogenetic and molecular genetic features of PEComas have highlighted the clonal nature of these tumors but without consistent findings. In the context of the TSC genes, allelic loss of the TSC2 locus on 16p13 has been found repeatedly in sporadic AMLs and PEComas suggesting a potential causal link8–11. However, many other recurrent chromosomal alterations including the loss of 1p, 17p, 18p, 19 as well as gain of 2q, 3q, 5, 12q and X have been identified in a recent comparative genomic hybridization study of PEComas12. To better appreciate the functional significance of 16p LOH in these tumors, we used a biochemical and immunohistochemical (IHC) approach to assess the potential role of the TSC2 pathway.
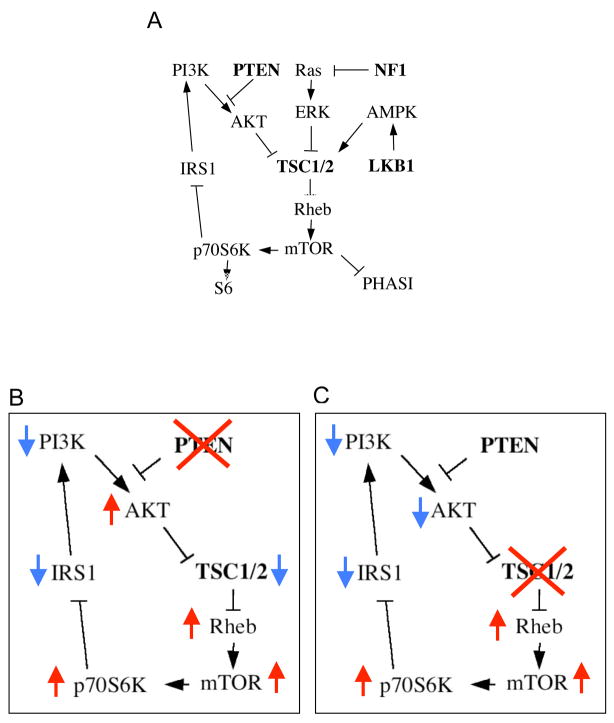
Proteins encoded by the TSC1 and TSC2 genes, hamartin and tuberin respectively, function as a complex to negatively regulate mTOR signaling in response to multiple environmental signals 13. In the presence of growth factors, PI3K-mediated AKT activity promotes Rheb-dependent mTOR function to increase protein synthesis and consequently, cell growth (Figure 1A). This pathway is under the regulation of three tumor suppressor proteins, PTEN, TSC1, and TSC2. PTEN functions as a phosphatase to reduce intracellular lipid products (e.g., PIP3) of PI3K, and in doing so, blocks the activation of AKT14. The TSC1/2 complex acts downstream of AKT to promote hydrolysis of Rheb-GTP via its GAP activity, which leads to the suppression of mTOR signaling. The mTOR serine/threonine kinase phosphorylates p70S6K and PHASI to promote ribosomal biogenesis and protein translation15. Recent studies identified other tumor suppressor proteins, LKB1 (Puetz-Jeghers syndrome) and NF1 (neurofibromatosis 1) that also regulate the mTOR pathway by modulating the activity of TSC1/2 16,17. Thus, dyregulation of the TSC/mTOR pathway may be central to a diverse group of human tumors.
Figure 1.
The TSC/mTOR signaling pathway. A) PI3K-dependent activation of AKT leads to the inhibition of TSC2 activity resulting in an increase in Rheb-GTP level and downstream activation of mTOR, which in turn phosphorylates p70S6K and PHASI to promote protein synthesis. LKB1 phosphorylates AMPK to enhance TSC1/2 inhibition of mTOR. Acting down-stream of Ras, ERK and RSK phosphorylates TSC2 to repress its GAP activity. In a negative feedback loop, p70S6K phosphorylates IRS1 to reduce insulin sensitivity and AKT activity. Tumor suppressor genes that inhibit mTOR activity are in bold. B, C) Simplified versions of the pathway to highlight the functional consequences of the loss of PTEN (B) and TSC1/2 (C) are shown to illustrate the unique difference in AKT activity of the two scenarios. Arrows indicate relative change in activities.
While the loss of PTEN, TSC1 or TSC2 leads to mTOR activation during tumor development, there is a unique difference that distinguishes the molecular events in the respective tumors. When PTEN is absent, AKT becomes constitutively activated (i.e., high phospho-AKT levels), whereas AKT phosphorylation is suppressed when either TSC1 or TSC2 is lost or mutated (Figures 1B,C). The opposing effects on AKT activity in these two scenarios have been implicated in the observed differences in phenotypic behavior (i.e., the benign nature of TSC-related tumors versus the frequent involvement of PTEN in human cancers) 18,19. The mechanism of AKT inhibition following disruption of TSC1/2 has been shown to be secondary to the negative feedback of p70S6K on the insulin receptor substrate 1, IRS1, leading to reduced insulin sensitivity20, 21. Therefore, the combined assessment of mTOR and AKT activities in tumors provides not only evidence for the possible involvement of the mTOR pathway but also clues to the underlying mechanisms. In this study, we show that sporadic forms of renal AML and non-renal PEComas possess high levels of phospho-p70S6K and low levels of phospho-AKT consistent with the inactivation of TSC1/2. Indeed, our data indicate the loss of TSC2 but not PTEN expression in sporadic AMLs.
