Abstract
One of the central questions in neuroscience is how refined patterns of connectivity in the brain generate and monitor behavior. Genetic mutations can influence neural circuits by disrupting differentiation or maintenance of component neuronal cells or by altering functional patterns of nervous system connectivity. Mutagenesis screens therefore have the potential to reveal not only the molecular underpinnings of brain development and function, but to illuminate the cellular basis of behavior. Practical considerations make the zebrafish an organism of choice for undertaking forward genetic analysis of behavior. The powerful array of experimental tools at the disposal of the zebrafish researcher makes it possible to link molecular function to neuronal properties that underlie behavior. This review focuses on specific challenges to isolating and analyzing behavioral mutants in zebrafish.
Keywords: zebrafish, behavior, mutagenesis
Biologists, like firefighters, spend a great deal of time inspecting the wreckage of broken things. From the careful analysis of defective biological processes, geneticists seek insight into the mechanisms of normal function. One of the most powerful tools for disrupting biological processes is to directly alter the genetic blueprint that directs the development and function of the organism. The rationale for this approach is clear for questions of cellular biology—genetic mutations that alter protein function can be expected to have consequences for cellular metabolism. However, genetic disruptions are also a powerful tool for analyzing animal behavior. Gene mutations can either directly impair neuronal or synaptic function, or influence behavior indirectly, by causing the miswiring of neural circuits during development.
When the mechanistic underpinnings of a biological process remain mysterious, a powerful and successful technique has been to screen randomly generated genetic mutants to identify individuals where the process in question is disrupted. Such unbiased screens make no assumptions about the mechanism involved making it possible to uncover a role for unknown or unsuspected molecular pathways. Key aspects of our understanding of nervous system function have developed out of an insight initially gleaned from identifying the genetic defect in a mutant. For example, the cellular mechanism of circadian rhythmicity remained elusive until the period gene was isolated in an unbiased genetic screen in Drosophila [1]. The study of genetic mutants with altered behavior gives investigators the opportunity to probe the anatomical substrates of neurobiological function without prior assumptions about where to look.
Mutational analysis of neural development in invertebrates has already established a solid foundation for studying the development of the vertebrate nervous system. Genetic experiments in invertebrates have taken the next step, tracing the intricate pattern of neuronal connectivity required for complex behavior [2]. But because invertebrate brains do not share the basic anatomical plan common to vertebrate species, these studies have a limited scope for informing our understanding of how neural circuits in the vertebrate brain control behavior. Until recently, practical considerations have made a forward genetic approach difficult to apply to vertebrate model organisms. Forward genetic screens in vertebrates are expensive, requiring a large number of mutant lines to be generated and maintained. Moreover, behavioral variability makes it desirable to be able to screen a large number of individuals from each mutant line, adding to the expense. In mammals, behavioral plasticity generated by the cerebral cortex complicates the design and interpretation of rigorous behavioral tests. Finally, mutations in genes controlling specific behaviors may also compromise survival to adulthood, pre-empting the possibility of running a behavioral test.
BEHAVIORAL GENETICS IN ZEBRAFISH
The emergence of the zebrafish as a genetic model organism, addresses many of these problems. Compared to mice, stock maintenance is relatively inexpensive, allowing a medium sized lab to raise the large number of families required to carry out a genetic screen. Clutch sizes are large, enabling mutants and siblings from a single cross to be compared providing a stringent control for genetic background effects (see below). And importantly, external development means that behavioral tests can be applied to larvae at the very onset of locomotor activity at 17 h post fertilization (hpf), thus minimizing the problem of early lethality. It is not mere serendipity that a popular laboratory organism should have all these assets for behavioral genetics—the zebrafish was cultivated as a vertebrate model system by George Streisinger precisely because he sought an animal with these features [3]. Just as Drosophila has proven to be the ‘Rosetta Stone’ for deciphering the mysteries of embryonic development, zebrafish promises to act as a blueprint for unraveling patterns of neural connectivity underlying behavior in the vertebrate brain.
An impediment to genetic analysis of behavior in any organism is the possibility that redundant genes and compensatory biochemical or cellular networks may occlude the effect of a single gene mutation (reviewed in [4, 5]). There is no reason to believe that zebrafish are exempt from this obstacle, although the opportunity to study behavior in early larvae may preempt compensatory changes in neuronal networks that suppress behavioral deficits in adults. The ancient duplication of the genome that preceded the teleost radiation might in some cases enhance the likelihood that genetic redundancy will mask the effect of a single gene mutation. However the flip side of this is the potential for paralogous genes to acquire restricted expression patterns through spatial and temporal partitioning of the ancestral gene expression domain [6, 7]. This raises the enticing possibility that single gene mutations in zebrafish will in some cases allow an even more selective dissection of their contribution to neural function.
