Abstract
The centromere is a specialised chromosomal structure that regulates faithful chromosome segregation during cell division, as it dictates the site of assembly of the kinetochore, a critical structure that mediates binding of chromosomes to the spindle, monitors bipolar attachment and pulls chromosomes to the poles during anaphase. Identified more than a century ago as the primary constriction of condensed metaphase chromosomes, the centromere remained elusive to molecular characterisation for many years owed to its unusual enrichment in highly repetitive satellite DNA sequences, except in budding yeast. In the last decade, our understanding of centromere structure, organisation and function has increased tremendously. Nowadays, we know that centromere identity is determined epigenetically by the formation of a unique type of chromatin, which is characterised by the presence of the centromere-specific histone H3 variant CenH3, originally called CENP-A, which replaces canonical histone H3 at centromeres. CenH3-chromatin constitutes the physical and functional foundation for kinetochore assembly. This review explores recent studies addressing the structural and functional characterisation of CenH3-chromatin, its assembly and propagation during mitosis, and its contribution to kinetochore assembly.
Keywords: CenH3, CENP-A, centromere, chromatin
Introduction
The centromere is a highly differentiated chromosomal structure that fulfils multiple functions during cell division, governs kinetochore assembly and ensures equal chromosome segregation to daughter cells during mitosis and meiosis. Apart from its intrinsic biological interest, the analysis of the centromere is also relevant from a biomedical perspective, as alterations in centromere function lead to aneuplody (gain or loss of chromosomes) that most frequently results in lethality. Aneuploidy is also a common event in various diseases, both congenital and acquired, including tumour progression, infertility and birth defects. Centromere biology has been a very intense area of research in the past few years and, though still incomplete, a general picture of the centromere, its structure and regulation, is emerging.
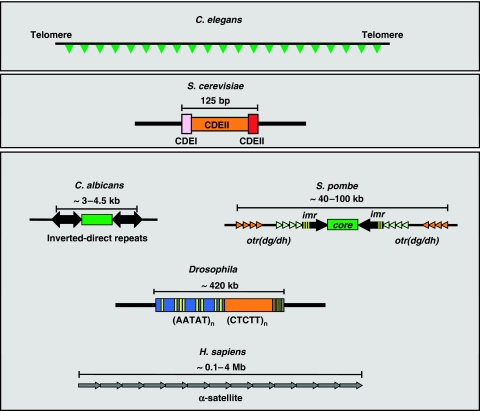
Eukaryotic centromeres come in quite different flavours (reviewed in Sullivan et al, 2001; Allshire and Karpen, 2008) (Figure 1). Monocentric eukaryotes contain ‘localised' centromeres, in which centromere formation is restricted to a specific chromosomal locus. On the other hand, in holocentric organisms such as the nematode Caenorhabditis elegans, a ‘diffuse' centromere forms along the entire chromosome. In addition, ‘localised' centromeres are highly variable in size and sequence, ranging from the simple ‘point' centromeres of the budding yeast Saccharomyces cerevisiae to the large ‘regional' centromeres found in the fission yeast Schizosaccharomyces pombe, the pathogenic fungus Candida albicans, Drosophila, plants and human cells. In the 16 chromosomes of S. cerevisiae, centromeric function resides in an essential 125 bp long CEN sequence that comprises three conserved functional elements (CDEI, II, III). However, this quite simple sequence-based organisation of S. cerevisiae centromeres is not conserved in most monocentric eukaryotes that, instead, contain large ‘regional' centromeres. In S. pombe, centromeres localise to 40–100 kb long regions consisting of non-homologous 4–5 kb central-core sequences flanked by repeated imr and otr(dg/dh) elements present in the three S. pombe chromosomes. Centromeres of C. albicans, though not embedded in long tracts of repetitive DNA, show a similar structural organisation, in which a core sequence is located near short inverted/direct repeats. In Drosophila, centromeres are also located in repeated DNA. Actually, the only Drosophila centromere characterised at the DNA level corresponds to a 420 kb long region composed of tandem arrays of short satellite DNA repeats interrupted by transposable elements. Similarly, in humans cells, centromeres consist of long α-satellite arrays extending for 0.1–4 Mb. Plant centromeres too are ‘regional' containing variable amounts of tandem arrays of satellite repeats and transposable elements.
Figure 1.
Structural organisation of the different classes of eukaryotic centromeres. In holocentric organisms (C. elegans), centromeres form along the entire chromosome. Most eukaryotes, however, contain monocentric chromosomes, in which the centromere forms at a single, generally large, chromosomal region (C. albicans, S. pombe, Drosophila, H. sapiens). In S. cerevisiae, centromeric function resides in a small 125 bp long conserved DNA sequence. See text for details.
Lack of conservation of centromeric DNAs suggests that DNA sequence is not the main determinant of centromere identity and function. Actually, centromeric DNAs are neither necessary nor sufficient to support centromere function. On one hand, in stably transmitted dicentric chromosomes, one of the two centromeres is functionally inactivated, indicating that the presence of centromeric DNA does not necessarily lead by itself to the formation of a functional centromere (Earnshaw and Migeon, 1985; Sullivan and Schwartz, 1995; Warburton et al, 1997; Agudo et al, 2000). Moreover, the formation of neocentromeres at non-centromeric sites shows that, under some circumstances, non-centromeric DNAs can also acquire centromere activity (Williams et al, 1998; Choo, 2001; Lo et al, 2001; Warburton, 2004). All these observations led to the proposition that, rather than by the DNA sequence itself, centromere identity and function is regulated epigenetically through the formation of a specialised chromatin structure.
As a matter of fact, centromere structure and organisation shows a high degree of conservation at the chromatin level, as centromeric chromatin shares a number of common features. In particular, all eukaryotic centromeres, from S. cerevisiae to humans, are characterised by the presence of the centromere-specific histone H3 variant, CenH3 (Earnshaw and Migeon, 1985; Palmer et al, 1991; Meluh et al, 1998; Buchwitz et al, 1999; Henikoff et al, 2000; Takahashi et al, 2000). In addition, most centromeres map to highly condensed heterochromatic regions. CenH3-containing chromatin and heterochromatin form structurally and functionally distinct domains. For instance, in S. pombe, the central-core region is characterised by the presence of the centromere-specific CenH3 variant, CenH3Cnp1. On the other hand, imr/otr-regions show the characteristic features of heterochromatin, enrichment in H3K9me2 and binding of HP1(Swi6). Boundary elements, encoded by tRNA genes, insulate inner CenH3-chromatin from flanking heterochromatin. More complex ‘regional' centromeres also seem to adopt a similar structural organisation (Sullivan and Karpen, 2004). These two distinct domains, which are both required for proper chromosome segregation and inheritance, mediate different functions. CenH3-chromatin is mainly responsible for kinetochore assembly whereas, on the other hand, the surrounding heterochromatin domain seems to have a determinant function in sister-chromatid cohesion. This review focuses on CenH3-chromatin. Aspects related to heterochromatin assembly and function, on the other hand, are out of the scope of this review, but are discussed in another review by Bühler and Gasser (2009), also contained within this focus series.