Materials and Methods
Tissue samples
Kidney AML samples (formalin fixed, paraffin embedded) were obtained from Elizabeth Henske MD, Fox Chase Cancer Center (Philidelphia, PA) and from the University of Washington Medical Center (Seattle, WA). PEComa samples (formalin fixed, paraffin embedded) were obtained from Emory University (Atlanta, GA) and the University of Washington Medical Center (Seattle, WA). Fresh AML and kidney tissues were obtained from the University of Washington Medical Center, Seattle, WA. Samples were obtained through protocols approved by the respective Institutional Review Boards.
Chemicals and Reagents
The following antibodies were purchased from the respective suppliers: rabbit anti-PHAS-I (4E-BP1) from Zymed (San Francisco, CA), rabbit anti-tuberin (C-20) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), rabbit anti-LKB1 from Calbiochem (La Jolla, CA), and mouse anti-actin from Sigma Chemical Co. (St. Louis, MO). All other antibodies were purchased from Cell Signaling (Beverly, MA). Secondary antibodies and ECL reagent were purchased from Amersham Pharmacia Biotech (Piscataway, NJ). The Elite ABC kits, DAB, and Hematoxylin QS were purchased from Vector Laboratories (Burlingame, CA). Lamda Protein Phosphatase (λ-Ppase) was purchased from NE Biolabs (Ipswich, MA).
Immunohistochemistry
Five-micrometer tissue sections on glass slides were deparaffinized, rehydrated, and washed with PBS. Following antigen retrieval in 0.1 mM sodium citrate (pH 6.0) and quenching of endogenous peroxidase activity with 3% H2O2, samples were blocked with 5% normal goat serum (NGS) or 5% normal horse serum (NHS) prior to incubation with primary antibodies overnight at 4 °C. Negative controls were treated with 5% NGS or NHS without primary antibodies. Signals were processed according to supplied protocol (Elite ABC Kit). Slides were counterstained with Hematoxylin QS, dehydrated and mounted using Permount (Fischer Scientific, Santa Clara, CA).
Results of the immunohistochemistry were interpreted using the following scale: 0, no staining; 1, focal (<50%), weak staining; 2, diffuse (>50%), weak staining; 3, focal, intense staining; 4, diffuse, intense staining. Staining intensity was graded with respect to the adjacent non-tumor elements.
Western Blotting
Both AML and corresponding normal kidney tissue were homogenized in ice-cold radioimmunoprecipitation (RIPA) buffer (1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 10 mM Tris (pH 7.2), 0.025 M β-glycophosphate (pH 7.2), 2mM EDTA, and 50mM sodium fluoride) with protease and kinase inhibitors (0.05 mM AEBSF, 10 μg/ml aprotinin, 10 μg/ml pepstatin, 1 mM orthovanadate, 10 μg/ml leupeptin, 1 mM microcystin LR). The protein concentration was measured using the BCA Protein Assay (Pierce, Rockford, IL). Equal amounts of protein were separated by SDS-PAGE, transferred to Immobilon-P membranes (Millipore, Bedford, MA) and blotted with antibodies according to manufacturer recommendations. Densitometry of bands was performed using Image J. For phosphatase treatment experiments, tissue lysates from human AML and corresponding normal kidney containing 80 μg of protein were treated with 400 units of λ-PPase and incubated at 30°C for 20 minutes prior to SDS-PAGE.
Results
mTOR signaling is up-regulated in sporadic AMLs
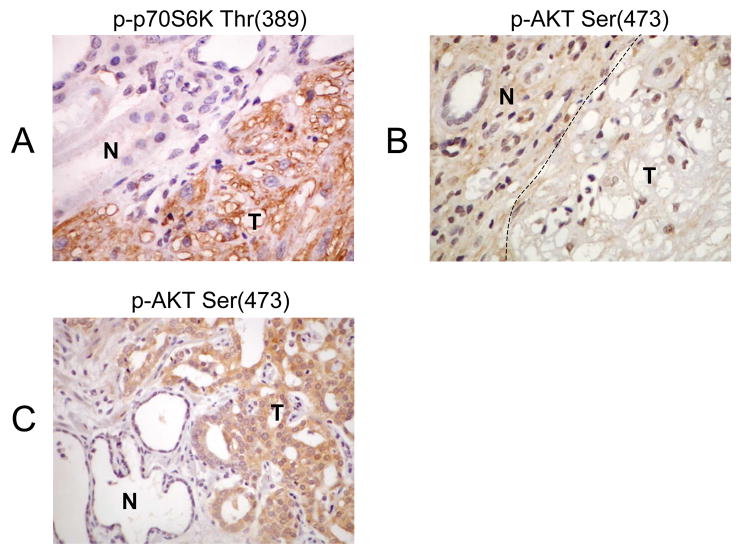
The levels of p70S6K and AKT phosphorylation are commonly used to indicate their activities. In this study, we used phospho-specific antibodies in immunohistochemical (IHC) analyses to dissect the mTOR pathway. Previously, we have shown that TSC-related AMLs exhibit evidence of mTOR activation 22, 23. As an example, Figures 2A and 2B illustrate the use of phospho-specific antibodies in a case of renal AML from a TSC patient with known TSC2 mutation. With the loss of TSC2 function, mTOR signaling in the tumor was increased as illustrated by the strong immunoreactivity to anti-phospho-p70S6K (Thr389) antibody (Figure 2A). Consequently, this led to a feedback inhibition of AKT (see Figure 1) resulting in the lack of phospho-AKT (Ser473) expression (Figure 2B). In contrast, a sample of human prostate cancer, which frequently incurs PTEN loss, stained strongly for phospho-AKT (Ser 473) indicating incrased AKT activity. Thus, the immunophenotype based on reactivity to phospho-p70S6K and phospho-AKT antibodies can provide an assessment of mTOR activity as well as clues to the underlying mechanism.