Successful genetic screens have been carried out in adult zebrafish [8, 9]. However, the behavior of adult zebrafish remains less well characterized than that of larvae. This review will therefore focus on practical challenges faced in performing a genetic screen on zebrafish larvae. Nevertheless, the opportunity to apply genetic techniques in adult zebrafish for studying truly complex behaviors such as aggression [10], fear [11], sociality [12, 13], courtship [14], spatial cognition and learning [9] is exciting and will no doubt be of increasing interest.
Zebrafish larvae engage in a variety of locomotor activities. Some movements are initiated in direct response to acoustic [15], tactile [16], olfactory [17] and a variety of visual stimuli (reviewed in [18]). However, larval activity also follows an extended program after a change in the environment [19] or can be driven by intrinsic cues such as the circadian clock [20]. This sophisticated behavioral repertoire provides a broad substrate for exploring how neural circuits interpret sensory cues to initiate motor commands.
The earliest behavioral screens in zebrafish used gamma irradiation to induce mutations and scored for failure to respond to a touch stimulus. Paralyzed mutants with neuromuscular [21] and muscle defects [22] were identified, establishing the zebrafish as a vertebrate model for behavioral screens. The next generation of zebrafish screens used ethylnitrosourea (ENU) to mutagenize germ cells in adult fish [23, 24]. ENU almost exclusively induces point mutations, avoiding the large chromosomal deletions often associated with irradiation, and therefore has the dual advantages of allowing single gene effects to be studied and being able to generate both hypomorphs and null mutations. The large-scale ENU mutagenesis screen performed in Tubingen in 1996 included an assay for locomotor function yielding mutants with abnormal responses to tactile and vibratory stimuli [25]. Since then photosensory mutants have been isolated using both the optokinetic and the optomotor assays [26–29], which respectively measure eye movement and whole body displacement in response to moving bars.
Thus most behavioral screens in zebrafish have been directed toward isolating sensory or motor mutants. It is challenging to design a screen to isolate mutants with normal sensory and motor performance, but with specific deficits in higher order processes. Such mutants have the potential to shed new light on the function of the vast brain territory that lies between sensory reception and motor neuron firing, and illuminate fundamental aspects of brain function such as learning and memory, behavioral choice, attention, motivation, emotion and biological rhythms. Indeed, the first such screen to be carried out in zebrafish identified a semi-dominant mutant with a reduced circadian period [30].
This type of screen requires particular care to be exercised in establishing a robust screening system in which quantitative measures of behavior can rapidly and accurately distinguish mutants from normal fish. We have recently carried out such a screen [31] and will describe some of the challenges encountered.
ESTABLISHING A SCREENING PROTOCOL
A particular challenge in the design and execution of behavioral screens is the variability of normal behavior. In order to minimize environmental contributions to behavioral variability, a major effort must be devoted to controlling the raising and testing conditions. Before carrying out a genetic screen, it is critical to be confident that one can detect a mutant phenotype above the distribution of normal behaviors.
A key step is selecting a developmental age at which the behavior to be assayed is stable. Over the first week of development, several transient locomotor behaviors are manifest, including spontaneous and touch-evoked coiling [32, 33], olfactory aversion [17] and cyclic swimming [34]. Other motor behaviors mature at different time points of development. For instance zebrafish larvae have two modes of acoustic startle response distinguished by latency to movement initiation [31]. Short latency acoustic startle responses begin to appear after 72 hpf, whereas long latency acoustic startle responses are robustly elicited after 96 hpf (Burgess and Granato, unpublished results). Similarly, a weak optokinetic response is observed at 3 days post fertilization, but becomes reliable only at 5 days post fertilization (dpf) [26]. In an ENU mutagenesis screen, developmental delay can affect as many of 25% of families [35]. It is therefore advisable to test fish the day after the behavior stabilizes and it is critical to exclude developmental delay as the cause of a behavioral phenotype.
Another source of developmental delay is suboptimal raising conditions. Particular care needs to be exercised over water quality in an ENU mutagenesis screen, which achieve an average of one lethal mutation per genome [35, 36]. One quarter of families harbor lethal mutations causing embryonic death before behavioral testing can take place. Degenerating embryos provide fodder for micro-organisms which deplete oxygen from the water. Under reduced oxygen conditions, larval development is retarded [37]. It is therefore critical to promptly remove necrotic embryos from the pool in order to maintain water quality for the remaining fish.
