Abstract
Despite their ubiquity and impact, psychiatric illnesses and other disorders of the central nervous system remain among the most poorly treated diseases. Most psychiatric medicines were discovered due to serendipitous observations of behavioural phenotypes in humans, rodents and other mammals. Extensive behaviour-based chemical screens would likely identify novel psychiatric drugs. However, large-scale chemical screens in mammals are inefficient and impractical. In contrast, zebrafish are very well suited for high-throughput behaviour-based drug discovery. Furthermore, the vast amounts of data generated from large-scale behavioural screens in zebrafish will facilitate a systems-level analysis of how chemicals affect behaviour. Unlike serendipitous discoveries in mammals, a comprehensive and integrative analysis of zebrafish chemobehavioural phenomics may identify functional relationships that would be missed by more reductionist approaches. Thus, behaviour-based chemical screens in the zebrafish may improve our understanding of neurobiology and accelerate the pace of psychiatric drug discovery.
Keywords: phenomics, chemical genetics, zebrafish
SERENDIPITY AND THE HISTORY OF PHENOTYPE-BASED NEUROACTIVE DRUG DISCOVERY
Psychiatric disorders such as depression, anxiety and schizophrenia are widespread and devastating illnesses. Despite the need for improved psychiatric medicines, drug discovery success rates for psychiatric illnesses and other disorders of the central nervous system (CNS) are lower than for other therapeutic areas [1]. To meet the vast unmet need for novel psychiatric drugs, it will be essential to develop new approaches to neuroactive drug discovery. Because the complexity of CNS diseases is likely to preclude us from arriving at a mechanistic understanding of disease pathology in the near term, drug discovery approaches that can be effective in the absence of complete understanding of disease pathology will be of particular value [2, 3]. Identifying novel neuroactive chemicals is an important first step toward developing psychiatric medicines. But, lacking a detailed understanding of the biochemical mechanisms that cause psychiatric disease, how can novel neuroactive drugs be discovered?
Many of the successes of modern biology have resulted from the advent of systematic methodologies such as genome sequencing, microarray expression analysis and high-throughput screening. Bringing systematic approaches to bear on neuroactive drug discovery is an appealing goal, particularly if it can be done while retaining the advantages of the in vivo approaches that have been so powerful historically.
Behaviour-based approaches to neuroactive drug discovery are not new. Ethanol, caffeine, opium and other naturally occurring psychoactive substances have been used since antiquity and were undoubtedly discovered due to their rapid perceptual and behavioural effects on humans and other animals. Today, psychiatric medicines are among the most commonly prescribed drugs. However, many ‘new’ medications are actually analogues of a few prototypic molecules that were originally discovered decades ago (Table 1). As the founders of large classes of drugs, these prototypes have played a key role in the field of psychiatric drug discovery. Thus, it is instructive to consider how they were originally discovered.
Table 1:
Psychiatric drug prototypes
| Therapeutic class | Prototype | Discovery date |
|---|---|---|
| Anti-psychotic | Chlorpromazine | 1952 |
| Haloperidol | 1958 | |
| Stimulant | Amphetamine | 1937 |
| Mood stabilizer | Lithium | 1949 |
| Lamotrigine | 1990 | |
| Valproate | 1962 | |
| Anti-depressant | Iproniazid | 1957 |
| Imipramine | 1956 | |
| Fluoxetine | 1988 | |
| Anxiolytic | Chlordiazepoxide | 1958 |
In the following section, we briefly review how the first anti-psychotics, stimulants, anti-depressants, anxiolytics and mood stabilizers were discovered via behavioural phenotypes in humans and other animals. These examples underscore the power of behaviour-based psychiatric drug discovery, and suggest that high-throughput behaviour-based chemical screens in the zebrafish will be a valuable approach for identifying novel psychiatric drugs.
Anti-psychotics
Chlorpromazine, the prototype of phenothiazine anti-psychotics (and one of the first ‘blockbuster’ psychiatric drugs), was discovered due to its unexpected behavioural effects on surgical patients. In the early 1950s, anti-histamines were found to improve surgical outcomes by reducing anaphylactic shock. Pre-surgical administration of anti-histamines also lowered patients’ body temperature, which slowed metabolism and reduced the amount of anaesthetic needed during operations. Efforts to identify anti-histamines with optimal properties for surgery led to the unexpected discovery that chlorpromazine made patients feel unusually relaxed and unconcerned during the normally stressful pre-operative period. Further investigations into these side effects eventually led to chlorpromazine's evaluation on a wide variety of psychiatric disorders and its identification as a remarkably effective anti-psychotic drug [4].