Figure 2.
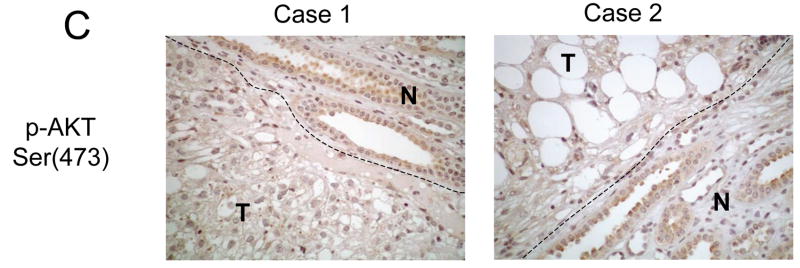
Immunophenotype of a TSC2-related AML. The loss of TSC2 function results in A) up-regulation of mTOR as illustrated by increased levels of phospho-p70S6K(Thr389) expression in the tumor (T) compared to adjacent normal kidney (N), and B) down-regulation of AKT as shown by the lack of phospho-AKT(Ser473) immuoreactivity in the tumor (T). C) In contrast, prostate carcinoma, which frequently undergo PTEN loss, expressed high levels of phospho-AKT(Ser473) compared with adjacent atrophic gland (N). Magnification 400x.
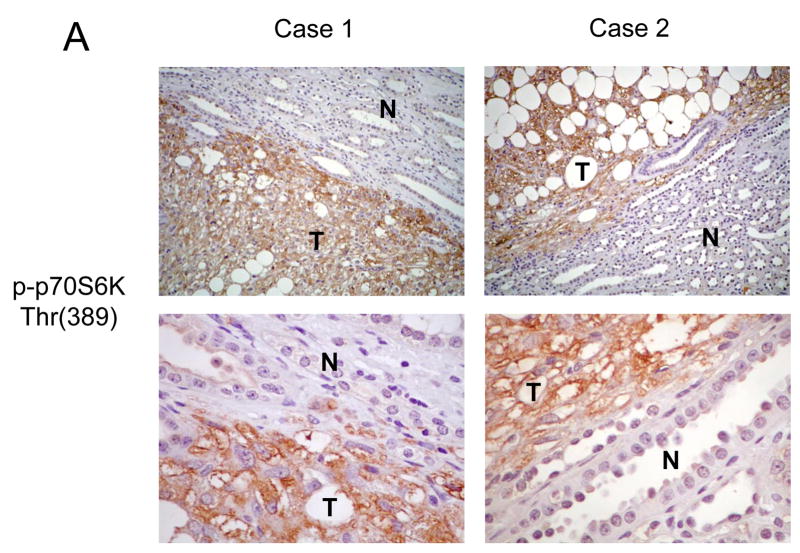
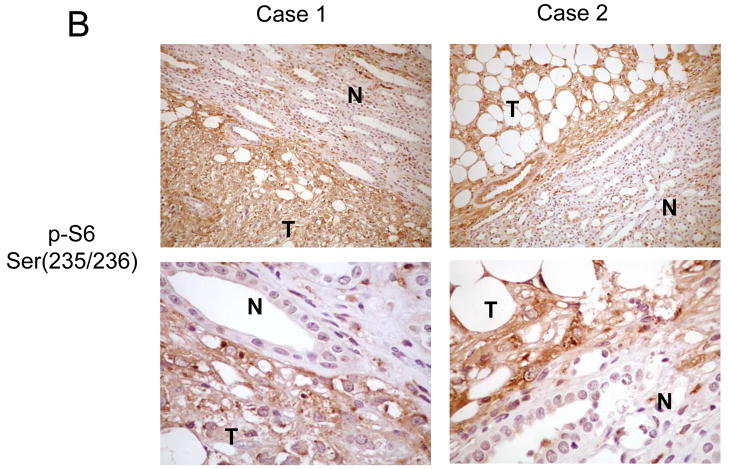
Using this approach, we examined the status of p70S6K and AKT in a set of 15 sporadic renal AMLs. The formalin-fixed, paraffin-embedded tissues were obtained from patients with histologically confirmed AMLs with no personal or family history of TSC or LAM. In accordance with previous reports, the majority of the AMLs were derived from women (12 of 15), ages ranging between 29 and 80. The staining intensity and distribution within the AMLs were compared to adjacent normal kidneys and scored from 0 to 4+ (see Materials and Methods). Diffuse strong immunoreactivity (4+) to anti-phospho-p70S6K antibody was seen in all 15 samples of sporadic AMLs examined. Within each tumor, all three components (epithelioid cells, adipocyte-like cells and cells of abnormal blood vessels) expressed phospho-p70S6K. Figure 3A illustrates two examples of phospho-p70S6K staining specifically in the tumors but not the adjacent kidneys. As previously noted, a population of cells within the normal collecting ducts of the kidney expressed phospho-p70S6K 22. To confirm that p70S6K activity in these lesions is elevated, we analyzed the expression of the phosphorylated form of its substrate, ribosomal S6 protein, in a random subset of these AMLs. In the 5 cases examined, all displayed significant phospho-S6 staining (3 or 4+) consistent with increased p70S6K activity (Figure 3B). In terms of AKT, none of the 15 sporadic AMLs showed significant immunoreactivity to anti- phospho-AKT(Ser473) antibody (0+) (Figure 3C), while staining for total AKT remained unchanged compared to adjacent normal kidneys (data not shown). These findings demonstrate a highly consistent immunophenotype of sporadic AMLs showing increased mTOR and decreased AKT activities that are consistent with the loss of TSC1/2 function.