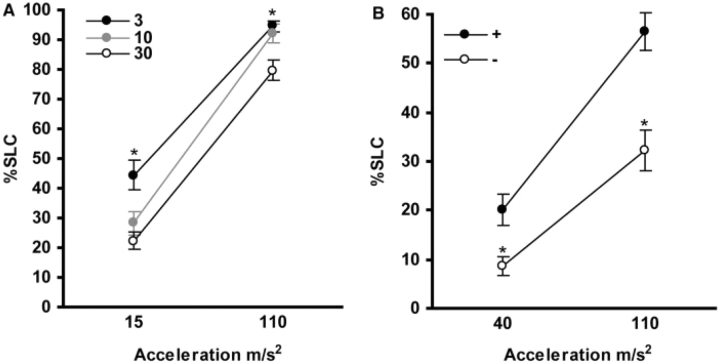
However, the removal of a significant fraction of necrotic larvae from the pool introduces a source of variability into the raising conditions in that it reduces the density of the remaining fish. We found that when raised at low density, zebrafish larvae exhibit heightened startle sensitivity (Figure 1A), complicating our effort to perform a 'hypersensitivity' screen. Increased startle sensitivity in low-density raised fish is specific to short latency acoustic startle responses, making it unlikely that this reflects adjustment of sensory acuity. As increased startle has been described in a variety of animals raised at low density, we were alert to the possibility of this also occurring in larval zebrafish. The multitude of factors with the potential to influence behavior underscores the importance of adhering to a strict raising protocol and noting even trivial changes to the regime.
Figure 1:
Environmental manipulations alter startle responsiveness in zebrafish larvae. (A) Raising density alters startle responsiveness. Larvae were raised at a density of 3, 10 or 30 per 7 ml in a 6 cm dish, with water changes every 2 days. Startle responsiveness was tested using a vibratory stimulus at two intensities as previously described [31]. Larvae raised at the low density (closed circles) showed significantly greater startle responsiveness (measured as the percentage of trials where larvae perform a short latency C-bend response ‘%SLC’) than larvae raised at the ‘standard’ density of 30 per 7 ml (open circles) at both stimulus intensities. (B) Reduced startle responsiveness in wildtype larvae lacking swim bladders. Swim bladder inflation in one group of larvae was prevented by raising larvae under a wire mesh just below the air-water interface (open circles). Control larvae (closed circles) were raised under similar conditions but with the wire mesh just above the water—all such larvae developed swim bladders. Larvae were tested at the indicated stimulus intensities. Larvae without swim bladders showed reduced startle sensitivity at both intensities. All graphs show mean ± SEM (p < 0.01).
It is not always clear whether to exclude larvae with abnormal morphology from testing. Genetic pleiotropy makes it inevitable that many genes with important contributions to behavior, also act outside the brain in organogenesis and metabolism [38]. ENU mutagenesis screens generate a high rate of visible mutations [36] some of which, like brain necrosis or general retardation, are clearly likely to affect behavior. Nevertheless, common phenotypes like mild edema or pigmentation defects are worth including in the screen with the caveat that morphology must be excluded as the source of altered motor activity. A good example of this is the large class of mutants which fail to inflate the swim bladder [39]. Larvae initially inflate their swim bladders by ingesting small bubbles of air. The pharynx opens by 74 hpf [40] and later on the third day post fertilization, larvae begin to inflate their swim bladder, requiring access to the air-water interface to do so [41]. As swim bladder inflation is itself a complex behavior requiring sensory, neural and muscular integrity, it is no surprise that a very large proportion of mutant larvae fail this task. Indeed in the Tubingen screen, 95% of mutants did not inflate their swim bladder. Similarly almost all mutants identified in a large scale insertional mutagenesis screen failed to inflate their swim bladder [42]. Failure to inflate the swim bladder results in skeletal malformations and delayed growth [41]. Behavioral abnormalities also result from failure to inflate the swim bladder. We found that when wildtype larvae are prevented from inflating their swim bladder by physically blocking their access to the air-water interface, they show a 50% reduction in startle sensitivity (Figure 1B). Thus, it is important either to exclude swim bladder mutants from testing, or to demonstrate that failure to inflate the swim bladder does not cause a relevant behavioral phenotype.
Behavioral variability can also be introduced from the genetic background of the mutagenized stocks. While some zebrafish lines have been bred to remove embryonic lethal mutations [43], most are not maintained as inbred stocks in order to avoid unhealthiness. As a result there is a great deal of polymorphism within zebrafish lines. Consistent with this, we find significant behavioral variability within stocks. Thus before initiating a screen, individuals to be mutagenized should be carefully tested for the behavior assayed, to ensure that the population of founders is relatively homogeneous.
