Abstract
Extracellular stimuli regulate neuronal differentiation and subtype specification during brain development, although the intracellular signaling pathways that mediate these processes remain largely unclear. We now show that the PDK1-Akt pathway regulates differentiation of telencephalic neural precursor cells (NPCs). Active Akt promotes differentiation of NPC into γ-aminobutyric acid-containing (GABAergic) but not glutamatergic neurons. Disruption of the Pdk1 gene or expression of dominant-negative forms of Akt suppresses insulin-like growth factor (IGF)-1 enhancement of NPC differentiation into neurons in vitro and production of neocortical GABAergic neurons in vivo. Furthermore, active Akt increased the protein levels and transactivation activity of Mash1, a proneural basic helix-loop-helix protein required for the generation of neocortical GABAergic neurons, and Mash1 was required for Akt-induced neuronal differentiation. These results have unveiled an unexpected role of the PDK1-Akt pathway: a key mediator of extracellular signals regulating the production of neocortical GABAergic neurons.
Keywords: GABAergic neuronal differentiation, neural precursor cells, telencephalon
The mammalian central nervous system (CNS) arises from common precursor cells (neural precursor cells, or NPCs), which proliferate and generate both neurons and glia (1, 2). The development of the CNS involves sequential waves of neurogenesis and gliogenesis (3–5) and requires an appropriate balance between the proliferation and differentiation of NPCs and their progeny (6). Accumulating evidence has shown that extrinsic cues, including cell-cell interactions and secreted molecules, are key determinants of NPC fate. For instance, extrinsic factors such as Wnt-7a, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-1, bone morphogenetic protein (BMP), erythropoietin, and the neurotrophic factors brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), and neurotrophin-3 promote neuronal differentiation by several mechanisms, including the selective expansion of neuronal progenitors, enhancement of the survival of neurons (or their progenitors), and favoring neuronal fate (7–10). However, few studies have investigated the underlying mechanisms that mediate the neurogenic effects of these extrinsic factors.
Diverse types of neurons are produced during CNS development. Two major types of neurons arise during development of telencephalon: excitatory, glutamatergic neurons in the dorsal region, and inhibitory, GABAergic neurons in the ventral region (11, 12). A key issue in developmental neurobiology concerns the mechanisms responsible for determining the generic and specific characteristics of neurons. These mechanisms are mediated by combinations of transcription factors including proneural basic helix-loop-helix (bHLH) and homeodomain proteins (10, 13, 14). The bHLH proteins neurogenin1 (Ngn1), Ngn2, and Mash1 are important for neuronal fate determination of undifferentiated NPCs (15–17). Ngns and Mash1 are expressed in complementary patterns in the dorsal and ventral parts of the telencephalon, contributing to the generation of glutamatergic and GABAergic neurons, respectively (17, 18). Although some transcriptional/posttranscriptional regulations on these transcription factors have been revealed, it largely remains unclear how they are regulated by neurogenic extrinsic cues during CNS development (7, 19–24).
A signaling module comprised of phosphoinositide-dependent kinase 1 (PDK1) and Akt is activated via phosphoinositide 3-kinase (PI3K) by various extrinsic cues. This signaling module plays a pivotal role in the regulation of cell survival, proliferation, and size in many systems (25–28). In fact, a number of studies has suggested the roles of the PI3K-Akt pathway in NPC proliferation and/or differentiation (29–33). However, even though PDK1 and all 3 members of the Akt family are expressed in the developing CNS, genetic evidence for their importance in CNS development is lacking. Mice deficient in 1 or 2 Akt family members exhibit little embryonic defects in the CNS, possibly because of functional compensation among the family members (28, 34, 35). Pdk1 knockout mice, in which Akt activity is almost completely absent, die before CNS development starts (26). We therefore generated mice in which Pdk1 is conditionally ablated in the CNS to examine the role of the PDK1-Akt pathway in CNS development.
Here we show that PDK1 is essential for regulation of NPC differentiation during CNS development. Disruption of Pdk1 or expression of dominant-negative forms of Akt reduced neuronal differentiation of NPCs. Moreover, constitutively active Akt promoted NPC differentiation into GABAergic but not glutamatergic neurons. Consistently, CNS-specific ablation of Pdk1 impaired production of GABAergic but not glutamatergic neurons in the telencephalon. We further show that Akt activates Mash1, a transcription factor important for the differentiation of a subset of GABAergic neurons, and that Akt has little effect on neuronal differentiation in Mash1-deficient NPCs. Together, these results demonstrate that the PDK1-Akt pathway regulates, through activation of Mash1, neuronal differentiation and subtype specification during telencephalic development.
Results
Activation of Akt in NPCs of the Developing Telencephalon.
We first examined where the PDK1-Akt pathway is activated in the developing brain by immunohistochemical analysis with antibodies that react specifically with the phosphorylated form of Akt substrates (anti-phospho-Akt substrate antibodies). In the developing telencephalon, immunoreactivity was detected in the mantle of the cortex and the ventricular (VZ) and subventricular (SVZ) zones of both the dorsal (pallium) and ventral (subpallium) regions of the telencephalon [supporting information (SI) Fig. S1 A–D ]. While staining of the mantle was not affected, staining of the VZ and SVZ was greatly reduced in mice having a CNS-specific disruption of Pdk1 (Fig. S1 A–D) (see below). This confirms antibody specificity, and shows that PDK1 is active in the VZ and SVZ, where proliferating NPCs reside.
Reduced Potential of Pdk1−/− NPCs to Differentiate into Neurons.