Figure 3.
Immunohistochemical analysis of the mTOR pathway in sporadic AMLs. Examples of two sporadic, non-TSC AMLs analyzed by immunohistochemistry for A) phospho-p70S6K, B) phospho-S6, and C) phospho-AKT. T, tumor; N, normal kidney. Magnification: for A and B, top panel: 100x; bottom panel: 400x; for C, 200x.
Loss of TSC1/2 in sporadic AMLs
To further define the mTOR signaling in AMLs, we examined other components of the pathway as outlined in Figure 1 in four independent AML cases by Western blot analyses. Fresh tissues were obtained from nephrectomy specimens. The indications for surgery were symptom-related in three (i.e., pain, hemorrhage) and to rule out malignancy in one. None of the four patients has personal or family history of TSC or LAM. Ages ranged from 32 to 80; all were females. The tumor sizes were 1.2 cm, 7.5 cm, 13 cm and 15 cm.
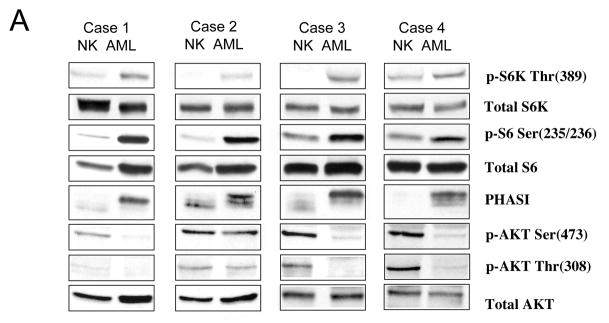
First, to confirm the findings of our IHC analysis, we analyzed the expression of AKT and down-stream effectors of mTOR: S6K, S6 and PHASI. As shown in Figure 4A, each of the sporadic AMLs expressed higher levels of phospho-p70S6K(Thr389) and phospho-S6(Ser235/236) compared to adjacent normal kidneys. Further, AMLs expressed slower mobility forms of PHASI indicative of its hyper-phosphorylation state compared to normal kidney. The combined presence of phosphorylated S6, p70S6K and PHASI strongly implicates elevated mTOR activity in these tumors.
Figure 4.
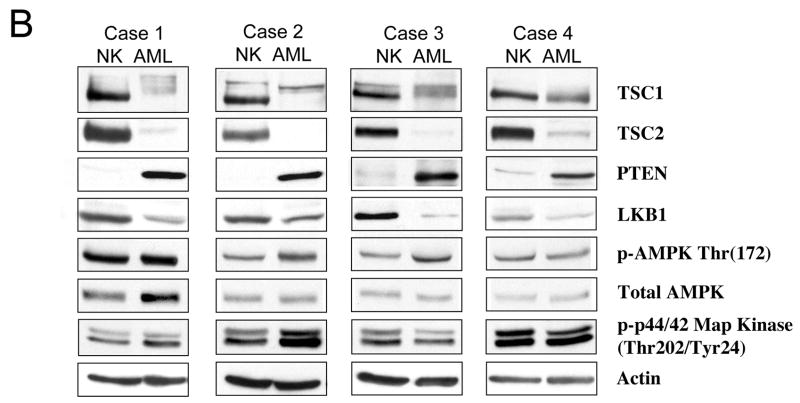
Biochemical analysis of the mTOR pathway in sporadic AMLs. Four independent, non-TSC AMLs were analyzed by Western blotting using antibodies indicated. Panel A highlights the targets of mTOR: p70S6K, ribosomal S6, and PHASI, as well as AKT. Panel B illustrates the up-stream negative regulators of mTOR: TSC1, TSC2, PTEN, LKB1, AMPK, and ERK. Each sample is represented by normal kidney (NK), and tumor (AML). Actin serves as loading control.
To determine the state of AKT function, phosphorylation of Ser473 and Thr308, two sites that are necessary for AKT activity, were examined. Figure 4A shows that all but one AML tested have significantly reduced levels of phospho-AKT expression despite comparable amounts of total AKT between AML and normal kidney. Case 2 displayed 67% of the normal kidney level when normalized to total AKT, also indicating reduced AKT activity in the AML compared to normal kidney. Thus, the Western blotting results are in keeping with the IHC analyses confirming the up-regulation of mTOR and down-regulation of AKT in these lesions. The inverse relationship between AKT and mTOR is highly suggestive of a functional loss of TSC1/2.
