Abstract
Neuronal accumulation of α-synuclein and Lewy body formation are characteristic to many neurodegenerative diseases, including Parkinson's disease (PD). This Lewy pathology appears to spread throughout the brain as the disease progresses. Furthermore, recent studies showed the occurrence of Lewy pathology in neurons grafted into the brains of PD patients, suggesting the spread of pathology from the host tissues to the grafts. The mechanism underlying this propagation is unknown. Here, we show that α-synuclein is transmitted via endocytosis to neighboring neurons and neuronal precursor cells, forming Lewy-like inclusions. Moreover, α-synuclein was transmitted from the affected neurons to engrafted neuronal precursor cells in a transgenic model of PD-like pathology. Failure of the protein quality control systems, especially lysosomes, promoted the accumulation of transmitted α-synuclein and inclusion formation. Cells exposed to neuron-derived α-synuclein showed signs of apoptosis, such as nuclear fragmentation and caspase 3 activation, both in vitro and in vivo. These findings demonstrate the cell-to-cell transmission of α-synuclein aggregates and provide critical insights into the mechanism of pathological progression in PD and other proteinopathies.
Keywords: Lewy body, neurodegeneration, Parkinson's disease, protein aggregation
The neuronal protein α-synuclein is a protein whose function is not yet completely understood. Although this protein has been suggested to play a role in synaptic vesicle biogenesis, modulation of synaptic transmission, and brain lipid metabolism, involvement of α-synuclein in the pathogenesis of neurodegenerative diseases has been established clearly (1). In several forms of inherited Parkinson's disease (PD), three missense mutations and gene multiplications have been linked to SNCA, the gene encoding α-synuclein protein (2). Amyloid fibril forms of α-synuclein aggregates are the main constituents of Lewy bodies (LBs) and Lewy neurites in PD and several other LB diseases (3). Overproduction of the wild-type protein or mutant variants in several model organisms results in neurodegeneration and Lewy-like inclusion formation (4). The tight association between α-synuclein aggregation and neurodegenerative phenotypes in human patients and animal models and the fact that accelerated aggregation is a common outcome of all known α-synuclein mutations (5) strongly highlight the importance of abnormal aggregation of this protein in the pathogenesis of PD and related LB diseases.
Parkinson's disease primarily is defined as a movement disorder associated with degeneration of dopaminergic neurons in the nigrostriatal system (6). However, its pathology involves the progressive neuronal accumulation of aggregated α-synuclein, and the formation of LBs affects various functional structures throughout the human nervous system (7), leading to serious cognitive and behavioral alterations. In a large proportion of PD cases, accumulation of aggregated α-synuclein undergoes an ascending and highly predictable pattern of progression, spreading from the lower brainstem and olfactory bulb into the limbic system and, eventually, to the neocortex (8), suggesting a mechanism involving pathological propagation, similar to the one observed in prion diseases. The idea of pathological propagation recently has gained much attention after two studies showed the host-to-graft propagation of α-synuclein-positive Lewy-like pathology in long-term mesencephalic transplants in PD (9, 10), results that have a profound impact on cell-based therapies. Despite this, the underlying mechanism of the initiation and propagation of α-synuclein pathology is unknown, and whether the previously proposed direct interneuronal transmission of α-synuclein aggregates actually occurs has not been determined (11, 12).
We previously have found that a small but significant proportion of α-synuclein and its aggregates are secreted from neuronal cells via exocytosis (13). Moreover, upon exposure, neurons in culture are capable of taking up α-synuclein aggregates via endocytosis (14, 15). On the basis of these observations, we tested the hypothesis that α-synuclein pathology can be propagated by direct neuron-to-neuron transmission of α-synuclein aggregates.
Results
Direct Host-to-Graft Transmission of α-Synuclein in Vivo.