CenH3-chromatin: distinct composition, structure and organisation
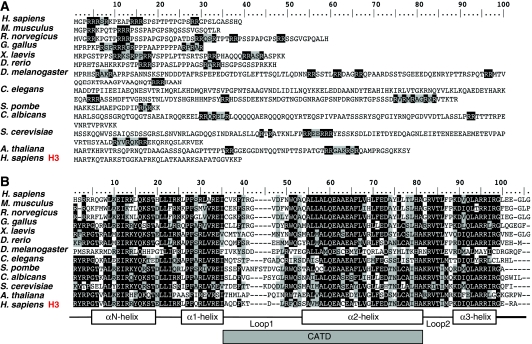
CenH3 is a highly divergent histone H3 variant (Figure 2). The most striking feature of CenH3 resides in the presence of a highly variable N-terminal domain that, ranging in size from 20 to 200 amino acids, shows essentially no sequence homology to the N-tail of histone H3 or across different eukaryotic lineages (Figure 2A). On the other hand, the C-terminal histone-fold domain (HFD) of CenH3, which shows significant homology to histone H3, shares only an average 48% identity across phylogeny (Figure 2B). All these observations indicate that CenH3 evolves very rapidly, which is in contrast to the high evolutionary conservation of canonical histone H3. Fast evolutionary rate of CenH3 is likely reflecting its functional specialisation. Histone H3 interacts with essentially all different classes of genomic DNA sequences and contributes to the regulation of multiple aspects of chromatin structure and function, resulting in strong evolutionary constraints. On the opposite, CenH3 is only required to interact with centromeric DNAs that, being amongst the most rapidly evolving DNA sequences in the genome, seem to drive adaptive evolution of CenH3 in both Drosophila and Arabidopsis (Malik and Henikoff, 2001; Talbert et al, 2002). In mammals, the essential centromere protein CENP-C also shows adaptively evolving domains that overlap with regions of DNA-binding activity though, in this case, there is no evidence for adaptive evolution of CenH3CENP-A (Talbert et al, 2004). Despite their differences, CenH3s from different species share some common structural features. In particular, a consistent feature of CenH3 is the presence of a 2–6 amino acids insertion in the first loop (L1) of the HFD, between helices α1 and α2. A second conserved feature is the presence of arginine (R)-rich motives at the N-tail, which results from a significant enrichment in R-residues in comparison to histone H3.
Figure 2.
CenH3 is a highly divergent histone H3 variant that evolves very rapidly. Sequence comparison of the N-terminal domain (A) and the histone-fold domain (HFD) (B) of CenH3 proteins from different species, ranging from S. cerevisiae to humans, is shown. The sequence of canonical histone H3 is shown at the bottom for comparison. R-rich motives are indicated in A. Secondary structure of the HFD is indicated in B. The position of the CATD, which mediates centromeric targeting of CenH3 and confers distinct structural properties to CenH3-nucleosomes, is indicated.
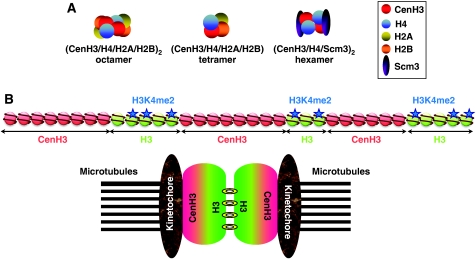
It is generally assumed that CenH3 incorporates into nucleosomes. The actual composition and structure of CenH3-containing nucleosomes is, however, a matter of debate (Figure 3A). In vitro reconstitution experiments showed that human CenH3CENP-A can replace histone H3 in nucleosomes that, otherwise, show a canonical histone composition and stoichiometry (Yoda et al, 2000). In addition, their pattern of DNase I digestion shows a characteristic 10 bp-repeat ladder, similar to what is observed when DNA wraps around a histone octamer. Affinity purification of CenH3-nucleosomes, both from human and fly cells, is also consistent with the formation of ‘canonical' (CenH3/H4/H2A/H2B)2 octamers (Blower et al, 2002; Foltz et al, 2006). Recent results, however, challenged this model. In S. cerevisiae, centromeres contain a single CenH3Cse4-nucleosome that seems to lack H2A/H2B dimers. Instead, CenH3Cse4-nucleosomes contain Scm3, a non-histone protein that is required to recruit CenH3Cse4 to centromeres and, in vitro, displaces H2A/H2B dimers from preassembled CenH3Cse4-containing octamers, suggesting that CenH3Cse4-nucleosomes are composed of (CenH3Cse4/H4/Scme3)2 hexamers (Camahort et al, 2007; Mizuguchi et al, 2007; Stoler et al, 2007). It is, however, uncertain whether this situation is general, as the presence of Scm3 seems restricted to fungi. Moreover, in S. pombe, Scm3 localises at centromeres independently of CenH3Cnp1 and dissociates during mitosis (Pidoux et al, 2009; Williams et al, 2009). On the other hand, on the basis of intranucleosomal cross-linking experiments and atomic-force microscopy measurements, a very provocative model was proposed suggesting that, in Drosophila, CenH3CID-nucleosomes exist as (CenH3CID/H4/H2A/H2B) tetramers, or ‘half-nucleosomes', rather than as octamers (Dalal et al, 2007a, 2007b). It must be noticed, however, that formation of such heterotypic tetramers is, in general, less favourable than the formation of octamers. It is possible that these alternative models reflect behaviour of CenH3-nucleosomes during dynamic assembly/disassembly processes (see below).
Figure 3.
Structural organisation of CenH3-chromatin. (A) CenH3-nucleosomes can be composed by (CenH3/H4/H2A/H2B)2 octamers as are canonical nucleosomes. Alternatively, in Drosophila, the formation of ‘half-nucleosomes', composed by unusual (CenH3/H4/H2A/H2B) tetramers, has been proposed, and, in S. cerevisiae, it was reported that H2A/H2B dimers are replaced by Scm3 to form unusual (CenH3/H4/Scm3)2 hexamers. (B) In centromeric chromatin, blocks of CenH3-nucleosomes are found interspersed with blocks containing canonical histone H3-nucleosomes. H3-blocks show a peculiar pattern of post-translational histone modifications being hypoacetylated and, at the same time, enriched in H3K4me2. During mitosis, these blocks can direct folding of centromeric chromatin into a specific higher-order structure, in which H3-blocks locate in the interface between sister chromatids and CenH3-blocks face out towards the kinetochore, an arrangement that could facilitate bipolar attachment.
Large sequence deviation from histone H3 strongly suggests that, whether ‘canonical' or not, CenH3-containing nucleosomes have differential structural properties. Deuterium exchange experiments indicate that reconstituted (CenH3CENP-A/H4)2 tetramers are more compact and rigid than (H3/H4)2 tetramers (Black et al, 2004, 2007). These differential structural properties seem to be functionally relevant as they depend on region L1/α2 of CenH3CENP-A, which accumulates many amino acid changes with respect to histone H3 and mediates targeting of CenH3 to centromeres, both in humans and Drosophila (Vermaak et al, 2002; Black et al, 2004). L1/α2 region locates at the predicted interface with histone H4, indicating an important functional contribution of CenH3/H4 interaction. Additional support for this hypothesis comes from genetic analyses in S. cerevisiae, as CenH3Cse4 was identified as a suppressor of the mitotic defects associated to a specific histone H4 mutation (Smith et al, 1996) and, in addition, three out of four identified temperature-sensitive CenH3Cse4 mutations map to the predicted CenH3Cse4/H4 interface, the fourth mapping to the CenH3Cse4/CenH3Cse4 interface (Glowczewski et al, 2000).
CenH3-chromatin adopts a peculiar organisation, in which blocks of CenH3-nucleosomes are found interspersed with blocks containing canonical histone H3-nucleosomes (Blower et al, 2002) (Figure 3B). These blocks, which vary in size from 200 to 500 kb in flies to 500–1500 kb in humans, seem to mediate folding into a specific higher-order structure so that, in mitotic chromosomes, H3-blocks locate in the interface between sister chromatids whereas CenH3-blocks are facing out, towards the kinetochore. This arrangement could facilitate bipolar attachment during mitosis by ensuring that kinetochores form on opposite sites of sister chromatids. Complex regional centromeres of rice, flies, mouse and human adopt this structural organisation. It is, however, uncertain whether the more simple regional centromeres of S. pombe and C. albicans, which contain a single CenH3-block, or the point centromeres of S. cerevisiae, which are formed by a single CenH3-nucleosome, also conform to this model.