To explore the mechanism of mTOR activation in these tumors, we evaluated the status of four pathways that have an influence on mTOR signaling including TSC1/2, PTEN, LKB1/AMPK and NF1/Ras/ERK (see Figure 1). With respect to TSC1/2, Western blotting analysis showed a dramatic reduction in TSC2 expression relative to the adjacent kidney tissues suggestive of the loss of TSC2 (Figure 4B). The faint TSC2 band found in the AML samples represents the non-tumor elements of the AMLs (e.g., connective tissues, non-tumor vessels). On the contrary, TSC1 was expressed in all four AMLs, but the immunoreactive bands representing TSC1 migrated slower on SDS-PAGE analyses, thus giving the appearance of broader and less distinct bands (Figure 4B). This is a reflection of the phosphorylation-dependent mobility shifts of TSC1 when it is unbound to TSC2. In support of this, when we treated the tumor lysates with λ phosphatase, the tumor TSC1 bands reverted to the form found in the non-tumor tissues (data not shown). We observed the same phenomenon in Tsc2−/− embryonic fibroblasts or when TSC1 alone was over-expressed in Cos7 cells (data not shown). Together, these observations showed the consistent loss of TSC2 but not TSC1 expression in sporadic AMLs.
To assess other plausible causes for mTOR activation in AMLs, we examined other components of the pathway outlined in Figure 1. Loss of PTEN would result in activation of mTOR, but we found PTEN to be expressed at significantly higher levels in all 4 AMLs compared to normal kidneys (Figure 4B), therefore unlikely to contribute to mTOR deregulation. LKB1 expression was more variable in these lesions with a slight, overall reduction in its levels. To evaluate the significance of this observation, we examined the functional status of AMPK. Since AMPK is the substrate of LKB1 that negatively regulates mTOR activity via TSC1/2 24, 16, the level of phospho-AMPK(Thr172) can be used as an indicator of its activity. In the AMLs, the expression of phospho-AMPK was at least equivalent to that of normal kidney (cases 1 and 4), if not stronger (cases 2 and 3) (Figure 4B). This indicates that AMPK was active in the tumors, and therefore should suppress mTOR activity. Since the opposite was observed, we can conclude that the LKB1/AMPK pathway was not the primary event leading to mTOR activation in these lesions. Functioning through ERK phosphorylation of TSC2, the Ras/MAPK pathway can also modulate mTOR25. However, ERK phosphorylation was found to be elevated in only 1 of 4 AMLs (i.e., case 2) (Figure 4B). Collectively, the Western blotting analyses suggest the loss of TSC2 as a common underlying mechanism for mTOR activation in sporadic AMLs.
PEComas exhibit aberrant mTOR activity
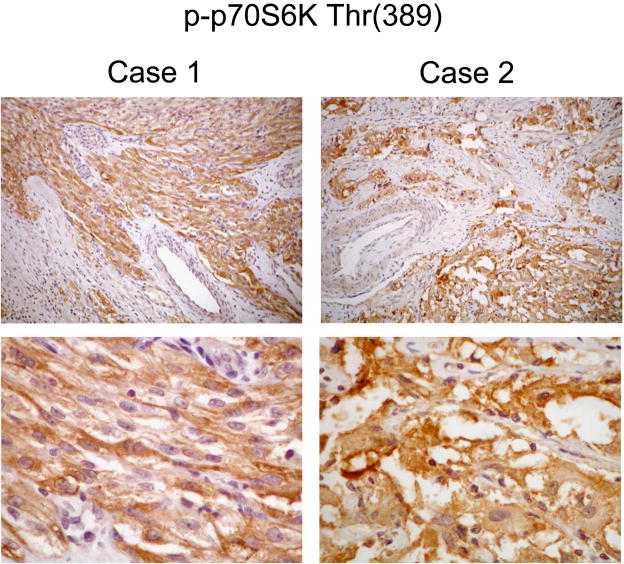
Next, we determined if the broader group of PEComas, of which AML represent a subset, shared a similar functional aberration in mTOR signaling. Fifteen cases of PEComas were chosen among 26 previously reported cases based on availability of formalin-fixed, paraffin-embedded tumor materials26. The clinicopathologic features of these tumors were summarized in a recent publication26. Of note, none of these cases had a history of TSC; this is consistent with the observation that less than 10% of non-AML, non-LAM PEComas are associated with TSC. Here, we examined the expression of phospho-p70S6K and phospho-AKT by IHC. Our results show that all 15 tumors exhibited immunoreactivity of varying degrees to the anti-phospho-p70S6K antibody. Eight tumors had diffusely weak staining (2+) while 3 had focally strong (3+) and 4 had diffusely strong (4+) immunoreactivities (Figure 5). All but one PEComa failed to stain (0+) with anti-phospho-AKT antibody, and one had a few focal areas of weak staining (1+). Due to the lack of fresh tissue, we were unable to confirm these findings by Western blot analysis. Nonetheless, these results suggest that the PEComas behave similarly to AMLs with respect to mTOR signaling. Increased p70S6K and decreased AKT activities in these tumors would support the loss of TSC1/2 function as the underlying mechanism but we cannot exclude other mechanisms of mTOR activation.
Figure 5.
Immunohistochemical analysis of p70S6K in PEComas. Two examples of PEComas immunostained for phospho-p70S6K. Magnification: 100x (top) and 400x (bottom).