We then examined the in vivo role of PDK1 during brain development by generating mice in which Pdk1 is conditionally ablated in the CNS (Fig. S1E). Compared with control (Pdk1flox/+) mice, the amount of PDK1 in forebrain extracts was markedly reduced in heterozygous mutant mice (Pdk1flox/+ mice harboring the nestin enhancer-Cre transgene), and the protein was virtually undetectable in homozygous mutant mice (Pdk1flox/flox mice harboring the transgene), at postnatal day 0 (P0) (Fig. S1F). For the purposes of this study, these 3 types of mice will be referred to hereafter as Pdk1+/+, Pdk1+/−, and Pdk1−/−, respectively. The in vitro kinase activity of Akt immunoprecipitates prepared from brain extracts of Pdk1−/− mice was reduced by 60% compared with that in wild-type or heterozygous mutant mice, and this reduction in Akt activity was accompanied by reduced phosphorylation of Akt on Thr-308, which is catalyzed by PDK1 (Fig. S1G). In contrast, phosphorylation of Akt on Ser-473, which is catalyzed by TORC2, was unaffected (Fig. S1G). The intensity of the bands detected by anti-phospho-Akt substrate in NPC culture extracts of Pdk1−/− mice was also lower than that in the corresponding wild-type extracts (Fig. S1H). These results suggested that the abundance and activity of PDK1 was substantially reduced in the brains of Pdk1−/− mice.
Given that PDK1 is active in NPCs, we next investigated the differentiation potential of NPCs obtained from Pdk1−/− mice. NPCs freshly isolated from the ganglionic eminence (GE) as well as GE-derived NPC aggregates (so-called neurospheres) prepared from floating culture were differentiated. The numbers of neuronsproduced from Pdk1−/− NPCs were decreased in both cultures (Fig. 1 A and Fig. S1 I–K). Since the percentage of dying cells was small (3%) even in the absence of Pdk1 gene under the condition used (Fig. 1B and Fig. S1L), the reduction of neuronal number by Pdk1 gene deletion was unlikely to be due to an increased cell death. These results suggest that inhibition of PDK1 signaling reduces the potential of NPCs to differentiate into neurons.
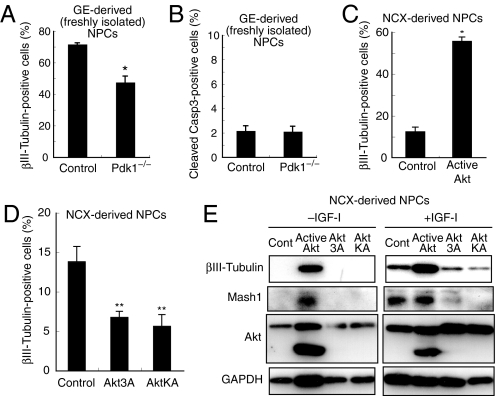
Fig. 1.
Role of PDK1-Akt signaling in neuronal differentiation. (A and B) NPCs freshly isolated from the E14.5 MGE were infected with GFP retroviruses (see also Fig. S1I), and differentiated for 2 days. The percentage of βIII-tubulin- (A) or cleaved caspase-3- (B) positive (+) cells among GFP+ cells was determined. Data are from 3 independent experiments. (C) NPCs prepared from 3-day neurosphere cultures of the E12.5 NCX were infected with GFP (Control) or active Akt-IRES-GFP (Active Akt) retroviruses, and differentiated for 2 days. The percentage of βIII-tubulin+ cells among GFP+ cells was determined. Data are from 4 independent experiments. (D) Primary neuroepithelial cells prepared from the NCX were infected with the indicated retroviruses. The cells were further cultured for 3 days in suspension and then induced to differentiate and analyzed as in C. (E) NPCs prepared from 6-day neurosphere cultures of the NCX were infected with the indicated retroviruses and cultured for 2 days in the absence of FGF-2, EGF and insulin, and either the absence or presence of IGF-1. The cell lysates were then immunoblotted with antibodies to the indicated proteins. Asterisks indicate a statistical difference between experimental groups (*, P < 0.0001; **, P < 0.03).
Akt Promotes Neuronal Differentiation of Telencephalic NPCs In Vitro.
We next examined whether Akt regulates neuronal differentiation of NPCs. Infection of neocortex (NCX)-derived NPCs with a retrovirus encoding a constitutively active form of Akt markedly increased the proportion of cells that express the neuronal markers βIII-tubulin, MAP2, and NeuN (Fig. 1C and Fig. S2 A–C). Conversely, dominant-negative forms of Akt (Akt3A or AktKA) reduced the proportion of βIII-tubulin-positive cells (Fig. 1D). These results suggest a pivotal role for Akt in the neuronal differentiation of embryonic NPCs.
At least 2 possibilities might account for the increase in the proportion of neurons induced by Akt activation: Akt might instruct commitment of undifferentiated NPCs to neuronal fate, or it might selectively promote the proliferation of committed neuronal progenitors. To distinguish between these possibilities, we performed an in vitro clonal assay by infecting NPCs with retroviruses at a low titer and classifying each single cell-derived clone on the basis of its fate as a neuronal clone, nonneuronal clone, or mixed clone. This method enabled us to examine neuronal commitment directly, given that the extent of cell death in our culture system was negligible (3%, revealed by immunocytochemical analysis with antibody to cleaved caspase-3) (7). Expression of activated Akt increased the percentage of pure neuronal clones (Fig. S2D), suggesting that Akt promotes neuronal commitment of NPCs. The number of neurons in the pure neuronal clones was also increased (Fig. S2E), suggesting that Akt also promotes the proliferation of neuron-producing progenitor cells. Together, these results indicate that Akt affects neuronal fate and the proliferation of neuronal progenitor cells simultaneously, resulting in a large increase in the size of the neuronal population.