Haloperidol, the first butyrophenone anti-psychotic drug, was discovered because of its behavioural effects on rodents. Haloperidol's discovery can be traced to pethidine, the first synthetic opiate analgesic. Pethidine was initially identified as a potential muscle relaxant due to its anti-spasmodic activity on the isolated guinea pig colon. Its analgesic activity was discovered during toxicology tests in mice when it unexpectedly induced the ‘straub tail phenomenon’, a characteristic tail posture known to occur in response to morphine [5]. A decade after its discovery, pethidine analogues were screened in rodent pain assays to identify improved analgesics. One of these analogues caused an unusual calmness and sedation in addition to reducing responses to noxious stimuli. Efforts to identify more potent analogues of this compound led to the discovery of haloperidol, a potent tranquilizer in rodents and an effective anti-psychotic in humans [4].
Stimulants
Amphetamine, a synthetic analogue of ephedrine, is the original prototype of many analogous drugs that are used to treat attention-deficit hyperactivity disorder (ADHD). Amphetamine was originally synthesized as a cheap ephedrine-like decongestant. Its modern use for treating ADHD was serendipitously discovered in experiments designed to investigate the hypothesis that it might ameliorate severe headaches and vomiting in children who had undergone pneumoencephalography [6–8]. In this painful (and now defunct) medical procedure, a small amount of cerebrospinal fluid is drained and replaced with gas to improve the quality of X-ray pictures of the brain [9]. Amphetamine did not do much for the headaches but surprisingly, children treated with the drug showed ‘remarkable improvement’ in their school assignments [7]. Today, methylphenidate (Ritalin), a structural analogue of amphetamine, is frequently prescribed to treat symptoms of ADHD. Interestingly, methylphenidate shares very similar pharmacology with cocaine, another well-known stimulant originally discovered because of its behavioural effects [10].
Anti-depressants and anxiolytics
Iproniazid, the first monoamine oxidase inhibitor (MAOI) anti-depressant, was originally used as an antibiotic to treat tuberculosis. Its anti-depressant activity was serendipitously discovered due to its behavioural effects on ‘hopeless’ tuberculosis patients on whom it conferred ‘therapeutic benefit beyond any of the chemotherapeutics or antibiotic agents previously utilized’ [11]. The odd observation that patients often felt better before any objective improvement in their disease eventually led its investigation as an anti-depressant.
Imipramine, the prototype of tricyclic anti-depressants, is a phenothiazine-like analogue of chlorpromazine. Imipramine was originally investigated as a ‘me too’ drug to profit from the financial and therapeutic successes of chlorpromazine (see above). Imipramine and many other chlorpromazine-like molecules were tested for a wide variety of activities on psychiatric patients. Unexpectedly, imipramine was not an effective antipsychotic drug, but it did relieve symptoms of depression [12].
Chlordiazepoxide, the prototype of benzodiazepine anxiolytics, was discovered in a series of serendipitous events. Like imipramine, chlordiazepoxide was also developed in an attempt to capitalize on the success of chlorpromazine (see above). A series of about 40 chlorpromazine-like compounds were synthesized and tested for tranquilizing properties in rodents. The majority of these molecules were behaviourally inactive. The final compound in the series, chlordiazepoxide, sat untested on a shelf in the laboratory for more than a year before it was sent, almost as an afterthought, for animal testing. Remarkably, chlordiazepoxide caused interesting psycho-sedative effects in rodents and was even found to calm a colony of vicious monkeys. Subsequent investigation into the structure of this molecule showed that it was structurally unrelated to chlorpromazine, and had been formed from an unexpected chemical side reaction [4].
Today, benzodiazepines (such as Valium and Xanax) have largely replaced barbiturates and meprobamate as the most commonly prescribed anxiolytic drugs. However, it is interesting to note that these earlier anxiolytics were also discovered based on their behavioural effects on animals. Barbital, the first barbiturate, was discovered in 1864 via its unique and potent sedating effects on a dog [4]. The psychosedative properties of meprobamate, which was originally developed as an antibiotic, were discovered via its unexpected behavioural effects on mice in the course of toxicity tests [13].