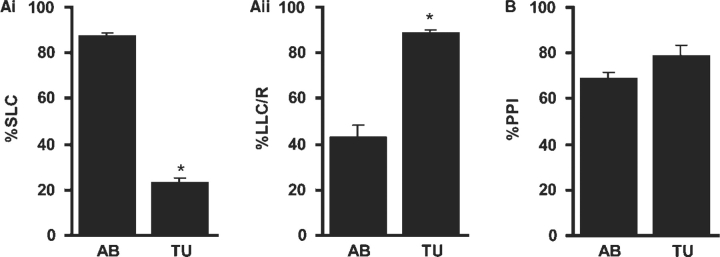
Single nucleotide polymorphisms occur at a rate 10-fold higher than in other vertebrate model species [44]. It is therefore not surprising that large variation in behavior exists between strains. For example, acoustic startle responsiveness varies significantly between our wildtype Tubingen and AB stocks (Figure 2A), while prepulse inhibition of startle does not differ significantly (Figure 2B). Inter-strain variability has consequences both for the design of the screen itself and for planning subsequent genetic mapping experiments [45]. For mapping to be feasible, the wildtype behavior should be similar in at least two genetic backgrounds. It is also worth checking that the trait remains stable in the F2 offspring of the two strains, to ensure that mutation induced changes in behavior will not be masked by variability. However, even if wildtype strains show similar normal behavior, the mutant phenotype can vary on different genetic backgrounds, presumably because of differing degrees of genetic redundancy and the presence of modifier genes. We encountered this obstacle in mapping the twitch twice mutant (Burgess and Granato, unpublished results). On a Tubingen background the phenotype was a highly penetrant set of multiple tail flips to the same side in response to a tap stimulus. On a background mixed with the WIK mapping strain, only occasional multiple same-side tail flips are seen—mutants generally respond to a tap stimulus with a ‘rolling’ startle response. Thus, strain effects on behavior do not impose an insuperable obstacle to genetic studies in zebrafish, but do demand that vigilance be exercised in designing both the screening and mapping protocols.
Figure 2:
Genetic background alters startle responsiveness in zebrafish larvae. AB strain (n = 108) and Tubingen strain (n = 99) larvae were raised in identical conditions and subjected to the startle and prepulse inhibition conditions used for screening [31]. The two strains differed significantly in the mode of startle response used. AB strain larvae predominantly used ‘SLC’ (Ai), while Tubingen larvae tended to produce long latency ‘LLC’ responses (Aii). In contrast, the inhibitory effect of a weak prepulse (PPI) was similar in both groups (C), making these strains compatible for genetic screening purposes. All graphs show mean ± SEM (p < 0.01).
Behavioral assays are notoriously sensitive to small changes in environmental conditions during testing. It is impossible to systematically test the effect of every factor that can vary in the testing protocol. Thus extreme care needs to be taken to ensure consistency of the testing arena. When renovations forced us to relocate our startle apparatus to a new room, we were aghast to find a 25% increase in startle C-bend amplitude during the first week of testing. It transpired that the difference was attributable to a 3°C drop in temperature in our new environment. Other factors requiring attention include pH, illumination and time of day. Light–dark transitions begin to entrain the larval zebrafish circadian system by the second day post fertilization with profound effects on diurnal variation in locomotor activity [46]. It is therefore advisable to raise larvae in a light cycle incubator and pre-adapt them to a set intensity of light before beginning testing.
Before embarking on a behavioral screen, it is critical to consider what types of mutants the assay is likely to yield. Behavioral assays provide a readout of the function of multiple brain systems. For instance, we anticipated that a failure to respond to an acoustic startle stimulus could reflect abnormalities of the sensory apparatus, motor systems or central integration. It is helpful to have a battery of complimentary tests to assess the integrity of these systems in order to quickly exclude mutant lines unlikely to be informative. Even simple observations can be informative. Visually impaired fish often show darker pigmentation due to expanded melanophores [26]. Paralysis due to disorganized muscle is easy to assess by checking muscle birefringence [25]. Any hint of postural instability or circling behavior makes it important to examine the morphology of the inner ear and otoliths [47]. During our screen for mutants with defects in startle modulation, we found quantification of the kinematics of movement helpful. Mutants with reduced startle amplitude also often showed reduced amplitude of tail-flips during swim bouts. This combination of features suggested that the primary defect lay at the level of the muscle or neuromuscular junction. Other mutants had defective acoustic startle responses but behaved normally to a touch stimulus, indicating that the defect was likely to represent a sensory abnormality. As we were primarily interested in modulation of normal startle responses, we excluded both these categories from further consideration.
IDENTIFYING BEHAVIORAL GENES
Henry Thoreau wrote, ‘Many men go fishing all of their lives without knowing it is not fish they are after’ Indeed, while study of a mutant fish itself can reveal much about the neural architecture underlying a behavior, a critical further goal is to isolate the genetic lesion responsible. The first step in identifying the mutant locus is to determine its position within the genome. Unfortunately, behavioral phenotypes are more difficult to map than morphological abnormalities. This is because behavioral variability makes it likely that sibling fish will occasionally be misidentified as mutants. Even robust behavioral phenotypes require repeated testing to confidently sort mutants from sibling fish. Fortunately, sorting errors can usually be spotted during recombination mapping. The real problem is that mapping the mutation to a small genomic interval usually requires the genotyping of several hundred mutant fish. Collecting this large number using a behavioral assay is arduous and presents a serious bottleneck for cloning behavioral mutants.