IGF-1 is expressed in the embryonic telencephalon (36) and has been shown to enhance neuronal differentiation in NPC cultures (8). Since IGF-1 is a well-known activator of the PDK1-Akt pathway, we asked whether this pathway mediates the effects of IGF-1 on neuronal differentiation. IGF-1 treatment indeed increased the levels of Akt phosphorylation (Fig. S2F) and the amounts of βIII-tubulin (Fig. 1E). Importantly, expression of dominant-negative forms of Akt (Akt 3A or Akt KA) suppressed this increase (Fig. 1E). These results suggest that Akt may mediate the effects of IGF-1 in promoting neuronal differentiation.
Selective Induction of GABAergic Neurons by Akt In Vitro.
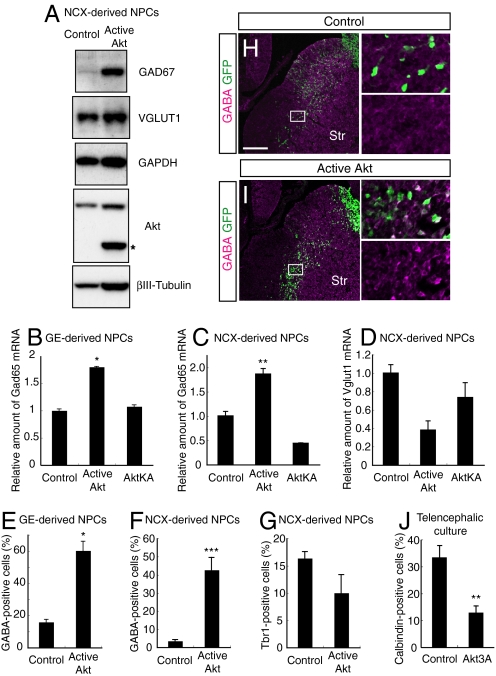
Most NPCs residing on the dorsal side of the telencephalon give rise to excitatory, glutamatergic neurons, whereas those on the ventral side produce inhibitory, GABAergic neurons (11, 12). Several reports, however, have shown that cultured NPCs derived from the NCX have ventralized characteristics with marker expression patterns intermediate between the NCX and GEs (cf. up-regulation of Mash1 in Fig. 1E) and generate both glutamatergic and GABAergic neurons (37–39). NCX-derived NPCs, which are prepared as neurospheres for 3–6 days from the E12.5 NCX, therefore enabled us to examine which type of neurons is produced in response to Akt in the same cell source. Expression of active Akt markedly increased the amount of GAD67 (a GABAergic neuronal marker) but only slightly increased the amount of VGLUT1 (a glutamatergic neuronal marker) (Fig. 2A). The slight increase of VGLUT1 is likely to reflect the increased total protein synthesis (see GAPDH bands), and active Akt did not significantly increase the amount of Vglut1 protein relative to that of GAPDH protein in these cells. In addition, reverse transcription (RT) and real-time polymerase chain reaction (PCR) analysis revealed that expression of active but not inactive Akt increased the amount of mRNA for Gad65 (another GABAergic neuronal marker) but slightly reduced that of Vglut1 mRNA (Fig. 2 C and D). Furthermore, the proportion of GABA-positive but not Tbr1 (a marker of the glutamatergic lineage)-positive neurons was greatly increased by expression of active Akt (Fig. 2 F and G and Fig. S3 A and B). Since GABAergic neurons are normally produced from the GEs, we also confirmed that the proportion of GABA-positive neurons, the levels of Gad65 mRNA and GAD67 protein were significantly increased when active Akt was expressed in GE-derived NPCs (Figs. 2 B and E and 5C). Together, these results suggest that Akt selectively promotes the generation of GABAergic neurons from NPCs.
Fig. 2.
Role of the PDK1-Akt pathway in the generation of GABAergic neurons. (A–G) NPCs prepared from 3-day neurosphere cultures of the NCX (A, C, D, F, and G) or the GE (B and E) were infected with the indicated retroviruses and differentiated for 2 days. The cells were then subjected to immunoblot analysis with antibodies to the indicated proteins (A), assayed for Gad65 and Vglut1 mRNAs by real-time RT-PCR analysis (B–D) or subjected to immunostaining with antibodies to GABA or Tbr1 and to GFP (E–G). The band indicated by the asterisk corresponds to active Akt. Data in (B–D) are from 3 independent experiments. The percentage of GABA+ or Tbr1+ cells among GFP+ cells was determined (E–G). Data are from 4 different fields. (H and I) GFP or active Akt-IRES-GFP plasmid vectors were introduced into the E12.5 VZ by in utero electroporation. After 2 days, the fate of the GFP-positive cells was examined by immunohistochemistry with anti-GFP and anti-GABA. Str, striatum. (J) Telencephalic hemisphere cultures were used to introduce the indicated vectors focally into the MGE by electroporation. After 3 days, the telencephalic hemispheres were immunostained with antibodies to calbindin, which is readily detectable in immature GABAergic neurons in this culture system. The percentage of calbindin+ cells among GFP+ cells within the MGE was determined. Data are from 4 hemispheres. (*, P < 0.0001; **, P < 0.01; ***, P < 0.001) (Scale bar, 200 μm.)
