Abstract
Neural circuits are generated by precisely ordered synaptic connections among neurons, and this process is thought to rely on the ability of neurons to recognize specific partners. However, it is also known that neurons promiscuously form synapses with nonspecific partners, in particular when cultured in vitro, causing controversies about neural recognition mechanisms. Here we reexamined whether neurons can or cannot select particular partners in vitro. In the cerebellum, granule cell (GC) dendrites form synaptic connections specifically with mossy fibers, but not with climbing fibers. We cocultured GC neurons with pontine or inferior olivary axons, the major sources for mossy and climbing fibers, respectively, as well as with hippocampal axons as a control. The GC neurons formed synapses with pontine axons predominantly at the distal ends of their dendrites, reproducing the characteristic morphology of their synapses observed in vivo, whereas they failed to do so when combined with other axons. In the latter case, synaptic proteins could accumulate between axons and dendrites, but these synapses were randomly distributed throughout the contact sites, and also their synaptic vesicle recycling was anomalous. These observations suggest that GC dendrites can select their authentic partners for synaptogenesis even in vitro, forming the synapses with a GC-specific nature only with them.
Keywords: cerebellum, neuronal recognition, pontine axon, synapse
The brain circuitries are established by specific connections between neurons. These neuronal connections develop via multiple cellular processes. At early developmental stages, the axon of a neuron is extended to the region where its target neurons are differentiating, relying on axon guidance mechanisms; then it becomes associated with these neurons to form initial synaptic contacts. Further refinement of the synaptic contacts is then made by activity-dependent selection of synapses (1, 2). During these processes, axons need to recognize their specific partners for connections, as a large number of heterogeneous neurons are present along their migration pathways as well as at their destinations. Molecular mechanisms to ensure this recognition process have been extensively studied (3–5).
Despite the apparent accuracy of the neuronal connectivities in the mature brain, it is thought that synaptogenesis during development is promiscuous (6). This has particularly been observed in neurons cultured in vitro. For example, in cultures of isolated hippocampal pyramidal neurons, mismatching of presynaptic and postsynaptic components, such as the localization of GABA receptors at non-GABAergic terminals, can occur (7). Clustering of presynaptic elements can be induced even by nonspecific polybasic substrates (8). Nonneuronal cells, which have been engineered to express certain synaptic adhesion molecules (9, 10), can induce the accumulation of presynaptic proteins in axons when they contact one another. These observations suggest that synapses can be generated irrespective of the neuronal types combined, when appropriate molecular systems that can link the pre- and postsynaptic membranes are provided on the cellular surfaces. The question then arises as to how neurons compromise the nonspecific vs. specific nature of synaptogenesis in their establishment of specific circuitries.
In the present study, we re-examined whether synaptogenesis is a nonselective process in vitro, seeking to determine whether neurons can still recognize their specific partners even under in vitro culture conditions. To this end, we used neurons involved in the formation of the cerebellar circuitries (Fig. 1A), which had been well studied. The major afferent axons in the cerebellum are mossy and climbing fibers. The mossy fibers, the major sources of which are the pontine nuclei, form synapses with cerebellar granule cells (GCs). The dendrites of the GCs are connected to the mossy fibers, assuming a characteristic claw-like morphology at their distal ends and organizing synaptic glomeruli (11–13). On the other hand, the climbing fibers, derived from the inferior olivary nuclei, selectively associate with Purkinje cells for synaptogenesis (2). During development, these two classes of fibers are spatially able to touch inappropriate partners, that is, the mossy fibers contacting Purkinje cells and the climbing fibers, GCs. In fact, these fibers do form transient synapses with the respective inappropriate partners (14), although these synapses are abandoned with maturation. Here, we tested whether the GCs could distinguish between the mossy fibers and other axons for synaptogenesis in in vitro cultures. Our results show that the GC dendrites can indeed discriminate their real partners from others even in vitro.
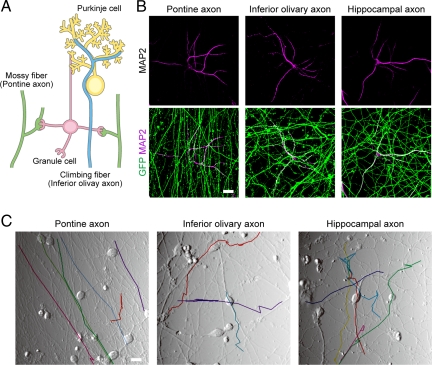
Fig. 1.