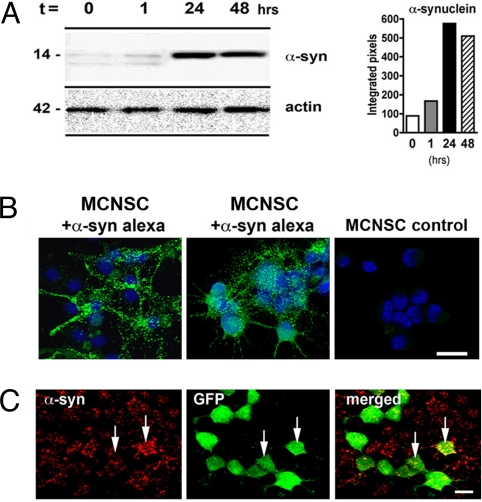
Recent reports showed host-to-graft progression of α-synuclein pathology in mesencephalic transplants in PD (9, 10), which ultimately may affect the beneficial outcome of stem cell grafting over the long term. However, the mechanism underlying this pathological propagation remains unknown (11). We therefore directly tested whether α-synuclein is transmitted from host neurons to grafted neural stem cells. Previously, we have shown that neurons and other brain cells can take up extracellular α-synuclein aggregates (15). To determine if a similar mechanism is acting in neural stem cells, mouse cortical neuronal stem cells (MCNSCs) were incubated with media containing Alexa-Fluor-488-labeled α-synuclein. Both Western blot and immunofluorescence analyses showed the ability of stem cells to take up extracellular α-synuclein (Fig. 1 A and B). To demonstrate whether α-synuclein released from neuronal cells can be directly transferred to MCNSCs, we cocultured neuronal cells overexpressing human α-synuclein with GFP-labeled MCNSCs as acceptors. After 24 h, 47% of the GFP-positive MCNSCs displayed punctate patterns of cytoplasmic accumulation of human α-synuclein (Fig. 1C).
Fig. 1.
α-Synuclein uptake by mouse cortical neuronal stem cells (MCNSCs). (A) Immunoblot analysis of extracellular α-synuclein uptake after 24 and 48 h in MCNSCs. (B) Confocal microscopy analysis demonstrating the MCNSC uptake of Alexa-Fluor-488-tagged extracellular α-synuclein (green). (C) The MCNSCs infected with a lentivirus-GFP (green) were cocultured with a neuronal cell line (B103) infected with a lentivirus expressing α-synuclein (red). Punctate and diffuse red staining represents the transmitted α-synuclein in acceptor cells (arrows). (Scale bars, 10 μm.)
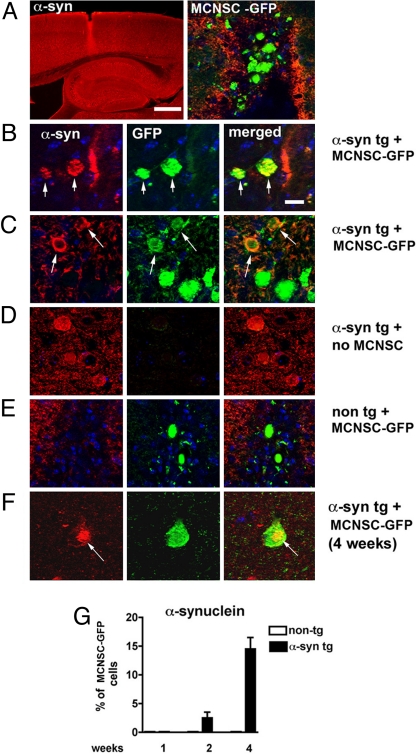
Next, we analyzed the propagation of α-synuclein to transplanted stem cells in vivo. For this purpose, GFP-labeled MCNSCs were injected into the hippocampus (Fig. 2A) of transgenic mice (line 61) expressing human α-synuclein under the control of the Thy-1 promoter (16). One week postinjection, ≈2.5% of the grafted GFP-labeled MCNSCs showed human α-synuclein immunoreactivity (Fig. 2 B and C), whereas the MCNSCs grafted into nontransgenic mice tested negative for human α-synuclein (Fig. 2 D and E). After 4 weeks, the percentage of human α-synuclein-positive MCNSCs increased to 15% (Fig. 2G), and a few of these cells developed compact inclusion bodies within the cytoplasm (Fig. 2F). These results suggest that α-synuclein pathology can be transmitted directly from host to grafted cells.
Fig. 2.