Centromeric deposition of CenH3: being in the right place at the right moment
CenH3 exclusively localises to centromeres. Our understanding about how CenH3-nucleosomes are targeted to and assembled at centromeres, though still incomplete, is increasing very rapidly. Differential structural properties of CenH3-nucleosomes seem to have an important contribution to targeting, as centromeric localisation of CenH3 depends on L1/α2 region that, as discussed above, confers distinct structural properties to CenH3-nucleosomes (Figure 2B). Swapping experiments showed that this region, called CATD for CENP-A targeting domain, is necessary and sufficient for centromeric localisation of human CenH3CENP-A (Black et al, 2004), and similar domains have been identified as required for centromeric localisation of CenH3 in both Drosophila and S. cerevisiae (Keith et al, 1999; Vermaak et al, 2002; Black et al, 2004). How does CATD determine centromeric localisation of CenH3? It is possible that CATD mediates interaction with specific CenH3 recruiting and/or chromatin assembly complexes (see below), which could directly bind to CATD or, alternatively, recognise the distinct structural features of CenH3-nucleosomes. It is also possible that CATD influences stability, and/or specificity, of the interaction with DNA, as loop L1 in histone H3 forms a DNA-binding domain together with loop L2 in histone H4. Swapping experiments performed with CenH3CID from distant Drosophila species are consistent with this hypothesis, as centromeric localisation of CenH3CID from Drosophila bipectinata, which does not target centromeres in Drosophila melanogaster, is restored when L1 of D. bipectinata is replaced by that of D. melanogaster (Vermaak et al, 2002). At this respect, it must be noticed that CenH3 can go to essentially any site on the genome, as shown by transient-expression experiments in different organisms (Van Hooser et al, 2001; Heun et al, 2006; Moreno-Moreno et al, 2006). However, when bound to non-centromeric DNA, CenH3-nucleosomes seem to be more unstable (Conde e Silva et al, 2007), so that they are evicted and rapidly degraded by proteolysis (Moreno-Moreno et al, 2006). On the contrary, CenH3-nucleosomes are tightly bound at centromeres, showing an extremely low turnover (Hemmerich et al, 2008). In addition, though neocentromeres can form over different types of DNA sequences, having no similitude to centromeric DNAs, some chromosomal regions seem to be more prone to form neocentromeres in humans (Marshall et al, 2008).
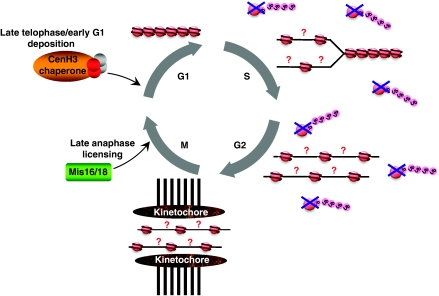
Contrary to canonical histones, which are deposited during DNA replication, CenH3 incorporates into chromatin independently of DNA replication, similar to other histone variants (Shelby et al, 2000; Ahmad and Henikoff, 2001) (Figure 4). In human cells, CenH3CENP-A levels peak in G2 (Shelby et al, 1997, 2000), and deposition of new CenH3CENP-A occurs during mitosis, at late telophase, and early G1 (Jansen et al, 2007; Hemmerich et al, 2008). In syncytial Drosophila embryos, in which no G1/G2-phases are observed, CenH3CID deposition also takes place during mitosis, at anaphase (Schuh et al, 2007). Yeasts constitute an exception to this rule as, in S. cerevisiae, all pre-existing CenH3Cse4 is evicted from centromeres and replaced by newly synthesised CenH3Cse4 during S-phase (Pearson et al, 2004) whereas, in S. pombe, in which G1-phase is exceedingly short, CenH3Cnp1 deposition takes place in two phases, during S and in late G2 (Takayama et al, 2008). In Arabidopsis, it was reported that loading of CenH3 also occurs mainly in G2 (Lermontova et al, 2006). As a consequence of its loading outside of S-phase, CenH3 concentration at centromeres is diluted during DNA replication, generating ‘gaps' that could remain nucleosome-free, ready for CenH3 deposition during mitosis, or be filled by replicative H3-nucleosomes that, later, would be replaced by CenH3-nucleosomes (Figure 4). It might also be possible that, during DNA replication, CenH3-nucleosomes are disassembled into heterotypic tetramers or ‘half-nucleosomes'.
Figure 4.
Assembly and dynamic behaviour of CenH3-chromatin during cell cycle. Like other histone variants, CenH3 incorporates into chromatin independently of DNA replication. Deposition of newly synthesised CenH3 takes place during mitosis, at late telophase, or early G1. Specific CenH3 chaperones localise to the centromere coincidentally with deposition of new CenH3 and mediate assembly of CenH3-nucleosomes. During assembly, CenH3 might become resistant to proteolysis that, otherwise, degrades CenH3 and prevents deposition at non-centromeric sites. Before deposition, at late anaphase, specific complexes (Mis16/Mis18) seem to modify centromeric chromatin to allow assembly of new CenH3-nucleosomes. During DNA replication at S-phase, CenH3 concentration at centromeres is diluted and kinetochore assembly takes place before replenishment with new CenH3-nucleosomes. It is unclear whether ‘gaps' generated during DNA replication remain nucleosome-free or are filled by replicative H3-nucleosomes. It might also be possible that CenH3-nucleosomes are disassembled into ‘half-nucleosomes' to compensate for this deficit.
Loading of CenH3 late in mitosis raises some intriguing questions. First, it means that kinetochore assembly actually takes place before centromeres are fully replenished with CenH3-nucleosomes. Second, it also means that loading occurs in close coincidence to chromosome segregation, suggesting that signalling events occurring during segregation might actually trigger CenH3 deposition. And third, it raises the question of how CenH3-assembly could take place during mitosis, when chromatin is believed to be more inaccessible and, in general, refractory to transactions. At this respect, work carried out in S. pombe identified a number of factors and complexes that are required for centromeric localisation of CenH3Cnp1 (Table I). Amongst those, Mis16/Mis18 complex is required to maintain histone acetylation status at the central-centromere region, indicating that it has a central function in modifying centromeric chromatin (Hayashi et al, 2004) (Figure 4). Mis16 is the S. pombe homologue of RbAp46/48, a general histone H3/H4 chaperone that forms part of several chromatin assembly, remodelling and modifying complexes. On the other hand, Mis18 is widely conserved in eukaryotes. In humans, hMis18 also cooperates with RbAp46/48 and localises to centromeres only at late telophase/early G1, when newly synthesised CenH3 is deposited (Fujita et al, 2007). Similarly, in S. pombe, Mis16/Mis18 complex dissociates from centromeres from early mitosis until anaphase, when it again localises to centromeres. Mis16/Mis18 complex, however, does not seem to directly interact with CenH3. These observations suggest that, rather than directly recruiting CenH3 to centromeres, Mis16/Mis18 complex is involved in modifying centromeric chromatin to allow deposition of new CenH3. Consistent with this hypothesis, treatment with the HDAC-inhibitor TSA restores centromeric localisation of CenH3CENP-A in the absence of hMis18 (Fujita et al, 2007). Additional factors affecting CenH3 localisation might also be involved in licensing centromeric chromatin for deposition of new CenH3.
Table 1.