After mapping and cloning a genetic mutation in a behavioral mutant, it is important to verify that the observed mutation is indeed responsible for the behavioral phenotype. For mutations introducing a translational stop codon, there is little doubt that the appropriate gene was cloned. Some researchers have raised the theoretical concern that due to the high load of mutations in ENU mutagenized fish, the abnormal behavior could actually result from a mutation in a tightly linked locus—however to our knowledge, no case has been reported in which a nonsense mutation has proven to be the wrong gene. If sequencing reveals a missense mutation, then this could merely represent a polymorphism. This is unlikely if a genetic mutation can be identified in the same gene in an independent, non-complementing mutant line. If only one mutant allele is available, sequencing the putative lesion site from DNA isolated from the originally mutagenized fish can confirm if the mutation was induced, in which case the base-pair change should not be present in the founder fish, or if it represents a polymorphism already present in the founder fish [48]. In addition, rescue experiments can serve as independent evidence. While mRNA rescue of behavioral mutants is limited to defects that are observed in the first 24 h of development, rescue using a transgenic approach, using either a cell-type specific promoter or an inducible promoter such as the HSP70 heat shock promoter, is very feasible. Another useful strategy for early behavioral defects is to attempt to phenocopy the effects of a mutation using morpholinos. A last resource is to perform a small non-complementation screen to isolate a second allele where an independent mutation in the same gene gives rise to the same behavioral phenotype [49].
After establishing the identity of the genetic lesion, the next step is to establish the nexus between mutation and behavior. Genetic mutations can alter any aspect of neuronal physiology with potential for modifying behavior. Indeed the optokinetic assay alone has identified mutants with defects in axon guidance [50], retinal differentiation [51] and maintenance [27], synaptic structure [52], retinal physiology [26] and neuronal metabolism [53]. In the latter case the identity of the mutant gene may predict that a pharmacological intervention should phenocopy the behavioral effect. In an elegant study, Page-McCaw et al.demonstrated that sandy harbors a mutation in the tyrosinase gene. Pharmacological inhibition of tyrosinase phenocopies the reduced optokinetic response and rate of light adaptation in the mutant fish [53]. In other cases a clear anatomical defect in the mutant fish can be found and the problem is to demonstrate that this defect underlies the behavioral abnormality. Variable penetrance of the behavioral phenotype can actually be helpful in this regard. If behavior in mutants is consistently accompanied by a specific anatomical variation, the latter is likely to mediate the behavioral defect. For example, some belladonna mutants have an ipsilateral retinotectal projection and the same individuals show a reversed optokinetic response [54]. In mutants that display multiple neuroanatomical abnormalities, lesion analysis can help to pinpoint which neural circuit is required for the behavior. In the space cadet mutant, spiral fiber neuron projection defects were a strong candidate for the escape response phenotype. Severing the spiral fiber commissures phenocopied the mutant providing direct evidence for their role in modulating startle behavior [55]. The optical transparency of zebrafish larvae makes laser ablation of neurons an attractive tool for establishing an even stronger link between specific neurons and behavior [56, 57]. If the expression pattern of a behavioral gene is sufficiently restricted, laser ablation will sometimes allow elucidation of the entire causal chain from gene to circuit to behavior.
PROSPECTIVES
A number of developments make the prospect of performing forward genetic screens for zebrafish behavior even more attractive. Several platforms for automated quantitative analysis of behavior are now available [19, 58–62], making robust quantitation readily accessible without a large up-front investment in programming.
To date, most behavioral screens in zebrafish have employed chemical mutagens. While chemical mutagenesis offers an efficient way of introducing a high load of mutations with an unbiased distribution, subsequent genetic mapping and cloning of the mutation is laborious. Insertional methods for generating mutants offer an attractive alternative (reviewed in [63]). Both pseudotyped retroviruses [42, 64–66] and more recently transposons [67, 68] have been used to generate mutant libraries and enjoy the advantage of the integration-site carrying a molecular tag for ease of cloning. It has been estimated that the mutational load generated by retroviral insertion, is approximately 7-fold lower than that created by ENU mutagenesis, necessitating a concomitantly greater investment in screening [65]. In addition, a lesson from P element mutagenesis in Drosophila is that insertional systems show significant bias with regard to mutational sites (reviewed in [69]). Screening efficiency is reduced by the propensity of retroviruses to repeatedly target ‘hot-spots’ [65]. Perhaps of greater concern are ‘cold–spots’ where few integration events are observed—indeed in Drosophila P element transposons may only target 30% of genes required for embryonic development. While these considerations might continue to make ENU mutagenesis the method of choice for generating mutants in most biological processes, the ease of cloning insertional mutants presents a strong temptation for behavioral geneticists.