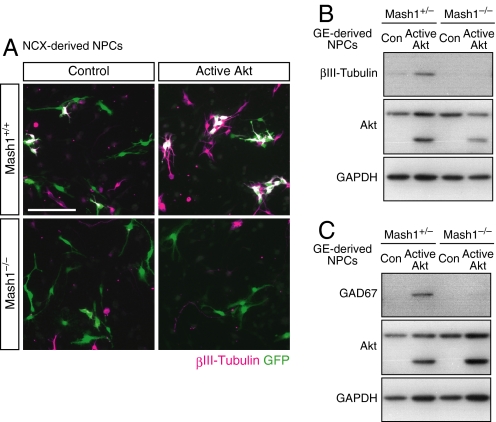
Fig. 5.
Requirement of Mash1 for Akt-mediated neuronal differentiation. (A) NPCs prepared from 6-day neurosphere cultures generated from the NCX of E13.5 Mash1+/+ or Mash1−/− mice were infected for 24 h with the indicated retroviruses, differentiated for 2 days, and immunostained for βIII-tubulin and GFP. Expression of active Akt increased the proportion of neurons generated by Mash1+/− NPCs from 3.0 ± 0.9 to 14.2 ± 1.1% but had little effect on neuronal differentiation of Mash1−/− NPCs (4.1 ± 0.3% in control cells versus 5.5 ± 1.1% in active Akt−expressing cells). (B and C) NPCs prepared from 6-day neurosphere cultures of the GE were infected with the indicated retroviruses, and differentiated for 2 days (B) or 4 days (C). The cell lysates were then immunoblotted with antibodies to the indicated proteins. (Scale bar, 100 μm.)
Akt Promotes Production of GABAergic Neurons in the Developing GEs.
Given that Akt promotes GABAergic neuronal differentiation in vitro, we next asked whether forced expression of active Akt might affect GABAergic neuronal differentiation in the developing GEs in utero. Expression plasmids were injected into the lateral ventricles of mouse embryos at E12.5, and were introduced into NPCs in the VZ of the GEs by electroporation. Electroporation of plasmids encoding active Akt and GFP, but not control plasmid encoding GFP alone, markedly increased the number of cells strongly positive for GABA, as determined by immunohistochemical analysis (Fig. 2 H and I). In contrast, forced expression of active Akt in the NCX by in utero electroporation did not induce ectopic differentiation of GABAergic neurons (Fig. S3C). This suggests that Akt on its own is not sufficient to convert NPC fate from glutamatergic to GABAergic, and that a target or cofactor of Akt might be missing in the NCX for inducing GABAergic neurons.
We further tested whether inhibition of Akt might affect the differentiation of GABAergic neurons. To test this, a dominant-negative form of Akt was introduced into NPCs at the medial ganglionic eminence (MGE) by electroporation in the telencephalic explant culture. We found that the number of the cells positive for calbindin (a GABAergic neuron marker) was greatly reduced by expression of dominant-negative Akt (Fig. 2J). These observations support the notion that the Akt pathway promotes NPC differentiation into GABAergic neurons in vivo. Importantly, these in utero experiments, in addition to in vitro results, suggest that Akt regulates NPC differentiation in a cell-autonomous manner.
Reduced Number of GABAergic, But Not Glutamatergic, Neurons in the NCX of Pdk1−/− Mice.
Given that Akt promoted the differentiation of GABAergic but not glutamatergic neurons in vitro, we investigated whether disruption of Pdk1 selectively affected the generation of GABAergic neurons in the developing telencephalon by immunohistochemistry using antibodies to neuronal subtype-specific markers. Subpopulations of GABAergic neurons generated in the ventral telencephalon migrate to the NCX, olfactory bulb, and striatum (11, 12), and a large proportion of neocortical interneurons are produced in a Mash1-dependent manner (16). The number of GABA-positive neurons in the NCX was markedly decreased inPdk1−/− mice compared with wild-type mice at all rostrocaudal levels analyzed (Fig. 3 A and B and Fig. S4 A and B). The number of calbindin-positive neurons was also reduced in the NCX of Pdk1−/− mice (Fig. S4C).
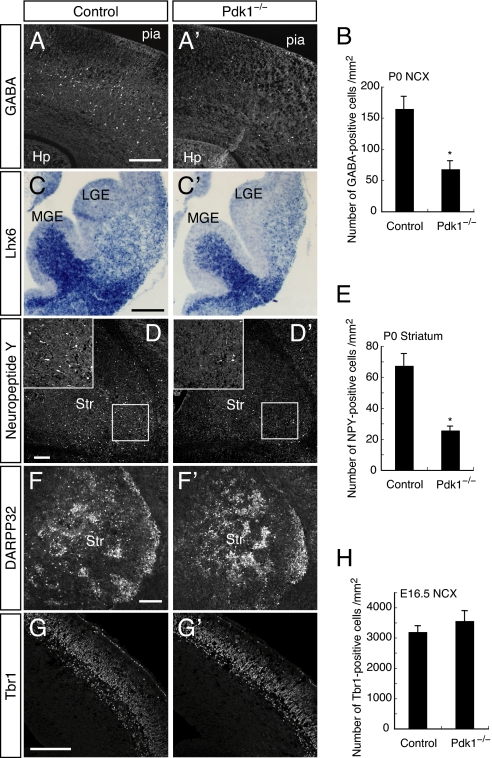
Fig. 3.