Factors required for centromeric localisation of CenH3
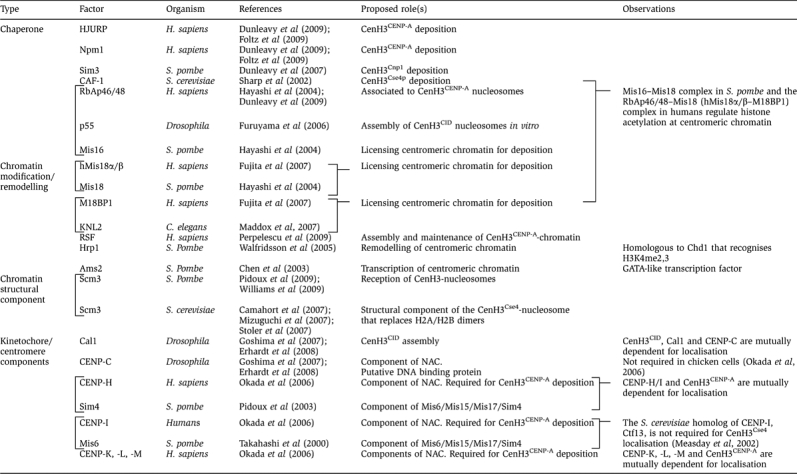 |
Recent studies identified chromatin-assembly complexes that mediate incorporation of prenucleosomal CenH3 to centromeres in human cells (Dunleavy et al, 2009; Foltz et al, 2009) (Figure 4). These complexes contain a unique CenH3CENP-A histone chaperone, HJURP (Holliday junction-recognising protein), which directly interacts with CenH3CENP-A and localises to centromeres coincidentally with deposition of newly synthesised CenH3CENP-A. Interaction with HJURP is mediated through the CATD (Foltz et al, 2009) and, most interesting, this interaction seems to prevent proteolytic degradation of CenH3CENP-A as removal of HJURP results, after a few cell divisions, in a strong reduction in CenH3CENP-A levels (Dunleavy et al, 2009; Foltz et al, 2009). It must be noted that, both in S. cerevisiae and Drosophila, proteolytic degradation tightly regulates CenH3 expression to ensure its centromere-only deposition (Collins et al, 2004; Moreno-Moreno et al, 2006). It is possible that CenH3 proteolysis is also cell-cycle regulated, so that CenH3 becomes resistant to degradation only when it gets incorporated into specific chromatin-assembly complexes. Prenucleosomal CenH3 can also interact with the general histone chaperone RbAp46/48, which is part of CAF-1 and interacts with histone H4 (Furuyama et al, 2006; Dunleavy et al, 2009), and Npm1 (Nucleophosmin 1), which acts as a chaperone for both H3:H4 and H2A:H2B (Dunleavy et al, 2009; Foltz et al, 2009). Interestingly, in Drosophila, Npm1 acts as an ATP-dependent chromatin remodelling complex (Ito et al, 1996), suggesting a link to nucleosome remodelling/exchange. Recently, the contribution of the chromatin remodelling complex RSF to assembly of CenH3CENP-A-chromatin in human cells was reported (Perpelescu et al, 2009). In addition, in S. pombe, CenH3Cnp1 was found to physically interact with Sim3, a NASP-related histone chaperone that was proposed to escort CenH3Cnp1 to centromeres (Dunleavy et al, 2007). At present, little is known about how CenH3-assembly complexes are recruited to centromeres. They could directly recognise pre-existing CenH3-nucleosomes, specific histone modifications or, alternatively, be recruited through factors that would act as receptor complexes for incoming CenH3. In S. pombe, Scm3 was proposed to play such a role, receiving at the centromere incoming CenH3Cnp1 from the Sim3 escort chaperone. Scm3 interacts with CenH3Cnp1 and is required for its centromeric localisation but not vice versa (Pidoux et al, 2009; Williams et al, 2009). Interestingly, Mis16/Mis18 complex seems to directly recruit Scm3 to centromeres (Pidoux et al, 2009; Williams et al, 2009), suggesting that, in addition to regulate histone acetylation at centromeric chromatin, Mis16/Mis18 complex might also be involved in recruitment of receptor complexes.
Additional factors are known to be required for centromeric localisation of CenH3, although their precise role in assembly of centromeric chromatin is not fully understood (Table I). These include, some inner kinetochore components (i.e. CENP-C, -H/Sim4, -I/Mis6, -K, -L and -M) that, in turn, are dependent on CenH3 for centromere localisation (Takahashi et al, 2000; Pidoux et al, 2003; Okada et al, 2006; Goshima et al, 2007; Erhardt et al, 2008). In Drosophila, CAL1 also shows reciprocal dependency with CenH3CID and, in addition, with CENP-C (Goshima et al, 2007; Erhardt et al, 2008). Mutual dependency for centromere localisation between CenH3 and inner kinetochore components indicate the existence of cross-talk mechanisms acting at the initial stages of assembly.
Factors and mechanisms discussed above provide a general picture of how centromere identity is propagated during mitosis. Centromeres, however, are also formed de novo in the absence of a pre-existing centromere. The formation of neocentromeres, as well as changes in centromere localisation that occur during evolution, are examples of de novo centromere formation (Choo, 2001; Warburton, 2004). De novo establishment of centromeres has also been observed in fungi, plants and mammals, when appropriate DNAs carrying centromeric DNA sequences are transfected into cells (Clarke and Carbon, 1980; Hahnenberger et al, 1989; Harrington et al, 1997; Carlson et al, 2007). Recent studies addressed the determination of the requirements for de novo centromere formation in S. pombe, in which centromere formation on naked DNA templates occurs efficiently only in the presence of heterochromatin (Folco et al, 2008). Notice that, in most organisms, centromeres are embedded into large heterochromatic regions. Heterochromatin, however, is not required for centromere maintenance, as mutations disrupting heterochromatin do not impair mitotic propagation of pre-existing centromeres. On the other hand, in mammals, the CENP-B protein also seems to contribute to de novo centromere formation (Okada et al, 2007). Again, CENP-B is dispensable for centromere maintenance.
CenH3-chromatin and kinetochore assembly: a ‘complex' affair
CenH3 is essential for centromere function. In all eukaryotes analysed to date, deletion of CenH3 is lethal, both at the cell and organism level (Stoler et al, 1995; Howman et al, 2000; Blower and Karpen, 2001; Oegema et al, 2001; Régnier et al, 2005). Cells deficient in CenH3 fail to localise kinetochore proteins and show strong chromosome segregation defects, indicating that CenH3 is necessary for kinetochore formation. Is it also sufficient? Over-expression experiments performed both in flies and human cells showed that CenH3 misincorporates throughout chromatin (Van Hooser et al, 2001; Heun et al, 2006; Moreno-Moreno et al, 2006) and, although most of it is rapidly removed by proteolytic degradation (Moreno-Moreno et al, 2006), a small fraction remains stably associated to a few non-centromeric sites resulting in recruitment of kinetochore proteins at these ectopic sites and, concomitantly, cells showed delayed mitosis, chromosome segregation defects, aneuploidy and growth defects. However, in mammals, only a subset of kinetochore proteins are detected at these ectopic sites, with some essential kinetochore proteins being excluded (Van Hooser et al, 2001). Moreover, in flies, not all ectopic sites seem to be equally efficient in recruiting kinetochore proteins and, moreover, spindle attachments could be detected only in a small subset (Heun et al, 2006). It is likely that not all ectopic sites contain enough CenH3, or in the appropriate organisation, to support formation of a fully functional kinetochore. Alternatively, it is also possible that, independent of CenH3, additional factors have an essential contribution to kinetochore formation. At this respect, it was proposed the existence of a second pathway that, depending on the conserved Mis12 protein, contributes to kinetochore assembly (Goshima et al, 1999, 2003). In S. pombe, Mis12 is required for equal chromosome segregation, localises to centromeres throughout the cell cycle and, moreover, its centromeric localisation is independent of CenH3Cnp1 and vice versa (Takahashi et al, 2000). A similar situation is observed in human cells, in which depletion of hMis12 results in loss of some kinetochore proteins without affecting centromeric localisation of CenH3CENP-A though, in this case, depletion of CenH3CENP-A seems to partially affect centromeric localisation of hMis12 (Liu et al, 2006). As a matter of fact, both in C. elegans and Drosophila, Mis12 localisation depends on CenH3 (Cheeseman et al, 2004; Goshima et al, 2007; Przewloka et al, 2007).
