Abstract
Background/Aims
The impact of highly active antiretroviral therapy (HAART) on progression to end-stage liver disease (ESLD) in human immunodeficiency virus (HIV)/ hepatitis C virus (HCV) co-infection remains controversial.
Methods
We studied 157 HCV+ hemophilic men (85 HIV+, 72 HIV−), on whom dates of HIV and HCV serovconversion and clinical outcomes were known. Time to ESLD was determined by Kaplan Meier product-limit methods, and risk factors for ESLD progression were analyzed by a Cox proportional hazards model.
Results
Among HIV+ men, ESLD was more common, 17 of 85 (20.0%) than in HIV−, 8 of 72 (11.1%) and median ESLD-free survival significantly shorter, p = 0.009, hazard ratio 3.00 [95%CI: 1.27–7.08]. HAART treated HIV+ had longer ESLD-free survival than HIV+ untreated, 30.3 vs. 20.0 yr, p = 0.043, hazard ratio, 3.14 [95% CI: 1.27–7.08], comparable to survival in HIV-men, p = 0.13, hazard ratio 2.20 [95% CI: 0.76–2.35]. Progression was unrelated to HAART toxicity (n=0) or HCV antiviral therapy (n=7). HIV+ HAART Rx and HIV− did not differ in HCV duration, age at ESLD, age at death or present, overall or AIDS mortality, all p > 0.05.
Conclusions
These data suggest HAART improves ESLD-free survival, approaching that in HIV− men.
Keywords: Highly active antiretroviral therapy, hepatitis C, endstage liver disease, HIV infection, survival, HIV-HCV co-infection
Introduction
It is well established that the rate of progression of hepatitis C virus (HCV) infection is rapidly accelerated in individuals with HIV co-infection. Among individuals with hemophilia, there is a 4-to 8-fold increase in progression to end-stage liver disease (ESLD) in HIV-positive, as compared with HIV-negative [1–3], a risk that is increased by HBsAg+, alcohol use, and increasing duration of HCV infection [1]. Since the introduction of highly active antiretroviral therapy (HAART), immune function has improved and morbidity and mortality associated with HIV infection have decreased [4]. Among those with HIV/HCV co-infection, there is evidence that HAART also slows progression to ESLD [5–7] and fibrosis progression [8–10], although this remains controversial. While it has been postulated that HIV viral suppression [10] and immune reconstitution possible with HAART [11] are critical factors influencing the rate of HCV fibrosis progression, available data are conflicting. Some studies demonstrate that HAART may adversely affect hepatitis C outcome by increasing HCV viral load, hepatotoxicity, and fibrosis progression [7, 9, 12–16]. It is important to recognize that some of the differences in published findings may relate to the populations studied, specifically with variable or unknown duration of HIV and/or HCV infection, heterogeneous populations with variable HCV disease severity, variable routes of HCV and HIV exposure, and lack of a comparable HIV-negative control group.
Individuals with hemophilia are a unique group in whom the dates of HCV infection and the dates of HIV infection are known. Specifically, HCV occurred in individuals with hemophilia with their first clotting factor exposure [17], and HIV seroconversion peaked in 1982 [1]. The availability of a group of HIV/HCV co-infected and a comparable group of HCV mono-infected individuals provided the unusual opportunity to study the natural history of HCV infection in the setting of superimposed HIV infection. We, therefore, evaluated the impact of HAART on progression to end-stage liver disease in a well-defined cohort of HCV+ hemophilic men, comparing HIV-infected and HIV-negative.
