Graphical abstract
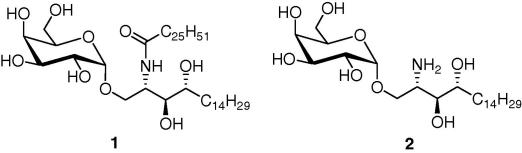
The synthesis and biological activity of α-galactosyl ceramide (α-GalCer) and Gal(α1→2)GalCer is reported.
Keywords: CD1d, KRN7000, iNKT, Galactosyl(α1-2)galactosyl
Abstract
We herein report a faster and less cumbersome synthesis of the biologically attractive, α-galactosyl ceramide (α-GalCer), known as KRN7000, and its analogues. More importantly, the use of a silicon tethered intramolecular glycosylation reaction gave easy access to the diglycosyl ceramide Gal(α1→2)GalCer, which has been shown to require uptake and processing to the biologically active α-GalCer derivative.
CD1d is a nonpolymorphic glycoprotein expressed on the surface of antigen-presenting cells (APCs). It is specifically associated with presenting lipid antigens that activate the distinctive class of T cells, known as invariant Natural Killer T (iNKT) cells. iNKT cells display characteristics of both T cells and NK cells and play a crucial role in diverse immune responses and other pathologic conditions.1–4 When the synthetic glycolipid α-galactosyl ceramide (α-GalCer),5 known as KRN7000 (1), is bound to CD1d and presented to the T cell receptors (TCRs) on the surface of iNKT cells, the latter are activated to release diverse cytokines, including both Th1 and Th2 cytokines.6–8 It is believed that the release of Th1 cytokines may contribute to antitumour and antimicrobial functions while that of Th2 cytokines may help alleviate autoimmune diseases9–11 such as multiple sclerosis12 and arthritis.13 α-GalCer and its derivatives have proved to be and remain invaluable tools in understanding the functioning of CD1d and NKT cells in a wide range of immune responses. As part of various ongoing studies, easy access to relatively extensive quantities of these substrates is, therefore, required.
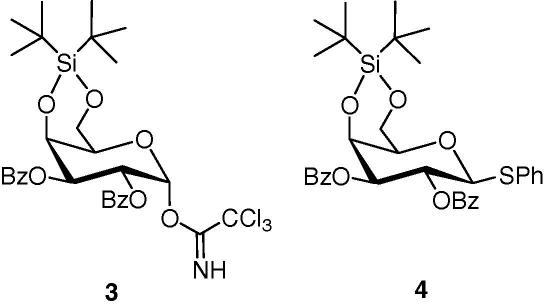
Several elegant syntheses of α-GalCer are present in the literature.14 Most of these syntheses make use of benzyl ether protecting groups on the sugar moiety. We, along with many other research groups, have encountered difficulties in the hydrogenolysis of benzyl ethers, thereby leading to low yields of α-GalCer. Therefore, synthetic routes circumventing the problematic hydrogenolysis step are highly desired. Kiso and co-workers15 recently reported such a synthesis, where interestingly they also made use of the bulky 4,6-O-di-tert-butylsilylene (DTBS) group as α-directing in galactosylation donor 3.
We have employed two different synthetic strategies, including that of Kiso and co-workers to obtain the glycosylated sphingoid base template 2, which can readily be converted to α-GalCer and other derivatives. Initially, to improve on the reported synthesis we resorted to the use of the thioglycosyl donor 4 as it is easier to handle and has a better shelf life than the trichloroacetimidate donor 3. Significantly, the second route describes a novel synthesis of α-GalCer and has the additional benefit of providing a crucial intermediate, which can be further modified to yield other biologically active α-GalCer derivatives.
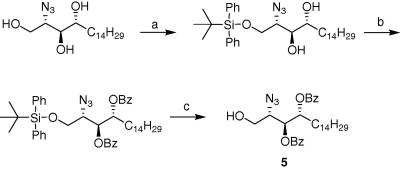
We first synthesized the phytosphingosine acceptor 516 from (2S,3S,4R)-2-azido-1, 3, 4-octadecanetriol17 in three steps as described in Scheme 1.
Scheme 1.