Mood stabilizers
John Cade discovered lithium's therapeutic effects on bipolar disorder in the course of experiments designed to investigate the ultimately incorrect hypothesis that the causative agent for bipolar disorder could be found in urine [4]. To investigate this hypothesis, Cade injected guinea pigs with urine from patients with various psychiatric disorders in an attempt to phenocopy mania in the rodents. These injections were universally lethal. However, animals injected with the urine from manic patients appeared to die faster than animals injected with control urine. Encouraged by these results, Cade identified urea as the likely lethal factor in this assay and began to study the effects of uric acid. Uric acid is highly insoluble and was therefore difficult to work with, so he experimented with more soluble urate salts. Lithium urate, the most soluble of the urates, suppressed lethality, however so did other lithium salts, suggesting that lithium itself may have interesting physiological effects. To investigate the physiological effects of lithium, Cade injected large amounts into the guinea pigs. After about a 2 h latency period, they became lethargic for 1–2 h before once again becoming ‘normally active and timid’. This prompted Cade to test for lithium toxicity on himself and then begin clinical trials for a wide variety of psychiatric indications [14].
Valproate, lamotrigine and other drugs used to treat bipolar disorder were originally identified as anti-convulsants in non-hypothesis-driven random drug screens on animal models of seizures induced by electrical and chemical shock [15]. The anti-convulsant activity of valproate was accidentally discovered when it was used as a solvent for other agents being tested in rabbits [16]. Another anti-convulsant, lamotrigine, was originally identified in vitro as an inhibitor of dihydrofolate reductase, based on the therapeutic hypothesis that folate contributes to epilepsy. Remarkably, it was found to have anti-convulsant activity in animal models even though it does not substantially lower folate levels in vivo and is believed to act through an unknown mechanism [17]. Unexpectedly, these anti-convulsants were also found to improve mood and communicativeness in humans and were therefore investigated for treating mood disorders.
HIGH-THROUGHPUT BEHAVIOUR-BASED DRUG SCREENING
As illustrated above, traditional behaviour-based neuroactive drug discovery is very effective. It is also very low-throughput (Table 2). Large-scale behaviour-based chemical screens would likely identify novel drug prototypes. However, it is prohibitively laborious, time consuming and expensive to systematically expose humans, rodents and other mammals to the small amounts of chemicals found in modern chemical libraries. Thus, despite its proven efficacy, behaviour-based drug discovery has largely been limited to serendipitous observations. Due to these drawbacks, phenotype-based drug discovery approaches have largely been replaced over the past 50 years by in vitro assays that are very high-throughput [18].
Table 2:
Drug discovery approaches
| Screening system | Advantages | Drawbacks |
|---|---|---|
| Mammalian | • In vivo relevance | • Low-throughput |
| • High cost | ||
| In vitro | • High-throughput | • Cannot be used to identify new targets |
| • Known target | • Undetermined relevance in vivo | |
| Zebrafish | • High-throughput | • Imperfect model of human biology |
| • In vivo relevance |
Ironically, target-based assays are especially problematic for psychiatric disorders, which are poorly understood and difficult to model in vitro. Unlike phenotype-based chemical screens, target-based in vitro screens are designed to identify compounds that act on pre-defined molecular targets [19, 20]. In vitro assays are incredibly valuable. However, they cannot be applied to poorly understood illnesses for which the appropriate therapeutic targets are unknown [18]. Biological understanding is a prerequisite, not a result, of in vitro screens. In vitro approaches to developing analogues of existing drugs are often profitable and sometimes provide therapeutic benefits. However, truly significant advances in psychiatric pharmacotherapy will require the discovery of novel drug classes and therapeutic reagents.
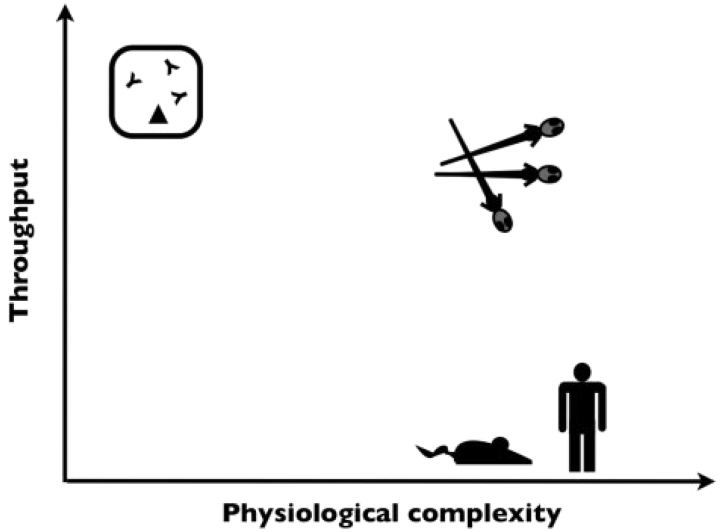
Unlike other approaches, behavioural assays in larval zebrafish have the potential to combine the advantages of phenotype-based drug discovery with high-throughput chemical screening methodologies (Figure 1). Zebrafish assays are lower-throughput than in vitro assays; however, they are much higher-throughput than behavioural tests in mammals. Like other chemical screens in zebrafish, behavioural screens will depend on larval phenotypes because only larvae are small enough to be easily arrayed in multi-well plates with chemicals from a chemical library (adult animals are too large). Today, most zebrafish chemical screens evaluate fewer than ten thousand chemicals. In future, the number of chemicals screened will likely increase to tens and even hundreds of thousands as automated robotic screening technologies become more accessible. Thus, zebrafish can make large-scale behaviour-based drug screens possible at a throughput that would be unattainable using mice and other large model organisms.
