Abstract
Dendritic spine morphogenesis contributes to brain function, cognition, and behavior, and is altered in psychiatric disorders. Kalirin is a brain-specific guanine-nucleotide exchange factor (GEF) for Rac-like GTPases and is a key regulator of spine morphogenesis. Here, we show that KALRN-knockout mice have specific reductions in cortical, but not hippocampal, Rac1 signaling and spine density, and exhibit reduced cortical glutamatergic transmission. These mice exhibit robust deficits in working memory, sociability, and prepulse inhibition, paralleled by locomotor hyperactivity reversible by clozapine in a kalirin-dependent manner. Several of these deficits are delayed and age-dependent. Our study thus links spine morphogenic signaling with age-dependent, delayed, disease-related phenotypes, including cognitive dysfunction.
Keywords: prefrontal cortex, kalirin-7, schizophrenia, Alzheimer's disease, synaptic plasticity
Most excitatory synapses in the mammalian forebrain are located on dendritic spines. Structural and functional modifications of spiny synapses are central to brain development, plasticity, and contribute to cognitive functions and behavior (1). Conversely, spine morphogenesis is altered in neuropsychiatric diseases (2).
Small GTPases are central regulators of dendritic spine morphogenesis. Kalirin, a brain-specific GEF for Rho-like small GTPases (3, 4), is a key regulator of spine morphogenesis, with expression restricted mainly to the cerebral cortex and hippocampus (5). The KALRN gene produces several kalirin isoforms generated by alternative splicing (6). Kalirin-7 is the most abundant isoform in the adult brain, with expression undetectable at birth and increasing during synaptogenesis (7). Kalirin-7 directly activates Rac1 and is concentrated in the postsynaptic densities (PSD) of spines through interactions with scaffold proteins, including PSD-95 (8, 9). In pyramidal neurons, kalirin-7 regulates postsynaptic actin dynamics, spine maturation (10), maintenance (11, 12), and activity-dependent plasticity (7).
Reduced forebrain kalirin mRNA expression has been detected in neuropsychiatric disorders including schizophrenia (13, 14) and Alzheimer's disease (15, 16), and genome-wide studies have found an association of the KALRN gene with adult attention deficit hyperactivity disorder (ADHD) and schizophrenia (17, 18). Interestingly, impaired working memory is a core feature of all of these disorders (19–23). We thus hypothesized that the absence of KALRN will result in specific behavioral phenotypes with relevance for psychiatric disorders, including deficient spatial working memory. To model the effects of kalirin loss on forebrain synaptic structure and behavior, we ablated the KALRN gene in mice. Surprisingly, we found that the knockout of KALRN produces striking, yet selective, age-dependent, anatomical, functional, and behavioral deficits, which resemble disease-related phenotypes.
Results
Kalirin Affects Cortical Spine Morphogenesis In Vivo.
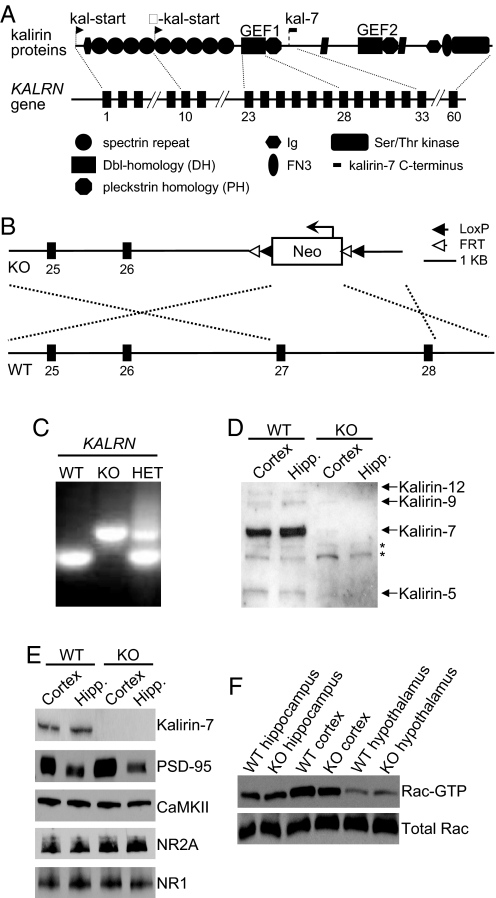
To investigate kalirin-dependent phenotypes, we knocked out the KALRN gene in mice (Fig. 1 A–C). KALRN-knockout mice (KO) are viable, fertile, and display no gross anatomical deficits. All kalirin proteins were absent from the KO brains (Fig. 1D). The abundance of kalirin-interacting proteins and glutamate receptor subunits in cortical and hippocampal homogenates did not differ between KO and WT mice (Fig. 1E, and Fig. S1 A–D). Surprisingly, although kalirin was absent from both the KO cortex and hippocampus, Rac1 activity (Rac1-GTP) was only reduced in the cortex of KO mice (WT cortex, 1 ± 0.13; KO cortex, 0.73 ± 0.12; P < 0.05; WT hippocampus, 1 ± 0.25; KO hippocampus, 1.09 ± 0.17; P > 0.05; Fig. 1F). As an additional control, we assessed Rac1-GTP levels in the hypothalamus as this region expresses low levels of kalirin (5). Indeed, we found that hypothalamic Rac1-GTP levels did not differ between KO and WT animals (WT, 1 ± 0.12; KO, 1.08 ± 0.06; P > 0.05; Fig. 1F).
