Abstract
Orangutans are the largest habitually arboreal mammal. For them, as for all arboreal mammals, access to the abundant fruits and narrowest gaps found among the thin peripheral branches of tree crowns poses considerable safety risks and energetic demands. Most arboreal primates use flexed-limb postures to minimize problems caused by branch compliance and instability. Here, we show that Sumatran orangutans employ unique locomotor strategies to control compliance and allow access to the terminal branch niche for feeding and gap crossing. We calculated a “stiffness score,” which is a measure of the flexibility of the supports on which orangutans moved. We found that certain locomotor behaviors clearly are associated with the most compliant supports; these behaviors appear to lack regular limb sequences, which serves to avoid the risk of resonance in branch sway caused by high-frequency, patterned gait. Balance and increased stability are achieved through long contact times between multiple limbs and supports and a combination of pronograde (horizontal) and orthograde (vertical) body postures, used both above branches and in suspension underneath them. Overall, adult females seem to be the most conservative in their travel, selecting more solid and secure supports than males and adolescents. These results have implications for understanding locomotor diversity in fossil and extant apes and for orangutan conservation and reintroduction programs.
Keywords: biomechanics, hominoid evolution, locomotor ecology, positional behavior, compliance
In recent years, researchers have discovered a great deal about the way in which animals move during steady locomotion on flat terrain, but remarkably little is known about how animals move in complexly structured environments (see, e.g., ref. 1). The most structurally complex, and speciose, land environment may be the canopy of tropical rainforest, which presents a three-dimensional meshwork containing unpredictable changes in the continuity and nature of the supports available for locomotion. In this habitat, arboreal animals, such as primates, must cope with one problem in particular that rarely exists for terrestrial animals: the flexibility (compliance) of the supports against which they must exert forces to support or propel themselves.
Flexible branches effectively add another spring in series with the animal's locomotor system and therefore will significantly disturb normal movement dynamics. Compliant supports have been shown to increase the energetic cost of locomotion in monkeys and lemurs (2, 3) and the cost of takeoff in birds (4). They further may affect energetics by forcing arboreal animals to adopt longer travel routes to circumvent gaps in the canopy. There are also significant, size-related safety implications; falls from great heights likely will be fatal to large animals (5, 6), and even falls between levels of the canopy may result in serious injury. The risk of these will be greater on the fine, terminal branches of trees (the terminal branch niche), where resources such as fruits and the narrowest gaps between tree crowns most often are found, because the vibrations caused by the body mass of even small animals will be much greater (7).
Thus, orangutans may be unusual in an ability to benefit from branch compliance, by using elastic energy stored in compliant branches during tree sway (a locomotor mode where they oscillate vertical, compliant tree trunks with increasing magnitude to cross gaps). Tree sway crosses given gaps for less than half of the cost of jumping and an order of magnitude less than the cost of crossing terrestrially (8). Our recent studies also have demonstrated the surprising result that orangutans frequently use hand-assisted bipedal locomotion to access the fine terminal branches of trees (9), just where the greatest problems with compliance are likely to occur. This allows orangutans to maximize stability by using their long prehensile toes to grip multiple small branches and a hand to further aid balance, while freeing the remaining hand to reach out for feeding or weight transfer. Because the Sumatran orangutan (Pongo abelii) is the largest habitually arboreal primate (10) and has one of the broadest repertoires of positional behavior of all of the primates [consisting of >100 biomechanically distinct postural and locomotor behaviors (11)], we hypothesize that this repertoire includes unique locomotor strategies for dealing with the flexibility of supports during arboreal travel. However, we expect that thresholds must exist below which supports become too compliant for safe locomotion. Our hypothesis further predicts that orangutans will use locomotion and support combinations close to these thresholds to minimize both the length of travel paths between food sites and the energetic cost of travel. Because falls from great heights are of greater risk for large animals, we further expect that adult males will choose to travel on more stable supports than adult females and adolescents. In testing these hypotheses, this article expands on our previous specific studies (8, 9) to explore the dynamic between compliant arboreal supports and the full range of orangutan locomotion.