Reagents and conditions: (a) TBDPSCl, Pyr, quant; (b) BzCl, Pyr, 88%; (c) TBAF, THF, 82%.
The thioglycoside 4 was obtained in large scale from commercially available β-d-galactopyranose pentaacetate after standard procedures, as described previously.18The critical glycosylation was then attempted on 2 g of the donor 4. NIS/TfOH activation18,19 of thioglycoside 4 (Scheme 2) in anhydrous CH2Cl2 at −78 °C afforded the glycosylated compound 6 in 71% yield, as the α-anomer exclusively after two hours. Subsequent sequential removal of the silyl group with TBAF and Zemplen’s deprotection of the benzoate protecting groups produced the azide intermediate in quantitative yields after purification by flash chromatography. Different methods for the reduction of the azide group were attempted, including the use hydrogen sulfide, but the best results were obtained via hydrogenation in methanol. 800 mg of the amine 2 was hence isolated as a white solid.
Scheme 2.
Reagents and conditions: (a) NIS/TfOH, CH2Cl2, 67%; (b) TBAF, THF, quant; (c) NaOMe/MeOH, 92%; (d) H2, Pd, 88%.
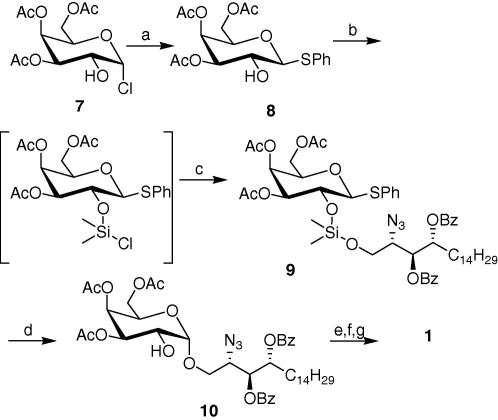
Scheme 3 illustrates our novel strategy to synthesise α-GalCer and its derivatives. Silicon tethered intramolecular glycosylation20 is a particularly attractive method for generating glycosidic bonds stereoselectively, but not attempted by many research groups due to the difficulty in handling and relative instability of the silylene-tethered sugar derivative. However, successful examples have been reported and we were motivated by Bols21 work on the synthesis of disaccharides containing α-galactosyl linkages. Hence, 3, 4, 6-tri-O-acetylgalactopyranosyl chloride (7)22 was obtained from β-d-galactopyranose pentaacetate and converted to the corresponding thioglycoside 8 by reacting with thiophenol in the presence of caesium carbonate. The tethered compound 9 was then synthesised following the procedure described by Bols.21 Rearrangement of the silylene 9, catalyzed by N-iodosuccinimide (NIS) in anhydrous nitromethane at 80 °C,21 yielded the desired product 10 along with some small amounts of 5 after 2 h. It was observed that by careful monitoring and quenching of the reaction as soon as compound 9 was consumed, helped in minimizing the regeneration of phytosphingosine derivative 5 and hence enhanced the yield of the glycosylated product. Methanolysis, followed by hydrogenation of the azide then afforded compound 2. Finally, N-acylation with the fully saturated fatty acid, hexacosanoic acid, was achieved via reaction of the corresponding acid chloride with the free amine 2 in a 1:1 mixture of THF and saturated sodium acetate solution. Target compound 1 was obtained as a white solid after concentration of the organic phase and purification of the residue by flash chromatography. The spectroscopic data of the latter were consistent with the literature.14
Scheme 3.
Reagents and conditions: (a) PhSH, DMF, Cs2CO3, 75%; (b) (CH3)2Si(Cl)2, Pyridine, CH2Cl2, quant; (c) 5, Pyridine, DMF, 72%; (d) NIS, CH3NO2, 66%; (e) NaOMe/MeOH, quant; (f) H2, Pd/C, MeOH, 80%; (g) C25H51COCl, THF, NaOAc, 75%.