Fig. 1.
Absence of kalirin leads to a cortex-specific reduction in Rac1 activation. (A) Domain structure of major kalirin proteins and schematic structure of the KALRN gene with exons corresponding to key domains. Arrows indicate alternative translational start sites. (B) Gene targeting strategy. Exons 27–28, containing the active site of the GEF1 domain, have been replaced with the neo cassette. (C) PCR genotyping of KALRN-KO mice. (D) Kalirin isoforms are absent from the KALRN-KO mouse forebrain. *Indicate nonspecific bands (E) The expression of kalirin-7 and synaptic proteins in the hippocampus and frontal cortex of KO and WT mice. (F) KALRN-KO mice have reduced Rac1-GTP levels in the frontal cortex (P < 0.05), but no alteration in Rac1-GTP levels in the hippocampus or hypothalamus.
Cdc42 is a Rho-like small GTPase with an established role in facilitating spine morphogenesis (24). However, unlike Rac1, Cdc42 is not activated by kalirin (9). We found that relative to WT mice, active Cdc42 levels were not altered in either the hippocampus or cortex of KO animals (P > 0.05; Fig. S1 E–G). This further indicates that kalirin loss produces a specific deficit in cortical Rac1-GTP levels.
Because kalirin is a key regulator of spine morphogenesis and maintenance in forebrain pyramidal neurons, we compared spine density in the frontal cortex (layer V) and hippocampus (CA1) in 12-week-old KALRN KO and WT mice. Golgi staining revealed a significant reduction in spine density in the KO frontal cortex (spines per 10 μm of dendrite: WT, 9.33 ± 0.29; KO, 6.23 ± 0.14; P < 0.0001; Fig. 2 A and B) but not in the hippocampus (spines per 10 μm of dendrite: WT, 8.96 ± 0.24; KO, 8.97 ± 0.25; P > 0.05; Fig. 2 C and D). This paralleled the reduced Rac1-GTP levels in the cortex, but not in the hippocampus, of KO mice.
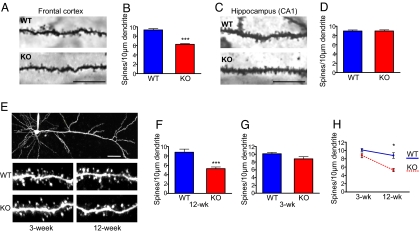
Fig. 2.
Brain region specific and age-dependent alterations in spine density in KALRN-KO mice. (A) Golgi-stained pyramidal neurons from frontal cortex layer V in KO and WT mice. (B) Quantification of (A) indicates that KO mice have a robust reduction in spine density in the frontal cortex relative to WT mice (***, P < 0.0001). (C) Golgi-stained hippocampal neurons from the CA1 field in KO and WT mice. (D) Quantification of (C) shows that spine density is unaffected in the CA1 field of KO mice relative to WT mice. (E) Two-photon imaging of layer V frontal cortical neurons in slices from 3- and 12-week-old mice. (F) Quantification of (E) shows that frontal cortex spine density was severely reduced in 12-week-old KALRN-KO mice (***, P < 0.0001). (G) Frontal cortical spine density was unaffected in 3-week-old KO mice. (H) KO mice show an age-mediated delayed reduction in spine density in the frontal cortex (*, P < 0.05). [Scale bars, 10 μm (A and C), 50 μm (E).] Data are mean ± SEM
We thus focused our further investigations on the frontal cortex. By imaging live dye-filled layer V pyramidal neurons in frontal cortex slices using 2-photon laser scanning microscopy (2P-LSM), we compared dendritic spine density on oblique dendrites in 12-week-old KO and WT mice. We detected a reduction in spine density in 12-week-old KALRN KO mice (spines per 10 μm of dendrite: WT, 8.79 ± 0.67; KO, 5.28 ± 0.37; P < 0.0001; Fig. 2 E and F), similar to that observed by Golgi staining. Neurons from 12-week-old KO and WT mice did not differ in mean spine area or length (Fig. S2A).
We also examined the spine density of frontal cortical layer V pyramidal neurons in 3-week-old KO and WT mice using 2P-LSM. Interestingly, the spine density of 3-week-old KO mice did not significantly differ from that of 3-week-old WT mice (spines per 10 μm of dendrite: WT, 10.13 ± 0.35; KO, 8.81 ± 0.57; P > 0.05 Fig. 2 E and G), and the spine density reduction in KO mice became more prominent with age (age × genotype: F (1, 38) = 5.08; P < 0.05; Fig. 2H). Similar to 12-week-old animals, neurons from 3-week-old KO and WT mice did not differ in mean spine area or length (Fig. S2B).