Materials and Methods
Study subjects
A group of 157 HCV-infected hemophilic men, representing all HCV-infected men who were HCV positive in 1978 [1, 18] and cared for at the Hemophilia Center Western PA were available for study. These included 85 HIV-infected and 72 HIV-negative men on whom clinical outcomes and laboratory results were obtained as part of standard care, including all treatment regimens and dates, including date of first clotting factor exposure, HIV seroconversion, highly active antiretroviral therapy (HAART), onset of HCV infection, and HCV treatment, as previously described [1]. These patients belong to a well-defined cohort which has participated in previous studies [1, 18], and in whom all clinical outcomes, including ESLD and death through October 2005 were known. ESLD was defined by the presence of ascites, hepatic encephalopathy, variceal bleeding, jaundice, spontaneous bacterial peritonitis, and/or hepatorenal syndrome. HIV-positive patients were placed on antiretroviral therapy (ART) and highly active antiretroviral therapy (HAART) as it became available. Alcohol misuse was by self-report or family report during routine clinic visits, and defined as exceeding two drinks (24 g) wine, beer, liquor) daily. Since HCV infection occurred with the first exposure to clotting factor, we estimated the onset of HCV infection as the first year of factor treatment, beginning in 1970 for those born before 1970, or year of birth for those born after 1970, as previously described [1, 18]. HIV-positive patients were placed on antiretroviral therapy as it became available: the 66 who did not receive HAART died before it became available. Only seven patients, including one HIV-positive and six HIV-negative, received antiviral treatment for HCV: four received interferon alone and three received combination interferon plus ribavirin. All of those alive at the time of this analysis, 78 patients, including 19 HIV-positive and 59 HIV-negative, 20 of whom underwent liver biopsies, provided informed consent as part of the NHLBI-funded HIV Impact on Hepatitis C in Hemophilia Study (HHH Study) [19]. The protocol and informed consent documents for this study were approved by the Clinical Translational Research Center (CTRC) Advisory Committee (formerly General Clinical Research Center GCRC) and the Institutional Review Board (IRB) at the University of Pittsburgh.
Liver histopathology
Liver biopsies, obtained for clinical reasons pre-transplant or pre-HCV treatment (n=7), autopsy (n=10), or research (HHH study) (n=14), were scored by Ishak fibrosis progression score, 0–6 [20]. The fibrosis progression rate was calculated as the ratio of the fibrosis stage over the duration of HCV infection. All but one of the biopsies were independently reviewed by a single pathologist, MN, who was blinded to HIV status and HIV treatment. The single unavailable biopsy, not independently reviewed, was on a patient not receiving anti-HIV therapy: there were no statistical differences between the independent reviewer, MN, and the single original reviewer, in individual biopsy Ishak scores, nor in median group Ishak scores
Statistical methods
Time from HCV acquisition to ESLD was estimated by Kaplan Meier product-limit methods, using the log-rank test for significance. Independent predictors of ESLD were determined, with adjustment for covariates, using the Cox proportional hazards model. Other cross-sectional comparisons were done by non-parametric Wilcoxon rank-sum test. For all analyses, p < 0.05 was used to determine statistical significance.
Results
Comparison of baseline characteristics
The 157 HCV-positive patients in this cohort included 137 (87.3%) with hemophilia A and 20 (12.7%) with hemophilia B, of whom 85 (54.1%) had HIV co-infection. By race, 151 (96.2%) were Caucasian and six (3.8%) were African-American (Table 1). Twenty (23.5%) were treated with HAART (Group 1), including protease inhibitor (PI) in 19 and non-nucleoside reverse transcriptase inhibitor (NNRTI) in one; and, of these all 20 had received ART (antiretroviral therapy) prior to HAART. Of the 65 non-HAART treated HIV-infected men (Group 2), 40 (61.5%) received ART only and 25 (38.5%) received no treatment, as they were unwilling or died before drugs were available. The comparison group included 72 HIV-negative patients (Group 3).
TABLE 1.