Discussion
The pathogenesis of AMLs has intrigued pathologists for many years. Genetic analyses of sporadic AMLs have demonstrated that these tumors are clonally derived, but the studies have not provided consistent results with respect to an underlying genetic mechanism27–29. Trisomy 7 has been independently reported in sporadic AMLs. Its significance remains uncertain given that non-neoplastic renal tissues often exhibit the same karyotypic change30–33. The association between AML and TSC has led several investigators to examine alterations at the TSC1 and TSC2 loci, 9q34 and 16p13, respectively. Henske et al. found LOH on 16p13 in 5 of 8 TSC-associated AMLs and 3 of 29 sporadic AMLs8. Similarly, Smolarek et al. reported TSC2 LOH in 7 of 13 AMLs associated with sporadic LAM9. In two reports by Martignoni et al., LOH on 16p was found in 2 of 4 cases of epithelioid AMLs in non-TSC patients10,11. However, Kattar et al. was unable to detect loss of 16p in 11 sporadic AMLs using comparative genomic hybridization 34. Nonetheless, these studies suggest the possible role of TSC2 in sporadic AMLs unrelated to TSC or LAM, but the functional significance of this pathway has not been determined.
In this study, we discovered that sporadic AMLs uniformly exhibit activation of the mTOR cascade. This is similar to the findings reported for TSC and LAM-associated AMLs suggesting a common pathogenetic mechanism22,35,36. We found robust expression of phospho-p70S6K and lack of phospho-AKT expression in all 15 cases examined by immunohistochemistry. This implies that sporadic AMLs represent a homogenous class of neoplasm. In the subset of four lesions where fresh tissues were obtained, our results indicate the loss of TSC2 as the primary event in their pathogenesis. This is in keeping with the findings of TSC2 LOH in genetic analyses of sporadic AMLs. The apparent lack of TSC1 involvement is unclear since patients with TSC1-related tuberous sclerosis also develop AMLs. However, it has been reported that TSC2 is more commonly affected in non-familial cases of TSC 37. Further, genotype-phenotype studies suggest that TSC2-related pathologies are more severe than the TSC1 cases 37. Thus, we speculate that TSC2-affected AMLs are more likely to be detected and/or clinically symptomatic than TSC1-affected AMLs, although larger studies are needed to confirm our findings.
The observed increase in p70S6K and PHASI phosphorylation along with reduced AKT phosphorylation represents the molecular signature of cells lacking a functional TSC1/2 complex (Figure 1C). This is distinct from the pattern observed following the loss of PTEN, which results in increased AKT phosphorylation (Figures 1B). The unique suppression of AKT activity observed in the TSC2-null state is secondary to the negative feedback of p70S6K on IRS1 and insulin signaling 21. It has been suggested that the reduced AKT activity in TSC-related tumors may be relevant to their benign behavior 19. Conversely, AKT activation in the setting of PTEN deficiency has been commonly observed in human malignancies 38. In this study, we also observed an inverse relationship between TSC2 and PTEN expression not previously reported. Despite increased PTEN expression in the AMLs, mTOR activity remains elevated due to the loss of TSC2. This up-regulation of PTEN may represent a compensatory mechanism of the cell to normalize its elevated mTOR activity. Serving as a phosphatase, PTEN antagonizes the activity of PI3K 14 and could further suppress AKT function in these lesions. Currently, PTEN activity and the mechanism of its regulation and expression in AMLs have not been elucidated.
Multiple pathways converge on mTOR to regulate its function. Besides TSC1/2 and PTEN, the tumor suppressor proteins, LKB1 and NF1, inhibit mTOR through their influence on AMPK and the Ras/MAPK pathways, respectively 16,17,25. Based on the levels of phosphorylated AMPK and ERK, we failed to find evidence that either of these signaling cascades could account for the mTOR activities found in the AMLs. Instead, our collective data favor TSC2 loss as the primary mechanism of mTOR dysregulation.
Non-AML PEComas share common histologic and immunophenotypic characteristics with AMLs. Their relative scarcity provides few clues to their pathogenesis. A recent review of the literature found that a minority (<10%) of soft tissue and gynecologic PEComas are associated with TSC26, thus providing a plausible link to an underlying genetic pathway. This is supported by a comparative genomic hybridization study by Pan et al. showing multiple recurrent chromosomal alterations including the loss of 16p12. In this study, we show for the first time that p70S6K is phosphorylated while AKT is non-phosphorylated in all cases examined except for one lesion with limited phospho-AKT expression. Once again, these results point to the involvement of the TSC/mTOR pathway in non-AML, non-LAM PEComas. However, given the lack of fresh tissues, we were unable to perform immunoblot analyses to confirm or characterize the mTOR pathway in these tumors. Compared to the AMLs, the immunostaining results were more variable, and this may be due to technical differences in the fixation process, the age and storage of the specimens, and/or a reflection of the involvement of alternative molecular pathways. Further biochemical and genetic analyses are necessary to define the mechanisms of mTOR activation in these tumors. Nonetheless, the highly consistent finding of p70S6K phosphorylation provides a novel target for therapy.
Besides surgery, there is no effective medical treatment for AMLs or PEComas. The results of this study suggest that mTOR hyperactivity may contribute to tumor growth and progression. Rapamycin is a potent and specific inhibitor of mTOR by acting through a cellular chaperone, FKBP12 39. It is approved by the FDA for immunosuppression therapy following renal transplant 40. Pre-clinical studies in animal models of TSC have shown significant in vivo response to rapamycin and its derivatives41, 42. Results in TSC and LAM patients are pending. These drugs are also being tested in a wide array of human malignancies and have been recently approved for use in renal cell carcinomas and acute myeloid leukemia43,44. Given the relative low toxicity profile, rapamycin and its derivatives may provide therapeutic benefits in the treatment of AMLs and non-renal PEComas.