Individual mature cortical pyramidal neurons (DIV28) from KO mice had a significantly lower spine density compared with WT neurons and the overexpression of kalirin-7 rescued this deficit (Fig. S2 C and D).
Impaired Cortical AMPAR-Mediated Synaptic Transmission in KALRN KO Mice.
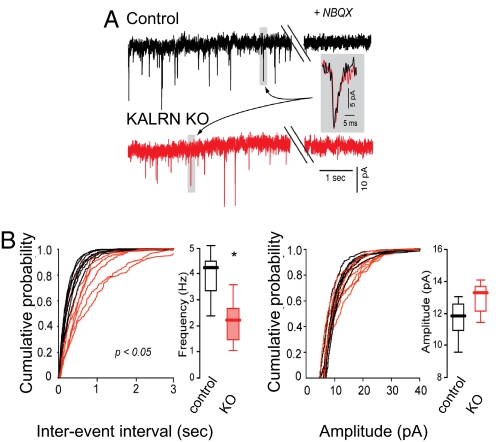
Kalirin modulates AMPA receptor (AMPAR) maintenance in spines and AMPAR-mediated synaptic transmission in cortical pyramidal neurons (7). Indeed, AMPAR-mediated synaptic transmission was significantly reduced in layer V KO pyramidal neurons from acute frontal cortical slices (Fig. 3A). KO neurons showed a significant reduction in AMPA mEPSC frequency (WT, 4.26/s, range 2.39–5.15; n = 7 cells; KO, 2.25/s, range 1.14–3.61; n = 7 cells; P < 0.05; Fig. 3B and Fig. S3C), but no significant difference in mEPSC amplitudes (WT, 11.8 pA, range 9.5–13.1; n = 7 cells; KO, 13.3 pA, range 9.7–17.5; n = 7 cells; P > 0.05; Fig. 3B and Fig. S3D).
Fig. 3.
Reduced AMPAR-mediated synaptic transmission in KALRN-KO mice. (A) Traces of AMPA mEPSC recordings from layer V pyramidal neurons in frontal cortical slices from WT and KO mice. (B) Quantification of (A) indicates that KO mice have a reduction in mEPSC frequency (*, P < 0.05), but no alteration in mEPSC amplitude. Data are median ± data distribution (box plots)
Expression levels of GluR1 and GluR2/3 in cortical and hippocampal lysates were not different between WT and KO (Fig. S3A and B). Furthermore, the expression levels of the presynaptic proteins VGLUT, VGAT, and synaptoporin were unaltered in KO mice (Fig. S3A and B). There was no difference in mEPSC rise (P > 0.05) or decay times (P > 0.05; Fig. S3E and F). Whereas WT neurons showed an increase in spine area 30-min post-APV withdrawal relative to basal conditions (area in μm2: WT basal, 0.977 ± 0.05; WT activated, 1.330 ± 0.08; P < 0.001), KO neurons failed to show this increase (area in μm2: KO basal, 0.841 ± 0.062; KO activated, 0.901 ± 0.07; P > 0.05).
Specific Behavioral Alterations in KALRN KO Mice.
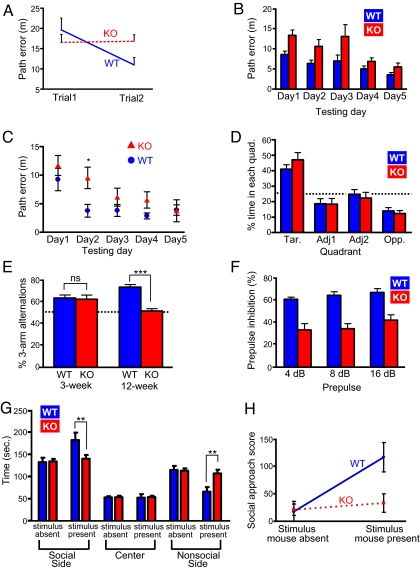
Spine morphogenesis and its regulators are associated with cognitive functions in humans (25), and deficits in cognitive functions, especially working memory, occur in certain psychiatric disorders, such as schizophrenia, ADHD, and Alzheimer's disease (19–23). To determine whether the absence of KALRN affected specific cognitive functions, we tested spatial working and reference memory in 12-week-old animals using established methods for the Morris water maze (26). Before testing, all animals underwent training with a visible platform, which indicated that KO animals did not have gross deficits that would preclude accurate memory assessment (Fig. S4A). Using a 2-trial matching-to-sample Morris water maze task to test spatial working memory (26), we found that unlike WT mice, KO mice failed to improve their performance in the second trial vs. the first trial across the 5 testing days, indicative of impaired spatial working memory in the KO mice [trial × genotype: F (1, 22) = 8.07; P = 0.01; Fig. 4A].
