Abstract
Introduction
The National Association of Children’s Hospitals (NACHRI) and the Centers for Medicare and Medicaid Services (CMS) recently introduced 30-day hospital readmission rate as a quality care indicator in children with Sickle Cell Disease (SCD). Based on previous research identifying risk factors for 30-day readmission in our patient population, we designed and implemented a multi-modal intervention to reduce 30-day readmission rate in children with SCD and pain.
Methods
A before-and-after study design was performed to evaluate an intervention containing three components: 1) standardized SCD-pain admission orders; 2) monthly SCD-pain in-service for house physicians for the first 6-months; and 3) continuous patient/caregiver education. Following order implementation, we prospectively collected data on all children admitted for SCD-pain over a 6-month period. We compared the 30-day readmission rate after the intervention to the rate during the same 6-month interval in the previous calendar year prior to the availability of pre-specified SCD-pain orders.
Results
A total of 89 admissions, in 68 individuals, were eligible for the standardized orders during the prospective time period and were compared to 85 admissions in 56 individuals during the control period. Pre-specified SCD-pain orders were used in 93% of eligible admissions during the intervention. Readmission rate within 30-days was lower for the intervention cohort than the control cohort, 11% (10/89) vs 28% (24/85), p = 0.007, 95% CI 0.1–0.7.
Conclusions
A multi-modal intervention was successful in decreasing 30-day hospital readmission rate for children with SCD and pain. Provider education was the most important component of the multi-modal intervention.
Keywords: Readmission Rate, Sickle Cell Disease, Children
Introduction
The National Association of Children’s Hospitals (NACHRI) and the Centers for Medicare and Medicaid Services (CMS) currently use the 30-day hospital readmission rate to define quality care in children with Sickle Cell Disease (SCD)1. Quinn, in a recent editorial, questions whether readmission is a true proxy of quality care or a product of the underlying chronic disease in patients with SCD2. The editorial critiques the measure further as it addresses only two of the six performance dimensions recognized by the Institute of Medicine (IOM)3 as necessary to produce quality improvement: effectiveness and efficiency while excluding safety, patient-centeredness, timeliness and equity. A low 30-day readmission rate has not yet been associated with improved patient or caregiver satisfaction, decreased morbidity or decreased cost. Such factors will need to be validated for the 30-day readmission rate to become a meaningful standard to families, healthcare providers and the health care industry.
Given this new benchmark for quality care in children with SCD, we set out to better understand the barriers to behavior change and how to develop targeted strategies for decreasing the 30-day readmission rate. Previous research suggests single interventions, especially those employing only information dissemination, are less effective than multi-modal interventions in producing behavior change4, 5. Interventions are most successful when they incorporate the end-user in development, include a participatory educational component, and integrate recommendations into the regular process of care6. Based on a systematic review of the data from our hospital7 and the existing evidence suggesting that more than one strategy is needed to produce a significant change in physician behavior, we designed and implemented a multi-modal intervention. The primary objective of this study was to prospectively reduce the 30-day readmission rate in children with SCD and pain.
Methods
The protocol was reviewed by the Washington University School of Medicine Human Research Protection Office and approved. The intervention focused on addressing risk factors for readmission identified in a retrospective cohort published separately, and contained three components: 1) standardized, evidence-based SCD-pain admission orders (Supplemental Appendix 1 and 2); 2) a 30-minute small group in-service on SCD-pain given monthly to all St. Louis Children’s Hospital house staff physicians for 6 months (Supplemental Appendix 3); and 3) patient/caregiver education by an inpatient nurse with expertise in SCD (Supplemental Appendix 4). A 5-month run-in period occurred before the prospective cohort study was started to increase awareness among nurses, unit secretaries and attending physicians, to prevent lack of knowledge as a barrier to implementation.
All hospital admissions for children with SCD-pain were evaluated prospectively for six-months from July 1, 2007 to December 31, 2007. Inclusion criteria were any diagnosis of SCD as confirmed by hemoglobin analysis; age > 12 months, as pain requiring opioid administration is rarely the primary reason for admission in young children; and admission to the inpatient unit for further pain management. Exclusion criteria were pain not requiring use of intravenous (IV) opioids such as dactylitis, headache, Group A β-hemolytic Streptococcus infection, costochondritis, or priapism as the primary reason for admission; and ≥ 12 hospitalizations for SCD-related morbidity in the previous 12 months. We chose a similar seasonal time period (July 1, 2006 to December 31, 2006) prior to the intervention for the control cohort to help exclude influenza and other infections as a cause of readmission. The same inclusion and exclusion criteria were used to identify patients in the control cohort.