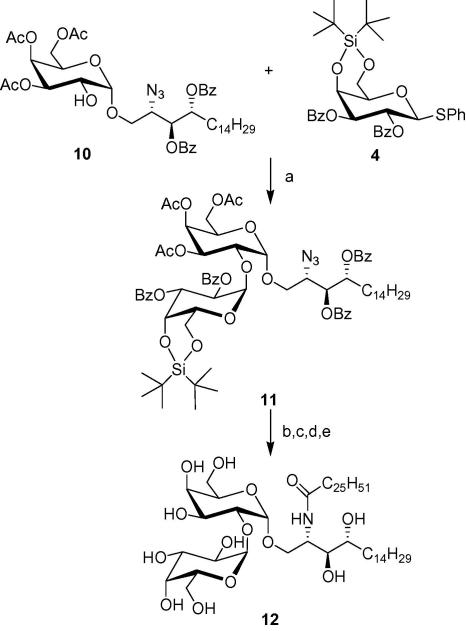
While our first approach (Scheme 2) is more direct and higher yielding, the alternative route (Scheme 3) also provides the additional benefit of freeing the hydroxyl group at C-2 on the sugar residue (compound 10). This allows for selective modification on α-GalCer; such as the introduction of an additional sugar residue. Specifically, the diglycosyl ceramide, Gal(α1→2GalCer) 12 has been used to study lysosomal glycolipid processing.23 Briefly, galactosidases from lysosomes are responsible for truncating oligoglycosyl ceramides to monoglycosyl ceramides before they can bind to CD1d and be presented to iNKT cells.23 Scheme 4 depicts a new strategy for synthesizing disaccharide 11 via NIS/TfOH activation of sugar donor 4 at −78 °C in anhydrous CH2Cl2 for 3 h. Once more, the directing effect of the bulky silyl group ensured the formation of the desired α-linkage, as confirmed by the H-1 and C-1 signals in 1H and 13C NMR. Compound 12 was obtained after routine procedures, similar to those described above and exhibited spectroscopic data consistent with the literature.24
Scheme 4.
Reagents and conditions: (a) NIS/TfOH, CH2Cl2, 67%; (b) TBAF, THF, quant; (c) NaOMe/MeOH, quant; (d) H2, Pd, MeOH, 80%; (e) C25H51COCl, THF, NaOAc, 78%.
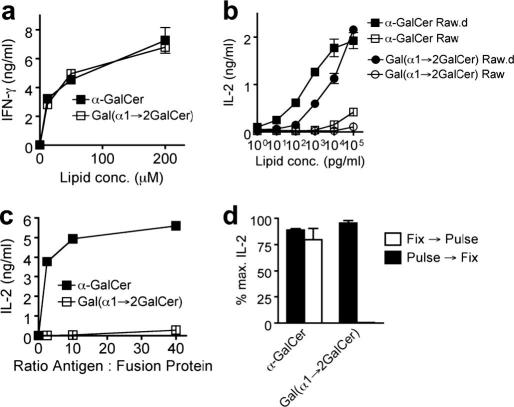
We next tested the biological activity of α-GalCer and Gal(α1→2GalCer). Both lipids stimulated human and mouse iNKT cells in the presence of CD1d-expressing antigen-presenting cells (APC) (Fig. 1a and b). α-GalCer, but not Gal(α1→2GalCer), stimulated iNKT cells in an APC-free CD1d-Fc fusion protein plate assay (Fig. 1c). In fix/pulse, pulse/fix experiments α-GalCer stimulated an iNKT cell response under both conditions, whereas Gal(α1→2GalCer) resulted in cytokine release only under the pulse/fix condition (Fig. 1d). Together, these data suggest that α-GalCer and Gal(α1→2GalCer) described here can stimulate human and mouse iNKT cells. Furthermore, Gal(α1→2GalCer), in contrast to α-GalCer, required uptake and processing to generate the biologically active monoglycosyl ceramide.
Figure 1.