Figure 1:
Zebrafish combine high-throughput screening and physiologically complex phenotyping. Behavioural assays in mammals are a physiologically complex but low-throughput approach to drug discovery. Conversely, in vitro target-based assays are a high-throughput but low-content approach. Behaviour-based chemical screens in the zebrafish combine the advantages of behavioural assays with high-throughput screening methodologies and are a promising way to identify novel neuroactive drugs.
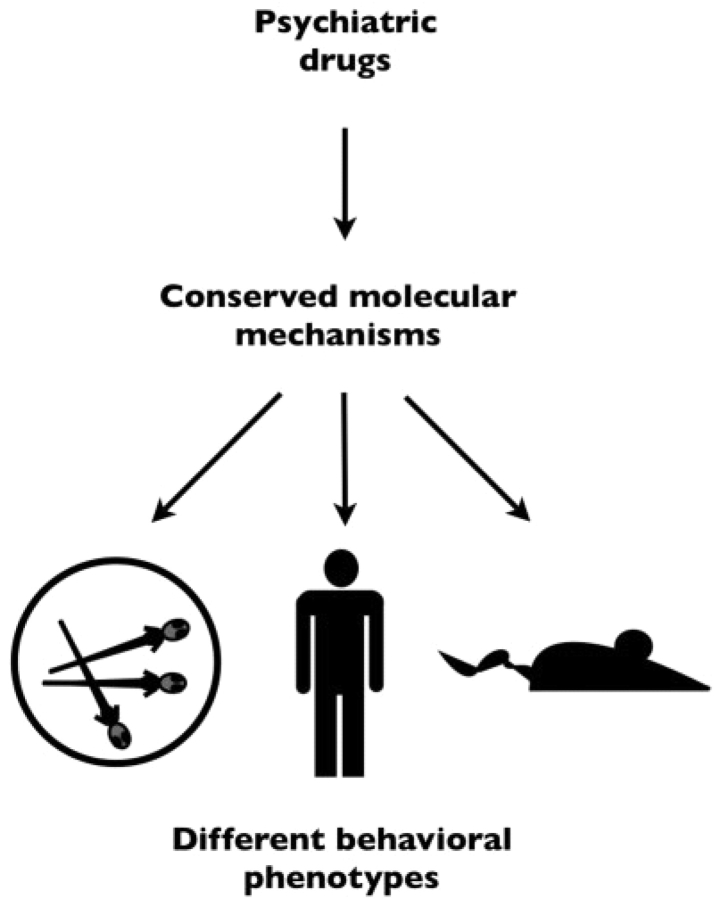
Fish do not suffer from schizophrenia or depression. So how can they be used to identify psychiatric drugs? Because drugs act on highly conserved molecular targets to affect neuronal signaling, behaviours in other species can be used to identify chemicals with potential psychoactive properties in humans (Figure 2). Behavioural tests in model organisms need not simulate human behaviours or illnesses to be useful for drug discovery. To be useful for drug screening, behaviour only needs to be sensitive and specific to neuroactive drugs [21]. In theory, almost any behaviour in any animal could be used to discover neuroactive drugs; even simple behaviours in larval zebrafish [22].
Figure 2:
Psychiatric drugs act though conserved mechanisms to alter behavioural phenotypes. Evolutionarily conserved drug targets mediate different behavioural phenotypes in a variety of organisms.