At the very outset of the modern era of genetics it was possible to posit a crucial role for inheritance in nervous system function and neurological disorders. Soon after rediscovering Mendel's principles of heredity, William Bateson recognized that at least one form of mental illness, Hereditary Chorea, was transmitted in a simple Mendelian pattern [70]. In conjunction, Archibald Garrod argued that certain inborn disorders of metabolism should be regarded as alternate modes of normal function and that human variation could thus be at least in part attributed to ‘chemical individuality’ [71]. With the explosive growth in our understanding of genetics, development and brain function in the last 100 years, it has now become possible to finally join the links in the causal chain between genes and the function of the nervous system. Thus behavioral genetics is a young field with old questions. Mutagenesis experiments in zebrafish have demonstrated that this system has tremendous potential to finally provide an answer to some of those questions.
Key Points.
Practical considerations make zebrafish larvae a highly attractive vertebrate system for performing mutagenesis screens for neural circuits underlying sensory, motor and integrative aspects of behavior.
Behavioral screens require vigilance in maintaining constant environmental conditions during raising and testing larvae, as seemingly trivial deviations can introduce unanticipated quantitative changes in responses.
Genetic mutations altering behavior provide insights into a broad spectrum of neurobiological processes required for normal behavior, from development to neuronal cell biology and patterns of connectivity.
FUNDING
NRSA postdoctoral fellowship (H.A.B.); National Institutes of Health (NS-048258 and MH-075691 to M.G.).
Biographies
Harold Burgess is a postdoctoral fellow at the University of Pennsylvania, USA.
Michael Granato is a Professor at the University of Pennsylvania, USA.
References
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–6. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2005;102:3184–91. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald DJ, Eisen JS. Headwaters of the zebrafish – emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–24. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Gene targeting in neuroscience: the systemic approach. Trends Neurosci. 1996;19:188–9. [Google Scholar]
- Gingrich JA, Hen R. Commentary: The broken mouse: the role of development, plasticity and environment in the interpretation of phenotypic changes in knockout mice. Curr Opin Neurobiol. 2000;10:146–52. doi: 10.1016/s0959-4388(99)00061-6. [DOI] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, et al. Preservation of Duplicate Genes by Complementary. Degenerative Mutations. 1999;151:1531–45. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait J, Amores A, Cresko W, et al. Subfunction partitioning, the teleost radiation and the annotation of the human genome. Trends Genet. 2004;20:481–90. doi: 10.1016/j.tig.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Li L, Dowling JE. A dominant form of inherited retinal degeneration caused by a non-photoreceptor cell-specific mutation. Proc Natl Acad Sci USA. 1997;94:11645–50. doi: 10.1073/pnas.94.21.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc Natl Acad Sci USA. 2001;98:11691–6. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Zebra fish: an uncharted behavior genetic model. Behav Genet. 2003;33:461–8. doi: 10.1023/a:1025762314250. [DOI] [PubMed] [Google Scholar]
- Waldman B. Quantitative and developmental analysis of the alarm reaction in the Zebra Danio, Brachydanio rerio. Copeia. 1982;1:1–9. [Google Scholar]
- Engeszer R, Ryan M, Parichy D. Learned Social Preference in Zebrafish. Curr Biol. 2004;14:881–4. doi: 10.1016/j.cub.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio) Behav Brain Res. 2007;184:157–66. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Darrow K, Harris W. Characterization and Development of Courtship in Zebrafish, Danio rerio. Zebrafish. 2004;1:40–5. doi: 10.1089/154585404774101662. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Patterson J, Kimmel RO. The development and behavioral characteristics of the startle response in the zebra fish. Dev Psychobiol. 1974;7:47–60. doi: 10.1002/dev.420070109. [DOI] [PubMed] [Google Scholar]
- Eaton R, Farley R, Kimmel C, et al. Functional development in the Mauthner cell system of embryos and larvae of the zebra fish. J Neurobiol. 1977;8:151–72. doi: 10.1002/neu.480080207. [DOI] [PubMed] [Google Scholar]
- Vitebsky A, Reyes R, Sanderson M, et al. Isolation and characterization of the laure olfactory behavioral mutant in the zebrafish, Danio rerio. Dev Dyn. 2005;234:229–42. doi: 10.1002/dvdy.20530. [DOI] [PubMed] [Google Scholar]
- Neuhauss S. Behavioral genetic approaches to visual system development and function in zebrafish. J Neurobiol. 2003;54:148–60. doi: 10.1002/neu.10165. [DOI] [PubMed] [Google Scholar]