Reduction of GABAergic neurons in Pdk1−/− mice. (A and B) Immunohistochemistry for GABA in the P0 control or Pdk1−/− NCX (A). Hp, hippocampus; pia, pial surface. The numbers of GABA+ cells per mm2 in the NCX (indicated in Fig. S4A) were determined (B). Data are from 4 sections of each genotype. (C) Coronal sections at E13.5 were subjected to in situ hybridization with Lhx6 probes. (D–F) Coronal sections of the P0 striatum were immunostained with antibodies to NPY (D) and to DARPP32 (F). The boxed regions in D are shown at higher magnification in the Insets. The numbers of NPY+ cells per mm2 in the striatum (indicated in Fig. S4A) were determined (E). Data are from 4 sections of each genotype. (G and H) Immunohistochemistry for Tbr1 in the E16.5 NCX (G). The numbers of Tbr1+ cells per mm2 in the NCX were determined (H). Data are from 8 sections of each genotype. (*, P < 0.005) (Scale bars, 200 μm.)
We further examined differentiation of GABAergic neurons in Pdk1−/− mice at earlier stages. We found that Pdk1 deletion substantially reduced expression levels of Lhx6 at E13.5, a marker for interneurons derived from the MGE (Fig. 3C) as well as the number of calbindin-positive cells in the MGE at E14.5 (Fig. S4 D–F). These results indicate that PDK1 already affects the production of GABAergic neurons, before their migration to the NCX(see also Fig. 4 E–G and Fig. S6D for early phenotypes of Pdk1−/− mice).
Fig. 4.
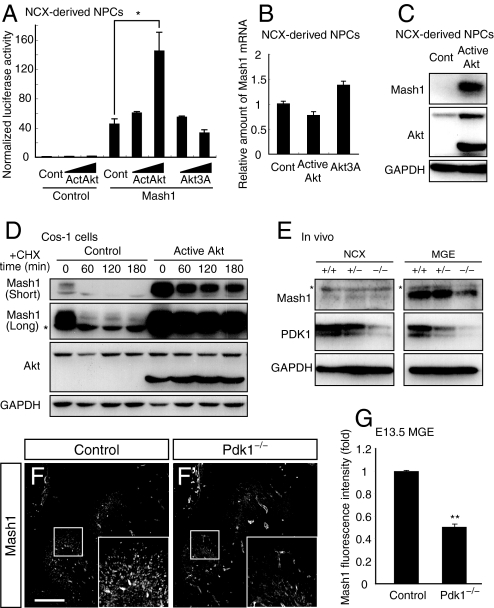
Effects of Akt on the transactivation activity and expression of a proneural bHLH factor Mash1. (A) NPCs prepared from 6-day neurosphere cultures of the NCX were transfected for 24 h with a luciferase reporter plasmid containing E-box repeats (Mash1 binding sites) together with the indicated vectors. The normalized luciferase activity of cell lysates was determined. Data are from 3 independent experiments. (B) NPCs prepared from 6-day neurosphere cultures of the NCX were infected with the indicated retroviruses, incubated for 24 h, and assayed for Mash1 mRNA by real-time RT-PCR analysis. (C) NPCs prepared from 3-day neurosphere cultures of the NCX were infected with the indicated retroviruses, incubated for 24 h in the absence of FGF-2, EGF and Insulin, and subjected to immunoblot analysis with antibodies to the indicated proteins. (D) Cos-1 cells were transfected for 24 h with a Mash1 expression plasmid together with the indicated plasmids. The cells were then treated with cycloheximide (CHX) and subjected to immunoblot analysis with antibodies to the indicated proteins. The asterisk indicates a nonspecific band. (E) Lysates prepared from the E13.5 NCX or MGE were immunoblotted with antibodies to the indicated proteins. The asterisks indicate nonspecific bands. (F and G) Immunohistochemistry for Mash1 in the E13.5 basal telencephalon. The boxed regions are shown at higher magnification in the Insets. Fluorescence intensity of Mash1 protein per area in the VZ/SVZ of the MGE was determined (G). Data are from 4 different regions. (*, P = 0.034; **, P < 0.0001) (Scale bar, 200 μm)
We also examined whether striatal neurons are affected by Pdk1 ablation. Staining of brain sections from newborn (P0) mice with antibodies against neuropeptide Y (NPY) or choline acetyltransferase (ChAT), markers of subsets of inhibitory striatal interneurons, revealed that both types of interneurons were greatly reduced in Pdk1−/− mice compared with control animals (Fig. 3 D and E and Fig. S4 G and H). In contrast, the overall proportion of striatal cells positive for DARPP32, a D1 receptor-associated protein found in striatal projection neurons, was not markedly affected by Pdk1 deletion (Fig. 3F). Consistently, the expression patterns of Dlx1 and Gsh2, regional markers of the lateral ganglionic eminence (LGE) (which gives rise to striatal and olfactory GABAergic neurons), were unaffected by Pdk1 deletion (Fig. S5A). These results together suggested that PDK1 is essential for the generation of some, but not all, GABAergic neurons.
In contrast to interneurons, cortical projection neurons were largely unaffected by disruption of Pdk1. Expression of the glutamatergic neuron markers Vglut1 and Tbr1 was relatively normal (Fig. 3 G and H and Fig. S5B). These results suggest that PDK1 is important for producing interneurons but not projection neurons in the cortex.
Akt Increases the Transcription Activity and Protein Levels of Mash1.