The behaviour of larval zebrafish is a relatively small but exciting field that is developing rapidly. Zebrafish larvae exhibit a variety of behaviours including the optokinetic response [23], the optomotor response [24], prepulse inhibition [25] and sleep [26,27]. In the optokinetic response (OKR) assay, smooth-pursuit and saccadic eye movements are measured in partially immobilized ∼5-day-old larvae as they respond to movements in their visual field [28]. Abnormal smooth-pursuit eye movements are an endophenotype of schizophrenia, suggesting that this behaviour has great potential for identifying potential anti-psychotic drugs. Although the OKR assay requires some labour-intensive manual steps, further refinements to this assay may increase its throughput for large-scale chemical screens. In the optomotor (OMR) assay, changes in swimming direction are measured in response computer-animated patterns of moving images. The OMR assay is high-throughput, capable of evaluating the behaviour of hundreds of larvae in a few minutes, and would likely be a powerful approach for identifying neuroactive chemicals that affect motion perception [24, 29]. Prepulse inhibition (PPI) is a phenomenon in which a startle reflex to a high intensity stimulus (the pulse) is inhibited by a lower intensity prepulse. An automated behavioural assay for PPI in zebrafish larvae has recently been described that can evaluate hundreds of larvae per trial [25, 30]. Deficits in PPI are an endophenotype of schizophrenia in humans and, haloperidol, an anti-psychotic drug, can suppress prepulse inhibition defects in larval zebrafish [25, 30]. Thus, PPI is a promising behavioural test on which to base large-scale chemical screens. Sleep-like behaviour has also recently been described in zebrafish, and may be useful for identifying stimulants, hypnotics and other drugs that affect arousal. For example, benzodiazepines modulate sleep in larval zebrafish [27, 31].
Zebrafish larvae exhibit additional behaviours that may be amenable to high-throughput chemical screens including the startle response [32], feeding [33, 34], learning [35] and (of course) swimming [36]. Zebrafish larvae exhibit a robust startle response to high intensity sound stimuli [32]. Startle magnitude may be a useful model for post-traumatic stress disorder. Feeding assays may be useful for modeling aspects of eating disorders and zebrafish behaviours that undergo habituation, sensitization and other simple forms learning may be useful models for identifying cognitive enhancers. Finally, simple locomotor swimming behaviours may also be useful for identifying neuroactive drugs. For example, anti-psychotics and anti-depressants produce locomotor defects [37, 38], and anti-convulsants can suppress seizures in zebrafish larvae [39, 40]. Like behavioural assays in all model organisms, new behavioural assays in larval zebrafish will be difficult to develop. However, the unique ability to perform large-scale chemical screens in larval zebrafish provides strong motivation to pursue these developments.
Considering the strengths of zebrafish as a developmental and genetic model organism one can imagine many additional potential applications for high-throughput behavioural assays beyond forward chemical screens in wild-type animals. For example, high-throughput behavioural assays would be a valuable tool for phenotyping genetic mutants, performing chemical and genetic suppressor/enhancer screens and analysing large-scale morpholino loss-of-function experiments. Because zebrafish larvae are transparent, transgenic animals expressing fluorescent markers could also be used to identify chemicals that affect neuronal morphology in vivo, or to correlate structural changes in the nervous system with behavioural outcomes. Overall, these examples suggest that larval behaviours have great potential to be used for investigating psychopharmacolgy in general.
CHEMOBEHAVIOURAL PHENOMICS
Most psychiatric drugs have complex pharmacologies and are thought to exert their therapeutic effects by acting on multiple biological targets simultaneously [41]. By focusing on single molecular targets, target-based in vitro assays fail to capture this complexity. In contrast, behavioural phenotypes in zebrafish are much more likely to identify chemicals with complex pharmacological interactions.