Fig. 4.
Behavioral phenotypes of KALRN-KO mice. (A) Two-trial matching-to-sample performance in the Morris water maze (MWM) shows that WT mice, but not KO mice, have a reduced path error on trial 2 relative to trail 1 (trial × genotype, P = 0.01). Data are means of each trial across 5 testing days. (B) MWM spatial reference memory (fixed platform test). Data are means of 6 trials for each day; main effects are observed for day and genotype (P < 0.001), but no effect of genotype by day (P > 0.05). (C) Additional analysis of MWM spatial reference memory. Means of trail 1 for each testing day; KO mice show an increased path error relative to WT mice on day 2 (*, P < 0.05), whereas performance between genotypes did not differ on any other days. (D) Water maze probe trail as a test of spatial reference memory shows the percentage of time animals search the target quadrant (Tar), the 2 adjacent quadrants (Adj1 and Adj2), and the quadrant opposite the target quadrant (Opp) for the removed platform. KO and WT mice spent a similar percentage of time searching in the target quadrant (P > 0.05). Line indicates chance level (25%). (E) Y-maze spontaneous alternation in 3- and 12-week-old KO and WT mice. Whereas the performance of 3-week-old KO and WT mice did not differ from each other, 12-week-old KO mice showed a deficit in spontaneous alternation relative to 12-week-old WT mice (***, P < 0.001). Line indicates chance performance (50%). Further analysis indicated a significant interaction of genotype by age (P < 0.001). (F) Prepulse inhibition: KO mice have a deficit in sensory motor gating across all prepulse intensities (P < 0.01). (G) Social behavior: In the presence of a stimulus mouse, KO mice spent less time in the social side and more time in the nonsocial side of the chamber relative to WT mice (**, P < 0.01); WT and KO mice show similar behavior in the absence of the stimulus mouse. (H) Additional testing for social behavior: social approach scores. Whereas WT mice show an increased social approach during the testing phase in which the stimulus mouse was present, relative to the habituation phase in which the stimulus mouse was absent, KO mice fail to show an increase in social approach between the 2 trials (P < 0.05). Data are mean ± SEM.
Using a trial-independent Morris water maze task to assess spatial reference memory (26), we compared the learning capability of adult KO and WT mice over a 5-day period (6 trials per day). We found main effects for day [F(2.5, 55.7) = 9.36; P < 0.001], trial [F(3.6, 80.4) = 22.49; P < 0.001], and genotype [F (1, 22) = 13.07; P < 0.005]; however, there was no interaction of genotype by trial or day (P > 0.05) in this task, indicating that both KO and WT mice improved across trials and testing days (Fig. 4B). To assess long-term capabilities across testing days, we compared the reference memory performance for trial 1 only on all 5 testing days. We found that the performance of KO mice differed from WT mice on day 2 only (P < 0.05), with no interaction of genotype by day (P > 0.05) (Fig. 4C). Last, a probe trial conducted on the final testing day showed that KO and WT mice spent a similar percentage of time searching the target pool quadrant for the removed platform (WT, 41 ± 2.85; KO, 47 ± 4.66; P > 0.05; Fig. 4D), and both groups of animals showed an above chance preference for the target quadrant (P < 0.0005).
We used the Y-maze as an additional means of testing reference and working memory (27). Using an arm recognition task in the Y-maze, we further confirmed that KO mice have intact reference memory (Fig. S4B). We assessed spontaneous alternation behavior as a test of working memory in the Y-maze and further confirmed a deficit in adult (12-week-old) KO mice (% of 3-arm alternations: WT, 73 ± 2.40%; KO, 51 ± 2.14%; P < 0.001; Fig. 4E). Because spine loss in KO mice was age-dependent, we determined whether working memory deficits were also age-dependent by assessing 3-week-old mice in the spontaneous alternation task. KO and WT mice of this age performed similarly (% of 3-arm alternations: WT, 63 ± 2.77%; KO, 59 ± 2.80%; P > 0.05, Fig. 4E) and both groups performed above chance levels (P < 0.05). Furthermore, we found a significant interaction of genotype by age for spontaneous alternation [F (1, 31) = 14.48; P < 0.001] indicating an age-dependent progressive impairment in working memory in KO mice.
Sensory-motor gating deficits are common in mice with genetically-induced glutmatergic hypofunction (28–30). In addition, sensory-motor gating deficits are prevalent in schizophrenia patients and are characteristic of schizophrenia animal models (31–35). Prepulse inhibition (PPI) reflects the dampening of the mammalian startle response to an intense auditory stimulus when it is preceded by an innocuous auditory prepulse stimulus by an innocuous auditory prepulse stimulus (36). We found that KALRN KO animals showed a significantly reduced PPI response relative to WT mice across a range of prepulse intensities [F (1, 15) = 8.81, P < 0.01; Fig. 4F], indicative of impaired sensory-motor gating.