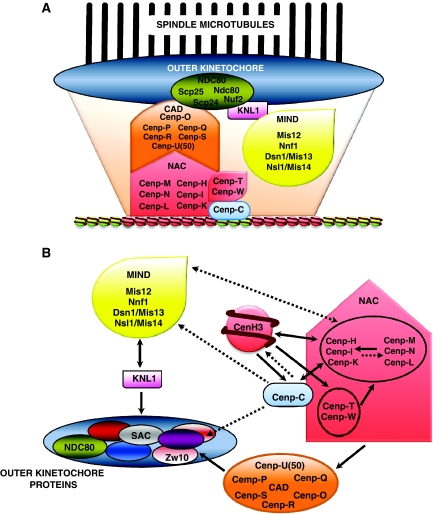
Kinetochore assembly at the centromere involves complex pathways of hierarchical, sometimes reciprocal, interactions (Figure 5). CenH3 is at the bottom of such network of interactions. At present, we are only beginning to understand its actual contribution to kinetochore assembly. Kinetochores are large macromolecular entities that, depending on the organisms, are composed by dozens to more than a hundred different protein components (for a comprehensive overview, see the accompanying focus review by Santaguida and Musacchio, 2009). Kinetochore architecture is well understood only in S. cerevisae, in which binding of a single microtubule requires co-operation of at least six different protein complexes (Mif2, COMA, Spc150, MIND, NDC80 and Dam-DASH), resulting in more than 500 protein molecules participating in formation of the relatively simple S. cerevisiae kinetochore, as deduced from quantitative fluorescence microscopy analyses (Joglekar et al, 2006). In the rest of eukaryotes, kinetochore composition remains poorly understood. Morphologically, electron microscopic studies show the kinetochore as a trilaminar structure consisting of an inner-plate, which is in direct contact with centromeric chromatin, and the central- and outer-plates that mediate spindle attachment. Biochemical studies have identified a number of protein complexes/networks that act at different stages of assembly, some being constitutively associated to the centromere throughout the cell cycle, whereas others localise to the kinetochore only transiently during mitosis (Figure 5). Identified complexes include the KNM network (KNL1, NDC80 and MIND), which is involved in microtubule binding, and, in particular, the NAC/CAD network that directly associates to centromeric chromatin (Foltz et al, 2006; Okada et al, 2006). In addition, a third protein network that regulates chromosome movement, Dam1/DASH, has been identified both in S. cerevisiae and S. pombe. Components of the NAC/CAD network are good candidates to be directly recruited by CenH3-chromatin. As a matter of fact, in human cells, NAC (nucleosome associated complex) was isolated on the basis of its co-purification with CenH3CENP-A-nucleosomes (Foltz et al, 2006). In the same study, CAD (CenH3CENP-A distal complex) was purified using NAC components as baits. CAD components do not seem to directly associate to CenH3CENP-A-nucleosomes and, moreover, centromeric localisation of some CAD components seems to depend on NAC. NAC/CAD are essential for stabilising microtubule attachments but not for recruitment of some SAC components (Foltz et al, 2006). Hierarchical NAC/CAD interactions are, however, complex as NAC components are also required for centromeric localisation of CenH3CENP-A (Okada et al, 2006) and NAC/CAD interactions might be cell-cycle regulated (Kwon et al, 2007). In addition, some NAC components (CENP-T/W) might directly bind DNA, as they contain HFDs that mediate DNA-binding in vitro (Hori et al, 2008). In chicken cells, CENP-T/W seem to preferentially associate to centromeric regions containing canonical histone H3, though their centromeric localisation depends on CenH3CENP-A. Most interestingly, CENP-T/W do not seem to directly influence centromeric localisation of CenH3CENP-A. It must be noticed that CENP-C is also a putative DNA-binding protein (Yang et al, 1996).
Figure 5.
Kinetochores are large macromolecular entities. (A) Various protein complexes/networks are known to act at different stages of kinetochore assembly. These include the KNM network (KNL1, NDC80 and MIND), which is involved in microtubule binding, and the NAC/CAD network that directly associates to centromeric chromatin. (B) CenH3 is essential for kinetochore assembly. CenH3 is at the bottom of a complex network of interactions that, ultimately, leads to assembly of a fully functional kinetochore. Dependencies for centromeric/kinetochore localisation are indicated by solid arrows. Possible interactions, observed only in some species or not fully confirmed, are indicated by dotted arrows.
The molecular basis of the association of NAC/CAD with CenH3-nucleosomes is, however, not known. Which are the components that directly bind CenH3-nucleosomes? What features of CenH3-nucleosomes they recognise? As mentioned above, some NAC components might bind DNA whereas others could recognise CenH3-nucleosomes on the basis on their distinct structural features or directly bind CenH3. Support for this hypothesis comes from studies performed in S. cerevisiae, in which deletion of the N-domain of CenH3Cse4 causes lethality (Keith et al, 1999; Chen et al, 2000), which is in contrast to deletion of the N-domain of canonical histone H3 that is viable. Mutation analyses identified within the N-domain of CenH3Cse4 a 33 amino acids motif (END) that, being essential for viability, seems to interact with COMA, a kinetochore complex that is functionally related to NAC/CAD and mediates protein–protein interactions with other kinetochore/centromere proteins, including the essential CBF3 complex. Interestingly, END corresponds to an R-rich motif, whose presence at the N-domain is conserved in CenH3s from distant species (Figure 2). It is possible that, also in other organisms, N-domain is involved in interactions with NAC/CAD and/or other kinetochore proteins.
Concluding remarks
In the last few years, we have learned much about the components and mechanisms that determine centromere identity and function. There are, however, several aspects that remain poorly understood. A major challenge for the future is to describe molecularly the pathway(s) leading from centromeric chromatin to assembly of a fully functional kinetochore. To a large extent, the kinetochore is a puzzle that, composed by hundreds of pieces, needs to be pieced together by sorting out individual protein–protein interactions. For this purpose, extensive information needs to be obtained about kinetochore composition and organisation in various organisms by combining both molecular and imaging technologies.
To what extent the structural organisation of CenH3-chromatin changes during cell cycle is another question that needs to be addressed. As mentioned above, centromeric chromatin is actually deficient on CenH3 during a significant part of the cell cycle, from replication of centromeric DNA to deposition of new CenH3. Is this deficit compensated through the deposition of H3-nucleosomes, the formation of ‘half-nucleosomes' or, simply, by spacing CenH3-nucleosomes? At this respect, it must be noticed that, in contrast to the regular nucleosomal ladder observed in bulk chromatin, CenH3-chromatin shows a smeared pattern of microccocal nuclease digestion both in S. pombe and C. albicans, suggesting a more irregular nucleosomal spacing (Polizzi and Clarke, 1991; Takahashi et al, 1992; Baum et al, 2006).
It is anticipated that histone modifications would have important functions in regulating centromere biology, as histones are extensively modified post-translationally and covalent histone modifications have an essential contribution to the regulation of chromatin functions, they correlate with different functional states and are involved in chromatin assembly/disassembly processes. Little is known, however, about the actual pattern of post-translational modifications of CenH3-chromatin. Is CenH3 subjected to post-translational modifications? Are they regulated during cell cycle? How? Are they involved in regulating CenH3 deposition, kinetochore assembly or other aspects of centromere/kinetochore function? At this respect, it was reported that human CenH3CENP-A is phosphorylated at residue S7 in a manner dependent on both Aurora-A and -B (Zeitlin et al, 2001; Kunitoku et al, 2003). Phosphorylation of human CenH3CENP-AS7 is required for normal progression through mitosis and, unexpectedly, cytokinesis. Expression of non-phosphorylatable mutants disrupts localisation of the chromosomal passenger complex, leads to chromosome misalignment during mitosis and delays cell separation during cytokinesis. Although this residue is not conserved, most CenH3s contain S-residues at the N-domain that, being in a similar sequence context, could be susceptible to phosphorylation by Aurora-A/B.