The aim of the multi-modal intervention was to reduce 30-day hospital readmission in the same population while maintaining patient-focused quality measures such as: reduction in pain score upon discharge, reduction in complications associated with admission for pain, and PCA-use when appropriate. The primary outcome measure for the study was the 30-day hospital readmission rate. Secondary outcome measures included markers of quality care (PCA-use, duration of IV pain medications, early weaning of analgesics within the first 24 hours not due to respiratory depression or side effects, absolute admission and discharge pain scores); risk factors identified in the retrospective study (hematology follow-up appointment); and opioid-induced side effects (including itching and nausea); excessive sedation; decreased room air saturations; and respiratory depression. The house staff educational component was completed in the first six months of the academic year (July 1 through December 31) where 98% of residents received the educational in-service (one resident missed due to illness).
Sustainability of Intervention
All SCD patients admitted for pain during the six-month period from January 1, 2008 to June 30, 2008 after termination of the educational component of the intervention were followed prospectively to determine if the order sets alone were sufficient maintain a lower readmission rate. The same inclusion and exclusion criteria and definitions were applied to this cohort. The 30-day readmission rate during the second six month period was compared to the readmission rate throughout the previous seasonal time period.
Definitions in the Study
A second admission for acute SCD-pain that occurred within 30-days of a readmission visit but was greater than 30-days from the original admission for acute SCD-pain was considered an independent admission. A readmission was defined as a hospital admission for any SCD-related morbidity, occurring ≤ 30-days after the primary admission for SCD-pain. SCD-pain was defined as acute pain in the extremities, back, abdomen, or chest that is presumed to be due to SCD, with no other identified cause. Disease severity was defined as patients with ≥ 3 hospitalizations for SCD-related morbidity in a one-year period8. Asthma symptoms during admission were defined as the presence of chest pain, cough, hypoxia, wheezing, respiratory distress such as tachypnea or increased work of breathing, or decreased breath sounds in a patient with a history of asthma. Respiratory depression was defined as a decrease in PCA due to respiratory rate ≤ 8 breaths per minute or naloxone administration. Itching was defined as a patient complaint plus administration of an anti-pruritic agent and nausea was defined as a patient complaint plus administration of an anti-emetic. Excessive sedation was defined as a decrease in PCA due to inability to arouse the patient and decreased room air saturations were defined as a decrease in the PCA for room air oxygen saturations ≤ 92%.
Data Collection Methods
Prospective data were obtained directly from inpatient medical records and from the hospital-based computer system using an a priori data collection that was developed and refined for the retrospective study designed to identify risk factors associated with hospital re-admission. After collection was complete, 10% of the patient records were additionally reviewed by a second extractor for accuracy. The following data fields were checked: SCD genotype, number of hospitalizations in the previous 12 months, primary admission diagnosis, hematology follow-up, PCA-use and whether or not the patient was readmitted within 30 days. These variables were found to be 96% concordant between the two extractors.
Baseline characteristics including type of SCD, co-morbid diagnoses, hemoglobin level, oxygen saturations, and frequency of hospitalization were collected for each patient. For each primary admission and readmission, the following were collected: admission and discharge diagnoses, length of stay, admission and discharge hemoglobin, presence of asthma symptoms, steroid administration, presence of fever, antibiotic administration, type and method of opioid administration, timing of opioid weaning, duration of oral analgesics, use of oxygen, discharge oxygen saturations, admission and discharge pain scores, use of standardized orders and adherence to hospital follow-up.
Statistical Methods
The statistical software package, SPSS 15.0.0, was used to perform statistical analysis. Dichotomous primary and secondary outcomes were analyzed using Chi square and Fisher’s Exact Test to compare the outcomes of interest between groups before and after the intervention. Continuous variables were analyzed using Student’s T-test. A Mann Whitney U test was used for data not normally distributed such as change in pain scores. Baseline characteristics of the cohort are presented in descriptive tables. A second statistician reviewed the analysis to ensure accuracy of the final analysis. Because the primary and secondary outcomes were defined a priori, alpha was set at 0.05 to avoid a type I error. Therefore, no adjustment in p-value was made for multiple testing.
Sample size calculations were performed using a readmission rate in the control population of 0.3 or 30%. Setting alpha at 0.05 with 80% power, 65 patients per group would be needed to detect a clinically relevant reduction in readmission rate to 20%. Based on our patient population, we would expect to have more than 65 patients in a six-month time period and chose to enroll all eligible patients during a the first six-months of the academic calendar year.