Results and Discussion
During a year-long field study of wild Sumatran orangutans, we obtained 2,811 instantaneous visual observations of orangutan locomotion from 10 individuals and of the supports on which they moved (see Materials and Methods). To assess the effect of support compliance on locomotion in a diverse locomotor habitat, we calculated a “stiffness score” (SS), which is essentially a measure of the mean support diameter used for each bout in which up to four supports were used (see Materials and Methods). A small SS indicates that the supports are more compliant, and a large score indicates that they are more rigid. We used a general linear model (GLM) with ln(SS) (the natural log of the stiffness score) as the response variable to adjust for the range of physical, ecological, and behavioral factors that may influence orangutan locomotion (12), including the number, types, and angles of supports used, contextual behavior, height in the canopy, direction of travel, body size, and individual, here represented by a summary age–sex variable (see ref. 12 and Materials and Methods). The effect of each variable on ln(SS) was tested, and to explore further the relationship between ln(SS) and locomotion, we also tested the effect of two-way interactions between locomotion and all other variables on ln(SS). The final model shows that locomotion, the number, type, and angle of supports used, height in the canopy, age–sex category, and the interaction term (locomotion × number of supports) combine significantly to explain ln(SS) (Table 1). Tukey's difference between means tests were performed on the GLM interaction term and main effects to identify significant patterns in the dataset (Figs. 1–5).
Table 1.
General linear model for dependent variable: Natural logarithm of stiffness score
| Source | Type III sum of squares | Degrees of freedom | Mean square | F | Significance |
|---|---|---|---|---|---|
| Corrected model | 454.080* | 40 | 11.352 | 34.105 | 0.000 |
| Intercept | 275.975 | 1 | 275.975 | 829.116 | 0.000 |
| Locomotion | 48.330 | 9 | 5.370 | 16.133 | 0.000 |
| Support type | 113.230 | 7 | 16.176 | 48.597 | 0.000 |
| Support angle | 8.470 | 9 | 0.941 | 2.827 | 0.003 |
| No. of supports | 8.313 | 1 | 8.313 | 24.975 | 0.000 |
| Age–sex | 15.103 | 2 | 7.552 | 22.687 | 0.000 |
| Height | 26.885 | 4 | 6.721 | 20.193 | 0.000 |
| Locomotion × no. of supports | 10.220 | 8 | 1.278 | 3.838 | 0.000 |
| Error | 492.293 | 1,479 | 0.333 | ||
| Total | 8,434.962 | 1,520 | |||
| Corrected total | 946.373 | 1,519 |
*R2 = 0.480 (adjusted R2 = 0.466).
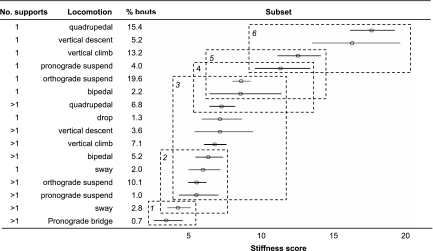
Fig. 1.
Tukey's homogenous subsets and confidence intervals for stiffness scores (SSs) for the association “locomotion × number of supports”. The SS is a measure of the mean support diameter used for each bout of locomotion. A small SS indicates that the supports are more compliant, and a large score indicates that they are more rigid. Mean values and significance levels have been calculated for ln(SS), but presented means are back-transformed values. The stippled, numbered boxes indicate subsets of the SSs that do not differ significantly from each other, because there is a significant difference between the mean of two SSs only if they do not appear in any of the same subsets. For example, pronograde bridge on >1 support and sway on >1 support are in subset 1, but sway on >1 support is also in subset 2. Thus, pronograde bridge on >1 support has a SS that is significantly different than all those in subset 2, but sway on >1 has a SS that does not differ significantly from pronograde bridge on >1 support or from bipedal on >1 support, sway on 1 support, orthograde suspend on >1 support, and pronograde suspend on >1 support. Note that orangutans are able to navigate on branches with low SSs when using multiple supports.
Fig. 2.
Tukey's homogenous subsets and confidence intervals for age–sex category. For explanation of figure, see caption to Fig. 1. Adult females are the most conservative in their travel, selecting more solid and secure supports than males and adolescents
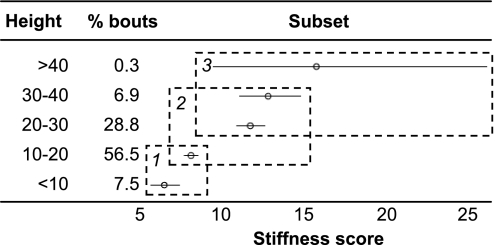
Fig. 3.
Tukey's homogenous subsets and confidence intervals for height (m). For explanation of figure, see caption to Fig. 1. The mean stiffness score increases with height in the canopy.