Abnormal social behavior is prevalent in certain psychiatric disorders and associated animal models (35, 37, 38). To assess the sociability of KALRN KO mice, we used a 3-chamber social behavior apparatus to quantify the preference of a test mouse for either a live mouse or an inanimate object (39). For each animal, the test consisted of a habituation phase, in which no stimulus mouse was present, and a testing phase in which a stimulus mouse and an inanimate object were present in separate chambers. The end chamber containing the stimulus mouse was designated the social side, and the end chamber containing the inanimate object was designated the nonsocial side. We found that WT mice spent more time in the social side when the stimulus mouse was present than KO mice (time in seconds: WT, 182.00 ± 16.82; KO, 139.80 ± 8.70; P < 0.01) and less time in the nonsocial side when the stimulus mouse was present than KO mice (time in seconds: WT, 65.70 ± 10.25; KO, 106.70 ± 8.38; P < 0.01) (Fig. 4G). We also calculated a social approach score (Methods) for the habituation phase and testing phase for KO and WT mice. We found main effects for genotype [F (1, 36) = 4.39; P < 0.05] and trial [F (1, 36) = 8.50; P < 0.01]. Furthermore, we found a significant social approach interaction of trial × genotype [F (1, 36) = 5.17; P < 0.05; Fig. 4H]. This indicates that in the presence of the stimulus mouse, KO mice show a reduced social approach relative to WT mice.
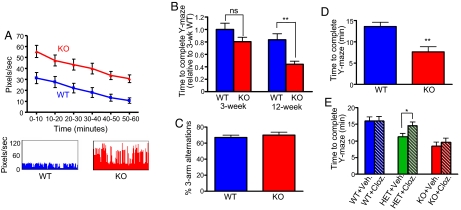
Locomotor hyperactivity is a common feature of mice hypomorphic for genes associated with glutamatergic signaling (29, 40). Locomotor hyperactivity is also a common feature of mice with genetic abnormalities associated with schizophrenia (31, 41). Interestingly, KALRN KO mice showed more spontaneous motor activity in an automated cage than WT mice over a 1-h period (pixels/s: WT, 22.32 ± 3.70; KO, 38.99 ± 4.03; P = 0.001; Fig. 5A).
Fig. 5.
Locomotor hyperactivity in KALRN KO mice. (A) Twelve-week-old KO mice exhibit hyperactive behavior in an open-field environment (P = 0.001). Graphs show mean activity during 10-min bins for 1 h. Activity plots are shown below. (B) Choice-driven activity analysis in the Y-maze among 3- and 12-week-old WT and KO mice. Three-week-old WT and KO mice showed similar choice-driven activity levels. Twelve-week-old KO mice required less time to complete the Y-maze task than 12-week-old WT mice (**, P < 0.01), indicative of choice-driven hyperactivity in 12-week-old KO mice. (C) In the presence of intra-maze cues, Y-maze spontaneous alternation in 12-week-old WT and KO mice did not differ from each other (P > 0.05). (D) In the presence of intra-maze cues in the Y-maze, KO mice required less time to complete the task than WT mice, indicative of hyperactivity in KO mice (**, P < 0.01). (E) Heterozygous mice (HET) showed activity levels that were intermediate between KO and WT mice (P < 0.05). Clozapine (1 mg/kg) reversed the hyperactivity of HET mice (*, P < 0.05), but did not affect KO or WT mouse activity (P > 0.05). Data are mean ± SEM
To determine whether hyperactive behavior was age-dependent, we compared the spontaneous motor activity of 3-week-old KO and WT mice in an automated cage for 1 h and found no differences in activity between juvenile mice (pixels/s: WT, 61.31 ± 3.85; KO, 61.42 ± 6.23; P > 0.05; Fig. S4C). In addition, we compared the activity levels of 3-week-old and 12-week-old KO and WT mice in a choice-driven Y-maze task (42). Interestingly, whereas the activity level of 3-week-old WT and KO mice did not significantly differ from each other (time to complete task relative to 3-week-old WT: WT, 1 ± 0.10; KO 0.80 ± 0.07; P > 0.05; Fig. 5B), 12-week-old KO mice showed higher activity levels than 12-week-old WT mice (time to complete task relative to 3-week-old WT: WT, 0.83 ± 0.097; KO, 0.44 ± 0.05; P < 0.001; Fig. 5B), indicating age-dependent delayed occurrence of hyperactivity.
We showed that KALRN KO mice perform at chance levels in spontaneous alternation in the Y-maze, and KO mice also exhibited hyperactive choice-driven behavior in this task. In these tasks, the Y-maze was surrounded by distal, extra-maze cues in the absence of proximal, intra-maze cues. To assess Y-maze performance in the presence of intra-maze cues, we tested KO and WT mice in the Y-maze in which a single unique intra-maze cue was placed in each arm. We found that the performance of KO and WT mice did not differ in this task (% of 3-arm alternations: WT, 67 ± 2.94%; KO, 70 ± 3.63%; P > 0.05; Fig. 5C), suggesting that KO mice are able to use intra-maze cues to guide spontaneous alternation behavior. Interestingly, KO mice still exhibited hyperactive behavior in this task (time to complete task: WT, 13.59 ± 1.00 min; KO, 7.62 ± 1.22 min; P < 0.01; Fig. 5D), suggesting a disassociation between Y-maze spontaneous alternation performance and activity levels.