Results
Study Population for Intervention and Control Cohorts
During the six-month intervention period, a total of 89 inpatient admissions were eligible for the pre-printed standardized SCD-pain admission order sets. Pre-specified SCD pain orders were used in 93% of eligible admissions during the intervention period. The admissions were among 68 unique individuals with 9% (6/68) of patients composing 20% (18/89) of the primary admissions. The control group was composed of 85 primary inpatient admission for SCD-pain, occurring among 56 individual patients also with 9% (5/56) comprising 20% (17/85) of the admissions. Demographic data for the intervention and control cohorts are presented in Table I.
Table I.
Demographics and Clinical Characteristics of Individual Patients in the Prospective and Control Cohorts
| Prospective Cohort n=68 |
Control Cohort n=56 |
p-value | |
|---|---|---|---|
| Mean Age (Range years) | 11.2 (1.5–19) | 12.7 (2–20) | 0.11 |
| Gender (males) | 31 (46%) | 29 (52%) | 0.49 |
| SCD Genotype | |||
| SS | 44 | 34 | 0.71 |
| SC | 17 | 17 | 0.55 |
| Sβ+ Thalassemia | 3 | 2 | 1 |
| Sβº Thalassemia | 3 | 3 | 1 |
| SHPFH | 1 | 0 | 1 |
| Co-morbidities | |||
| Asthma | 36 (53%) | 26 (46%) | 0.001 |
| Stroke | 8 | 7 | 1 |
| Hydroxyurea | 9 | 8 | 1 |
| Baseline Labs | |||
| Hemoglobin (gm/dl) | 9.6 | 9.3 | 0.19 |
| Room Air Oxygen Saturations (%) | 98 | 98 | 0.30 |
| Number of Hospitalizations in Previous 12 months (Range) | 2.4(0–10) | 2.7(0–11) | 0.26 |
| Insurance Status | |||
| Medicaid or other State-sponsored Insurance | 49(72%) | 40 (71%) | 1 |
30-Day Readmission Rate During Intervention
Readmission rate within 30-days was significantly lower for children admitted with SCD-pain during the intervention period than the control period, 11% vs 28%; p &< 0.002; 95% CI 0.1–0.6. For patients who were readmitted in the intervention cohort, 50% (5/10) were within 14-days of the primary admission. Average length of stay (LOS) increased by less than one day after the intervention which was statistically significant but probably clinically irrelevant, (5 vs 4 days; p = 0.03; 95% CI −1.8 to −0.1). An increase of one hospital day likely does not outweigh the economic, physical or emotional impact of a full hospital readmission.
A history of asthma was found in 52% (36/68) of the intervention cohort, and 35% (31/89) of pain admissions were accompanied by an asthma exacerbation. Corticosteroid administration for an asthma exacerbation was 51% (16/31) and patients who received corticosteroids accounted for 20% (2/10) of the readmissions during the intervention.
Importance of Educational Component of Multi-modal Intervention
Including the follow-up time period after completion of the educational intervention, a total of 173 inpatient admissions were eligible for the pre-printed standardized SCD-pain admission order sets. Pre-specified SCD pain orders were used in 94% (162/173) of eligible admissions during the intervention period as opposed to 32% (51 of 159) during the previous year. The significant reduction in 30-day readmission rate for children admitted with SCD-pain during the educational intervention disappeared, with overall 30-day readmission rate increasing to 19% (33/173) vs 28% (44/159), in the previous year, (p = 0.06; 95% CI 0.4–1). The difference in monthly 30-day readmission rate between cohorts is presented in Figure 1.
Figure 1.