Association patterns of axons and dendrites in cocultures. (A) Schematic drawing of the cerebellar circuits. (B) Dissociated cerebellar GCs were cocultured with explants of pontine nuclei, inferior olivary nuclei or hippocampi collected from EGFP-transgenic mice, and observed at 5 div. Axons derived from the explants are EGFP positive (green); and dendrites of GCs are visualized by immunostaining for MAP-2 (magenta). (C) Trace of axonal growth cones on time-lapse images (Movies S1–S3), taken at 3 to 5 div. The somas of GCs are visible as rounded cells. Scale bars, 10 μm.
Results
Initial Processes of Axon–Dendrite Association.
To study whether mossy fibers can specifically recognize cerebellar granule cells (GCs) during in vitro synaptogenesis, we used a neuronal coculture system (15, 16) in which dissociated GCs were cocultured with pontine explants. As controls, we cocultured GCs with explants of inferior olivary nuclei, or those of the hippocampus, the axons of which never meet with cerebellar GCs in vivo. These explants were collected from EGFP-transgenic mice, allowing us to distinguish between the explant-derived axons and GC neurites. During normal development, mossy and climbing fibers arrive in the cerebellum before the formation of the inner granular layer, and wait for the maturation of the GCs, before synapse formation with them (14). To mimic this developmental sequence, we first placed the explants into the plates, and then after 3 days, added dispersed GCs to the cultures (this day was defined as 0 day in vitro [div]).
In these cocultures, the explants radially extended their EGFP-positive axons, whereas GCs developed multiple dendrites, as visualized by immunostaining for MAP-2 (Fig. 1B). In cultures at two to four div, we observed the initial processes of axon-dendrite attachment by live-cell imaging (Fig. 1C). Growth cones of the pontine axons tended to migrate straight [see supporting information (SI) Movie S1]. When they collided with granule cell dendrites, the growth cones simply crossed the dendrites, as if these axon tips were ignoring the latter. The inferior olivary axons ran in more complex fashions (Movie S2). Some of them crossed the granule cell dendrites, whereas others coursed along them. As a whole, the interaction between inferior olivary axons and GC dendrites appeared to be random. The migratory behavior of hippocampal axons was similar to that of the olivary axons (Fig. 1C and Movie S3). In fixed samples, we observed that the olivary or hippocampal axons tended to fasciculate with granule cell dendrites (Fig. 1B), probably because some of these axons migrated along the dendrites, as mentioned above. Thus, in these early cultures, pontine axons did not exhibit any signs of preferentially associating with their in vivo targets, the GCs.
Synapse Formation Between Granule Cell Dendrites and Pontine Axons.
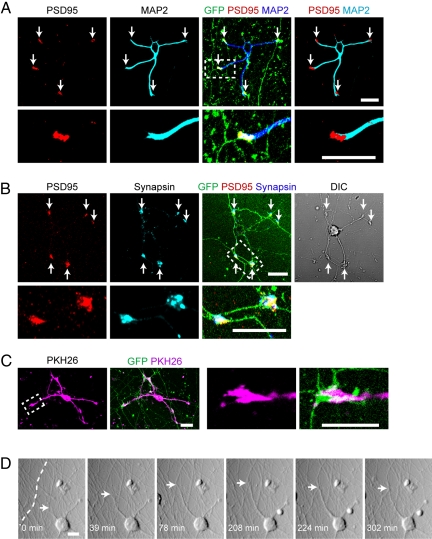
Next, we examined synapse formation in the pontine-GC cocultures by immunostaining for post- and presynaptic markers. These markers began to appear as punctate signals around 3 to 5 div, and became more distinctive in older cultures. When examined at 14 div, PSD95, a postsynaptic scaffold protein, was detected at the distal ends of GC dendrites as distinct patches (Fig. 2A). Another postsynaptic marker, NMDA receptor 2A, was also accumulated at the distal ends, colocalizing with the PSD95 signals (Fig. S1A). The PSD95 puncta were always associated with EGFP-labeled axons, which showed focal swelling at these specific contact sites. Presynaptic proteins, such as synapsin, were also recruited to these sites (Fig. 2B and Fig. S1B). As observed in early cultures, many axons crossed the dendrites at the nondistal portions of the latter, but their contact sites rarely accumulated synaptic markers (Fig. S2A). However, we occasionally detected a clustering of PSD95 and synapsin at such crossing points of axon and dendrite; and in these cases, the dendrites often showed a short protrusion or branching into the synaptic zones (Fig. S2B). To visualize more precisely the morphology of the GC dendrite terminals, we stained the cells with PKH26, a lipophilic dye to label the cell membranes (17) (Fig. 2C). The PKH26-positive dendrite terminals spread over the focal swelling of the axon, assuming a claw-like shape. These morphological features of axo-dendritic associations were reminiscent of those of the synaptic boutons observed in the inner GC layer in vivo (14).