Investigation of the precise relationship between centromeric chromatin and transcription, and non-coding RNAs, is likely to become an area of intense research in the future. Though centromeres correspond to essentially inactive regions, recent results indicate that centromeric DNAs might actually be transcribed, as a human neocentromere was shown to contain active genes (Saffery et al, 2003) and non-coding RNAs homologous to centromeric DNAs have been detected in mammals and plants (May et al, 2005; Bouzinba-Segard et al, 2006). Moreover, FACT (facilitates chromatin transcription) complex, which is required for efficient transcription through chromatin, is found associated to human CenH3CENP-A-nucleosomes (Foltz et al, 2006), and centromeric H3-blocks, which are found interspersed with CenH3 domains, show a distinct pattern of histone modifications, being enriched in H3K4me2 (Sullivan and Karpen, 2004), a modification normally associated with transcriptionally active chromatin domains. As a matter of fact, several observations indicate that transcription of centromeric chromatin might have a function in assembly of CenH3-chromatin. In S. pombe, centromeric deposition of CenH3Cnp1 is facilitated by Ams2, a GATA-like transcription factor (Chen et al, 2003), and Hrp1 (Walfridsson et al, 2005), which corresponds to the fission yeast homologue of Chd1, a chromatin remodelling factor that binds H3K4me2,3. It is also possible that non-coding RNAs themselves, centromeric or not, might physically associate with centromeric chromatin and regulate its structural and functional properties. In fact, early ultrastructural studies suggested the presence of RNA at the kinetochore (Rieder, 1979) and centromere-encoded RNAs have been shown to be integral components of the maize centromere/kinetochore complex (Topp et al, 2004).
Finally, it is striking that, despite its highly conserved cellular function, centromeres evolve so rapidly. Not only centromere components are diverse, but also kinetochore proteins seem to evolve adaptively as, most frequently, identifiable orthologs show no significant sequence similarity except for short regions. This high degree of structural diversity likely reflects a contribution to speciation. Epigenetics might facilitate fast evolution of centromeres, as epigenetic regulation permits to bypass strict sequence constraints. The formation of neocentromeres supports this view. The ability to form neocentromeres is conserved through evolution, from fungi to humans, and contributes to karyotype evolution and speciation both in vertebrates and plants. As a matter of fact, recent results indicate that neocentromere formation might be more frequent than previously thought as, in C. albicans, neocentromeres can form over multiple chromosomal locations at an extremely high frequency (Ketel et al, 2009). Also in S. pombe, neocentromeres form very efficiently (Ishii et al, 2008). Clearly, it seems as evolutionarily advantageous to ‘loosely' regulate centromere identity through epigenetics.
Acknowledgments
We apologise to colleagues whose work could not be cited here due to space limitations. Work in the authors' laboratory is supported by grants from MEC (BFU2006-1627 and CSD2006-49) and the Generalitat de Catalunya (SGR2005-678).
References
- Agudo M, Abad JP, Molina I, Losada A, Ripoll P, Villasante A (2000) A dicentric chromosome of Drosophila melanogaster showing alternate centromere inactivation. Chromosoma 109: 190–196 [DOI] [PubMed] [Google Scholar]
- Ahmad K, Henikoff S (2001) Centromeres are specialized replication domains in heterochromatin. J Cell Biol 153: 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire RC, Karpen GH (2008) Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nature Rev Genet 9: 923–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M, Sanyal K, Mishra PK, Thaler N, Carbon J (2006) Formation of functional centromeric chromatin is specified epigenetically in Candida albicans. Proc Natl Acad Sci USA 103: 14877–14882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BE, Brock MA, Bedard S, Woods VLJ, Cleveland DW (2007) An epigenetic mark generated by the incorporation of CENP-A into centromeric nucleosomes. Proc Natl Acad Sci USA 104: 5008–5013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BE, Foltz DR, Chakravarthy S, Luger K, Woods VL, Cleveland DW (2004) Structural determinants for generating centromeric chromatin. Nature 430: 578–582 [DOI] [PubMed] [Google Scholar]
- Blower MD, Karpen GH (2001) The role of Drosophila CID in kinetochore formation, cell-cycle progression and heterochromatin interactions. Nat Cell Biol 3: 730–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blower MD, Sullivan BA, Karpen GH (2002) Conserved organization of centromeric chromatin in flies and humans. Dev Cell 2: 319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzinba-Segard H, Guais A, Francastel C (2006) Accumulation of small murine minor satellite transcripts leads to impaired centromeric architecture and function. Proc Natl Acad Sci USA 103: 8709–8714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwitz BJ, Ahmad K, Moore LL, Roth MB, Henikoff S (1999) A histone H3-like protein in C. elegans. Nature 401: 547–548 [DOI] [PubMed] [Google Scholar]
- Bühler M, Gasser SM (2009) Silent chromatin in the middle and at the end: lessons from yeasts. EMBO J (e-pub ahead of print 23 July 2009; doi:10.1038/emboj.2009.185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camahort R, Li B, Florens L, Swanson SK, Washburn MP (2007) Scm3 is essential to recruit the histone H3 variant CSE4 to centromeres and to maintain a functional kinetochore. Mol Cell 26: 853–865 [DOI] [PubMed] [Google Scholar]
- Carlson SR, Rudgers GW, Zieler H, Mach JM, Luo S, Grunden E, Krol C, Copenhaver GP, Preuss D (2007) Meiotic transmission of an in vitro-assembled autonomous maize minichromosome. PLoS Genet 3: 1965–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR III, Oegema K, Desai A (2004) A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev 18: 2255–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ES, Saitoh S, Yanagida M, Takahashi K (2003) A cell cycle-regulated GATA factor promotes centromeric localisation of CENP-A in fission yeast. Mol Cell 11: 175–187 [DOI] [PubMed] [Google Scholar]
- Chen Y, Baker RE, Keith KC, Harris K, Stoler S, Fitzgerald-Hayes M (2000) The N terminus of centromere H3-like protein Cse4p performs an essential function distinct from that of the histone fold domain. Mol Cell Biol 20: 7037–7048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo KHA (2001) Domain organization at the centromere and neocentromere. Dev Cell 1: 165–177 [DOI] [PubMed] [Google Scholar]
- Clarke L, Carbon J (1980) Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature 287: 504–509 [DOI] [PubMed] [Google Scholar]
- Collins KA, Furuyama S, Biggins S (2004) Proteolysis contributes to the exclusive centromere localization of the yeast Cse4/CENP-A histone H3 variant. Curr Biol 14: 1968–1972 [DOI] [PubMed] [Google Scholar]
- Conde e Silva N, Black BE, Sivolob A, Filipski J, Cleveland DW, Prunell A (2007) CENP-A-containing nucleosomes: easier disassembly versus exclusive centromeric localization. J Mol Biol 370: 555–573 [DOI] [PubMed] [Google Scholar]
- Dalal Y, Furuyama T, Vermaak D, Henikoff S (2007a) Structure, dynamics, and evolution of centromeric nucleosomes. Proc Natl Acad Sci USA 104: 15974–15981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal Y, Wang H, Lindsay S, Henikoff S (2007b) Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells. PLoS Biol 5: 1798–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunleavy EM, Pidoux AL, Monet M, Bonilla C, Richardson W, Hamilton GL, Ekwall K, McLaughlin PJ, Allshire RC (2007) A NASP (N1/N2)-related protein, Sim3, binds CENP-A and is required for its deposition at fission yeast centromeres. Mol Cell 28: 1029–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunleavy EM, Roche D, Tagami H, Lacoste N, Ray-Gallet D, Nakamura Y, Daigo Y, Nakatani Y, Almouzni-Pettinotti G (2009) HJURP is a cell cycle dependent maintenance and deposition factor of CENP-A at centromeres. Cell 28: 1029–1044 [DOI] [PubMed] [Google Scholar]
- Earnshaw W, Migeon BR (1985) Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma 92: 290–295 [DOI] [PubMed] [Google Scholar]