Clozapine is an atypical antipsychotic with established efficacy in treating some schizophrenia symptoms (43), and acute administration attenuates hyperactive behavior in some animal models (31). To determine whether acute clozapine administration reversed the hyperactive behavior of KO mice, we administered clozapine (2 mg/kg) or vehicle to 12-week-old mice, and assessed choice-driven activity levels in the Y-maze (intra-maze cues were absent). Vehicle-treated KO mice were more active than vehicle-treated WT mice (P < 0.05); however, clozapine did not affect the activity level of WT mice (time to complete task: vehicle, 15.97 ± 1.22 min; treated, 15.95 ± 1.64 min; P > 0.05) or KO mice (vehicle, 8.4 ± 0.96 min; treated, 9.60 ± 0.91 min; P > 0.05; Fig. 5E). However, heterozygous (HET) mice showed intermediate activity levels relative to WT and KO mice (P < 0.05; Fig. 5E), and clozapine reversed hyperactivity in HET mice (vehicle, 11.25 ± 0.87 min; treated, 14.63 ± 1.11 min; P < 0.05; Fig. 5E).
We also determined the effects of clozapine treatment on working memory in the Y-maze and found that clozapine did not significantly alter the spontaneous alternation working memory of WT, KO, or HET mice (P > 0.05; Fig. S4D). This is consistent with human studies showing that clozapine has limited efficacy in improving cognition in schizophrenia subjects (44).
Discussion
Here we examined the structural, functional, and behavioral consequences resulting from ablation of the KALRN gene in mice, and characterize roles for KALRN in cortical Rac activation, cortical connectivity, and specific behavioral phenotypes in vivo. Our data present a clear relationship between regional loss of Rac activity and regional loss of spine connectivity in KALRN KO animals. We also find that mice with ablation of the KALRN gene show functional deficits and striking, yet selective, behavioral phenotypes. Moreover, several of the behavioral deficits showed an age-dependent manifestation.
We found that KALRN KO mice exhibited significant reductions in Rac1-GTP levels and spine loss in the frontal cotex, whereas Rac1-GTP levels and spine density were preserved in the hippocampus. Why was there a reduction of cortical but not hippocampal connectivity in KALRN KO mice? Kalirin is the only known RacGEF with significant expression levels in the cortex of adolescent and adult animals, whereas other RacGEFs retain high expression in the adult hippocampus but not cortex (3). The different regional and temporal expression of kalirin relative to other RacGEFs helps explain the selective cortical deficits in Rac-GTP and spine density in KALRN KO animals: Even in the absence of kalirin expression, other RacGEFs might be sufficient to activate Rac1 at normal levels in the KO hippocampus resulting in unaltered hippocampal spine connectivity, whereas in the cortex KALRN is necessary for normal Rac activity and spine connectivity.
We found that whereas working memory was impaired in KO animals, reference memory was intact. Working memory requires a reciprocal network connection between the prefrontal cortex and hippocampus (45, 46). Conversely, reference memory is hippocampal-dependent and persists even in the presence of prefrontal cortical inactivation (45). Because spines contribute to synaptic connectivity in cortical networks (47), the contribution of the frontal cortex to cognition may be reduced in KALRN KO mice.
The age-dependent delayed functional and behavioral impairments in schizophrenia have been difficult to explain in humans and to model in animals (48). The causes of delayed decline in schizophrenia are not known, but excessive synapse elimination, or defective synapse formation and turnover, are hypothesized to play a role (49). Here, we find that the synaptic and behavioral profile of KALRN KO mice is normal during early postnatal development (3-week-old), whereas synaptic and behavioral dysfunctions are apparent during young adulthood (12-week-old). This study may thus shed some light on this issue. The late onset of spine loss in KO mice suggests that rather than deficient spine formation, excessive synaptic pruning, possibly because of a reduction in spine stability, may occur in the developing KALRN KO cortex. The develomental expression profile of kalirin may explain this delayed and age-dependent synaptic and behavioral decline in KALRN KO mice (see SI Text).