Fig. 2.
Synapse formation of GC dendrites with pontine axons. (A, B) Double-immunostaining for PSD95 (red) and MAP-2 (cyan or blue) or synapsin-I (cyan or blue) in a GC-pontine coculture at 14 div. Arrows point to dendritic terminals. (C) Membranes of GCs are labeled with PKH26 (magenta) to outline the shape of a dendritic terminal at 14 div. Axons are visualized with EGFP signals (green). The squared areas in A and B are enlarged in the bottom photos (and also in other figures), and the squared area in C is enlarged at the right. (D) Time-lapse images of GCs cocultured with pontine axons, acquired at 2 to 3 div. A pontine axon is highlighted with a broken line on the image at 0 min. Arrows point to the migrating tip (growth cone) of a GC dendrite. (See also the corresponding Movie S4.) Scale bar, 10 μm.
Then, we asked how the GC dendrites came to be associated with axons in the above pattern. To visualize the association processes, we took time-lapse movies of cells at two to three div. The movie images revealed that a dendrite extending from a GC captured an axon with its distal end, resulting in a slight pulling of the axon toward the soma of the GC (Fig. 2D and Movie S4). These contacts sites likely mature to synapses at later stages, as observed above. These observations suggest that the dendritic terminals of GCs play an active role in recognizing the mossy fibers and making synaptic contacts with them, whereas mossy fiber growth cones have no such roles.
Synapse Formation Between Granule Cells and Nonauthentic Partners.
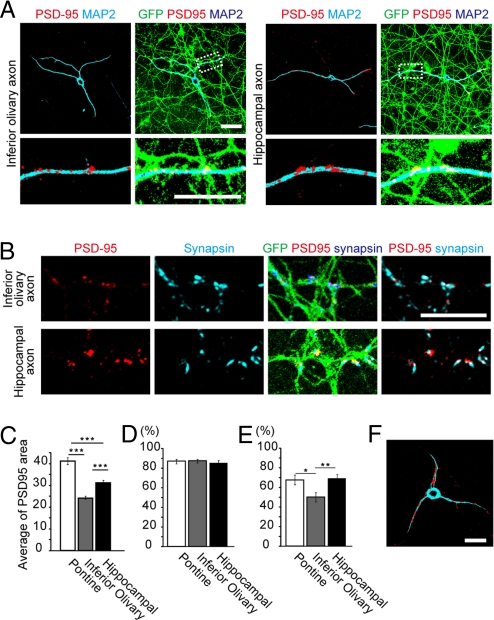
For comparison, we analyzed synapse formation in the cocultures of GCs and inferior olivary or hippocampal explants. As mentioned above, GC dendrites fasciculated with these axons. At these contact sites, PSD95 puncta were found to be scattered along the dendrites, not exclusively localized at their distal ends (Fig. 3A); and these puncta were smaller than those detected in the GC-pontine axon synapses (Fig. 3C). These PSD95 signals, in general, overlapped with synapsin signals (Fig. 3 B and D); although there were many PSD95-free synapsin puncta, particularly in the cocultures with olivary axons (Fig. 3E). These observations suggest that GC dendrites can accumulate pre- and postsynaptic proteins at their contact sites with olivary or hippocampal axons but that they cannot undergo the distal end-specific organization of synapses with these nonnatural partners. To further examine whether this pattern of synapse formation is regulated by axons, we cultured GCs alone without adding external axon sources and found that PSD95 was randomly distributed along the dendrites (Fig. 3F and Fig. S3), supporting the idea that the distal-end localization of synapses is generated via the specific interactions between GCs and pontine axons.