To address the mechanism by which the Akt pathway promotes the differentiation of GABAergic neurons, we focused on regulation of Mash1, a proneural bHLH protein that is required for the production of a subpopulation of GABAergic neurons in the NCX and striatum (ie, loss of Mash1 dramatically reduced GABAergic neocortical interneurons and striatal interneurons including NPY- and ChAT-positive cells but had little effect on striatal projection neurons) (16, 40). NCX-derived NPCs were transfected with vectors for Akt constructs, together with a vector encoding Mash1 and a Mash1-reporter gene construct containing the luciferase gene under the control of Mash1 binding sites (41). Expression of active Akt, but not that of Akt3A, increased Mash1-dependent transcriptional activity in a concentration-dependent manner (Fig. 4A).
Whereas active Akt increased the activity of ectopic Mash1 in NPCs (Fig. 4A), it did not increase the amounts of endogenous Mash1 mRNA in NPCs (Fig. 4B), suggesting that Akt modulates Mash1 activity at a post-transcriptional level. Indeed, expression of active Akt increased the amount of endogenous Mash1 protein in NPCs as judged by both immunoblot analysis (Fig. 4C) and immunostaining both in vitro and in vivo (Fig. S6 A and B). Furthermore, expression of active Akt markedly suppressed degradation of Mash1 protein in the presence of the protein synthesis inhibitor cycloheximide in Cos-1 cells (Fig. 4D). We also observed that active Akt did not markedly increase the amount of Mash1 protein in the presence of proteasome inhibitors (Fig. S6C). These results together indicate that Akt increases the amounts of Mash1 protein by increasing its stability.
As mentioned above, we found that IGF-1 treatment promotes neuronal differentiation through Akt activation. We also found that IGF-1 treatment of the NPC culture increased the amount of Mash1 protein, and that this was in part suppressed by the expression of dominant-negative forms of Akt (Fig. 1E). Thus, Akt may mediate the regulation of Mash1 protein abundance by extrinsic neurotrophic factors.
We next examined the abundance of Mash1 in the developing Pdk1−/− mouse brain. The amounts of Mash1 in the MGEs of Pdk1−/− mice were substantially reduced compared with those of the controls as judged by both immunoblot analysis and immunohistochemistry (Fig. 4 E–G). We further found that Pdk1 deletion reduced expression levels of Mash1-dependent genes Sp9 and Olig2 (Fig. S6D) (42). These results confirm that PDK1-Akt signaling regulates the abundance and activity of Mash1 in vivo.
Requirement of Mash1 for Akt-Induced Neuronal Differentiation.
Given that Akt activated Mash1 in NPCs, we next examined whether Mash1 is necessary for Akt-induced neuronal differentiation. NCX-derived or GE-derived NPCs prepared from mice that were either heterozygous or homozygous for Mash1 deletion (43) were infected with retroviruses encoding GFP alone or GFP together with active Akt. Whereas expression of active Akt markedly promoted neuronal differentiation of the Mash1+/+ or Mash1+/− NPCs as judged by the expression of βIII-tubulin, it had little effect on that of the Mash1−/− NPCs as revealed by immunocytochemistry (Fig. 5A) and immunoblot analysis (Fig. 5B). We also observed that expression of active Akt significantly increased the amounts of the GABAergic marker GAD67 in the Mash1+/− NPCs, but not in the Mash1−/− NPCs derived from GE (Fig. 5C). These results demonstrate that Mash1 is required for Akt-mediated GABAergic neuronal differentiation.
Discussion
The PDK1-Akt pathway contributes to regulation of the survival, proliferation, size, or metabolism of various cell types (27), but the precise role of this pathway in neural development has not been well understood (28). We have now provided several lines of evidence demonstrating a previously unrecognized role of this pathway in the promotion of neuronal differentiation, in particular that of a subset of GABAergic neurons, during mouse telencephalic development. First, disruption of Pdk1 resulted in the loss both of the neurogenic potential of NPCs in vitro and of a subset of GABAergic neurons in vivo. Second, ectopic expression of an active form of Akt promoted the production of (GABAergic) neurons in vitro and in vivo, whereas expression of dominant-negative forms of Akt inhibited this production, presumably in a cell-autonomous manner. Third, Akt was found to activate the bHLH protein Mash1, a key regulator of differentiation of GABAergic neurons (16, 17, 40). Akt signaling was highly active in the Mash1-positive NPCs localized in the VZ and SVZ of the MGE and LGE, which generate GABAergic neurons, supporting an in vivo role of PDK1-Akt signaling in the generation of such neurons. As development proceeds, this signal may also act in migrating GABAergic interneurons since PI3K has been reported to regulate tangential migration of neocortical interneurons (44).
Proneural bHLH proteins are key determinants of neuronal fate in the developing telencephalon (13, 15), and several studies have shown how these proteins are regulated posttranscriptionally by extrinsic cues (20–24). Our data now suggest that the PDK1-Akt pathway mediates regulation of Mash1 by extrinsic cues. Candidates for such extrinsic cues that activate the PDK1-Akt pathway during telencephalic development include secreted factors that are capable both of promoting differentiation of GABAergic neurons and of activating PDK1-Akt signaling, including IGF-1, Shh, GDNF, and BDNF (9). Other neurogenic factors such as erythropoietin, PDGF, and EGF are also potential candidates. Indeed, we found that IGF-1 promoted neuronal differentiation and increased Mash1 protein in our culture system in an Akt-dependent manner. Furthermore, any endogenous signals that induce Akt activation in the ventral telencephalon may control the timing and extent of the generation of GABAergic neurons.