Transmission of α-synuclein from host to grafted neural stem cells. (A) Thy-1 α-synuclein transgenic mice received stereotaxic intrahippocampal injections of mouse cortical neuronal stem cells (MCNSCs) (150,000 cells) infected with lentivirus-GFP. Confocal microscopy analysis demonstrates the site of the injection and the GFP-tagged MCNSCs. The α-synuclein is immunolabeled with tyramide red. (B and C) Colocalization of α-synuclein (red) in acceptor MCNSCs (green) (arrows) in areas adjacent to the injection site. (Scale bar, 10 μm.) (D) Control experiment showing the background fluorescence in the absence of MCNSC injection. (E) Control experiments showing lack of human α-synuclein in MCNSCs injected into nontransgenic mice. (F) A compact inclusion body with human α-synuclein immunoreactivity (red) 4 weeks postinjection. (G) Quantification of human α-synuclein-positive MCNSCs grafted into Thy-1 α-synuclein transgenic mice or nontransgenic control mice.
We then examined the ultrastructural features of the inclusion bodies formed in the grafted cells. The electron-dense inclusion bodies were apparent in the MCNSCs transplanted into human α-synuclein transgenic mice but not in the ones grafted into nontransgenic mice (Fig. S1). The number of grafted cells containing inclusion bodies increased with time after engraftment such that after 1 week cells rarely contained inclusion bodies; however, after 4 weeks, cells containing inclusion bodies were quite common (Fig. S1G). These structures were composed of vesicular and amorphous electron-dense material, with no apparent fibrils (Fig. S1F).
Inclusion Body Formation via Cell-to-Cell Transmission of α-Synuclein.
To further characterize the cell-to-cell transmission of α-synuclein, we established an in vitro coculture model. In this model, two populations of human dopaminergic neuronal cells (derived from SH-SY5Y neuroblastoma cells) were cocultured: one overexpressing myc-tagged α-synuclein (donor cells) and the other labeled with Qtracker without α-synuclein overexpression (acceptor cells). The myc tag was used to trace the donor-derived α-synuclein proteins. After 24 h of coculture, transmission of myc-tagged α-synuclein from the donor cells was detected in the acceptor cells (Fig. 3A). The number of acceptor cells that contain the donor-derived α-synuclein increased in proportion to the α-synuclein expression levels in donor cells. In addition, in some acceptor neuronal cells, donor-derived α-synuclein formed juxtanuclear inclusion bodies, with increasing numbers directly correlated with increasing expression levels in donor cells (Fig. 3A). Approximately half of these inclusion bodies displayed ubiquitin immunoreactivity and were labeled with thioflavin S, similar to human LBs (Fig. 3C). The inclusion bodies typically formed at later time points, suggesting that prolonged transmission of α-synuclein caused the inclusion body formation (Fig. 3B). To examine the potential involvement of donor cell membrane leakage, we performed the lactate dehydrogenase release assay, with the cells overexpressing β-galactosidase, α-synuclein, or myc-tagged α-synuclein. We were not able to detect a significant degree of membrane leakage in any of these cases, suggesting that cell-to-cell transmission occurs without cellular membrane damage due to toxic properties of overexpressed α-synuclein. However, longer incubation with high levels of α-synuclein expression did cause donor cell death, and in such a situation, the transmission of donor-derived α-synuclein to acceptor cells appeared more robust. This suggests that, although neuron-to-neuron transmission of α-synuclein occurs without apparent cell death, degenerating neurons may accelerate the transmission, probably by acutely providing large amounts of α-synuclein (see SI Discussion).
Fig. 3.