- Burgess H, Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. J Exp Biol. 2007;210:2526. doi: 10.1242/jeb.003939. [DOI] [PubMed] [Google Scholar]
- Cahill G, Hurd M, Batchelor M. Circadian rhythmicity in the locomotor activity of larval zebrafish. Neuroreport. 1998;9:3445–9. doi: 10.1097/00001756-199810260-00020. [DOI] [PubMed] [Google Scholar]
- Westerfield M, Liu D, Kimmel C, et al. Pathfinding and synapse formation in a zebrafish mutant lacking functional acetylcholine receptors. Neuron. 1990;4:867–74. doi: 10.1016/0896-6273(90)90139-7. [DOI] [PubMed] [Google Scholar]
- Felsenfeld A. Mutations affecting skeletal muscle myofibril structure in the zebrafish. Development. 1990;108:443–59. doi: 10.1242/dev.108.3.443. [DOI] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Haffter P, et al. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol. 1994;4:189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Schier AF, Driever W. Efficient recovery of ENU-induced mutations from the Zebrafish Germline. Genetics. 1994;136:1401–20. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, et al. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Brockerhoff S, Hurley J, Janssen-Bienhold U, et al. A behavioral screen for isolating Zebrafish mutants with visual system defects. Proc Natl Acad Sci USA. 1995;92:10545–9. doi: 10.1073/pnas.92.23.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockerhoff S, Hurley J, Niemi G, et al. A new form of inherited red-blindness identified in Zebrafish. J Neurol Sci. 1997;17:4236–42. doi: 10.1523/JNEUROSCI.17-11-04236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhauss S, Biehlmaier O, Seeliger M, et al. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in Zebrafish. J Neurol Sci. 1999;19:8603. doi: 10.1523/JNEUROSCI.19-19-08603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Orger MB, Wehman AM, et al. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005;1:e66. doi: 10.1371/journal.pgen.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyne J, Hurd MW, Gutierrez L, et al. Isolation and phenogenetics of a novel circadian rhythm mutant in zebrafish. J Neurogenet. 2004;18:403–28. doi: 10.1080/01677060490894540. [DOI] [PubMed] [Google Scholar]
- Burgess H, Granato M. Sensorimotor gating in larval Zebrafish. J Neurol Sci. 2007;27:4984. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol. 1998;37:622–32. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Downes G, Granato M. Supraspinal input is dispensable to generate glycine-mediated locomotive behaviors in the zebrafish embryo. J Neurobiol. 2006;66:437–51. doi: 10.1002/neu.20226. [DOI] [PubMed] [Google Scholar]
- Muller U, van Leeuwen J. Swimming of larval zebrafish: ontogeny of body waves and implications for locomotory development. J Exp Biol. 2004;207:853–68. doi: 10.1242/jeb.00821. [DOI] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Küster E, Altenburger R. Environ Toxicol. 2008. Oxygen decline in biotesting of environmental samples-Is there a need for consideration in the acute zebrafish embryo assay? doi:10.1002/tox.20377. [DOI] [PubMed] [Google Scholar]
- Miklos G, Rubin G. The role of the genome project in determining gene function: insights from model organisms. Cell. 1996;86:521–9. doi: 10.1016/s0092-8674(00)80126-9. [DOI] [PubMed] [Google Scholar]
- McCune AR, Carlson RL. Twenty ways to lose your bladder: common natural mutants in zebrafish and widespread convergence of swim bladder loss among teleost fishes. Evol Dev. 2004;6:246–59. doi: 10.1111/j.1525-142X.2004.04030.x. [DOI] [PubMed] [Google Scholar]
- Wallace K, Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003;255:12–29. doi: 10.1016/s0012-1606(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Goolish E, Okutake K. Lack of gas bladder inflation by the larvae of zebrafish in the absence of an air-water interface. J Fish Biol. 1999;55:1054–63. [Google Scholar]
- Amsterdam A, Nissen RM, Sun Z, et al. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci USA. 2004;101:12792. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G, Walker C, Dower N, et al. Production of clones of homozygous diploid zebra fish(Brachydanio rerio) Nature. 1981;291:293–6. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- Guryev V, Koudijs MJ, Berezikov E, et al. Genetic variation in the zebrafish. Genome Res. 2006;16:491–7. doi: 10.1101/gr.4791006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–81. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Hurd M, Cahill G. Entraining signals initiate behavioral circadian rhythmicity in larval Zebrafish. J Biol Rhythms. 2002;17:307–14. doi: 10.1177/074873002129002618. [DOI] [PubMed] [Google Scholar]
- Whitfield T, Granato M, van Eeden F, et al. Mutations affecting development of the zebrafish inner ear and lateral line. Development. 1996;123:241–54. doi: 10.1242/dev.123.1.241. [DOI] [PubMed] [Google Scholar]