The origin of PEComas remains unknown and controversial. To date, no progenitor cell has been identified and no normal counterpart displaying the dual phenotype of smooth muscle and melanocytic differentiation has been found. The involvement of the TSC pathway provides possible clues to this dilemma. It has been proposed that B-raf activity in cells lacking TSC2 may play a role in differentiation but the functional link and its mechanism remain unclear 45. Alternatively, we have previously reported that the TSC pathway negatively regulates the Wnt/β-catenin pathway by promoting the activity of its degradation complex 46. β-catenin is known to regulate transcription of crucial genes involved in proliferation and differentiation. Future studies are aimed at analyzing the contributions of these pathways in the pathogenesis of PEComas.
Acknowledgments
We thank Dr. Elizabeth Henske (Fox Chase Cancer Center) for providing kidney AML samples for this study, and Dr. Elizabeth Barnes (University of Washington) for critical reading of the manuscript.
Footnotes
Supported in part by a grant from NIH (CA102662)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Folpe AL. Neoplasms with perivascular epitheloid cell differentiation (PEComas) In: Fletcher CDMUK, Mertens F, editors. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of Soft Tissue and Bone. Lyon: IARC Press; 2002. pp. 221–222. [Google Scholar]
- 2.Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75–82. doi: 10.1111/j.1365-2559.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 3.Gomez MR. Definition and criteria for diagnosis. In: Gomez MR, Sampson JR, Whittemore VH, editors. Tuberous Sclerosis Complex. New York: Oxford University Press; 1999. pp. 10–23. [Google Scholar]
- 4.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 5.Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc. 2000;75:591–594. doi: 10.4065/75.6.591. [DOI] [PubMed] [Google Scholar]
- 6.Moss J, Avila NA, Barnes PM, Litzenberger RA, Bechtle J, Brooks PG, Hedin CJ, Hunsberger S, Kristof AS. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med. 2001;164:669–671. doi: 10.1164/ajrccm.164.4.2101154. [DOI] [PubMed] [Google Scholar]
- 7.Aubry MC, Myers JL, Ryu JH, Henske EP, Logginidou H, Jalal SM, Tazelaar HD. Pulmonary lymphangioleiomyomatosis in a man. Am J Respir Crit Care Med. 2000;162:749–752. doi: 10.1164/ajrccm.162.2.9911006. [DOI] [PubMed] [Google Scholar]
- 8.Henske EP, Neumann HP, Scheithauer BW, Herbst EW, Short MP, Kwiatkowski DJ. Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosomes Cancer. 1995;13:295–298. doi: 10.1002/gcc.2870130411. [DOI] [PubMed] [Google Scholar]
- 9.Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62:810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martignoni G, Pea M, Bonetti F, Zamboni G, Carbonara C, Longa L, Zancanaro C, Maran M, Brisigotti M, Mariuzzi GM. Carcinomalike monotypic epithelioid angiomyolipoma in patients without evidence of tuberous sclerosis: a clinicopathologic and genetic study. Am J Surg Pathol. 1998;22:663–672. doi: 10.1097/00000478-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Martignoni G, Pea M, Rigaud G, Manfrin E, Colato C, Zamboni G, Scarpa A, Tardanico R, Roncalli M, Bonetti F. Renal angiomyolipoma with epithelioid sarcomatous transformation and metastases: demonstration of the same genetic defects in the primary and metastatic lesions. Am J Surg Pathol. 2000;24:889–894. doi: 10.1097/00000478-200006000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Pan CC, Jong YJ, Chai CY, Huang SH, Chen YJ. Comparative genomic hybridization study of perivascular epithelioid cell tumor: molecular genetic evidence of perivascular epithelioid cell tumor as a distinctive neoplasm. Hum Pathol. 2006;37:606–612. doi: 10.1016/j.humpath.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris TE, Lawrence JC., Jr TOR signaling. Sci STKE. 2003:re15. doi: 10.1126/stke.2122003re15. [DOI] [PubMed] [Google Scholar]
- 16.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamji AF, Nghiem P, Schreiber SL. Integration of growth factor and nutrient signaling: Implications for cancer biology. Molecular Cell. 2003;12:271–280. doi: 10.1016/j.molcel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Manning BD, Logsdon MN, Lipovsky AI, Abbott D, Kwiatkowski DJ, Cantley LC. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, Olefsky JM, Kobayashi M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783–794. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- 21.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenerson HL, Aicher LD, True LD, Yeung RS. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res. 2002;62:5645–5650. [PubMed] [Google Scholar]