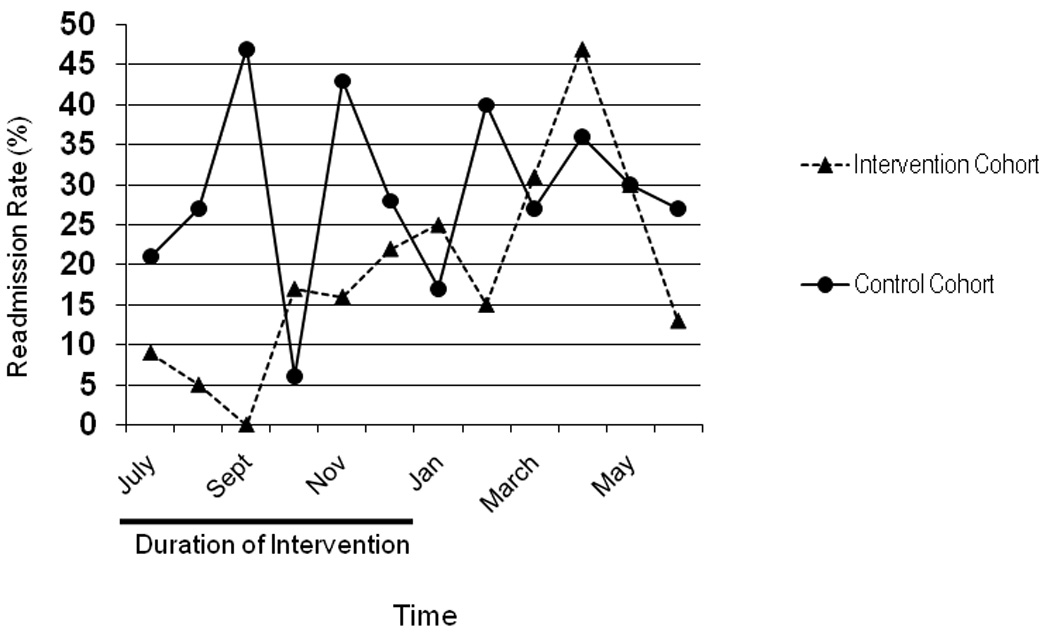
Monthly 30-day readmission rate (%) for the intervention and control cohorts (July 2006-June 2007 vs July 2007–June 2008).
Improvement in Other Quality Care Measures
When compared to the control cohort, patients in the intervention cohort experienced an improvement in quality of care. Children admitted for SCD-pain in the intervention cohort had a higher rate of patient controlled analgesia (PCA) use, demonstrated a greater change in discharge pain scores and more frequently received a discharge hematology appointment coupled with an actual outpatient hematology follow-up visit after discharge. After the intervention, a PCA was prescribed more often, 75%, versus 44%, p &< 0.0001. No patients in the intervention cohort were given as needed (prn) pain medications when admitted for pain compared to 10% of the control cohort.
Standard nursing assessment of pain, using a 10-cm visual analog scale, the Wong Baker FACES scale9, or the modified Children’s Hospital of Eastern Ontario Pain Scale (mCHEOPS) depending upon patient age10, demonstrated a greater change in pain scores between admission and discharge for patients treated following the pre-printed admission orders. For the intervention and control cohorts, the average change in pain score upon discharge was 5.3 and 6.4, respectively, (p= 0.02, 95% CI −2.1 to −0.15). Correspondingly, the discharge pain scores were lower in the intervention cohort when compared to the control cohort, 1.9 and 3.3, respectively (p=0.003, 95% CI 0.3–1.5). Admission pain scores were not different between the intervention and control cohorts (8.6 vs 8.3, p=0.3, 95% CI −0.2–0.6).
Risk factors for readmission in the retrospective analysis were decreased during the intervention period. The number of patients who received a discharge appointment was higher after the intervention (80% vs 54%, p&<0.001); and hematology-oncology follow-up improved from 37% to 69%; p &<0.001. In addition, after the intervention, patients were more likely to receive scheduled pain medications at discharge (72% vs 42%, p&<0.0001).
Characteristics of Opioid Administration
Variations in pain management strategy before and after the intervention are described in Table II. The use of standardized orders and education did not influence physician decision to wean opioids within the first 24 hours, duration of oral analgesics before discharge or opioid type administered. The prevalence of opioid-induced adverse events in the intervention and control cohort is described in Table III. Despite the increase in PCA-use, no difference was found in the adverse event rate between groups. Specifically, there was no increase in the rate of dose reduction for excessive sedation, decreased oxygen saturations, respiratory depression or the proportion of patients that received naloxone administration, had nausea or pruritus.
Table II.