Extensive neuronal diversity is central to the many complex functions of the CNS. The PDK1-Akt pathway appears to contribute to this diversity in the telencephalon, given that activation of PDK1-Akt signaling induced expression of the GABAergic neuronal markers GAD65, GAD67, and GABA but not that of the glutamatergic neuronal markers VGLUT1 and Tbr1. This selective action of the PDK1-Akt pathway in neuronal differentiation may depend on the selective requirement of Mash1 among proneural bHLH proteins for this pathway. The mechanism by which Akt activates Mash1 appears to involve the stabilization of Mash1 protein, but the direct target of Akt in this process remains to be determined. Although we detected a consensus sequence for Akt phosphorylation at Ser-90 of the rat Mash1 (RQRSSS), mutation of this residue to alanine did not affect Akt-induced activation of Mash1 (Fig. S7A). We found that 2 well-established downstream pathways of Akt, mTOR and GSK3, were not involved in Akt-induced neuronal differentiation (Fig. S7 B–D). PDK1 is also known to activate other downstream targets than Akt, including p70 S6 kinase and p90 Rsk, which might contribute, at least partly, to the Pdk1−/− phenotypes (25).
Pdk1 mutant mice did not exhibit a severe loss of MGE observed in Mash1 mutant mice (Fig. S8A) (16). We think that this difference might be ascribable to at least 2 possibilities, based on our findings that PDK1 deletion reduced Mash1 protein, but did not eliminate it. One possibility is that high protein levels of Mash1 are necessary for GABAergic neurogenesis but not for the maintenance of MGE cells, and PDK1 regulates only the former function of Mash1. The other possibility is that PDK1 regulates Mash1 qualitatively, such as the target specificity. In either case, although our results are consistent with the notion that the PDK1-Akt pathway promotes GABAergic neurogenesis through the increase of Mash1 protein, only a part of the functions of Mash1 appears PDK1-dependent.
Although Pdk1−/− mice were born alive, none of them survived for more than a few days after birth. The brains of the homozygous mutant mice were approximately 30% smaller than those of the wild-type controls (Fig. S8B). Our analysis of PDK1-deficient mice also revealed that PDK1 is essential for regulation of their size, proliferation, and survival (Fig. S8 C–E), the impairment of which likely contributes to the reduced brain size of the mutant animals. Consistent with these results, recent studies have shown that Akt3-deficient adult mice have a reduced brain size as well as a reduced size and number of brain cells (34, 35). Ectopic expression of wild-type Akt1 has also been shown to promote the survival, proliferation, and maintenance of neocortical NPCs (33). How does PDK1-Akt signaling promote both the proliferation and differentiation of NPCs, given that terminal neuronal differentiation is normally accompanied by cell cycle arrest? We propose that PDK1 exerts these effects differentially in a manner dependent on expression levels of Mash1. PDK1 may thus promote the proliferation and maintenance of NPCs irrespective of Mash1, while promoting neuronal differentiation only when Mash1 expression is above a threshold. Indeed, the size of the brain of Pdk1 mutant mice was reduced even in Mash1-negative regions, suggesting a Mash1-independent function for PDK1 in NPC population.
Recent studies have provided convergent evidence for impairment of Akt1 signaling in schizophrenia (45). A reduced abundance of Akt1 in the brain was found to be significantly associated with schizophrenia, and Akt1 deficiency conferred a greater sensitivity to the disruption of sensorimotor gating by amphetamine. Considering that a deficiency in a subpopulation of GABAergic neurons in the prefrontal cortex is also associated with schizophrenia (46), the loss of neocortical GABAergic neurons caused by the absence of PDK1-Akt signaling demonstrated in the present study might have important implications for the pathogenesis of schizophrenia.
Materials and Methods
Mouse Strains.
Pdk1flox/flox, Mash1−/− and nestin-CRE mice have been described (26, 43, 47, 48). All mice were maintained according to the protocol approved by the Animal Care and Use Committee of the University of Tokyo. Details on other methods are available in SI Methods.
Statistical Analysis.
Data are presented as means ± SEM, unless otherwise indicated. Values were compared with Student's t test. A P value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We thank J. A. Cooper for critical reading of the manuscript; D. Alessi for Pdk1flox/flox mice; K. Campbell, R. F. Hevner, T. Matsuda, C. L. Cepko, T. Kitamura, K. Tomita, R. Kageyama, M. Nakafuku, and M. E. Greenberg for reagents and advice; and the members of the Gotoh laboratory for helpful discussions and technical assistance. This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; Japan Society for the Promotion of Science; Toray Science Foundation; Ichiro Kanehara Foundation; Tokyo Biochemical Research Foundation; Sumitomo Foundation; Uehara Memorial Foundation; Takeda Science Foundation; Naito Foundation; Novartis Foundation; and Solution Oriented Research for Science and Technology of the Japan Science and Technology Corporation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808400106/DCSupplemental.