Demographics of the Hepatitis C (+) Hemophilia Cohort
| Group 1 | Group 2 | Group 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| HIV (+) |
HIV (+) Non-HAART Treated |
HIV (−) |
||||||
| HAART Rx | ALL | ART Rx | Untreated | p value | ||||
| (N=20) | (N=65) | (N=40) | (N=25) | (N=72) | 1 vs. 2 | 1 vs. 3 | ||
| Type Hemophilia | ||||||||
| A (Severe/Moderate/Mild) | 18 (17/1/0) | 58 (53/5/0) | 36(34/2/0) | 22(19/3/0) | 61(32/15/14) | p=0.321 | p=0.004 | |
| B (Severe/Moderate/Mild) | 2 (2/0/0) | 7 (6/1/0) | 4 (4/0/0) | 3 (2/1/0) | 11 (1/10/0) | |||
| Age | <29 yr | 0 | 18 | 11 | 7 | 2 | ||
| 29–40 yr | 7 | 23 | 16 | 7 | 24 | p=0.004 | p=0.611 | |
| >40 yr | 13 | 24 | 13 | 11 | 46 | |||
| Mean Age (Yr) | 42.5±1.4 (43) | 38.8±1.8 (36) | 37.4±1.9(35.5) | 41.2±3.5 (38) | 48.3±1.7 (43.5) | p = 0.05 | p = 0.29 | |
| Year of Birth | ||||||||
| After 1970 | 3 (15.0%) | 6 (9.2%) | 5 (12.5%) | 1 (4.0%) | 12 (16.7%) | p=0.003 | p=0.162 | |
| 1970 or before | 17 (85.0%) | 59 (90.8%) | 35 (87.5%) | 24 (96.0%) | 60 (83.3%) | |||
| Race | Caucasian | 20 | 63 | 38 | 25 | 70 | p=0.583 | p=0.611 |
| African American | 0 | 2 | 2 | 0 | 2 | |||
| Age at HIV Seroconversion (Yr) | 20.8 ± 1.6 | 29.9 ± 1.9 | 26.6 ± 2.0 | 35.7 ± 3.6 | - | p=0.03 | - | |
| CD4 | No./µl | 351 ± 56 | 109 ± 19 | 90 ± 19 | 145 ± 43 | - | p<0.01 | - |
| Range | 64 – 948 | 4 – 610 | 4 – 412 | 2 – 610 | - | |||
| HBV | HBsAg + ever | 15 (75.0%) | 64 (98.5%) | 39 (97.5%) | 25 (100%) | 65 (90.3%) | p=0.002 | p=0.040 |
| Anti-HBc+ only | 5 (25.0%) | 1(15.4%) | 1 (2.5%) | 0(0%) | 7(9.7%) | |||
| Alcohol misuse | 4 (20.0%) | 6 (9.2%) | 3 (7.5%) | 3 (12.0%) | 7 (9.7%) | p=0.128 | p=0.133 | |
| ESLD No.(%) | 6 (30.0%) | 11 (16.9%) | 6 (15.0%) | 5 (20.0%) | 8 (11.1%) | p=0.201 | p=0.040 | |
| Age at HCV Seroconversion (Yr) | 10.0 ± 1.2 | 18.1 ± 1.9 | 14.8 ± 1.9 | 25.2 ± 4.0 | 20.8 ± 2.1 | p=0.079 | p=0.94 | |