- Erhardt S, Mellone BG, Betts CM, Zhang W, Karpen GH, Straight AF (2008) Genome-wide analysis reveals a cell cycle-dependent mechanism controlling centromere propagation. J Cell Biol 183: 805–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco HD, Pidoux AL, Urano T, Allshire RC (2008) Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres. Science 319: 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz DR, Jansen LET, Bailey AO, Yates JR III, Bassett EA, Wood S, Black BE, Cleveland DW (2009) Centromere specific assembly of CENP-A nucleosomes is mediated by HJURP. Cell 137: 472–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz DR, Jansen LET, Black BE, Bailey AO, Yates JR III, Cleveland DW (2006) The human CENP-A centromeric nucleosome-associated complex. Nature Cell Biol 8: 458–469 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Hayashi T, Kiyomitsu T, Toyoda Y, Kokubu A, Obuse C, Yanagida M (2007) Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1. Dev Cell 12: 17–30 [DOI] [PubMed] [Google Scholar]
- Furuyama T, Dalal Y, Henikoff S (2006) Chaperone-mediated assembly of centromeric chromatin in vitro. Proc Natl Acad Sci USA 103: 6172–6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowczewski L, Yang P, Kalashnikova T, Santisteban MS, Smith MM (2000) Histone-histone interactions and centromere function. Mol Cell Biol 20: 5700–5711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G, Kiyomitsu T, Yoda K, Yanagida M (2003) Human centromere chromatin protein hMis12, essential for equal segregation, is independent of CENP-A loading pathway. J Cell Biol 160: 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G, Saitoh S, Yanagida M (1999) Proper metaphase spindle length is determined by centromere proteins Mis12 and Mis6 required for faithful chromosome segregation. Genes Dev 13: 1664–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G, Wollman R, Goodwin SS, Zhang N, Scholey JM, Vale RD, Stuurman N (2007) Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316: 417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnenberger KM, Baum MP, Polizzi CM, Carbon J, Clarke L (1989) Construction of functional artificial minichromosomes in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci USA 86: 577–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington JJ, Van Bokkelen G, Mays RW, Gustashaw K, Willard HF (1997) Formation of de novo centromeres and construction of first-generation human artificial chromosomes. Nature Genet 15: 345–355 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Fujita Y, Iwasaki O, Adachi Y, Takahashi K, Yanagida M (2004) Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell 118: 715–729 [DOI] [PubMed] [Google Scholar]
- Hemmerich P, Weidtkamp-Peters S, Hoischen C, Schmiedeberg L, Erliandri I, Diekmann S (2008) Dynamics of inner kinetochore assembly and maintenance in living cells. J Cell Biol 180: 1101–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Ahmad K, Platero JS, van Steensel B (2000) Heterochromatic deposition of centromeric histone H3-like proteins. Proc Natl Acad Sci USA 97: 716–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heun P, Erhardt S, Blower MD, Weiss S, Skora AD, Karpen GH (2006) Mislocalization of the Drosophila centromere-specific histone CID promotes formation of functional ectopic kinetochores. Dev Cell 10: 303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Amano M, Suzuki A, Backer CB, Welburn JP, Dong Y, McEwen BF, Shang WH, Suzuki E, Okawa K, Cheeseman IM, Fukagawa T (2008) CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell 135: 1039–1052 [DOI] [PubMed] [Google Scholar]
- Howman EV, Fowler KJ, Newson AJ, Redward S, MacDonald AC, Kalitsis P, Choo KHA (2000) Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proc Natl Acad Sci USA 97: 1148–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Ogiyama Y, Chikashige Y, Soejima S, Masuda F, Kakuma T, Hiraoka Y, Takahashi K (2008) Heterochromatin integrity affects chromosome reorganization after centromere dysfunction. Science 321: 1088–1091 [DOI] [PubMed] [Google Scholar]
- Ito T, Tyler JK, Bulger M, Kobayashi R, Kadonaga JT (1996) ATP-facilitated chromatin assembly with a nucleoplasmin-like protein from Drosophila melanogaster. J Biol Chem 271: 25041–25048 [DOI] [PubMed] [Google Scholar]
- Jansen LET, Black BE, Foltz DR, Cleveland DW (2007) Propagation of centromeric chromatin requires exit from mitosis. J Cell Biol 176: 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar AP, Bouck DC, Molk JN, Bloom K, Salmon ED (2006) Molecular architecture of a kinetochore-microtubule attachment site. Nature Cell Biol 8: 581–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith KC, Baker RE, Chen Y, Harris K, Stoler S, Fitzgerald-Hayes M (1999) Analysis of primary structural determinants that distinguish the centromere-specific functions of histone variant Cse4p from histone H3. Mol Cell Biol 19: 6130–6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketel C, Wang HS, McClellan M, Bouchonville K, Selmecki A, Lahav T, Gerami-Nejad M, Berman J (2009) Neocentromeres form efficiently at multiple possible loci in Candida albicans. PLoS Genet 5: e1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitoku N, Sasamaya T, Marumoto T, Zhang D, Honda S, Kobayashi O, Hatakeyama K, Ushio Y, Saya H, Hirota T (2003) CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev Cell 5: 853–864 [DOI] [PubMed] [Google Scholar]
- Kwon MS, Hori T, Okada M, Fukagawa T (2007) CENP-C is involved in chromosome segregation, mitotic checkpoint function, and kinetochore assembly. Mol Biol Cell 18: 2155–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lermontova I, Schubert V, Fuchs J, Klatte S, Macas J, Schubert I (2006) Loading of Arabidopsis centromeric histone CENH3 occurs mainly during G2 and requires the presence of the histone fold domain. Plant Cell 18: 2443–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ST, Rattner JB, Jablonski SA, Yen TJ (2006) Mapping the assembly pathways that specify formation of the trilaminar kinetochore plates in human cells. J Cell Biol 175: 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AW, Magliano DJ, Sibson MC, Kalitsis P, Craig JM, Choo KH (2001) A novel chromatin immunoprecipitation and array (CIA) analysis identifies a 460-kb CENP-A-binding neocentromere DNA. Genome Res 11: 448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox PS, Hyndman F, Monen J, Oegema K, Desai A (2007) Functional genomics identifies a Myb domain-containing protein family required for assembly of CENP-A chromatin. J Cell Biol 176: 757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik HS, Henikoff S (2001) Adaptive evolution of Cid, a centromere-specific histone in Drosophila. Genetics 157: 1293–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall OJ, Chueh AC, Wong LH, Choo KH (2008) Neocentromeres: new insights into centromere structure, disease development and karyotype evolution. Am J Hum Genet 82: 261–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May BP, Lippman ZB, Fang Y, Spector DL, Martienssen RA (2005) Differential regulation of strand-specific transcripts from Arabidopsis centromeric satellite repeats. PLoS Genet 1: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measday V, Hailey DW, Pot I, Givan SA, Hyland KM, Cagney G, Fields S, Davis TN, Hieter P (2002) Ctf3p, the Mis6 budding yeast homolog, interacts with Mcm22p and Mcm16p at the yeast outer kinetochore. Genes Dev 16: 101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meluh PB, Yang P, Glowczewski L, Koshland D, Smith MM (1998) Cse4p is a component of the core centromere of Saccharomyces cerevisiae. Cell 94: 607–613 [DOI] [PubMed] [Google Scholar]
- Mizuguchi G, Xiao H, Wisniewski J, Smith MM, Wu C (2007) Nonhistone Scm3 and histones CenH3-H4 assemble the core of centromere-specific nucleosomes. Cell 129: 1153–1164 [DOI] [PubMed] [Google Scholar]
- Moreno-Moreno O, Torras-Llort M, Azorín F (2006) Proteolysis restricts localization of CID, the centromere-specific histone H3 variant of Drosophila, to centromeres. Nucleic Acids Res 34: 6247–6255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema K, Desai A, Rybina S, Kirkham M, Hyman AA (2001) Functional analysis of kinetochore assembly in Caenorhabditis elegans. J Cell Biol 153: 1209–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Cheeseman IM, Hori T, Okawa K, McLeod IX, Yates JR III, Desai A, Fukagawa T (2006) The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nature Cell Biol 8: 446–457 [DOI] [PubMed] [Google Scholar]