Characteristics of Hospital Admissions and Pain Management Strategies Between the Intervention and Control Cohort
| Intervention Cohort n=89 |
Control Cohort n=85 |
p-value | |
|---|---|---|---|
| Primary Discharge Diagnosis | |||
| SCD-Painful Episode | 78 (88%) | 66 (78%) | 0.11 |
| Acute Chest Syndrome | 11 (12%) | 19 (22%) | 0.11 |
| Pain Management | |||
| Duration of IV Pain Meds (days) | 4.5 | 3.6 | 0.35 |
| Ibuprofen Prescribed | 86 (97%) | 68 (80%) | 0.002 |
| Type of Opioid | |||
| Morphine | 73 (82%) | 75 (88%) | 0.29 |
| Hydromorphone | 3 | 6 | 0.32 |
| Fentanyl | 8 | 3 | 0.21 |
| Weaning of Pain Medications | |||
| Wean in First 24 Hours | 26 (29%) | 26 (31%) | 0.87 |
| Average Time Before Wean (days) | 2.4 | 1.8 | 0.39 |
| Duration of Oral Meds Prior to Discharge | |||
| Less than 24 hours | 52 (58%) | 47 (55%) | 0.76 |
| Readmission Visits | 10 | 24 | 0.007 |
| Time to Readmission Visit (days) | 16 | 9.4 | 0.62 |
| Readmission Diagnosis | |||
| SCD-Painful Episode | 6 (60%) | 23 (96%) | 0.02 |
| Asthma | 0 | 1 | 0.49 |
| Fever | 2 | 0 | 0.50 |
Table III.
Opioid-Induced Adverse Events in the First Six-Months of the Intervention Compared to the Same Seasonal Time Period in the Previous Year
| Adverse Event | Intervention Cohort n=89 |
Control Cohort n=85 |
p-value |
|---|---|---|---|
| Dose reduction for: | |||
| Excessive Sedation | 3 (3%) | 5 (6%) | 0.50 |
| Decreased Oxygen | 2 (2%) | 0 | 0.50 |
| Saturations | 2 (3%) | 0 | 0.50 |
| Respiratory Depression | |||
| Naloxone Administration | 1 | 0 | 1 |
| Nausea | 25 (28%) | 20 (24%) | 0.60 |
| Pruritis | 58 (56%) | 58 (68%) | 0.75 |
Discussion
NACHRI11 recommended that hospital readmission within 30-days of discharge as the only quality measure to assess quality of medical for children with sickle cell disease. This edict is now part of the CMS Quality Care Compendium. Medicare, Medicaid and many other insurance and accreditation agencies, now use this measure in their comprehensive guide of medical care quality measures1. No published evidence exist linking 30-day hospital readmission to improved patient care in SCD. Whether or not readmission is an important outcome for patients, caregivers and healthcare professionals has not been established. In this study, we determined if a multi-model intervention targeting pre-specified risk factors would improve readmission rate and other potential disease-specific quality care measures we identified.
The multi-modal intervention, in our institution, we feel, succeeded in improving the care of children admitted with SCD-pain, not solely by improving the 30-day readmission rate, but also by improving adherence to recommended treatment guidelines for SCD-pain such as PCA-use and increasing pain relief during hospitalization. The methodology employed in our study showed a relevant improvement in other potential quality measures for children with SCD-pain.
Education was a key component of the multi-modal intervention, for healthcare providers, patients and caregivers. Evidence to support the critical component of education was based on the observation that cessation of the educational sessions resulted in a return to the readmission rate prior to the intervention. The most significant impact of the intervention appeared to be within the first three months after starting the educational sessions, and thereafter had limited impact. Healthcare providers in our institution, pediatric house staff physicians, were encouraged to attend a monthly, 30-minute in-service regarding the management of SCD-pain in children and how to use the pre-printed orders. A physician with expertise in SCD led the educational session for all pediatric house staff physicians directly assigned to the inpatient floors where children with SCD were admitted. In parallel, an inpatient nurse with expertise in SCD, hired to educate caregivers and patients about their medical care, met with each patient and caregiver during hospitalization and prior to discharge. During these sessions, the nurse educator reinforced a personalized pain action plan for use in future episodes and educated the families and patients about what to expect during their hospitalization. Pre-printed orders alone were ineffective at sustaining the reduction in readmission rate.
Previous research has found a single intervention does not increase the utilization of clinical guideline4, 6. Two retrospective studies evaluating the use of clinical practice guidelines to improve the care of patients with SCD reported some improvement in care but with low utilization rates. In Co et al. less than half of all eligible admissions were associated with pathway utilization. Improvement in pathway utilization occurred only when a second intervention was added. After a healthcare advocate began actively promoting the pathway, peak pathway utilization increased to more than 80%12. Recently, Morrissey et al. demonstrated a clinical practice guideline and standardized orders improved the use of pain scales, analgesic dosing, time to first analgesia and initiation of PCA in the emergency department (ED) when used. However, only 53% of eligible admissions were managed following the clinical practice guidelines13. These data further support the importance of multi-modal interventions and underscore the necessity of coupling education with implementation of standardized order sets.