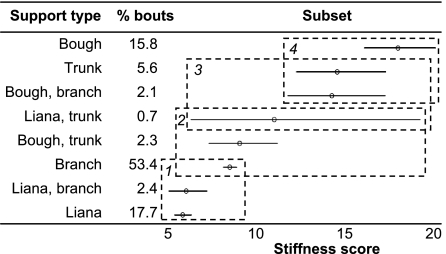
Fig. 4.
Tukey's homogenous subsets and confidence intervals for support type. For explanation of figure, see caption to Fig. 1. Branches account for >50% of locomotion, but lianas enable travel on the most compliant supports.
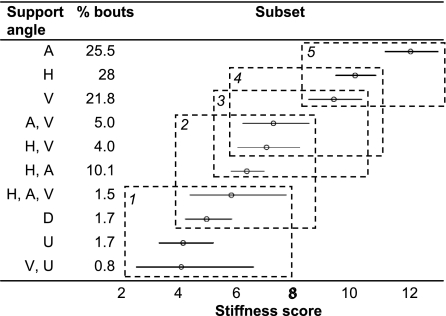
Fig. 5.
Tukey's homogenous subsets and confidence intervals for support angle. For explanation of figure, see caption to Fig. 1. Combining supports of different angles allows locomotion on supports with smaller mean stiffness scores. H, horizontal; A, angled; V, vertical; U, U-shaped; D, deforms heavily under body mass.
The great apes (including humans) are generally thought (reviewed in, e.g., ref. 13) to share morphological adaptations in the upper limb and trunk for orthograde (vertical trunk) posture and locomotion [but not necessarily suspension (14)], reflecting the demands of ripe-fruit-eating in the peripheral canopy of trees in tropical forests (15, 16). This hypothesis has been strongly supported by recently discovered fossil evidence for the great antiquity of orthogrady in hominoids [e.g., Morotopithecus dating to >20 mya (17) and Pierolapithecus dating to >12 mya (18)]. Nevertheless, although orthograde behaviors account for the greatest proportion of locomotion on supports with smaller stiffness scores in this study (Fig. 1), pronograde (horizontal trunk) bridging allows movement on the smallest mean support sizes and has a significantly smaller mean SS than orthograde suspension and bipedalism. Sway (which incorporates postures of all descriptions) also allows locomotion on smaller supports than orthograde behaviors, but this result is not statistically significant, because sway forms part of subset 2 as well as subset 1 in Fig. 1. Together, these results indicate that locomotor versatility is a crucial component of the ability of orangutans to navigate small supports.
Although pronograde bridging accounts for only 0.7% of the orangutan's locomotor repertoire, the musculoskeletal system of animals must adapt to the most strenuous activities in which they are used (19) as well as to the most frequent. Pronograde bridging places orangutan anatomy under very different stresses to those experienced in orthograde behaviors, and therefore, regardless of its frequency, selection for performance capability in this behavior must have influenced the evolution of orangutan morphology. Paradoxically, although the positional behavior of chimpanzees and gorillas is dominated by pronogrady during terrestrial quadrupedal knuckle-walking, the African apes have never been observed to use pronograde suspension in the trees, and pronograde bridging has been observed only very rarely (see, e.g., refs. 20 and 21 for descriptions of chimpanzee and gorilla locomotion). These behaviors appear to be the only major locomotor modes that distinguish orangutan locomotion from that of the other great apes (ref. 11 and K.D. Hunt, personal communication). Given the adaptive benefits of pronograde bridging for accessing the very smallest branches in the peripheral canopy of trees and given the very similar kinematics for pronograde bridging and suspension and knuckle-walking, that African apes should have lost any adaptations to these behaviors as a result of adaptations to terrestrial, pronograde knuckle-walking seems unlikely. Therefore, pronograde suspension and bridging probably evolved in orangutans after their evolutionary separation from the common great ape ancestor, in refinement of their arboreal adaptations, perhaps as a mechanism to cross increasing gaps in the canopy during mid-late Miocene canopy fragmentation (22). We further suggest (9) that chimpanzees and gorillas acquired their terrestrial knuckle-walking as a parallel response to canopy fragmentation to access their typically ground-based fallback foods (6).