Fig. 3.
Synapse formation of GC dendrites with inferior olivary or hippocampal axons. (A, B) GCs were cocultured with inferior olivary or hippocampal axons, and double-immunostained for PSD95 (red) and MAP-2 (cyan or blue) or synapsin-I (cyan or blue) at 14 div. (C) Comparison of PSD95-positive punctum areas. Punctum number (n) = 1,326 for pontine, 1,039 for inferior olivary, and 1,403 for hippocampal axons. Mean ± SEM; ***P < 0.001. (D) Percentage of the PSD95 puncta overlapping with synapsin signals to the total PSD95 puncta. The numbers of fields used for analysis were 25 for pontine, 25 for inferior olivary, and 21 for hippocampal axons. Data are mean ± SEM. P = 0.79 between pontine axon and inferior olivary axon, P = 0.62 between pontine axon and hippocampal axon, and P = 0.77 between inferior olivary axon and hippocampal axon. (E) Percentage of the synapsin puncta overlapping with PSD95 signals to the total synapsin puncta. The numbers of fields used for analysis were 25 for pontine, 25 for inferior olivary, and 21 for hippocampal axons. Data are mean ± SEM. P = 0.021 between pontine and inferior olivary axons, P = 0.90 between pontine and hippocampal axons, and P = 0.0046 between inferior olivary and hippocampal axons. (F) Granule cell cultured without any explants. Double-immunostaining for PSD95 (red) and MAP-2 (cyan). Scale bars, 10 μm.
In addition, we tested whether pontine axons could manage to induce the distal localization of synapses on the dendrites of other neurons, by preparing a coculture of pontine explants and dissociated hippocampal neurons. In these cultures, when pontine axons were in contact with hippocampal dendrites, PSD95 signals were more or less accumulated at the contact points. However, the hippocampal dendrites never accumulated synapse markers at their distal ends, even when these ends were located in close vicinity to pontine axons (Fig. S4). These results suggest that pontine axons have no universal ability to induce the distal end-localization of synapses on the dendrites of neurons.
Impairment of Synaptic Vesicle Recycling in Nonauthentic Axon-Dendritic Combinations.
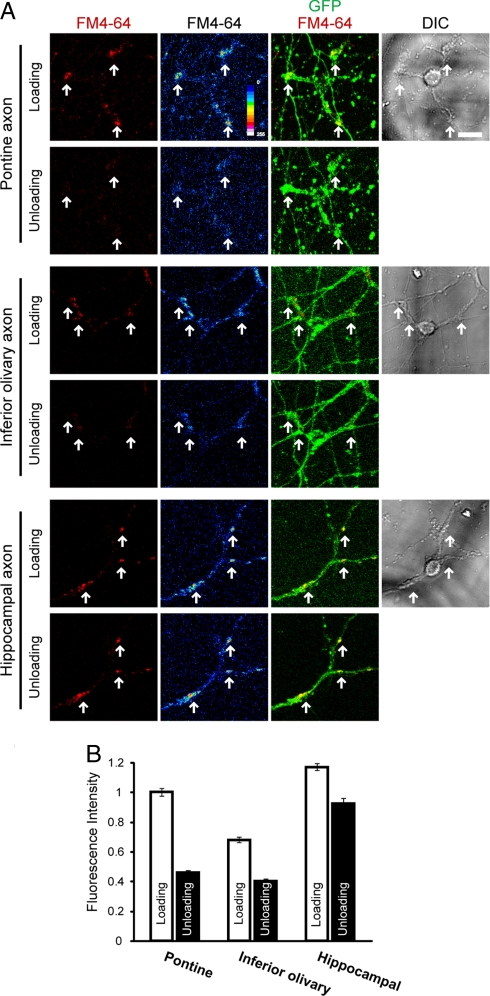
Although the pattern of synaptic marker distributions varied with the axon types combined, pre- and postsynaptic markers overlapped in all of the above cocultures, suggesting that GCs could organize synaptic contacts with any of these afferent partners. Hence, we asked whether these synapses were functional. To this end, we looked at synaptic vesicle recycling by conducting FM4–64 dye uptake experiments (18). When the cocultures with pontine explants were examined, the presynaptic recycling took place normally; that is, axons incorporated the dye upon stimulation with high potassium, and released it after re-stimulation (unloading stimulation, Fig. 4). Inferior olivary and hippocampal axons also incorporated FM4–64 dye in a high potassium-dependent way, although the loading intensity somewhat differed among the axon types (Fig. 4 and Fig. S5A). However, their responses to the unloading stimuli were anomalous. Only 41.1% and 21.1% of the once-incorporated dye was released from the inferior olivary and hippocampal axons, respectively, whereas 54.3% of it was released from the pontine axons (Fig. 4B). As a control, we treated cultures with high potassium in the absence of the dye and then re-stimulated them in the presence of the dye. In these experiments, the inferior olivary and hippocampal axons could incorporate the dye. These results suggest that synaptic vesicle endocytosis more or less proceeded normally in the inferior olivary and hippocampal axons but that its exocytotic process was impaired. As another control, we cocultured EFGP-labeled hippocampal explants with nonlabeled dissociated hippocampal neurons and found that these axons exhibited a much higher level of dye release (54.4%) than in the case of their cocultures with pontine axons (Fig. S5B). This result confirmed that hippocampal axons, used in these experiments, could undergo the ordinary vesicle recycling, when combined with appropriate partners. We did not perform similar control experiments for inferior olivary axons, because different culture conditions were required to produce differentiated Purkinje cells, which are the correct targets for these axons.