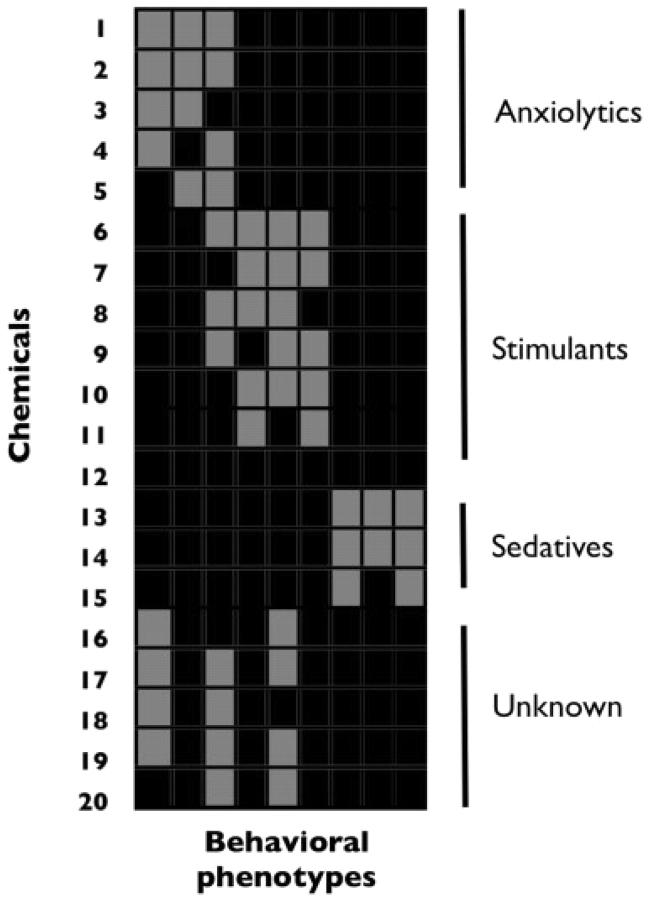
Comprehensive phenotypic profiling will be helpful for categorizing, understanding and identifying psychoactive drugs [42, 43]. Comprehensive analysis of behavioural profiles will be particularly useful for identifying drugs, and combinations of drugs, that have complex pharmacologies. Like transcriptional profiling, which has been invaluable for correlating biological phenotypes with changes in gene expression, chemobehavioural profiling will be invaluable for correlating phenotypes with drug pharmacology. Chemicals with similar phenotypic signatures are likely to share common mechanisms of action; large-scale analyses may be especially sensitive for identifying such similarities (Figure 3).
Figure 3:
Chemobehavioural phenomics—a vision for the future. In this hypothetical analysis, chemicals are clustered by their phenotypic signatures, which are generated from a battery of standardized behavioural tests. Chemicals annotated as anxiolytics, stimulants or sedatives cluster into three families. A group of chemicals with unknown mechanisms of action appears to partially phenocopy anxiolytics and stimulants, suggesting that they may represent a novel class of psychoactive molecules with some anxiolytic and stimulant properties. Grey boxes indicate drug activity in a given behavioural assay, black boxes indicates no activity.
Systems biology uses large-scale datasets to investigate molecular signalling networks from a comprehensive and integrative standpoint. Since the advent of genomics and proteomics, numerous genome-wide, data rich fields of biology (such as glycomics and metabolomics) have emerged based on the compilation of large-scale datasets. Today, large-scale phenotypic datasets generated in high-throughput behavioural screens are giving rise to the emerging field of chemobehavioural phenomics: the integrative analysis of how chemicals affect behaviour.
Like other sub-disciplines of systems biology, chemobehavioural phenomics depends on high-throughput technologies for collecting, organizing and analysing large-scale datasets [44]. Automated strategies for behavioural phenotyping are essential for the acquisition of large-scale phenotypic datasets. Fortunately, zebrafish behaviours are readily amenable to automated data acquisition and analysis. For example, automated high-throughput screens for chemicals that affect heart rate in larvae have been successfully used to discriminate known stimulants from other molecules [45]. Behavioural tracking programs have been used to identify zebrafish seizures [40, 46], circadian locomotor activity [26, 27] and other swimming behaviours [30]. High-throughput screens will give rise to large-scale behavioural datasets on a scale never before possible.
ENCOURAGING GOOD BEHAVIOUR
Phenotype-based drug discovery in the zebrafish is an exciting new approach for identifying psychiatric drug prototypes that may act on previously unidentified therapeutic targets [47]. Today, the first large-scale high-throughput behaviour-based chemical screens have yet to be described. However, recent successes using phenotype-based drug discovery in the zebrafish suggest that these screens cannot be far away. For example, the first known small-molecule inhibitor of BMP signalling, dorsomorphin, was recently identified based on its ability to perturb dorso-ventral axis formation in embryonic zebrafish [48]. Because conserved molecular pathways mediate axis formation in fish and iron homeostasis in humans, this drug may have therapeutic value for treating anaemia [48]. Phenotype-based chemical screens in the zebrafish have also been used to identify molecules that suppress cancer-related and cardiovascular disease phenotypes [49–52]. It is likely only a matter of time before high-throughput behaviour-based chemical screens in zebrafish are applied to psychiatric drug discovery.
Key Points.
The prototypes of most psychiatric drugs were discovered serendipitously via behavioural phenotypes.
Drugs act through conserved molecular mechanisms to affect different behaviors in different animals.
Chemobehavioural phenomics, the systems-level analysis of how chemicals affect behaviour, is based on large-scale phenotypic datasets collected from high-throughput behavioural screens.