Inclusion body formation via cell-to-cell transmission of α-synuclein. (A) Differentiated SH-SY5Y neuronal cells overexpressing myc-tagged α-synuclein (green) were cocultured with SH-SY5Y acceptor cells labeled with Qtracker 585 (Q; red). (Upper) Diffuse distribution of transmitted α-synuclein in acceptor cells. (Lower) Inclusion body formation (arrow) in acceptor cells. (Scale bars, 20 μm.) (Left) Percentage of acceptor cells with transmitted α-synuclein. (Right) Percentage of acceptor cells with compact inclusion body. Three independent experiments were performed, and on average, 300 cells per sample were analyzed. Error bars are SEM (*, P < 0.05; **, P < 0.01). Inclusion bodies were defined as distinct clusters of α-synuclein-positive structures near the nucleus with diameters exceeding 2 μm. (B) Time-dependent transmission of α-synuclein and inclusion body formation. The experiments were set up in the same way as in (A). The cocultures were examined at indicated times. For quantification, at least 200 cells (average 305 cells) were examined, and four independent experiments were performed. (C) Characterization of inclusion bodies in acceptor cells. (Upper) Ubiquitin-positive inclusion bodies in acceptor cells (arrows). (Scale bar, 10 μm.) (Lower) Thioflavin-S-positive inclusion bodies in acceptor cells (arrowhead, cell outlined with broken line). (Scale bar, 20 μm.) The graph represents the percentage of inclusion bodies labeled with anti-ubiquitin antibodies or thioflavin S. Three independent experiments were performed, examining 8–14 inclusions per experiment. Error bars are SEM. (D) Membrane leakage analysis. The cells overexpressing the indicated proteins were subjected to the lactate dehydrogenase (LDH) release assay. All of the infected cells show lower levels of LDH release compared with the noninfected cells.
α-Synuclein inclusion bodies also were produced when neuronal cells were incubated with the conditioned medium containing secreted forms of myc-tagged α-synuclein (Fig. S2A), further demonstrating the donor-to-acceptor transmission occurring in trans, not requiring cell-to-cell contact. Immunoblot analysis demonstrated similar effects when primary neuronal cultures were incubated with the conditioned medium containing myc-tagged α-synuclein released from the culture of differentiated SH-SY5Y cells (Fig. S2B). A large proportion of the transmitted α-synuclein was found in the Triton-insoluble fraction and appeared as SDS-resistant, large-molecular-mass complexes (Fig. S2B).
The neuronal cells incubated with the conditioned media from the β-galactosidase-expressing cells and the α-synuclein-expressing cells were examined under an electron microscope. The cells incubated with the α-synuclein medium developed electron-dense inclusion bodies in the cytoplasm, frequently near the nucleus, whereas these structures were absent in control cells or cells incubated with the β-galactosidase medium (Fig. S2C). The inclusion bodies were a mixture of various discernable structures, such as vesicles and filamentous structures tangled with amorphous electron-dense material.
To determine whether the transmission of α-synuclein aggregates is dependent on endocytosis, dynamin-1 K44A, a dominant-negative mutant that blocks endocytic vesicle formation, was expressed in acceptor cells. When donor neuronal cells were cocultured with the acceptor cells, transmission of α-synuclein was reduced considerably in the cells expressing dynamin-1 K44A compared with the cells not expressing the mutant dynamin (Fig. S3A), supporting a role for the endocytic pathway in cell-to-cell α-synuclein transfer. Consistently, >90% of the internalized myc-tagged α-synuclein was colocalized with endosomal GTPases rab5a and rab7 in the acceptor cells (Fig. S3B).
Failure of the Quality Control System Promotes the Accumulation of Transmitted α-Synuclein.
In PD, accumulation of α-synuclein is associated with impairment in the protein quality control systems, such as proteasomal and autophagic degradation (17, 18). To determine the role of quality control failure in the deposition of transmitted α-synuclein, cells were incubated with the conditioned medium containing secreted α-synuclein in the presence or absence of proteasomal or lysosomal inhibitors (MG132 and bafilomycin A1, respectively). Accumulation of internalized α-synuclein was increased with the failure of lysosomal function, whereas proteasomal inhibition had little effect (Fig. 4A and Fig. S4A). This is consistent with our previous finding that internalized α-synuclein aggregates are targeted to the lysosome for degradation (14). Inclusion formation, measured as the number of cells containing inclusion bodies, also was increased by lysosomal inhibition (Fig. 4B and Fig. S4A). Proteasomal inhibition, however, caused a slight, although not statistically significant, increase in the number of inclusions. Furthermore, we did not observe any additive effects of proteasomal and lysosomal inhibitors when combined in either transfer efficiency or inclusion formation, suggesting the specific role of lysosomes in the clearance of internalized α-synuclein (Fig. 4 and Fig. S4A). Lysosomal deficiency also caused increased inclusion body formation in primary neurons (Fig. S4B). Thus, the inclusion body formation through the uptake of extracellular α-synuclein is sensitive to the status of the quality control system of the cell.