Fig. 4.
Synaptic recycling in cocultures. (A) Cocultures at 14 div (for pontine and hippocampal axons) or 15 div (for olivary axons) were processed for the loading and unloading of FM4–64 (red and pseudocolor). Individual neurons were marked by culturing them on gridded cover glasses, and the same neurons were photographed at the loading and unloading steps. Arrows point to representative synapses, which have incorporated FM4–64. (B) Fluorescence intensity of FM4–64. Pixel intensity of each punctum was measured after background subtraction, and the intensity was normalized against the pontine sample. Punctum number (n) = 124 and 90 for pontine, n = 100 and 98 for inferior olivary, and 156 and 139 for hippocampal axons, at loading and unloading, respectively. Data are mean ± SEM. Scar bars, 10 μm.
Discussion
Our results demonstrated that cerebellar GCs could form morphologically and functionally normal synapses only with the pontine axons, out of the three different types of axons tested. Our culture systems did not provide any environmental cues for neuronal processes to search for specific targets, as cells and explants were plated randomly on a homogenous surface. Interneuronal recognition observed here, therefore, likely did not depend on axon guidance mechanisms, but solely depended on the autonomous properties of individual neurons.
Live-cell imaging analysis suggested that GCs and pontine axons were connected through a two-step process, i.e., initial extension of dendrites from GCs and the subsequent capturing of axons by the distal end of the dendrites. Recognition between the dendrite and axon, therefore, likely takes place during these processes. It remains unknown whether any attractants were released from the axons to GC dendrites, or whether these dendrites randomly migrated until they collided with axons. After the initial axon-dendrite contacts, some signals might be exchanged between the contacting neurites; for example, pontine axons may send signals to induce postsynaptic differentiation at the tip of GC dendrites. It should be noted that pontine axons failed to induce the distal synapse formation on hippocampal dendrites. This observation suggests that the machinery to receive such putative pontine signals is specifically provided by GC dendrites.
The growth cones of pontine axons, on the other hand, seem to have no active role in the recognition of GCs, as suggested by the observation that, when the pontine growth cones collided with GC dendrites, the former crossed the latter without any halting or collapsing. As a result, in older cocultures, many pontine axons that had crossed dendrites still showed no synapses at the crossing point. Occasionally, however, synaptic proteins were detectable at these crossing points. In these cases, a protrusion of the dendrite was generally observed at the synaptic sites, suggesting that the dendrite had branched to form a new distal end at these local sites. It should be recalled that, during excitatory synapse formation, dendrites produce an array of filopodia, and that these filopodia function to capture axons for making their connections (19), suggesting that the dendrites, rather than axons, play an active role in the final step of axon-dendrite linkage (20–22). Similar principles seem to be at work in the processes of GC-pontine connections.