Behaviour-based assays in the zebrafish are an exciting new tool for psychiatric drug discovery.
FUNDING
National Institutes of Health (HL079267, CA118498, HL07208).
Acknowledgements
The authors thank Peter Schlueter, Chetana Sachidanandan, Arpita Mukhopadhyay, Joanna Yeh, Sonia Kim, David Healey and Amber Bradley for helpful comments on the article.
Biography
David Kokel and Randall Peterson are affiliated with Harvard Medical School, Massachusetts General Hospital, and the Broad Institute of MIT and Harvard.
References
- Pangalos MN, Schechter LE, Hurko O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat Rev Drug Discov. 2007;6:521–32. doi: 10.1038/nrd2094. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–51. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Lansbury PTJ. Back to the future: the ‘old-fashioned’ way to new medications for neurodegeneration. Nat Med. 2004;10(Suppl):S51–7. doi: 10.1038/nrn1435. [DOI] [PubMed] [Google Scholar]
- Sneader W. Drug Discovery: A History. Chichester: Wiley-Interscience; 2005. [Google Scholar]
- Michaelis M, Schölkens B, Rudolphi K. An anthology from Naunyn-Schmiedeberg's archives of pharmacology. Naunyn-Schmiedeberg's Arch Pharmacol. 2007;375:81–4. doi: 10.1007/s00210-007-0136-z. [DOI] [PubMed] [Google Scholar]
- Feldman SA. Confessions of a managed behavioral health care physician. J Dev Behav Pediatr. 2002;23:S51–6. doi: 10.1097/00004703-200202001-00009. [DOI] [PubMed] [Google Scholar]
- Brown WA. Images in psychiatry: Charles Bradley, M.D., 1902-1979. Am J Psychiatr. 1998;155:968. [Google Scholar]
- Bromley E. Stimulating a normal adjustment: misbehavior, amphetamines, and the electroencephalogram at the Bradley Home for children. J Hist Behav Sci. 2006;42:379–98. doi: 10.1002/jhbs.20192. [DOI] [PubMed] [Google Scholar]
- Dandy WE. Ventriculography following the injection of air into the cerebral ventricles. Ann Surg. 1918;68:5–11. doi: 10.1097/00000658-191807000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends Pharmacol Sci. 2007;28:588–96. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Maxwell RA, Eckhardt SB, editors. Drug Discovery: A Casebook and Analysis. Totowa: Humana Press; 1990. [Google Scholar]
- Kuhn R. The treatment of depressive states with G 22355 (imipramine hydrochloride) Am J Psychiatr. 1958;115:459–64. doi: 10.1176/ajp.115.5.459. [DOI] [PubMed] [Google Scholar]
- Ramchandani D, López-Muñoz F, Alamo C. Meprobamate-tranquilizer or anxiolytic? A historical perspective. Psychiatr Quart. 2006;77:43–53. doi: 10.1007/s11126-006-7960-z. [DOI] [PubMed] [Google Scholar]
- Walter G. John Cade and lithium. Psychiatr Serv. 1999;50:969. doi: 10.1176/ps.50.7.969. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Molecular targets versus models for new antiepileptic drug discovery. Epilepsy Res. 2006;68:22–8. doi: 10.1016/j.eplepsyres.2005.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W. Birkhauser (Architectural). 1999. Valproate (Milestones in Drug Therapy) [Google Scholar]
- Weisler RH, Calabrese JR, Bowden CL, et al. Discovery and development of lamotrigine for bipolar disorder: a story of serendipity, clinical observations, risk taking, and persistence. J Affect Disord. 2008;108:1–9. doi: 10.1016/j.jad.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Berg EL, Kunkel EJ. Systems biology in drug discovery. Nat Biotechnol. 2004;22:1253–9. doi: 10.1038/nbt1017. [DOI] [PubMed] [Google Scholar]
- Fishman MC, Porter JA. Pharmaceuticals: a new grammar for drug discovery. Nature. 2005;437:491–3. doi: 10.1038/437491a. [DOI] [PubMed] [Google Scholar]
- Benson JD, Chen YN, Cornell-Kennon SA, et al. Validating cancer drug targets. Nature. 2006;441:451–6. doi: 10.1038/nature04873. [DOI] [PubMed] [Google Scholar]
- Willner P. Behavioural Models in Psychopharmacology: Theoretical, Industrial, and Clinical Perspectives. xiii. Cambridge; New York: Cambridge University Press; 1990. p. 540. [Google Scholar]
- Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther. 2007;82:70–80. doi: 10.1038/sj.clpt.6100223. [DOI] [PubMed] [Google Scholar]
- Brockerhoff SE, Hurley JB, Janssen-Bienhold U, et al. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc Natl Acad Sci USA. 1995;92:10545–9. doi: 10.1073/pnas.92.23.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhauss SC, Biehlmaier O, Seeliger MW, et al. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J Neurosci. 1999;19:8603–15. doi: 10.1523/JNEUROSCI.19-19-08603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci. 2007;27:4984–94. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Rihel J, Onah AA, et al. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26:13400–10. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanova IV, Wang SY, Leclair OU, Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–8. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