A recent study examined mice with a deletion of the exon encoding a short peptide targeting kalirin-7 to spines (Δkalirin-7 mice) (12). The removal of the exon encoding the C terminus of kalirin-7 resulted in a compensatory up-regulation of the nonsynaptic kalirin-8, -9, and -12 isoforms. This resulted in an approximate 25% reduction in total forebrain kalirin protein even though kalirin-7 accounts for ≈75% of total kalirin in the adult WT forebrain. Interestingly, Δkalirin-7 mutant mice and KALRN KO mice show several differences in neuronal ultrastructure. Notably, Δkalirin-7 mice exhibit a reduction in hippocampal spine density (≈15%), which contrasts with our finding that the ablation of the KALRN gene affected neither hippocampal Rac-GTP levels nor hippocampal spine density. The up-regulation of the kalirin-9 and -12 isoforms in the Δkalirin-7 mouse could contribute to the reduced hippocampal spine density: Kalirin-9 and kalirin-12 are not normally expressed at high levels in the adult forebrain, and they differ from kalirin-7 in that in addition to a RacGEF domain they also contain a RhoA domain (50), the latter of which has an established role in spine elimination (51). Δkalirin-7 and KALRN KO mice also showed differing behavioral phenotypes. Mainly, KALRN KO mice showed locomotor hyperactivity and deficits in spatial working memory, both of which were unimpaired in Δkalirin-7 mutants (12). This suggests that the complete ablation of KALRN produces additional behavioral deficits as compared with the targeted deletion of kalirin-7 which results in only an approximate 25% net reduction in kalirin protein. Differences in working memory capacity between Δkalirin-7 and KALRN KO mice might be due to the preservation of Rac1 activity in the cortex of Δkalirin-7 mutants and due to the different cognitive demands of the tasks used, notably the use of intramaze vs. extramaze cues (see SI Text).
Spines contribute to synaptic connnectivity in cortical networks, and neuropathological, imaging, and genetic studies support reduced synaptic connectivity as a key contributor to neuropsychiatric disease (52). Recent studies suggest potential roles for kalirin signaling in disorders that affect cognition. KALRN mRNA and kalirin protein expression are consistently underexpressed in the forebrain of Alzheimer's disease patients (15, 16). A recent genome-wide association study linked the KALRN gene with adult ADHD vulnerability (17). Last, reduced KALRN mRNA has been detected in the prefrontal cortex of schizophrenia patients (13, 14) and genome-wide association studies linked the KALRN gene with schizophrenia in 2 independent populations (18). Here we examined the structural, functional, and behavioral consequences resulting from KALRN loss and characterize a role for KALRN in cortical Rac activation, cortical connectivity, and specific behavioral phenotypes.
Methods
Supporting Information.
See SI Text for complete experimental reagents and procedures.
Behavioral Analyses.
For each individual behavioral test, behaviorally naïve animals were used.
Brain Slice Preparation and Electrophysiology.
The frequency, amplitude, and rise and decay times of mEPSCs were analyzed by using MINI ANALYSIS program (Synaptosoft). Deep-layer (V-VI) medial prelimbic/infralimbic pyramidal neurons were assessed: 1–2 slices per animal and 1–2 cells per slice. Recordings were obtained from 4 KO and 3 WT age-matched animals.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health-National Institute of Mental Health Grant R01MH071316, National Alliance for Autism Research, National Alliance for Research on Schizophrenia and Depression (P.P.), National Institutes of Health-National Institute of Mental Health Grant MH57014 and Evelyn F. McKnight (to J.D.S. and C.A.M.), National Institute on Aging Grants R37 AG08796 (to J. D.) and F31AG031621-01A2 (to M.E.C.), National Institutes of Health-National Institute of Mental Health Grant P01 MH074866, and National Institutes of Health-National Institute of Neurological Disorders and Stroke Grant R37 NS034696 (to D.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904636106/DCSupplemental.
References
- 1.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Ann Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 2.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 3.Penzes P, Cahill ME, Jones KA, Srivastava DP. Convergent CaMK and RacGEF signals control dendritic structure and function. Trends Cell Biol. 2008;18:405–413. doi: 10.1016/j.tcb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Penzes P, Jones KA. Dendritic spine dynamics–a key role for kalirin-7. Trends Neurosci. 2008;31:419–427. doi: 10.1016/j.tins.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma XM, Johnson RC, Mains RE, Eipper BA. Expression of kalirin, a neuronal GDP/GTP exchange factor of the trio family, in the central nervous system of the adult rat. neurology. Comp Neurol. 2001;429:388–402. doi: 10.1002/1096-9861(20010115)429:3<388::aid-cne3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RC, Penzes P, Eipper BA, Mains RE. Isoforms of kalirin, a neuronal Dbl family member, generated through use of different 5′- and 3′-ends along with an internal translational initiation site. J Biol Chem. 2000;275:19324–19333. doi: 10.1074/jbc.M000676200. [DOI] [PubMed] [Google Scholar]
- 7.Xie Z, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penzes P, et al. An isoform of kalirin, a brain-specific GDP/GTP exchange factor, is enriched in the postsynaptic density fraction. J Biol Chem. 2000;275:6395–6403. doi: 10.1074/jbc.275.9.6395. [DOI] [PubMed] [Google Scholar]