The GC dendrites were also able to make synaptic contacts with inferior olivary and hippocampal axons, but their contacts were not exclusively at the distal ends of GC dendrites. Through some unknown mechanisms, the growth cones of these axons occasionally migrated along the GC dendrites, although this type of migration was not observed in the GC-pontine cocultures. In older cultures, GC dendrites tended to fasciculate with these axons, and pre- and post synaptic proteins were clustered together along their contact sites. However, dye-uptake experiments suggested that, although synaptic vesicle endocytosis took place normally, its exocytosis was impaired in these synapses. The mechanisms for this failure in synaptic vesicle recycling remain unknown, but this finding suggests that the correct pairing of pre- and postsynaptic domains is required to ensure normal vesicle recycling.
In summary, GC dendrites can form normal synapses only with their authentic partners, mossy fibers, but not with other axons, in vitro. These findings predict the presence of molecular systems that lead GC dendrites to recognize mossy fibers; and identification of such systems is the most important subject for future research. Our results support the consensus that synaptogenesis can occur between nonselected partners but simultaneously have made us aware that such synapses might not be functionally equivalent to the synapses formed between the correct pairs of neurons.
Materials and Methods
Neuronal Cocultures.
Neuronal cocultures were prepared as described previously (16, 23), with some modifications. Briefly, pontine nuclei and hippocampi were excised from the brains of P0-P1 EGFP-transgenic mice, and inferior olivary nuclei, from those of E16 transgenics (24), and were cut into fragments to make microexplants. These microexplants were plated on cover glasses (Fisher Scientific) coated with poly-l-lysine and laminin in NeuroBasal medium (Invitrogen) with B27 supplements. When necessary, gridded cover glasses (GC1300, Matsunami) were also used. After 3 days, cerebellar granule cells of P6–8 mice or hippocampal neurons of E16 mice were isolated by trypsin treatment and plated at a density of 5,000 cells/cm2 onto the cultures containing the microexplants.
Immunocytochemistry.
Neuronal cultures were fixed with 2% paraformaldehyde and 4% sucrose in Hepes-buffered Hanks' balanced salt solution (HBSS) for 10 min at 37 °C. Fixed cells were permeabilized with 0.25% Triton X-100 in TBS for 5 min and blocked with 5% skim milk. The cultures were then incubated at 4 °C overnight with primary antibodies diluted with 5% skim milk in TBS. The primary antibodies were visualized with fluorochrome-conjugated secondary antibodies (Alexa fluor secondary antibodies, Invitrogen). The following primary antibodies were used: rat polyclonal anti-GFP (Nacarai); mouse monoclonal anti-MAP2 (Sigma); rabbit polyclonal anti-MAP2 (Chemicon); mouse monoclonal anti-PSD95 (Affinity BioReagents); rabbit polyclonal anti-synapsin I (Chemicon); and rabbit polyclonal anti-NMDAR2A (Affinity BioReagents).
PKH26 Staining.
Granule cells were incubated with 5 μM PKH26 (Sigma) in HBSS at room temperature for 4 min. An equal volume of 1% BSA in HBSS was then added, and the cells were further incubated for 1 min to stop the staining reaction. The labeled cells were then centrifuged at 400 g for 10 min at 25 °C, resuspended in NeuroBasal medium, and plated.
Functional Labeling of Presynaptic Buttons with FM4–64.
In the loading process, cells were incubated for 1 min in a high-K+ (90 mM KCl), isosmotic HBSS (18), containing 16 μM FM4–64 (Invitrogen). Neurons were then washed for 10 min in HBSS without Ca2+ but with 5 μM Mg2+ to prevent unloading. A 1-mM quantity of ADVASEP-7 (Sigma) was subsequently added to quench nonspecific signals. In the unloading process, neurons loaded with the dyes were incubated with the same high-K+ solution for 1 min and washed for 10 min with NeuroBasal medium. Image acquisition was made in NeuroBasal medium.
Live-Cell Imaging.
Live-cell imaging was performed using a LCV100 (Olympus) equipped with a UAPO 40×/340 objective lens (Olympus), a LED light source, a CCD camera (Olympus, DP30), differential interference contact (DIC) optical components, and interference filters. The entire system was placed in an incubator kept at 37 °C and 5% CO2. Image acquisition was performed by using MetaMorph version 6.3r4. Tracing of growth cone movement was carried out with ImageJ (National Institutes of Health).
Imaging Acquisition and Quantification.