- Brockerhoff SE. Measuring the optokinetic response of zebrafish larvae. Nat Protocols. 2006;1:2448–51. doi: 10.1038/nprot.2006.255. [DOI] [PubMed] [Google Scholar]
- Muto A, Orger MB, Wehman AM, et al. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005;1:e66. doi: 10.1371/journal.pgen.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. J Exp Biol. 2007;210:2526–39. doi: 10.1242/jeb.003939. [DOI] [PubMed] [Google Scholar]
- Renier C, Faraco JH, Bourgin P, et al. Genomic and functional conservation of sedative-hypnotic targets in the zebrafish. Pharmacogenet Genomics. 2007;17:237–53. doi: 10.1097/FPC.0b013e3280119d62. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Bombardieri RA, Meyer DL. The Mauthner-initiated startle response in teleost fish. J Exp Biol. 1977;66:65–81. doi: 10.1242/jeb.66.1.65. [DOI] [PubMed] [Google Scholar]
- Borla MA, Palecek B, Budick S, O’Malley DM. Prey capture by larval zebrafish: evidence for fine axial motor control. Brain Behav Evol. 2002;60:207–29. doi: 10.1159/000066699. [DOI] [PubMed] [Google Scholar]
- Gahtan E, Tanger P, Baier H. Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J Neurosci. 2005;25:9294–303. doi: 10.1523/JNEUROSCI.2678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JD, Berghmans S, Hunt JJ, et al. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2007;33:1206–15. doi: 10.1038/sj.npp.1301489. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Saint-Amant L, Buss RR, et al. Development of the locomotor network in zebrafish. Progress Neurobiol. 2002;68:85–111. doi: 10.1016/s0301-0082(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Giacomini NJ, Rose B, Kobayashi K, Guo S. Antipsychotics produce locomotor impairment in larval zebrafish. Neurotoxicol Teratol. 2006;28:245–50. doi: 10.1016/j.ntt.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Airhart MJ, Lee DH, Wilson TD, et al. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC) Neurotoxicol Teratol. 2007;29:652–64. doi: 10.1016/j.ntt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 2005;131:759–68. doi: 10.1016/j.neuroscience.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Berghmans S, Hunt J, Roach A, Goldsmith P. Zebrafish offer the potential for a primary screen to identify a wide variety of potential anticonvulsants. Epilepsy Res. 2007;75:18–28. doi: 10.1016/j.eplepsyres.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–9. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Freimer N, Sabatti C. The human phenome project. Nat Genet. 2003;34:15–21. doi: 10.1038/ng0503-15. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Nestler EJ. Neurobehavioral assessment in the information age. Nat Neurosci. 2004;7:462–6. doi: 10.1038/nn1225. [DOI] [PubMed] [Google Scholar]
- Lussier YA, Liu Y. Computational approaches to phenotyping: high-throughput phenomics. Proc Am Thorac Soc. 2007;4:18–25. doi: 10.1513/pats.200607-142JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan DJ, Peterson TA, Ruskin JN, et al. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107:1355–8. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Dinday MT, Castro PA, et al. A large-scale mutagenesis screen to identify seizure-resistant zebrafish. Epilepsia. 2007;48:1151–7. doi: 10.1111/j.1528-1167.2007.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RT, Fishman MC. Discovery and use of small molecules for probing biological processes in zebrafish. Methods Cell Biol. 2004;76:569–91. doi: 10.1016/s0091-679x(04)76026-4. [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey RD, Stern HM, Straub CT, Zon LI. A chemical genetic screen for cell cycle inhibitors in zebrafish embryos. Chem Biol Drug Des. 2006;68:213–9. doi: 10.1111/j.1747-0285.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- Stern HM, Murphey RD, Shepard JL, et al. Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nat Chem Biol. 2005;1:366–70. doi: 10.1038/nchembio749. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Shaw SY, Peterson TA, et al. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol. 2004;22:595–9. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci USA. 2000;97:12965–9. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]