The results for orthograde locomotion also are interesting, because results here suggest that although the mean SS for bipedalism on single supports was smaller than that for orthograde suspension, the value for multiple supports was slightly larger, although the differences are not statistically significant. This is in apparent contrast to our previous log-linear analysis (on a subset of this dataset) that found that arboreal bipedalism allowed orangutans to move on smaller supports than did orthograde suspension and quadrupedalism (9). This may be explained, however, by the nature of the statistical techniques. The GLM focuses on predicting the magnitude of a single, quantitative, dependent variable (in this case SS), whereas in log-linear analysis all variables have equal status and the analysis seeks to explain the associations underlying actual observations. Thus, in the present study, SS is an estimate of the mean diameter of each support used in a bout, whereas in our log-linear study (9) diameter is classified into categories and accounts for all support diameters used in a bout. Taking a mean support size, as in the present study, underemphasizes the special role of bipedalism in utilizing very small supports.
General predictions suggest that unstable branches should be associated with either suspensory behavior or “compliant quadrupedalism.” In theory, suspension enhances stability because the animal has, in effect, already fallen off the support (23), whereas compliant quadrupedalism is used by many other primates and marsupials that are restricted to moving along the top of branches (e.g., refs. 8, 24, and 25). These species, including chimpanzees, keep their elbows and knees highly flexed and maintain longer contact times between the limbs and the branch to maximize stability and avoid the risk of resonance in branch sway that would be caused by high-frequency, patterned gait on compliant substrates (e.g., refs. 8, 24, and 25). Our results suggest that the orangutan case contests both of these predictions: Orangutans actually access the smallest mean supports by using pronograde bridging, which may combine both above-branch and suspensory postures [indeed, the mean SS for bridging on multiple supports was 37% (5.5/3.49 × 100) lower than that for purely suspensory behaviors on multiple supports], and they only exhibited quadrupedalism on the stiffest supports (Fig. 1). Instead (with the exception of vertical climbing and descent), their gait on compliant supports is characterized by unpatterned forms of locomotion, where the limbs are used in any order to grasp new supports and transfer body mass (see locomotor definitions in Table 2). They also travel at low speeds and maintain long contact times, often with multiple limbs, which also must result in low impact forces and thus further reduce the risk of support vibrations.
Table 2.
Data classification
| Category | Classification |
|---|---|
| 1 | Age–sex: adult/subadult male (n = 4), adult female (n = 3), adolescent male/female (n = 3) |
| 2 | Locomotion (after 11): quadrupedal walk (torso is pronograde; all limbs contact the support in a symmetrical or unpatterned sequence); bipedal walk (hindlimbs provide support and propulsion; includes hand-assisted bipedalism); vertical climb; vertical descent; torso-orthograde suspension (forelimb-dominated suspension; sequence is mostly unpatterned); torso-pronograde suspension (all limbs used in symmetrical or unpatterned sequence; torso is pronograde, and limbs are in tension); sway (oscillation of flexible supports to cross gaps); drop; bridge (torso-pronograde, unpatterned, gap crossing movement with limbs in compression, suspension, or both). |
| 3 | Direction of locomotion: horizontal; ± angled; ± vertical |
| 4 | Height: 10-m interval to 40 m, >40 m (vertical distance from the animal to the ground) |
| 5 | Number of supports: 1; 2; 3; 4; >4* |
| 6 | Weight-bearing support (WBS) angle: horizontal (±20° horizontal); angled (±21–70° horizontal); vertical (±20° vertical); U-shaped; deforms heavily under animal's body mass |
| 7 | WBS type: trunk; bough (originates from trunk); branch (originates from bough or branch); liana |
| 8 | WBS diameter: <2 cm; ≥2 to <4 cm; ≥4 to <10 cm; ≥10 to <20 cm; ≥20 to <40 cm; ≥40 cm |
| 9 | Behavior: feed; travel |
*Not included in the analysis because we were unable to record support characteristics when >4 supports were used per bout.
The remarkable versatility of orangutan locomotion (11), their ability to exert forces in a wide range of joint positions (26), and the lack of possibly conflicting adaptations for terrestrial locomotion (as in chimpanzees and gorillas) suggest that orangutans may have had a greater opportunity to evolve the most effective solutions to locomotion on compliant supports for large-bodied animals than the other great apes. If so, then our results suggest that slow, unpatterned locomotion that utilizes both suspensory and above-branch positions is the most effective strategy for large-bodied apes to deal with branch flexibility. The apparent tendency of the living African apes, in contrast to the Asian apes, to use compliant quadrupedalism rather than orthogrady to negotiate small arboreal supports may be a consequence of adaptive compromises acquired during their acquisition of terrestrial quadrupedalism as a major locomotor mode.