As with all studies of this nature, there were limitations. Several patients (n=8) had multiple admissions for SCD-pain during the study period. The inclusion of these patients into the analysis could potentially bias the results toward a higher readmission rate by giving one patient too much influence. However, we elected to include all admissions to increase generalizability. Our multi-modal intervention program may be considered too specific for our local site. Therefore, we chose modifiable risk factors with easily implemented interventions that may be implemented in any children’s hospital with health personnel that have expertise in SCD. Regularly scheduled education of the house staff physicians can be done by a nurse practitioner or attending physician and education of the family can be done by a nurse with expertise in SCD. However, the apparent need to continue intensive education for an indefinite period of time will likely be a significant barrier to implementation in smaller institutions.
The use of a multi-modal intervention combining education and evidence-based standardized admission orders successfully reduced the 30-day readmission rate for children with SCD hospitalized for pain. This strategy also increased physician adherence to recommended treatment guidelines. The multi-modal intervention was far less successful when direct provider education was eliminated.
Supplementary Material
Acknowledgments
Melissa Frei-Jones, MD participated in the following NIH funded programs at Washington University School of Medicine.
UL1RR024992 - (09/17/2007 to 06/30/2008) Polonsky (PI)
TL1RR024995 - (09/17/2007 to 06/30/2008)
K30 RR022251 - (07/01/2006- 09/16/2007) Evanoff (PI)
NIH/NCRR
Washington University Institute of Clinical and Translational Sciences (PI: Polonsky)
Research Education, Training and Career Development (Co-PI: Fraser)
The Clinical and Translational Science Award provides support to establish the WU Institute of Clinical and Translational Sciences (ICTS). By implementing 15 key Program Functions, the ICTS operationally reinvents clinical and translational research and clinical research training at WU and its regional partners. The Education sub-project of the ICTS integrates and enhances existing training programs, develops new clinical and translational courses, promotes multidisciplinary team training and provides improved tracking and evaluation for all clinical research training programs.
References
- 1.Quality Measures Compendium: Medicaid and SCHIP Quality Improvement: Centers for Medicare and Medicaid Services. 2007 [Google Scholar]
- 2.Quinn C. A question of quality in sickle cell disease. Pediatr Blood Cancer. 2009;00:1–2. doi: 10.1002/pbc.21904. [DOI] [PubMed] [Google Scholar]
- 3.Crossing the quality chasm: A new health system for the 21st century. Washington, DC: 2001. [PubMed] [Google Scholar]
- 4.Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA. 1995;274(9):700–705. doi: 10.1001/jama.274.9.700. [DOI] [PubMed] [Google Scholar]
- 5.Grimshaw J, Russell I. Effect of clinical guidelines on medical practice: A systematic review of rigorous evaluations., 00995355, 11/27/93, Vol., Issue 8883. Lancet. 1993;342:1317–1322. doi: 10.1016/0140-6736(93)92244-n. [DOI] [PubMed] [Google Scholar]
- 6.Moulding NT, Silagy CA, Weller DP. A framework for effective management of change in clinical practice: dissemination and implementation of clinical practice guidelines. Qual Health Care. 1999;8(3):177–183. doi: 10.1136/qshc.8.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frei-Jones MJ, Field JJ, DeBaun MR. Risk factors for hospital readmission within 30 days: A new quality measure for children with sickle cell disease. Pediatr Blood Cancer. 2008 doi: 10.1002/pbc.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 9.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14(1):9–17. [PubMed] [Google Scholar]
- 10.McGrath , Johnson G, Goodman J, Schillinger J, Dunn J, Chapman J. CHEOPS: a behavioral scale for rating post-operative pain in children. Adv Pain Research Therapy. 1985;9:395–402. [Google Scholar]
- 11.Schwalenstocker E. Telephone interview regarding quality measure: 30-day readmission rate in sickle cell anemia. 2007 [Google Scholar]
- 12.Co J, Johnson K, Duggan A, Casella J, Wilson M. Does a clinical pathway improve the quality of care for sickle cell anemia? Jt Comm J Qual Saf. 2003;29(4):181–190. doi: 10.1016/s1549-3741(03)29022-5. [DOI] [PubMed] [Google Scholar]
- 13.Morrissey LK, Shea JO, Kalish LA, Weiner DL, Branowicki , Heeney MM. Clinical practice guideline improves the treatment of sickle cell disease vasoocclusive pain. Pediatr Blood Cancer. 2009;52(3):369–372. doi: 10.1002/pbc.21847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