Fig. 4.
Increased deposition of transmitted α-synuclein by lysosomal failure. Differentiated SH-SY5Y cells were incubated with conditioned medium containing secreted myc-α-synuclein for 2 days. Lysosomal (Baf A1) and proteasomal (MG132) inhibitors were added at the final 16 h of treatment. The cells were immunostained with anti-myc antibody. (A) Fluorescence intensities measured from individual cells. (B) Number of cells with inclusion bodies. Three experiments were performed, and 100 cells were analyzed per sample. Error bars are SEM (*, P < 0.05; **, P < 0.01).
Apoptosis of Neurons upon Exposure to Neuron-Derived Extracellular α-Synuclein.
Neurotoxicity of the secreted/endocytosed α-synuclein has not been investigated previously. To examine toxic effects of the endocytosed α-synuclein, we cultured rat primary cortical neurons in the presence of conditioned medium obtained from differentiated SH-SY5Y cells overexpressing either α-synuclein or β-galactosidase, monitoring cell death by nuclear fragmentation and caspase 3 activation. Upon exposure to secreted forms of α-synuclein, neurons showed nuclear fragmentation and increased immunoreactivity of activated caspase 3 over the period of 3 days, demonstrating a progressive neuronal degeneration (Fig. 5 A and B and Fig. S5 A and B). The neurons exposed to the LacZ medium showed a slight increase in caspase 3 activation, but the difference was not statistically significant. No changes were observed in nuclear fragmentation with the LacZ medium. To determine the toxic effects of neuron-derived α-synuclein in vivo, we examined caspase 3 activation in the MCNSCs transplanted into the hippocampus of human α-synuclein transgenic mice. We found a progressive increase in the number of MCNSCs with activated caspase 3 immunoreactivity over the period of 4 weeks (Fig. 5C and Fig. S5C). These apoptotic changes were absent in MCNSCs grafted into wild-type control animals. Furthermore, we found that only the cells that tested positive for human α-synuclein showed caspase 3 activation, whereas the cells that were only GFP-positive did not show caspase 3 activation.
Fig. 5.
Degeneration of primary neurons and mouse cortical neuronal stem cells (MCNSCs) after exposure to the neuronal-cell-derived extracellular α-synuclein. (A) Nuclear fragmentation in the primary cortical neurons after incubation with α-synuclein medium and LacZ medium. Four independent experiments were performed, and on average, 190 cells were analyzed per sample. Error bars are SEM (*, P < 0.05; **, P < 0.01). (B) Quantitative immunofluorescence of activated caspase 3 in the primary cortical neurons after the same incubation as in (A). (C) Caspase 3 activation in the MCNSCs grafted into the Thy-1 α-synuclein transgenic mice. The proportions of the GFP-positive cells that exhibited activated caspase 3 were analyzed at the different time points.
Discussion
The data presented here suggest a mechanism leading to the formation and spread of neuronal α-synuclein aggregates, providing strong evidence for direct cell-to-cell propagation of α-synuclein. This study also shows how failure of quality control systems contributes to the transmission of abnormal α-synuclein and thus the propagation of synuclein pathology. These results may explain the emergence of α-synuclein-positive Lewy-like pathology recently reported in long-term mesencephalic transplants in PD (9, 10). If the transmission occurs through the neural connections, then this mechanism also may explain the topographical progression of Lewy pathology in PD suggested by Braak et al. (8). In addition, we have observed that glial inclusions, common features of PD and related disorders, are formed through neuron-to-glia transmission of α-synuclein proteins (33). Demonstrating the relevance of direct cell-to-cell protein transmission in the progression of PD and other synucleinopathies will be of great interest.