Classic predictions regarding suspension also suggest that larger animals and larger species should suspend more than their smaller counterparts when support size is controlled and should otherwise use larger supports (5, 10). In the other apes, the prediction is supported in gibbons and siamangs (27) but not in intraspecific studies of lowland gorillas (20) or interspecific studies of gibbons and chimpanzees (27). Observed overall frequencies of orangutan suspensory locomotion also fail to follow predictions from body mass, which may reflect the existence of arboreal pathways that all individuals attempt to follow (10, 12). In this study, although each age–sex group utilizes a significantly different mean SS (Fig. 2) and immature males and females utilize the most compliant supports, as would be expected, adult females rather than adult or subadult males travel on the most solid and secure supports. However, if we divide mean SS by body mass [86 kg for adult males, 38 kg for adult females (after ref. 28), and 25 kg for adolescents], then adult males have the lowest ratio (0.11 compared with 0.28 for adult females and 0.33 for adolescents). This suggests that adult males utilize supports that are considerably more compliant relative to their weight than adult females or immature animals. Because the type of locomotor mode exhibited does not differ substantially between the orangutan age–sex categories (figure 2 of ref. 11), adult males appear to be using smaller supports to exhibit broadly the same locomotor repertoires as adult females and adolescents, despite their large body size.
Because falls from great heights are of a greater risk for large animals, adult males might be expected to choose to travel lower in the canopy than adult females and adolescents. Fig. 3 indicates that mean SS increases with increasing height; orangutans use supports with a SS almost twice as large when above 40 m as they do below 10 m. However, most travel takes place between 10 and 30 m, where supports are similarly sized, with a mean diameter of ≈10 cm. Unfortunately, testing whether this differed between the age–sex categories was not possible here because of the small sample size, but the log-linear analysis in ref. 12 suggested that adult females actually tend to travel lower in the canopy, whereas adult and subadult males travel and feed above 20 m more than the other age–sex categories.
Previous studies (12) found that the diameter, type, and number of supports used showed stronger associations with orangutan locomotion than height in the canopy, behavior, or the age or sex of the individual. However, analysis of the relationship between locomotion and support characteristics is somewhat complicated, because the diameters, types, and angles of supports used may simply reflect the totality of support availability within the animal's range rather than preference for particular support characteristics for given locomotor behaviors. In the GLM modeling for this study, the two-way associations between locomotion and support type and locomotion and support angle were not found to be significant, which would seem to support this conclusion. Nevertheless, both support type and angle were found to influence mean SS independent of locomotion (Figs. 4 and 5), which might imply overall preferences for support use rather than for specific locomotor behavior and support combinations. Although single and multiple branches accounted for >50% of orangutan locomotor bouts (Fig. 4), lianas enabled travel on the most compliant supports. This might, however, relate to the angle of the support, because 72% of locomotion on single or multiple lianas used vertical lianas (although progression may be in any direction). In such cases, the force is applied along rather than perpendicular to the long axis of the liana, which will reduce vibrations compared with movement on angled or horizontal branches, where bending forces are applied. This demonstrates the importance of lianas to orangutan locomotion and may have implications for conservation and reintroduction programs, because the number of lianas (large enough to support an orangutan's weight) tends to be greatly depleted in logged forests (29). Sumatran orangutan numbers are in rapid decline, and (according to ref. 30) P. abelii could become the first great ape species to go extinct. Establishing their crucial habitat requirements is therefore of particular urgency.