| Age at ESLD (Yr) | 43.4 ± 2.8 | 42.9 ± 3.6 | 34.5 ± 2.3 | 53.0 ± 4.2 | 41.8 ± 2.5 | p=0.93 | p=0.61 | |
| Duration HCV Infection (Yr) | 33.2 v 0.3 | 20.6 ± 0.6 | 22.6 ± 0.5 | 17.1 ± 0.9 | 27.9 ± 1.0 | p=0.001 | p=0.14 | |
| Median Time to ESLD (Yr) | 30.3 | 20.0 | - | - | 20.2 | p=0.043 | p=0.059 | |
Comparison of ESLD and ESLD-free survival
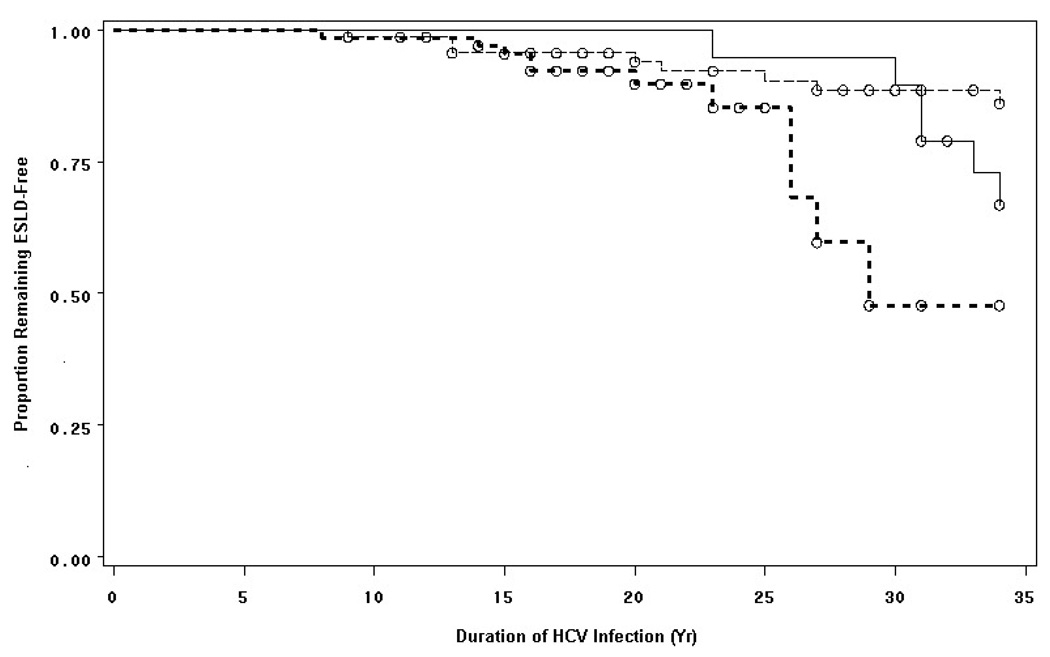
ESLD developed in 8 of 72 (11.1%) HIV-negative men (Group 3) and in 17 of 85 (20.0%) HIV-infected men (Groups 1 and 2). The latter included six of 20 (30.0%) HAART-treated (Group 1), six of 40 (15.0%) ART-only treated and five of 25 (20.0%) untreated HIV-infected men (Group 2) (Table 1). The median ESLD-free survival was significantly shorter in HIV-positive than HIV-negative men, p = 0.009, hazard ratio 3.00 [95%CI: 1.27–7.08] (Figure 1). By contrast, ESLD-free survival among HAART-treated HIV-infected men (Group 1) was significantly longer than in non-HAART-treated HIV-infected men (Group 2), 30.3 yr vs. 20.0 yr, p = 0.043, hazard ratio, 3.14 [95% CI: 1.27–7.08], but was similar to that in HIV-negative men (Group 3), 27.9 yr, p = 0.13, hazard ratio 2.20 [95% CI: 0.76–2.35]. The duration of HAART treatment among HIV-positive was not shorter in those developing ESLD than in those remaining ESLD-free, 6.7 vs. 7.2 years, p = 0.89, but the duration of HCV infection was somewhat shorter in those developing ESLD, 30.3 vs. 33.6 yr, p = 0.005.
Figure 1. Impact of HAART on End-Stage Liver Disease (ESLD)-Free Survival in Hemophilic Men with HIV/HCV Co-Infection.
The median time to ESLD among HIV-infected HAART-treated hemophilic men (Group 1) (solid line) was 30.3 years, significantly longer than in HIV-infected non-HAART-treated hemophilic men (Group 2) (bold dotted line), 20.0 years, p = 0.043; but not different from time to ESLD in HIV(−) hemophilic men (Group 3) (dashed line), 20.2 years, p = 0.059.