α-GalCer and Gal(α1→2GalCer) stimulate CD1d-restricted iNKT cells. (a) In vitro activation of 2.5 × 104 human iNKT cells (clone BM2a.3) in co-culture with 2.5 × 104 U937 cells and various concentrations of α-GalCer (filled squares) and Gal(α1→2GalCer) (open squares). After 16 h, cytokines were determined in culture supernatants by ELISA; (b) in vitro activation of 5 × 104 mouse iNKT cells (hybridoma DN32) in co-culture with 5 × 104 RAW cells transfected with CD1d (filled symbols) or untransfected (open symbols) and various concentrations of α-GalCer (squares) and Gal(α1→2GalCer) (circles). After 16 h, cytokine concentrations were determined in culture supernatants by ELISA; (c) Plate-bound murine recombinant CD1d-Fc fusion proteins were loaded with α-GalCer (filled squares) or Gal(α1→2GalCer) (open squares) for 16 h, washed, and 5 × 104iNKT cell hybridomas were added per well. Cytokines were determined in culture supernatants by ELISA; (d) RAW cells transfected with CD1d were pulsed with 100 ng/ml of α-GalCer or Gal(α1→2GalCer) for 3 h and then washed and fixed with glutaraldehyde (filled bars), or fixed and then pulsed for 3 h (open bars). 105 APCs were co-cultured with 105iNKT cell hybridomas for 16 h and cytokines were determined in culture supernatants by ELISA. Cytokine responses are expressed as percent of maximal response. Methods are described elsewhere.25
In conclusion, we have demonstrated the versatility of both compounds 4 and 10 as crucial intermediates in practical and high-yielding syntheses of α-GalCer and other biologically important derivative, such as Gal(α1→2GalCer).26
Acknowledgements
G.S.B. acknowledges support in the form of a Personal Research Chair from Mr. James Badrick, Royal Society Wolfson Research Merit Award, as a former Lister Institute-Jenner Research Fellow, the Medical Council and The Wellcome Trust (084923/B/08/7).
References and notes
- 1.Kronenberg M. Annu. Rev. Immunol. 2005;23:877. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 2.Brutkiewicz R.R. J. Immunol. 2006;177:769. doi: 10.4049/jimmunol.177.2.769. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac R., Rivera M.N., Park S.H., Roark J.H. Annu. Rev. Immunol. 1997;15:535. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 4.Hong S., Scherer D.C., Mendiratta S.K., Serizawa I., Koezuka Y., Van K.L. Immunol. Rev. 1997;169:31. doi: 10.1111/j.1600-065x.1999.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 5.Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E., Koseki H., Taniguchi M. Science. 1997;278:1626. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 6.Crowe N., Uldrich A.P., Kyparissoudis K., Hammond K.J.L., Hayakawa Y., Sidobre S., Keating R., Kronenberg M., Smyth M.J., Godfrey D.I. J. Immunol. 2003;171:4020. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 7.Burdin N., Brossay L., Kronenberg M. Eur. J. Immunol. 1999;29:2014. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Carnaud C., Lee D., Donnars O., Park S.H., Beavis A., Koezuka Y., Bendelac A. J. Immunol. 1999;163:4647. [PubMed] [Google Scholar]