- 9.Penzes P, Johnson RC, Kambampati V, Mains RE, Eipper BA. Distinct roles for the two Rho GDP/GTP exchange factor domains of kalirin in regulation of neurite growth and neuronal morphology. J Neurosci. 2001;21:8426–8434. doi: 10.1523/JNEUROSCI.21-21-08426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penzes P, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 11.Xie Z, et al. Coordination of synaptic adhesion with dendritic spine remodeling by AF-6 and kalirin-7. J Neurosci. 2008;28:6079–6091. doi: 10.1523/JNEUROSCI.1170-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma XM, et al. Kalirin-7 is required for synaptic structure and function. J Neurosci. 2008;28:12368–12382. doi: 10.1523/JNEUROSCI.4269-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- 14.Narayan S, et al. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res. 2008 doi: 10.1016/j.brainres.2008.08.023. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youn H, et al. Kalirin is under-expressed in Alzheimer's disease hippocampus. J Alzheimers Dis. 2007;11:385–397. doi: 10.3233/jad-2007-11314. [DOI] [PubMed] [Google Scholar]
- 16.Youn H, Ji I, Ji HP, Markesbery WR, Ji TH. Under-expression of kalirin-7 increases iNOS activity in cultured cells and correlates to elevated iNOS activity in Alzheimer's disease hippocampus. J Alzheimers Dis. 2007;12:271–281. doi: 10.3233/jad-2007-12309. [DOI] [PubMed] [Google Scholar]
- 17.Lesch KP, et al. Molecular genetics of adult ADHD: Converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm. 2008;115:1573–1585. doi: 10.1007/s00702-008-0119-3. [DOI] [PubMed] [Google Scholar]
- 18.St. Jean P. Smithkline Beecham Corporation. PA, United States: Philadelphia; 2008. [Google Scholar]
- 19.Germano C, Kinsella GJ. Working memory and learning in early Alzheimer's disease. Neuropsychol Rev. 2005;15:1–10. doi: 10.1007/s11065-005-3583-7. [DOI] [PubMed] [Google Scholar]
- 20.Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- 21.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 22.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(Suppl 8):21–26. [PubMed] [Google Scholar]
- 24.Kreis P, et al. The p21-activated kinase 3 implicated in mental retardation regulates spine morphogenesis through a Cdc42-dependent pathway. J Biol Chem. 2007;282:21497–21506. doi: 10.1074/jbc.M703298200. [DOI] [PubMed] [Google Scholar]
- 25.Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J Neurobiol. 2005;64:58–74. doi: 10.1002/neu.20153. [DOI] [PubMed] [Google Scholar]
- 26.Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarnyai Z, et al. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci USA. 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fradley RL, et al. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behav Brain Res. 2005;163:257–264. doi: 10.1016/j.bbr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Wiedholz LM, et al. Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry. 2008;13:631–640. doi: 10.1038/sj.mp.4002056. [DOI] [PubMed] [Google Scholar]
- 30.Duncan GE, et al. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Stefansson H, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry. 2003;160:815–824. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- 33.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 34.Van den Buuse M, Garner B, Koch M. Neurodevelopmental animal models of schizophrenia: Effects on prepulse inhibition. Curr Mol Med. 2003;3:459–471. doi: 10.2174/1566524033479627. [DOI] [PubMed] [Google Scholar]
- 35.Tarbox SI, Pogue-Geile MF. Development of social functioning in preschizophrenia children and adolescents: A systematic review. Psychol Bull. 2008;134:561–583. doi: 10.1037/0033-2909.34.4.561. [DOI] [PubMed] [Google Scholar]
- 36.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Tuathaigh CM, et al. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman JA, et al. The early stages of schizophrenia: Speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–897. doi: 10.1016/s0006-3223(01)01303-8. [DOI] [PubMed] [Google Scholar]
- 39.Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 40.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 41.Hikida T, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci USA. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savonenko AV, et al. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci USA. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchanan RW. Clozapine: Efficacy and safety. Schizophr Bull. 1995;21:579–591. doi: 10.1093/schbul/21.4.579. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg TE, et al. The effect of clozapine on cognition and psychiatric symptoms in patients with schizophrenia. Br J Psychiatry. 1993;162:43–48. doi: 10.1192/bjp.162.1.43. [DOI] [PubMed] [Google Scholar]
- 45.Yoon T, Okada J, Jung MW, Kim JJ. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learn Mem. 2008;15:97–105. doi: 10.1101/lm.850808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 47.Leuner B, Shors TJ. New spines, new memories. Mol Neurobiol. 2004;29:117–130. doi: 10.1385/MN:29:2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Lipska BK, Weinberger DR. Genetic mouse models of schizophrenia: From hypothesis-based to susceptibility gene-based models. Biol Psychiatry. 2006;59:1180–1188. doi: 10.1016/j.biopsych.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 49.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 50.Rabiner CA, Mains RE, Eipper BA. Kalirin: A dual Rho guanine nucleotide exchange factor that is so much more than the sum of its many parts. Neuroscientist. 2005;11:148–160. doi: 10.1177/1073858404271250. [DOI] [PubMed] [Google Scholar]
- 51.Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: Antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- 52.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.