Among untreated HIV-infected subjects, the short duration of HCV infection was related to their older age at first concentrate exposure (first available in 1970), which resulted in older age at ESLD in those who progressed, with the majority dying of AIDS before they could develop ESLD (Table 1). By contrast, the longer duration of HCV infection in ART-treated HIV-infected subjects reflected their younger age at first concentrate exposure, and resulting younger age at ESLD among those who progressed, the majority of whom died of AIDS before they could develop ESLD.
Other predictors of ESLD
In addition to HAART treatment, age at HIV SC was an independent predictor of ESLD, p =0.021, but not independent of HAART treatment. With each decade of HIV infection, ESLD risk increased 1.63-fold [95% CI: 1.14–2.35], p = 0.008. Comparing HAART-treated HIV-infected (Group 1) with HIV-negative (Group 3), there was no difference in HCV duration, age at death or present, median age at ESLD, overall mortality, or ESLD mortality, all p > 0.05. The age at HCV seroconversion was also an independent predictor of ESLD. With each year of HCV infection, ESLD risk increased 1.052 (95% CI, 1.024–1.080), p = 0.0002.
The development of ESLD was not related to mitochondrial toxicity or hepatotoxicity associated with HAART: none of the HIV-infected patients developing ESLD suffered mitochondrial toxicity with HAART; and hepatotoxicity, when it occurred, did so only after the onset of ESLD, following previous tolerance to HAART therapy. Treatment with HCV antiviral therapy was also not associated with development of ESLD: of the two subjects so treated who developed ESLD, HCV treatment predated ESLD by two and nine years, respectively.
Liver biopsy findings
Among a subset of 31 patients on whom liver biopsies were available, advanced fibrosis (Ishak score 3–6) was present in the majority, 22 (71.0%). These included 71.4% of the HIV non-HAART-treated (Group 2), 75.0% of the HAART-treated (Group 1), and 68.7% of the HIV-negative men (Group 3). Although the median Ishak score was similar, the fibrosis progression rate (FPR) [19], which takes into account duration of HCV infection, was significantly slower in the HAART-treated (Group 1) vs. non-HAART treated HIV+ men (Group 2), p < 0.01 (Table 2). Not one of the 31 individuals in this study who underwent liver biopsy had histopathologic light microscopy evidence of changes typically associated with mitochondrial toxicity.
TABLE 2.
Liver Biopsy Findings: Ishak Fibrosis Score in Hepatitis C (+) Hemophilia Cohort
| Group 1 | Group 2 | Group 3 | |||||
|---|---|---|---|---|---|---|---|
| HIV (+) |
HIV (+) Non-HAART Treated |
HIV (−) |
|||||
| HAART Rx | ALL | ART Rx | Untreated | p value | |||
| (N = 20) | (N=65) | (N=40) | (N=40) | (N = 72) | 1 vs. 2 | 1 vs. 2 | |
| Liver Biopsy (No.,%) | 8 (40.0%) | 7 (10.8%) | 0 (0%) | 7 (28.0%) | 16 (22.2%) | p=0.013 | p=0.378 |
| Reason for biopsy | |||||||
| Clinical | 1 (12.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (37.5%) | ||
| Autopsy | 1 (12.5%) | 7 (100%) | 0 (0%) | 7 (100%) | 2 (12.5%) | p=0.006 | p=0.184 |
| Research | 6 (75.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (50.0%) | ||
| Ishak Score (Median) | 4.5 | 5 | - | 5 | 5 | p=0.323 | p=0.413 |
| 0 | 2 (25.0%) | 0 (0%) | - | 0 (0%) | 2 (12.5%) | ||
| 1 | 0 (0%) | 2 (28.6%) | - | 2 (28.6%) | 3 (18.7%) | ||
| 2 | 0 (0%) | 0 (0%) | - | 0 (0%) | 0 (0%) | ||
| 3 | 1 (12.5%) | 1 (14.3%) | - | 0 (0%) | 2 (12.5%) | ||
| 4 | 1 (12.5%) | 0 (0%) | - | 1 (0%) | 0 (0%) | ||
| 5 | 3 (37.5%) | 1 (14.3%) | - | 1 (14.3%) | 3 (18.7%) | ||
| 6 | 1 (12.5%) | 3 (42.8%) | - | 3 (42.8%) | 6 (37.5%) | ||
| Advanced fibrosis (3–6) | 6/8 (75.0%) | 5/7 (71.4%) | - | 5/7 (71.4%) | 11/16 (68.7%) | p=0.431 | p=0.404 |
| Fibrosis progression rate (Median) | 0.104 | 0.333 | - | 0.333 | 0.143 (n=16) | p=0.023 | p=0.111 |
Mortality
The mortality rate associated with end-stage liver disease was greater in the non-HAART treated HIV-infected men (Group 2), 11 of 65 (16.9%), than in HAART-treated HIV-infected men (Group 1), 2 of 20 (10.0%), or HIV-negative men (Group 3), 6 of 72 (8.3%), but did not reach significance, p = 0.297 (Table 3).