- 9.Taniguchi M., Harada M., Kojo S., Nakayama T., Wakao H. Annu. Rev. Immnunol. 2003;21:483. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 10.Godfrey D.I., MacDonald H.R., Kronenberg M., Smyth M.J., Van K.L. Nat. Rev. Immunol. 2004;4:231. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Aseguinolaza G., Van Kaer L., Bergmann C.C., Wilson J.M., Schmieg J., Kronenberg M., Nakayama T., Taniguchi M., Koezuka Y., Tsuji M. J. Exp. Med. 2002;195:617. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto K., Miyake S., Yamamura T. Nature. 2001;413:513. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 13.Chiba A., Oki S., Miyamoto K., Hashimoto H., Yamamura T., Miyake S. Arthritis Rheum. 2004;50:305. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- 14.(a) Takikawa H., Muto S.E., Mori K. Tetrahedron. 1998;54:3141. [Google Scholar]; (b) Figueroa-Perez S., Schmidt R.R. Carbohydr. Res. 2000;328:95. doi: 10.1016/s0008-6215(00)00092-6. [DOI] [PubMed] [Google Scholar]; (c) Xia C., Yao Q., Schuemann J., Rossy E., Chen W., Zhu L., Zhang W., De Libero G., Wang P.G. Bioorg. Med. Chem. Lett. 2006;16:2195. doi: 10.1016/j.bmcl.2006.01.040. [DOI] [PubMed] [Google Scholar]; (d) Fan G.T., Pan Y.S., Lu K.C., Cheng Y.P., Lin W.C., Lin S., Lin C.H., Wong C.H., Fang J.M., Lin C.C. Tetrahedron. 2005;61:1855. [Google Scholar]; (e) Ndonye R.M., Izmirian D.P., Dunn M.F., Yu K.O.A., Porcelli S.A., Khurana A., Kronenberg M., Richardson S.K., Howell A.R. J. Org. Chem. 2005;70:10260. doi: 10.1021/jo051147h. [DOI] [PubMed] [Google Scholar]; (f) Michieletti M., Bracci A., Compostella F., De Libero G., Mori L., Fallarini S., Lombardi G., Panza L. J. Org. Chem. 2008;73:9192. doi: 10.1021/jo8019994. [DOI] [PubMed] [Google Scholar]; (g) Lee A., Farrand K.J., Dickgreber N., Hayman C.M., Juers S., Hermans I.F., Painter G.F. Carbohydr. Res. 2006;341:2785. doi: 10.1016/j.carres.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Kimura A., Imamura A., Ando H., Ishida H., Kiso M. Synlett. 2006;15:2379. [Google Scholar]
- 16.Akimoto K., Natori T., Morita M. Tetrahedron Lett. 1993;34:5593. [Google Scholar]
- 17.Van den Berg R.J.B.H.N., Boltje T.J., Verhagen C.P., Litjens R.E.J.N., Van der Marel G.A., Overkleeft H.S. J. Org. Chem. 2006;71:836. doi: 10.1021/jo0520240. [DOI] [PubMed] [Google Scholar]
- 18.Imamura A., Ando H., Korogi S., Tanabe G., Muraoka O., Ishida H., Kiso M. Tetrahedron Lett. 2003;44:6725. [Google Scholar]
- 19.Hada N., Oka J., Nishiyama A., Takeda T. Tetrahedron Lett. 2006;47:6647. [Google Scholar]
- 20.(a) Stork G., Kim G. J. Am. Chem. Soc. 1992;114:1087. [Google Scholar]; (b) Bols M. J. Chem. Soc., Chem. Commun. 1992;12:913. [Google Scholar]; (c) Bols M. J. Chem. Soc., Chem. Commun. 1993;9:791. [Google Scholar]; (d) Bols M. Acta Chem. Scand. 1993;47:829. [Google Scholar]
- 21.Bols M. Tetrahedron. 1993;49:10049. [Google Scholar]
- 22.Saito S., Ichinose K., Sasaki Y., Sumita S. Chem. Pharm. Bull. 1992;40:3261. doi: 10.1248/cpb.40.3261. [DOI] [PubMed] [Google Scholar]