- Lefebvre JL, Ono F, Puglielli C, et al. Increased neuromuscular activity causes axonal defects and muscular degeneration. Development. 2004;131:2605–18. doi: 10.1242/dev.01123. [DOI] [PubMed] [Google Scholar]
- Sidi S, Busch-Nentwich E, Friedrich R, et al. Gemini encodes a Zebrafish L-type calcium channel that localizes at sensory hair cell ribbon synapses. J Neurol Sci. 2004;24:4213–23. doi: 10.1523/JNEUROSCI.0223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Dowling JE. disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant Zebrafish. J Neurol Sci. 2000;20:1883–92. doi: 10.1523/JNEUROSCI.20-05-01883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J, Finger-Baier K, Roeser T, et al. Retinal ganglion cell genesis requires lakritz, a Zebrafish atonal homolog. Neuron. 2001;30:725–36. doi: 10.1016/s0896-6273(01)00312-9. [DOI] [PubMed] [Google Scholar]
- Allwardt BA, Lall AB, Brockerhoff SE, et al. Synapse Formation Is Arrested in Retinal Photoreceptors of the Zebrafish nrc Mutant. J Neurol Sci. 2001;21:2330–42. doi: 10.1523/JNEUROSCI.21-07-02330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw P, Chung S, Muto A, et al. Retinal network adaptation to bright light requires tyrosinase. Nat Neurosci. 2004;7:1329–36. doi: 10.1038/nn1344. [DOI] [PubMed] [Google Scholar]
- Rick JM, Horschke I, Neuhauss SC. Optokinetic behavior is reversed in achiasmatic mutant zebrafish larvae. Curr Biol. 2000;10:595–8. doi: 10.1016/s0960-9822(00)00495-4. [DOI] [PubMed] [Google Scholar]
- Lorent K, Liu KS, Fetcho JR, et al. The zebrafish space cadet gene controls axonal pathfinding of neurons that modulate fast turning movements. Development. 2001;128:2131–42. doi: 10.1242/dev.128.11.2131. [DOI] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–35. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Orger M, Kampff A, Severi K, et al. Control of visually guided behavior by distinct populations of spinal projection neurons. Nat Neurosci. 2008;11:327. doi: 10.1038/nn2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang P, Yelick P, Malicki J, et al. High-throughput behavioral screening method for detecting auditory response defects in zebrafish. J Neurosci Methods. 2002;118:177–87. doi: 10.1016/s0165-0270(02)00118-8. [DOI] [PubMed] [Google Scholar]
- Kato S, Nakagawa T, Ohkawa M, et al. A computer image processing system for quantification of zebrafish behavior. J Neurosci Methods. 2004;134:1–7. doi: 10.1016/j.jneumeth.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Blaser R, Gerlai R. Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods. Behavior Research Methods. 2006;38:456–69. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- Prober D, Rihel J, Onah A, et al. Hypocretin/Orexin Overexpression Induces An insomnia-like phenotype in Zebrafish. J Neurol Sci. 2006;26:13400. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine E, Lentink D, Kranenbarg S, et al. Automated visual tracking for studying the ontogeny of zebrafish swimming. J Exp Biol. 2008;211:1305–16. doi: 10.1242/jeb.010272. [DOI] [PubMed] [Google Scholar]
- Sivasubbu S, Balciunas D, Amsterdam A, et al. Insertional mutagenesis strategies in zebrafish. Genome Biology. 2007;8:S9. doi: 10.1186/gb-2007-8-s1-s9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N, Amsterdam A, Kawakami K, et al. Insertional mutagenesis and rapid cloning of essential genes in zebrafish. Nature. 1996;383:829–32. doi: 10.1038/383829a0. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Burgess S, Golling G, et al. A large-scale insertional mutagenesis screen in zebrafish. Genes & Development. 1999;13:2713. doi: 10.1101/gad.13.20.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golling G, Amsterdam A, Sun Z, et al. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat Genet. 2002;31:135–40. doi: 10.1038/ng896. [DOI] [PubMed] [Google Scholar]
- Sivasubbu S, Balciunas D, Davidson A, et al. Gene-breaking transposon mutagenesis reveals an essential role for histone H2afza in zebrafish larval development. Mech Dev. 2006;123:513–29. doi: 10.1016/j.mod.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Nagayoshi S, Hayashi E, Abe G, et al. Insertional mutagenesis by the Tol2 transposon-mediated enhancer trap approach generated mutations in two developmental genes: tcf7 and synembryn-like. Development. 2008;135:159–69. doi: 10.1242/dev.009050. [DOI] [PubMed] [Google Scholar]
- Ryder E, Russell S. Transposable elements as tools for genomics and genetics in Drosophila. Brief Funct Genomic Proteomic. 2003;2:57–71. doi: 10.1093/bfgp/2.1.57. [DOI] [PubMed] [Google Scholar]
- Bateson W. London: Cambridge University Press; 1909. Mendel's Principles of Heredity. [Google Scholar]
- Garrod AE. The incidence of alkaptonuria: a study in chemical individuality. Lancet. 1902;2:1616–20. [PMC free article] [PubMed] [Google Scholar]