Confocal images of neurons were obtained at 40×, 60× or 100× with an LSM510 (Carl Zeiss). For quantitative analysis, confocal images were processed with Adobe Photoshop (Adobe), immunofluorescence images were converted into binary images, and thresholds were chosen such that all naturally visible signals were included into the analysis. PSD95 area was measured from the binarized images with the “Analyze Particles” feature of ImageJ. Puncta with less than 10 pixels were excluded. For detecting the PSD95 and synapsin puncta that overlap each other, we first processed the images of each molecule with Analyze Particles, and then determined the overlapping puncta from these images. Multiple fields, each containing 23 puncta on average, were analyzed. FM4–64 fluorescence intensity was measured with the “Histogram” feature of ImageJ after selecting puncta with “Freehand selection.” Measured data were exported to Excel (Microsoft Corporation). All data were compared by using the Wilcoxon test.
Supplementary Material
Acknowledgments.
We are grateful to C. Yoshii, H. Saito, and H. Abe for maintaining the mice, and to all laboratory members for their + encouragement and discussion. This work was supported by a grant from the program Grants-in-Aid for Specially Promoted Research of the Ministry of Education, Science, Sports, and Culture of Japan (to M.T.), and by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (JSPS) for Junior Scientists (to S.I). S.I. is a recipient of a fellowship from the JSPS.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906653106/DCSupplemental.
References
- 1.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto K, Kano M. Postnatal development and synapse elimination of climbing fiber to Purkinje cell projection in the cerebellum. Neurosci Res. 2005;53:221–228. doi: 10.1016/j.neures.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 4.Garrity PA, Zipursky SL. Neuronal target recognition. Cell. 1995;83:177–185. doi: 10.1016/0092-8674(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 5.Ackley BD, Jin Y. Genetic analysis of synaptic target recognition and assembly. Trends Neurosci. 2004;27:540–547. doi: 10.1016/j.tins.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Jontes JD, Phillips GR. Selective stabilization and synaptic specificity: A new cell-biological model. Trends Neurosci. 2006;29:186–191. doi: 10.1016/j.tins.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Rao A, Cha EM, Craig AM. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J Neurosci. 2000;20:8344–8353. doi: 10.1523/JNEUROSCI.20-22-08344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burry RW. Formation of apparent presynaptic elements in response to poly-basic compounds. Brain Res. 1980;184:85–98. doi: 10.1016/0006-8993(80)90588-0. [DOI] [PubMed] [Google Scholar]
- 9.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 10.Biederer T, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 11.Cajal R. In: Histology of the Nervous System. Swanson L, editor. Vol 2. New York: Oxford Univ Press; 1995. pp. 28–44. [Google Scholar]
- 12.Altman J, Bayer S. Development of the Cerebellar System: In Relation to Its Evolution, Structure, and Functions. Boca Raton, Florida: CRC Press; 1997. pp. 470–497. [Google Scholar]
- 13.Shalizi A, et al. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- 14.Mason CA, Gregory E. Postnatal maturation of cerebellar mossy and climbing fibers: Transient expression of dual features on single axons. J Neurosci. 1984;4:1715–1735. doi: 10.1523/JNEUROSCI.04-07-01715.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manzini MC, Ward MS, Zhang Q, Lieberman MD, Mason CA. The stop signal revised: Immature cerebellar granule neurons in the external germinal layer arrest pontine mossy fiber growth. J Neurosci. 2006;26:6040–6051. doi: 10.1523/JNEUROSCI.4815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baird DH, Hatten ME, Mason CA. Cerebellar target neurons provide a stop signal for afferent neurite extension in vitro. J Neurosci. 1992;12:619–634. doi: 10.1523/JNEUROSCI.12-02-00619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell SK, Rivas RJ, Rodriguez-Boulan E, Hatten ME. Development of polarity in cerebellar granule neurons. J Neurobiol. 1997;32:223–236. doi: 10.1002/(sici)1097-4695(199702)32:2<223::aid-neu7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Gerrow K, et al. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49:547–562. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 20.Jontes JD, Smith SJ. Filopodia, spines, and the generation of synaptic diversity. Neuron. 2000;27:11–14. doi: 10.1016/s0896-6273(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 21.Togashi H, et al. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J Cell Biol. 2006;174:141–151. doi: 10.1083/jcb.200601089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohmann C, Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–260. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Togashi H, et al. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 24.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.