References
- 1.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DJ. Stem cells and pattern formation in the nervous system: The possible versus the actual. Neuron. 2001;30:19–35. doi: 10.1016/s0896-6273(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 3.Qian X, et al. Timing of CNS cell generation: A programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 4.Panchision DM, McKay RD. The control of neural stem cells by morphogenic signals. Curr Opin Genet Dev. 2002;12:478–487. doi: 10.1016/s0959-437x(02)00329-5. [DOI] [PubMed] [Google Scholar]
- 5.Miller FD, Gauthier AS. Timing is everything: Making neurons versus glia in the developing cortex. Neuron. 2007;54:357–369. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Hirabayashi Y, Gotoh Y. Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci Res. 2005;51:331–336. doi: 10.1016/j.neures.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Hirabayashi Y, et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- 8.Arsenijevic Y, Weiss S. Insulin-like growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: Distinct actions from those of brain-derived neurotrophic factor. J Neurosci. 1998;18:2118–2128. doi: 10.1523/JNEUROSCI.18-06-02118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pozas E, Ibanez CF. GDNF and GFRalpha1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron. 2005;45:701–713. doi: 10.1016/j.neuron.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 10.Guillemot F. Cell fate specification in the mammalian telencephalon. Prog Neurobiol. 2007;83:37–52. doi: 10.1016/j.pneurobio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Marin O, Rubenstein JL. A long, remarkable journey: Tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 12.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 13.Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- 14.Campbell K. Dorsal-ventral patterning in the mammalian telencephalon. Curr Opin Neurobiol. 2003;13:50–56. doi: 10.1016/s0959-4388(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 16.Casarosa S, Fode C, Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- 17.Fode C, et al. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- 18.Parras CM, et al. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16:324–338. doi: 10.1101/gad.940902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Israsena N, et al. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol. 2004;268:220–231. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Vinals F, et al. BMP-2 decreases Mash1 stability by increasing Id1 expression. EMBO J. 2004;23:3527–3537. doi: 10.1038/sj.emboj.7600360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma YC, et al. Regulation of motor neuron specification by phosphorylation of neurogenin 2. Neuron. 2008;58:65–77. doi: 10.1016/j.neuron.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shou J, Rim PC, Calof AL. BMPs inhibit neurogenesis by a mechanism involving degradation of a transcription factor. Nat Neurosci. 1999;2:339–345. doi: 10.1038/7251. [DOI] [PubMed] [Google Scholar]
- 23.Moore KB, Schneider ML, Vetter ML. Posttranslational mechanisms control the timing of bHLH function and regulate retinal cell fate. Neuron. 2002;34:183–195. doi: 10.1016/s0896-6273(02)00666-9. [DOI] [PubMed] [Google Scholar]
- 24.Hand R, et al. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48:45–62. doi: 10.1016/j.neuron.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 25.Williams MR, et al. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 26.Lawlor MA, et al. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21:3728–3738. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheid MP, Woodgett JR. PKB/AKT: Functional insights from genetic models. Nat Rev Mol Cell Biol. 2001;2:760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- 28.Yang ZZ, et al. Physiological functions of protein kinase B/Akt. Biochem Soc Trans. 2004;32:350–354. doi: 10.1042/bst0320350. [DOI] [PubMed] [Google Scholar]
- 29.Bai Y, et al. Ectopic expression of angiopoietin-1 promotes neuronal differentiation in neural progenitor cells through the Akt pathway. Biochem Biophys Res Commun. 2009;378:296–301. doi: 10.1016/j.bbrc.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 30.Han J, et al. Mammalian target of rapamycin (mTOR) is involved in the neuronal differentiation of neural progenitors induced by insulin. Mol Cell Neurosci. 2008;39:118–124. doi: 10.1016/j.mcn.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Otaegi G, et al. Modulation of the PI 3-kinase-Akt signalling pathway by IGF-I and PTEN regulates the differentiation of neural stem/precursor cells. J Cell Sci. 2006;119:2739–2748. doi: 10.1242/jcs.03012. [DOI] [PubMed] [Google Scholar]
- 32.Vojtek AB, et al. Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol Cell Biol. 2003;23:4417–4427. doi: 10.1128/MCB.23.13.4417-4427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinor AD, Lillien L. Akt-1 expression level regulates CNS precursors. J Neurosci. 2004;24:8531–8541. doi: 10.1523/JNEUROSCI.1470-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tschopp O, et al. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- 35.Easton RM, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayer-le Lievre C, Stahlbom PA, Sara VR. Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus. Development. 1991;111:105–115. doi: 10.1242/dev.111.1.105. [DOI] [PubMed] [Google Scholar]
- 37.Hack MA, et al. Regionalization and fate specification in neurospheres: The role of Olig2 and Pax6. Mol Cell Neurosci. 2004;25:664–678. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Machon O, Backman M, Krauss S, Kozmik Z. The cellular fate of cortical progenitors is not maintained in neurosphere cultures. Mol Cell Neurosci. 2005;30:388–397. doi: 10.1016/j.mcn.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Jo AY, Park CH, Aizawa S, Lee SH. Contrasting and brain region-specific roles of neurogenin2 and mash1 in GABAergic neuron differentiation in vitro. Exp Cell Res. 2007;313:4066–4081. doi: 10.1016/j.yexcr.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akazawa C, et al. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270:8730–8738. doi: 10.1074/jbc.270.15.8730. [DOI] [PubMed] [Google Scholar]
- 42.Long JE, et al. Dlx1&2 and Mash1 transcription factors control striatal patterning and differentiation through parallel and overlapping pathways. J Comp Neurol. 2009;512:556–572. doi: 10.1002/cne.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guillemot F, et al. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- 44.Polleux F, et al. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- 45.Emamian ES, et al. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 46.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 47.Kawaguchi A, et al. Nestin-EGFP transgenic mice: Visualization of the self-renewal and multipotency of CNS stem cells. Mol Cell Neurosci. 2001;17:259–273. doi: 10.1006/mcne.2000.0925. [DOI] [PubMed] [Google Scholar]
- 48.Isaka F, et al. Ectopic expression of the bHLH gene Math1 disturbs neural development. Eur J Neurosci. 1999;11:2582–2588. doi: 10.1046/j.1460-9568.1999.00699.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.