TABLE 3.
Age and Cause of Death in Hepatitis C (+) Hemophilia Cohort
| Group 1 | Group 2 | Group 3 | |||||
|---|---|---|---|---|---|---|---|
| HIV (+) |
HIV (+) Non-HAART Treated |
HIV (−) |
|||||
| HAART Rx | ALL | ART Rx | Untreated | p value | |||
| (N = 20) | (N=65) | (N=40) | (N=25) | (N = 72) | 1 vs. 2 | 1 vs. 3 | |
| Mean Age at Death (Yr) | 47.8 ± 1.7 | 38.8± 1.8 | 37.4 ± 1.9 | 41.2± 3.6 | 55.8 ± 3.4 | p<0.001 | p<0.05 |
| Cause of Death | |||||||
| ESLD | 2 (10.0%) | 11 (16.9%) | 6 (15.0%) | 5 (20.0%) | 6 (8.3%) | p=0.297 | p =0.670 |
| AIDS | 1 (5.0%) | 43 (66.1%) | 28 (70.0%) | 15 (60.0%) | 0 (0%) | p=0.061 | - |
| CNS Bleed | 1 (5.0%) | 6 (9.2%) | 4 (10.0%) | 2 (8.0%) | 8 (11.1%) | p=.335 | p=0.275 |
| Suicide | 0 (0%) | 3 (3.1%) | 1 (0%) | 2 (8.0%) | 1 (1.4%) | p=0.442 | p=0.783 |
| Accident | 0 (0%) | 1 (1.5%) | 1 (2.5%) | 0 (0%) | 0 (0%) | p=0.765 | p=1.000 |
| Other | 1 (5.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (9.7%) | p=0.235 | p=0.316 |
| All-Cause Mortality | 5 (25.0%) | 64 (98.5%) | 40 (100%) | 24 (96.0%) | 22 (30.6%) | p=0.001 | p =0.72 |
Discussion and Conclusion
The introduction of HAART therapy has greatly improved the outcomes of HIV infection by reducing opportunistic infection, improving cellular immune function, and decreasing mortality. The findings of this study suggest that among those with HIV/HCV co-infection, HAART therapy also slows HCV liver disease progression and improves end-stage liver disease (ESLD)-free survival. In fact, the duration of HAART therapy also appears to be an independent predictor of ESLD. Specifically, this study demonstrates that the rate of HCV liver disease progression in HAART-treated HIV/HCV co-infected hemophilic men approached that in their HIV-negative hemophilic counterparts.
Although the role of HAART in slowing ELSD progression has been controversial, previous studies have been limited by differences in risk factors for and duration of HIV and HCV infection in at-risk co-infected groups, lack of a comparable HIV-negative control group, inability to control for risk factors for ESLD, including hepato-toxicity associated with HAART and HCV antiviral therapy, or mitochondrial toxicity associated with HAART.