Although 63% of observed locomotion took place on single supports (Fig. 1), multiple-support use clearly allowed orangutans to move on much more flexible branches and lianas than were used singly, both overall and for the different types of locomotor behavior: The mean SS for each locomotor mode on multiple supports ranged from 26% (bipedalism) to 58% (quadrupedalism) lower than the mean SS for the same mode on single supports. (The difference is statistically significant for quadrupedalism, pronograde suspension, and vertical climb and descent.) This supports our hypothesis that stiffness thresholds exist below which orangutans cannot exhibit certain forms of locomotion on particular supports. Using multiple supports, however, clearly allows the threshold to be lowered by distributing the base of support more widely and reducing the risk of falling if one support breaks (e.g., ref. 23). This is greatly beneficial to orangutans, because it facilitates access to crucial resources in the terminal branch niche and minimizes the length of travel routes between food sources. The primary underlying mechanism is possibly simply a lower limit of the total diameter of supports used. However, from a mechanical perspective, multiple-support use also seems to confer additional benefits for the orangutans' abilities to control and utilize the effects of support compliance. When holding more than one support, slight changes of emphasis in which support bears the most weight or subtle adjustments of the position of the handhold or foothold along a tapered branch enable individuals to achieve subtle but fundamental changes in spatial position and dampen or increase oscillations according to requirements. The angles of the supports used also may be important in this strategy. Fig. 4 shows that in general orangutans can utilize branches and lianas with significantly smaller SSs by combining supports of different angles than by combining supports of the same angle: The SS of “horizontal, angled, vertical” is 38–52% smaller than that of single and multiple use of supports of the same angle. The ability to grasp several small branches is likely therefore to also be beneficial, because the different angles of the supports may help to counter the effects of compliance in each.
The energetic cost of locomotion is greatly influenced by the smallest diameter of supports that orangutans can utilize, because these dictate the distance that they must detour around (frequent) gaps in the canopy. Is there then a SS threshold below which orangutans are unable to utilize supports for locomotion? In this study, sway occurred on the smallest single supports that had a mean SS of 6 cm (Fig. 1); however, 3% of sway on single supports utilized a support <2 cm in diameter, and 13% utilized a diameter of 2–4 cm. In contrast, use of 2–4 supports allowed orangutans to bridge branches and lianas with a mean SS of 3.5 cm (Fig. 1). Overall 29% of orangutan locomotion occurred on >4 supports, and although recording detailed support characteristics when >4 supports were used was not possible, in many such cases body mass was supported only by handfuls of foliage (11, 12). If we take mean values as a broad indication of preference, then our results suggest that orangutans demonstrate a preference for single supports with a SS not much smaller than 6 cm and multiple supports with a SS not much smaller than 3.5 cm per support. This is remarkably small for an animal of such large body mass.
Conclusion
Orangutans cope with support compliance by a combination of orthograde and pronograde body postures, used both above branches and in suspension underneath them, and by employing multiple limbs and multiple supports to achieve balance and increased stability. These gaits are typically unpatterned, with long contact times and therefore low impact forces. These locomotor strategies allow orangutans to utilize the greatest range of mean support diameters, facilitating safe access to the terminal branch niche. They also allow orangutans to fine-tune their spatial position, dampening or increasing the effects of support compliance according to requirements. Such strategies differ from both predictions and those observed in other arboreal primates. These results have implications for orangutan conservation and reintroduction programs, because they aid in understanding the dynamic between orangutans and their habitat and thus in establishing crucial habitat requirements for orangutans.
Materials and Methods
Field Study.
The study took place in the Ketambe Research Station (3°41′N, 97°39′E) in the Gunung Leuser Ecosystem, Sumatra, comprising pristine rainforest on riverine terraces. Our method is described in detail elsewhere (11, 12). In brief, 1-min instantaneous sampling was used during whole-day focal visual observations to collect detailed support-use and behavioral-context data and positional behavior (see Table 2), backed up with video recordings of typical samples of some positional behaviors. Considerable self-training was carried out to verify accurate recording of positional behavior, height, and support diameter. The latter was achieved by estimating support diameters in the lower canopy (measurable from the ground) at horizontal distances of up to 50 m and confirming accuracy by subsequently measuring the supports. Because errors may be greater when estimating supports at increased heights, extra training in estimation of support diameters in the upper canopy was achieved by estimating diameters in tall “training” trees on which scales had been placed to allow accurate diameters to be subsequently obtained. Training sessions were carried out at monthly intervals and indicate an accuracy for correct classification of the diameter category of 97%. Individuals were followed for a maximum of five consecutive days on at least two occasions. We obtained 28,797 instantaneous observations of positional behavior, 2,811 being locomotion.
Support Compliance.
We calculated SS based on support diameter and the number of supports used, which allows us to quantify the broad relationships between positional modes and the compliance of the supports on which they were exhibited. The SS, calculated for bouts in which 1–4 supports were used, is
where Yi is the interval midpoint for each diameter category (see Table 2) for the ith support used and n is the number of supports used per bout. The SS is transformed by using a natural logarithm, giving a variable ln(SS), which in the GLM (Table 1) produces standardized residuals with an approximately normal distribution.