- 23.Mak BC, Kenerson HL, Aicher LD, Barnes EA, Yeung RS. Aberrant beta-catenin signaling in tuberous sclerosis. Am J Pathol. 2005;167:107–116. doi: 10.1016/s0002-9440(10)62958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558–1575. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 27.Paradis V, Laurendeau I, Vieillefond A, Blanchet P, Eschwege P, Benoit G, Vidaud M, Jardin A, Bedossa P. Clonal analysis of renal sporadic angiomyolipomas. Hum Pathol. 1998;29:1063–1067. doi: 10.1016/s0046-8177(98)90414-2. [DOI] [PubMed] [Google Scholar]
- 28.Saxena A, Alport EC, Custead S, Skinnider LF. Molecular analysis of clonality of sporadic angiomyolipoma. J Pathol. 1999;189:79–84. doi: 10.1002/(SICI)1096-9896(199909)189:1<79::AID-PATH366>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Cheng L, Gu J, Eble JN, Bostwick DG, Younger C, MacLennan GT, Abdul-Karim FW, Geary WA, Koch MO, Zhang S, Ulbright TM. Molecular genetic evidence for different clonal origin of components of human renal angiomyolipomas. Am J Surg Pathol. 2001;25:1231–1236. doi: 10.1097/00000478-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 30.de Jong B, Castedo SM, Oosterhuis JW, Dam A. Trisomy 7 in a case of angiomyolipoma. Cancer Genet Cytogenet. 1988;34:219–222. doi: 10.1016/0165-4608(88)90263-4. [DOI] [PubMed] [Google Scholar]
- 31.Dal Cin P, Sciot R, Van Poppel H, Baert L, Van Damme B, Van den Berghe H. Chromosome analysis in angiomyolipoma. Cancer Genet Cytogenet. 1997;99:132–134. doi: 10.1016/s0165-4608(97)00209-4. [DOI] [PubMed] [Google Scholar]
- 32.Wullich B, Henn W, Siemer S, Seitz G, Freiler A, Zang KD. Clonal chromosome aberrations in three of five sporadic angiomyolipomas of the kidney. Cancer Genet Cytogenet. 1997;96:42–45. doi: 10.1016/s0165-4608(96)00267-1. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg E, Dijkhuizen T, Storkel S, Molenaar WM, de Jong B. Chromosomal abnormalities in non-neoplastic renal tissue. Cancer Genet Cytogenet. 1995;85:152–154. doi: 10.1016/0165-4608(95)00035-6. [DOI] [PubMed] [Google Scholar]
- 34.Kattar MM, Grignon DJ, Eble JN, Hurley PM, Lewis PE, Sakr WE, Cher ML. Chromosomal analysis of renal angiomyolipoma by comparative genomic hybridization: evidence for clonal origin. Hum Pathol. 1999;30:295–299. doi: 10.1016/s0046-8177(99)90008-4. [DOI] [PubMed] [Google Scholar]
- 35.El-Hashemite N, Zhang H, Henske EP, Kwiatkowski DJ. Mutation in TSC2 and activation of mammalian target of rapamycin signalling pathway in renal angiomyolipoma. Lancet. 2003;361:1348–1349. doi: 10.1016/S0140-6736(03)13044-9. [DOI] [PubMed] [Google Scholar]
- 36.Robb VA, Astrinidis A, Henske EP. Frequent [corrected] hyperphosphorylation of ribosomal protein S6 [corrected] in lymphangioleiomyomatosis-associated angiomyolipomas. Mod Pathol. 2006;19:839–846. doi: 10.1038/modpathol.3800610. [DOI] [PubMed] [Google Scholar]
- 37.Cheadle JP, Reeve MP, Sampson JR, Kwiatkowski DJ. Molecular genetic advances in tuberous sclerosis. Hum Genet. 2000;107:97–114. doi: 10.1007/s004390000348. [DOI] [PubMed] [Google Scholar]
- 38.Xu G, Zhang W, Bertram P, Zheng XF, McLeod H. Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR pathway in common human tumors. Int J Oncol. 2004;24:893–900. [PubMed] [Google Scholar]
- 39.Cohen E. mTOR inhibitors. Clin Adv Hematol Oncol. 2006;4:38–39. [PubMed] [Google Scholar]
- 40.Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 2006;81:1234–1248. doi: 10.1097/01.tp.0000219703.39149.85. [DOI] [PubMed] [Google Scholar]
- 41.Kenerson H, Dundon TA, Yeung RS. Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr Res. 2005;57:67–75. doi: 10.1203/01.PDR.0000147727.78571.07. [DOI] [PubMed] [Google Scholar]
- 42.Lee L, Sudentas P, Donohue B, Asrican K, Worku A, Walker V, Sun Y, Schmidt K, Albert MS, El-Hashemite N, Lader AS, Onda H, Zhang H, Kwiatkowski DJ, Dabora SL. Efficacy of a rapamycin analog (CCI-779) and IFN-gamma in tuberous sclerosis mouse models. Genes Chromosomes Cancer. 2005;42:213–227. doi: 10.1002/gcc.20118. [DOI] [PubMed] [Google Scholar]
- 43.Pantuck AJ, Thomas G, Belldegrun AS, Figlin RA. Mammalian target of rapamycin inhibitors in renal cell carcinoma: current status and future applications. Semin Oncol. 2006;33:607–613. doi: 10.1053/j.seminoncol.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Recher C, Dos Santos C, Demur C, Payrastre B. mTOR, a new therapeutic target in acute myeloid leukemia. Cell Cycle. 2005;4:1540–1549. doi: 10.4161/cc.4.11.2159. [DOI] [PubMed] [Google Scholar]
- 45.Karbowniczek M, Henske EP. The role of tuberin in cellular differentiation: are B-Raf and MAPK involved? Ann N Y Acad Sci. 2005;1059:168–173. doi: 10.1196/annals.1339.045. [DOI] [PubMed] [Google Scholar]
- 46.Mak BC, Takemaru K, Kenerson HL, Moon RT, Yeung RS. The tuberin-hamartin complex negatively regulates Beta -catenin signaling activity. J Biol Chem. 2003;278:5947–5951. doi: 10.1074/jbc.C200473200. [DOI] [PubMed] [Google Scholar]