This study provided the unique opportunity to evaluate the impact of HAART in a cohort of well-characterized HIV/HCV+ hemophilic men on whom dates of HCV and HIV infection, HAART therapy, HCV therapy, and all clinical outcomes were known.
The impetus for this study was the unexpected observation in the NHLBI multicenter, prospective HHH study (Impact of HIV on Hepatitis C Liver Disease in Hemophilia) that progression to ESLD was uncommon and the severity of hepatic fibrosis, as measured by Ishak score, was similar between HIV/HCV co-infected and HIV-negative hemophilic men [19]. We hypothesized that this observation might be related to the introduction of HAART therapy. The findings of this study not only confirm this hypothesis but further demonstrate that the improvement in ESLD-free survival possible with HAART among the HIV-infected hemophilic men does not appear to be related to HCV antiviral treatment or to hepatotoxicity or mitochondrial toxicity associated with HAART.
The mechanism by which liver disease progression is reduced with HAART therapy is not known. It is possible, but not proven, that reduction of HIV viral load with HAART prevents the HIV-induced upregulation of cytokines that promote hepatic fibrosis, including interleukin (IL)-6 and transforming growth factor-β [19, 21]. Indeed, consistent with this hypothesis, the hepatic fibrosis progression rate (FPR) measured on biopsies from a subset of the hemophilic men (Table 1), was significantly longer in HAART-treated than non-HAART-treated HIV-infected men.
We recognize several potential limitations of this study. First, the findings of this study may be limited by small sample size and power, especially the HIV-infected group treated with HAART. A second limitation of this study is the observational nature of the data, potentially subject to recall bias. This hemophilia cohort, however, was unique in the inclusion of both HIV-infected and HIV-negative hemophilic men and the careful, prospective followup of this group since the start of the HIV epidemic [1], and, thus, bias is believed to be minimal.
In contrast to recent studies of FPR in HIV/HCV co-infected cohorts [10], almost all of whom were treated with HAART therapy, our study is unique as it compared HAART-treated and non-HAART-treated HIV-infected hemophilic men. We found that HIV viral load, but not CD4, was directly related to fibrosis progression rate, as has been shown in other at-risk populations [10], a finding consistent with the role of HIV viral load both as a predictor of AIDS survival [4] and as a predictor of survival in HCV/HIV co-infected liver transplant recipients [22].
In summary, the findings of this study suggest that HAART therapy is effective in slowing liver disease progression in HIV/HCV co-infected hemophilic men. This may also be true in other co-infected groups at risk for liver disease progression. Further studies are underway to evaluate the relationship of HIV viral load, HAART therapy, cytokines that cause fibrosis, as well as cytokine promoter genotypes in progression of liver disease among HIV/HCV co-infected hemophilic men.
Acknowledgements
This study was supported by the National Institutes of Health Grant R01 HL68429; CTRC/CTSI grant: NIH/NCRR/CTSA UL-1 RR024153; Centers for Diseases Control Prevention of the Complications of Hemophilia Grant, CDC Contract U24CCU318053; HHS Federal Region III Hemophilia Treatment Center Grant, Maternal and Child Health Bureau, HRSA, Contract #1-H-30-MC-00038-01; and the Pennsylvania State Department of Health Contract, SAP #04100000330.