- 23.Prigozy T.I., Naidenko O., Qasba P., Elewaut D., Brossay L., Khurana A., Natori T., Koezuka Y., Kulkarni A., Kronenberg M. Science. 2001;291:664. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 24.Kinjo Y., Pei B., Bufali S., Raju R., Richardson Stewart K., Imamura M., Fujio M., Wu D., Khurana A., Kawahara K., Wong C.H., Howell A.R., Seeberger P.H., Kronenberg M. Chem. Biol. 2008;15:654. doi: 10.1016/j.chembiol.2008.05.012. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Brigl M., Bry L., Kent S.C., Gumperz J.E., Brenner M.B. Nat. Immunol. 2003;4:1230. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]; (b) Gumperz J.E., Roy C., Makowska A., Lum D., Sugita M., Podrebarac T., Koezuka Y., Porcelli S.A., Cardell S., Brenner M.B., Behar S.M. Immunity. 2000;12:211. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]; (c) Sugita M., van Der Wel N., Rogers R.A., Peters P.J., Brenner M.B. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8445. doi: 10.1073/pnas.150236797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selected data for new compounds6: 1H NMR (CDCl3): δ 7.92–8.04 (10H, m, Ar–H), 7.27–7.64 (10H, m, Ar–H), 5.80 (1H, dd, H-2, J2,1 = 3.6), 5.55 (1H, dd, J3,2 = 10.6, J3,4 = 2.8 Hz, H-3), 5.42–5.50 (2H, m, H-3Cer, H-4Cer), 5.31 (1H, d, H-1), 4.87 (d, 1H, H-4), 4.13–4.27 (m, 3H, H-2Cer, H-6a,, H-6b), 4.00–4.13 (m, 1H, H-5), 3.85–3.90 (m, 1H, H-1aCer), 3.68 (dd, 1H, J1a,1b = 10.4, J1b,2 = 8.6 Hz, H-1bCer), 1.86 (m, 2H, H-5aCer, H-5bCer), 1.24 (m, 24H, CH2), 0.96, 1.12 (2s, 18H, 2 × t-Bu), 0.88 (3H, t, CH3); 13C NMR (75 MHz, CDCl3): δ 98.5 (C-1); HRMS calcd for C60H79N3O12Si [M+Na]+: 1084.5433, found 1084.5402. Compound 10: 1H NMR (CDCl3): δ 7.97–8.07 (4H, m, Ar–H), 7.54–7.67 (2H, m, Ar–H), 7.39–7.52 (4H, m, Ar–H), 5.63 (1H, dd, J3,4 = 4.8, J3,2 = 6.8 Hz, H-3Cer), 5.55 (1H, ddd, J4,5a = 4.2, J4,5b = 8.5 Hz, H-4Cer), 5.43 (d, 1H, J4,3 = 2.6 Hz, H-4), 5.13 (dd, 1H, J3,2 = 10.6 Hz, H-3), 4.85 (d, 1H, J1,2 = 3.8 Hz, H-1), 4.17–4.26 (2H, m, H-6a, H-6b), 4.07 (m, 2H, H-5, H-2Cer), 4.00 (m, 1H, H-1aCer), 3.94 (1H, dd, H-2), 3.71 (dd, 1H, J1a,1b = 10.4, J1b,2 = 6.5 Hz, H-1bCer), 2.40 (d, 1H, J2,OH = 2.8 Hz, OH), 2.13, 2.04, 2.00 (3s, 9H, 3 × OCOCH3), 1.88 (m, 2H, H-5aCer, H-5bCer), 1.21 (m, 24H, CH2), 0.88 (3H, t, CH3); 13C NMR (75 MHz, CDCl3): δ 97.8 (C-1); HRMS calcd for C44H61N3O13 [M+Na]+: 862.4204, found 862.4199. Compound 11: 1H NMR (CDCl3): δ 7.90–8.03 (8H, m, Ar–H), 7.30–7.59 (12H, m, Ar–H), 5.58–5.60 (2H, m, H-2′, H-3′), 5.41–5.46 (3H, m, H4′, H4, H-4Cer), 5.32–5.36 (m, 1H, H-3Cer), 5.32 (dd, 1H, J3,2 = 10.1, J3,4 = 3.5 Hz, H-3), 4.86 (d, 1H, J1,2 = 3.6 Hz, H-1), 4.76 (1H, br s, H-1’), 4.09–4.20 (3H, m, H-2, H-6a, H-6b), 3.88–4.06 (m, 6H, H-5, H-5′, H-6a′, H-6b′, H-1aCer, H-2Cer), 3.52 (dd, 1H, J1a,1b = 9.9, J1b,2 = 8.5 Hz, H-1bCer), 2.12, 2.09, 2.01 (3s, 9H, 3 × OCOCH3), 1.83 (m, 2H, H-5aCer, H-5bCer), 1.29 (m, 24H, CH2), 0.95, 1.09 (2s, 18H, 2 × t-Bu), 0.86 (3H, t, CH3); 13C NMR (75 MHz, CDCl3): δ 98.9 (C-1′), 97.9 (C-1); HRMS calcd for C72H95N3O20Si [M+Na]+: 1372.6278, found 1372.6251. 1: HRMS calcd for C49H97NO9 [M+Na]+: 880.7239, found 880.7218. Compound 12: HRMS calcd for C56H109NO14 [M+Na]+:1042.7848, found 1042.7837.