Statistical Analysis.
The GLMs, with ln(SS) as the response variable, are used to quantify the effect of support compliance on orangutan locomotion. Type III hypotheses are used to calculate sums of squares, F ratios, degrees of freedom, and significance levels. Because interaction terms substantially increase the complexity of the model, the contribution of the interaction in this model was quantified by using the modified F statistic of the model both with and without the interaction term (F8,1479 = 3.84; P > 0.0005).
The sample consists of repeated measures from 10 individuals. Because this might contravene assumptions of independence, the importance of the presence of an “individual” variable was compared with that of the presence of a combined age–sex variable, by using the modified F statistic. For the model of best fit, F7,1464 = 2.00 and P < 0.05, which suggests that the repeated measures made on individuals do not contravene assumptions of independence and that individual differences are adequately represented by the classification of a combined age–sex variable.
For analysis, height in the canopy and the number of supports used may be classified in a number of different ways. Here, we began the modeling process following ref. 9, which found that the relationships between locomotion and related variables were best described when height was classified as <20 m or >20 m and number of supports distinguished only between single- and multiple-support use (1 or >1). We then quantified the validity of these summary variables for the model of best fit by increasing the classification of height to 10-m intervals and number of supports to 1, 2, 3, or 4. Comparing the alternate models by using a modified F statistic on the error mean squares showed that number of supports used is adequately described by the summary variable (1 or >1) (F27,1447 = 1.39; P < 0.05), but height is better described when classified in 10-m intervals (F7,1467 = 3.69; P > 0.001).
The differences between mean ln(SS) for each level within a factor in the final model were tested for significance with Tukey's test of multiple pairwise comparisons (P < 0.05). Statistically similar means form homogeneous subsets. There is a significant difference between the mean of two factor levels only if they do not appear in any of the same subsets.
Acknowledgments.
We are grateful for the comments of two anonymous reviewers. We thank the Indonesian Institute of Science, the Indonesian Nature Conservation Service, and the Leuser Development Program for granting permission and giving support to conduct scientific research in the Ketambe Research Station in the Leuser Ecosystem. Our research into orangutan locomotion has been supported by the Leverhulme Trust, the Royal Society, the L.S.B. Leakey Foundation, and the Natural Environment Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Alexander RM. Principles of Animal Locomotion. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 2.Alexander RM. Elastic mechanisms in primate locomotion. Z Morphol Anthropol. 1991;78:315–320. [PubMed] [Google Scholar]
- 3.Demes B, Jungers WL, Gross TS, Fleagle JG. Kinetics of leaping primates: Influence of substrate orientation and compliance. Am J Phys Anthropol. 1995;96:419–429. doi: 10.1002/ajpa.1330960407. [DOI] [PubMed] [Google Scholar]
- 4.Bonser R. Branching out in locomotion: The mechanics of perch use in birds and primates. J Exp Biol. 1999;202:1459–1463. doi: 10.1242/jeb.202.11.1459. [DOI] [PubMed] [Google Scholar]
- 5.Cartmill M, Milton K. The lorisiform wrist joint and the evolution of ‘brachiating’ adaptations in the Hominoidea. Am J Phys Anthropol. 1977;47:249–272. doi: 10.1002/ajpa.1330470206. [DOI] [PubMed] [Google Scholar]
- 6.Pontzer H, Wrangham RW. Climbing and the daily energy cost of locomotion in wild chimpanzees: Implications for hominoid locomotor evolution. J Hum Evol. 2004;46:317–335. doi: 10.1016/j.jhevol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt D. Compliant walking in primates. J Zool. 1999;247:149–160. [Google Scholar]