References
- 1.Ragni MV, Belle SH. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C infection. J Infect Dis. 2001;183:1112–1115. doi: 10.1086/319273. [DOI] [PubMed] [Google Scholar]
- 2.Graham C, Baden L, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 3.Goedert JJ, Eyster ME, Lederman MM, et al. End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood. 2002;100:1584–1589. [PubMed] [Google Scholar]
- 4.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.Mehta SH, Thomas DL, Torbenson M, Brinkley S, Mirel L, Chaisson RE, et al. The effect of antiretroviral therapy on liver disease among adults with HIV and hepatitis C co-infection. Hepatology. 2005;41:123–131. doi: 10.1002/hep.20541. [DOI] [PubMed] [Google Scholar]
- 6.Qurishi N, Creuzberg C, Luchters G, Effenberger W, Kupfer B, Sauerbruch T, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus co-infection. Lancet. 2003;362:1708–1713. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 7.Bonacini M. Liver injury during highly active antiretroviral therapy: the effect of hepatitis C co-infection. Clin Infect Dis. 2004;38 Suppl 2:S104–S108. doi: 10.1086/381453. [DOI] [PubMed] [Google Scholar]
- 8.Benhamou Y, DiMartino V, Bochet M, Colombet G, Thibault V, Liou A, et al. Factors affecting liver fibrosis in HIV and HCV-co-infected patients: impact of protease inhibitor therapy. Hepatology. 2001;34:283–287. doi: 10.1053/jhep.2001.26517. [DOI] [PubMed] [Google Scholar]
- 9.Verma S, Wang CH, Govindarajan S, Kanel G, Squires K, Bonacini M. Do type and duration of antiretroviral therapy attenuate liver fibrosis in HIV-hepatitis C virus-co-infected patients. Clin Infect Dis. 2006;42:262–270. doi: 10.1086/499055. [DOI] [PubMed] [Google Scholar]
- 10.Brau N, Salvatore M, Riso-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriquez-Orengo JF, et al. Slower fibrosis progression in HIV/HCV-co-infected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Lange CG, Lederman MM. Immune reconstitution with antiretroviral therapies in chronic HIV-1 infection. J Antimicrobial Chemotherapy. 2003;51:1–4. doi: 10.1093/jac/dkg071. [DOI] [PubMed] [Google Scholar]
- 12.Verma S. HAART attenuates liver fibrosis in patients with HIV/HCV co-infection: fact or fiction? J Antimicrobial Chemotherapy. 2006;58:496–501. doi: 10.1093/jac/dkl280. [DOI] [PubMed] [Google Scholar]
- 13.Andersson K, Chung RT. Hepatitis C virus in the HIV-infected patient. Clin Liver Dis. 2006;10:303–320. doi: 10.1016/j.cld.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Sulkowski M, Mehta S, Torbenson M, et al. Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS. 2005;19:585–592. doi: 10.1097/01.aids.0000163935.99401.25. [DOI] [PubMed] [Google Scholar]
- 15.Backus L, Phillips B, Boothroyd D, et al. Effects of hepatitis C virus co-infection on survival in veterans with HIV treated with highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;39:613–618. [PubMed] [Google Scholar]
- 16.Kramer J, Giorano T, Souchek J, et al. Hepatitis C co-infection increases the risk of fulminant hepatic failure in patients with HIV in the HAART era. J Hepatol. 2005;42:309–314. doi: 10.1016/j.jhep.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Kasper CK, Kipnis SA. Hepatitis and clotting-factor concentrates. JAMA. 1972;221:510. [PubMed] [Google Scholar]
- 18.Ragni MV, Bontempo FA, Faruki H. Increase in hepatitis C virus load in hemophiliacs treated with highly active antiretroviral therapy. J Infect Dis. 1999;180:2027–2029. doi: 10.1086/315143. [DOI] [PubMed] [Google Scholar]
- 19.Soadwa K, Nalesnik M, Dang Q, Ragni M. Predictors of liver fibrosis in HCV(+) hemophilic men. Blood. 2005;106:319. (abstract) [Google Scholar]
- 20.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histologic grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 21.Rockstroh JK, Spengler U, Sudhop T, Ewig S, Theisen A, Hammerstein U, et al. Immunosuppression may lead to progression of hepatitis C virus-associated liver disease in hemophiliacs co-infected with HIV. Am J Gastroenterol. 1996;91:2563–2573. [PubMed] [Google Scholar]
- 22.Ragni MV, Im K, Neff G, Roland M, Stock P, Heaton N, Humar A, Fung JF. Survival in HIV-infected liver transplant recipients. J Infect Dis. 2003;118:1412–1420. doi: 10.1086/379254. [DOI] [PubMed] [Google Scholar]