- Okada T, Ohzeki J, Nakano M, Yoda K, Brinkley WR, Larionov V, Masumoto H (2007) CENP-B controls centromere formation depending on the chromatin context. Cell 131: 1287–1300 [DOI] [PubMed] [Google Scholar]
- Palmer DK, O'Day K, Trong HL, Charbonneau H, Margolis RL (1991) Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone. Proc Natl Acad Sci USA 88: 3734–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CG, Yeh E, Gardner M, Odde D, Salmon ED, Bloom K (2004) Stable kinetochore-microtubule attachment constrains centromere positioning in metaphase. Curr Biol 14: 1962–1967 [DOI] [PubMed] [Google Scholar]
- Perpelescu M, Nozaki N, Obuse C, Yang H, Yoda K (2009) Active establishment of centromeric CENP-A chromatin by RSF complex. J Cell Biol 185: 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux AL, Choi ES, Abbott JKR, Liu X, Kagansky A, Castillo AG, Hamilton GL, Richardson W, Rappsilber J, He X, Allshire RC (2009) Fission yeast Scm3: a CENP-A receptor required for integrity of subkinetochore chromatin. Mol Cell 33: 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux AL, Richardson W, Allshire RC (2003) Sim4: a novel fission yeast kinetochore protein required for centromeric silencing and chromosome segregation. J Cell Biol 161: 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizzi C, Clarke L (1991) The chromatin structure of centromeres from fission yeast: differentiation of the central core correlates with function. J Cell Biol 112: 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewloka MR, Zhang W, Costa P, Archambault V, D'Avino PP, Lilley KS, Laue ED, McAinsh AD, Glover DM (2007) Molecular analysis of core kinetochore composition and assembly in Drosophila melanogaster. PLoS ONE 2: e478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier V, Vagnarelli P, Fukagawa T, Zerjal T, Burns E, Trouche D, Earnshaw W, Brown W (2005) CENP-A is required for accurate chromosome segregation and sustained kinetochore association of BubR1. Mol Cell Biol 25: 3967–3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL (1979) Ribonucleoprotein staining of centrioles and kinetochores in newt lung cell spindles. J Cell Biol 80: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffery R, Sumer H, Hassan S, Wong LH, Craig JM, Todokoro K, Anderson M, Stafford A, Choo KHA (2003) Transcription within a functional human centromere. Mol Cell 12: 509–516 [DOI] [PubMed] [Google Scholar]
- Santaguida S, Musacchio A (2009) The life and miracles of kinetochores. EMBO J (e-pub ahead of print 23 July 2009; doi:10.1038/emboj.2009.173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh M, Lehner CF, Heidmann S (2007) Incorporation of Drosophila CID/CENP-A and CENP-C into centromeres during early embryonic anaphase. Curr Biol 17: 237–243 [DOI] [PubMed] [Google Scholar]
- Sharp JA, Franco AA, Osley MA, Kaufman PD (2002) Chromatin assembly factor I and Hir proteins contribute to building functional kinetochores in S. cerevisiae. Genes Dev 16: 85–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby RD, Monier K, Sullivan KF (2000) Chromatin assembly at kinetochores is uncoupled from DNA replication. J Cell Biol 151: 1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby RD, Vafa O, Sullivan KF (1997) Assembly of CENP-A into centromeric chromatin requires a cooperative array of nucleosomal DNA contact sites. J Cell Biol 136: 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MM, Yang P, Santisteban MS, Boone PW, Goldstein AT, Megge PC (1996) A novel histone H4 mutant defective in nuclear division and mitotic chromosome transmission. Mol Cell Biol 16: 1017–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler S, Keith KC, Curnick KE, Fitzgerald-Hayes M (1995) A mutation in CSE4, an essential gene encoding a novel chromatin-associated protein in yeast, causes chromosome nondisjunction and cell cycle arrest at mitosis. Genes Dev 9: 573–586 [DOI] [PubMed] [Google Scholar]
- Stoler S, Rogers K, Weitze S, Morey L, Fitzgerald-Hayes M, Baker RE (2007) Scm3, an essential Saccharomyces cerevisiae centromere protein required for G2/M progression and Cse4 localization. Proc Natl Acad Sci USA 104: 10571–10576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BA, Blower MD, Karpen GH (2001) Determining centromere identity: cyclical stories and forking paths. Nat Rev Genet 2: 584–596 [DOI] [PubMed] [Google Scholar]
- Sullivan BA, Karpen GH (2004) Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nature Struct Mol Biol 11: 1076–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BA, Schwartz S (1995) Identification of centromeric antigens in dicentric Robertsonian translocations: CENP-C and CENP-E are necessary components of functional centromeres. Hum Mol Genet 4: 2189–2197 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Chen ES, Yanagida M (2000) Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science 288: 2215–2219 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Murakami S, Shikashige Y, Funabiki H, Niwa O, Yanagida M (1992) A low copy number central sequence with strict symmetry and unusual chromatin structure in fission yeast centromere. Mol Biol Cell 3: 819–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama Y, Sato H, Saitoh S, Ogiyama Y, Masuda F, Takahashi K (2008) Biphasic incorporation of centromeric histone CENP-A in fission yeast. Mol Biol Cell 19: 682–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert PB, Bryson TD, Henikoff S (2004) Adaptive evolution of centromere proteins in plants and animals. J Biol 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert PB, Masuelli R, Tyagi AP, Comai L, Henikoff S (2002) Centromeric localization and adaptive evolution of an Arabidopsis histone H3 variant. Plant Cell 14: 1053–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp CN, Zhong CX, Dawe RK (2004) Centromere-encoded RNAs are integral components of the maize kinetochore. Proc Natl Acad Sci USA 101: 15986–15991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooser AA, Ouspenski II, Gregson HC, Starr DA, Yen TJ, Goldberg ML, Yokomori K, Earnshaw WC, Sullivan KF, Brinkley BR (2001) Specification of kinetochore-forming chromatin by the histone H3 variant CENP-A. J Cell Sci 114: 3529–3542 [DOI] [PubMed] [Google Scholar]
- Vermaak D, Hayden HS, Henikoff S (2002) Centromere targeting element within the histone fold domain of Cid. Mol Cell Biol 22: 7553–7561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfridsson J, Bjerling P, Thalen M, Yoo EJ, Park SD, Ekwall K (2005) The CHD remodeling factor Hrp1 stimulates CENP-A loading to centromeres. Nucleic Acids Res 33: 2868–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton PE (2004) Chromosomal dynamics of human neocentromere formation. Chromosome Res 12: 617–626 [DOI] [PubMed] [Google Scholar]
- Warburton PE, Cooke CA, Bourassa S, Vafa O, Sullivan BA, Stetten G, Gimelli G, Warburton D, Tyler-Smith C, Sullivan KF, Poirier GG, Earnshaw WC (1997) Immunolocalization of CENP-A suggests a distinct nucleosome structure at the inner kinetochore plate of active centromeres. Curr Biol 7: 901–904 [DOI] [PubMed] [Google Scholar]
- Williams BC, Murphy TD, Goldberg ML, Karpen GH (1998) Neocentromere activity of structurally acentric mini-chromosomes in Drosophila. Nature Genet 18: 30–37 [DOI] [PubMed] [Google Scholar]
- Williams JS, Hayashi T, Yanagida M, Russell P (2009) Fission yeast Scm3 mediates stable assembly of Cnp1/CENP-A into centromeric chromatin. Mol Cell 33: 287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Tomkiel J, Saitoh H, Johnson DH, Earnshaw WC (1996) Identification of overlapping DNA-binding and centromere-targeting domains in the human kinetochore protein CENP-C. Mol Cell Biol 16: 3576–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda K, Ando S, Morishita S, Houmura K, Hashimoto K, Takeyasu K, Okazaki T (2000) Human centromere protein A (CENP-A) can replace histone H3 in nucleosome reconstitution in vitro. Proc Natl Acad Sci USA 97: 7266–7271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin SG, Shelby RD, Sullivan KF (2001) CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J Cell Biol 155: 1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]