- 8.Thorpe SKS, Crompton RH, Alexander RM. Orangutans use compliant branches to lower the energetic cost of locomotion. Biol Lett. 2007;3:253–256. doi: 10.1098/rsbl.2007.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorpe SKS, Holder R, Crompton RH. Origin of human bipedalism as an adaptation for locomotion on flexible branches. Science. 2007;316:1328–1331. doi: 10.1126/science.1140799. [DOI] [PubMed] [Google Scholar]
- 10.Cant JGH. Positional behavior and body size of arboreal primates: A theoretical framework for field studies and an illustration of its application. Am J Phys Anthropol. 1992;88:273–283. doi: 10.1002/ajpa.1330880302. [DOI] [PubMed] [Google Scholar]
- 11.Thorpe SKS, Crompton RH. Orangutan positional behavior and the nature of arboreal locomotion in Hominoidea. Am J Phys Anthropol. 2006;131:384–401. doi: 10.1002/ajpa.20422. [DOI] [PubMed] [Google Scholar]
- 12.Thorpe SKS, Crompton RH. Locomotor ecology of wild orangutans (Pongo pygmaeus abelii) in the Gunung Leuser ecosystem, Sumatra, Indonesia: A multivariate analysis using log-linear modelling. Am J Phys Anthropol. 2005;127:58–78. doi: 10.1002/ajpa.20151. [DOI] [PubMed] [Google Scholar]
- 13.Crompton RH, Vereeke E, Thorpe SKS. Locomotion and posture from the common hominoid ancestor to fully modern hominins, with special reference to the last common panin/hominin ancestor. J Anat. 2008;212:501–543. doi: 10.1111/j.1469-7580.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson SG. Parallel evolution in the Hominoid trunk and forelimb. Evol Anthropol. 1998;87:87–99. [Google Scholar]
- 15.Pilbeam D. Genetic and morphological records of the Hominoidea and hominid origins: A synthesis. Mol Phylogenet Evol. 1996;5:155–168. doi: 10.1006/mpev.1996.0010. [DOI] [PubMed] [Google Scholar]
- 16.Pilbeam D. In: The Primate Fossil Record. Hartwig WC, editor. Cambridge, UK: Cambridge Univ Press; 2002. pp. 303–310. [Google Scholar]
- 17.Gebo D, et al. A hominoid genus from the early Miocene of Uganda. Science. 1997;276:401–404. doi: 10.1126/science.276.5311.401. [DOI] [PubMed] [Google Scholar]
- 18.Moyà-Solà S, et al. Pierolapithecus catalaunicus, a new middle Miocene great ape from Spain. Science. 2004;306:1339–1344. doi: 10.1126/science.1103094. [DOI] [PubMed] [Google Scholar]
- 19.Alexander RM. Mechanics of skeleton and tendons. In: Brooks VB, editor. Handbook of Physiology: The Nervous System. Bethesda: Am Physiol Soc; 1981. pp. 17–42. [Google Scholar]
- 20.Hunt KD. Positional behavior of Pan troglodytes in the Mahale Mountains and Gombe Stream National Parks, Tanzania. Am J Phys Anthropol. 1992;87:83–105. doi: 10.1002/ajpa.1330870108. [DOI] [PubMed] [Google Scholar]
- 21.Remis M. Effects of body size and social context on the arboreal activities of lowland gorillas in the Central African Republic. Am J Phys Anthropol. 1995;97:413–433. doi: 10.1002/ajpa.1330970408. [DOI] [PubMed] [Google Scholar]
- 22.Elton S. The environmental context of human evolutionary history in Eurasia and Africa. J Anat. 2008;212:377–393. doi: 10.1111/j.1469-7580.2008.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cartmill M. Climbing. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional Vertebrate Morphology. Cambridge, MA: Harvard Belknap Press; 1985. pp. 73–88. [Google Scholar]
- 24.Alexander RM, Maloiy GMO. Stride lengths and stride frequencies of primates. J Zool. 1984;202:577–582. [Google Scholar]
- 25.Larson SG, Schmitt D, Lemelin P, Hamrick M. Limb excursion during quadrupedal walking: How do primates compare to other mammals? J Zool. 2001;255:353–365. [Google Scholar]
- 26.Payne RC, et al. Morphological analysis of the hindlimb in apes and humans. I. Muscle architecture. J Anat. 2006;208:709–724. doi: 10.1111/j.1469-7580.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt KD. Positional behavior in the Hominoidea. Int J Primatol. 1991;12:95–118. [Google Scholar]
- 28.Markham R, Groves CP. Weights of wild orangutans. Am J Phys Anthropol. 1990;81:1–3. doi: 10.1002/ajpa.1330810102. [DOI] [PubMed] [Google Scholar]
- 29.Gerwing JJ. Degradation of forests through logging and fire in the eastern Brazilian Amazon. For Ecol Manage. 2002;157:131–141. [Google Scholar]
- 30.Wich S, et al. Distribution and conservation status of the orang-utan (Pongo spp.) on Borneo and Sumatra: How many remain? Oryx. 2008;42:329–339. [Google Scholar]