This unexpected behavior of α-synuclein raises the intriguing possibility that a subset of early α-synuclein aggregates and LBs may not necessarily be cell-autonomous structures. Perhaps LBs represent not only the production of its own misfolded protein but also the production of misfolded α-synuclein in neighboring cells. Further research on intercellular transmission of α-synuclein is likely to provide insights into mechanisms of disease progression and enable us to dissect the roles of external and internal α-synuclein proteins in inclusion body formation. The results derived from such studies may identify therapeutic targets for PD and related synucleinopathies.
Intercellular transmission of exogenous protein aggregates has been well documented in prion disorders (19). A similar mechanism of pathological propagation has been suggested in a mouse model of Alzheimer's disease, in which injection of brain extracts from human patients with Alzheimer's disease or from older amyloid precursor protein (APP) transgenic mice with plaque pathology induced amyloid deposition in young APP mice (20). More recently, Ren et al. showed that externally applied polyglutamine fibrils “seed” the aggregation of otherwise soluble polyglutamine proteins in the cytoplasm (21). A similar mode of aggregate transmission also has been shown with extracellular tau aggregates, which internalized and induced the aggregation of intracellular protein (22). Therefore, direct transmission of aggregated proteins may be the common fundamental principle underlying many progressive neurological diseases.
Deposition of α-synuclein in the human brain takes several decades. The progression of Lewy-like inclusions from host to grafted neurons in human PD patients is also age-dependent and takes >10 years. The reasons for the long latency and rate of progression in humans, compared with the animal models, are unknown. Overproduction of α-synuclein in our models might contribute to the accelerated transmission observed in our study compared with the human grafts. Another factor might be the way that recipient cells handle the transmitted proteins. We previously showed in cell culture that extracellular α-synuclein aggregates were taken up by endocytosis, transported through the endosomal pathway, and finally degraded by lysosomes (14). In the current study, we confirmed that cell-to-cell transmission of α-synuclein also was mediated by endocytosis of recipient cells and that the transmitted α-synuclein accumulated when the recipient's lysosomes were inhibited. These results suggest that reduced lysosomal capacity of recipient cells may be in part responsible for the deposition of transmitted α-synuclein. Lysosomal activity decreases with aging, and mutations in lysosomal hydrolases cause neurodegeneration in several inherited lysosomal storage disorders (23, 24). Moreover, neuronal and glial accumulation of α-synuclein was observed in several human lipidoses and a mouse model of GM2 gangliosidosis (25, 26). Recently, in yeast, Caenorhabditis elegans, and rat midbrain dopamine neurons, α-synuclein toxicity was shown to be antagonized by ATP13A2/PARK9 (27), whose gene product is localized in the lysosome and suspected in lysosomal function (28). Therefore, age-associated decline of lysosomal function might be a contributing factor for inefficient clearance, and hence deposition, of transmitted α-synuclein.
The demonstration that stem cells are targets for cell-to-cell transmission of α-synuclein has profound implications in stem-cell-based therapies for PD. Although soon after grafting the transplanted cells may differentiate, migrate, and become integrated in the affected areas, thus alleviating motor symptoms, the long-term beneficial effects of the grafts may be hampered by endocytosis and accumulation of extracellular α-synuclein derived from host neurons. Our study showed that transmission of α-synuclein induced what appears to be apoptosis in the grafted cells. This is consistent with previous studies showing the toxic effects of extracellular recombinant α-synuclein on cells (12). Whether the cells die due to cytoplasmic accumulation of the transmitted α-synuclein or whether mere exposure to the host-derived α-synuclein might be sufficient to induce cell death is not known. However, we also should consider the possibility that the cell death observed in our study is an exaggerated phenotype only present in overexpression-based systems, because no apparent cell death was observed in human transplantation cases (9, 10, 29). Nevertheless, our data suggest that functional and structural deterioration of the grafted cells by long-term exposure to the host-derived extracellular α-synuclein in the disease milieu cannot be ruled out. In the human graft studies, Kordower and colleagues reported a reduction in dopamine transporter and tyrosine hydroxylase in grafted dopamine neurons (9, 30), implicating a functional decline in the long-term graft. Knowledge of the molecular basis of the intercellular transmission of α-synuclein therefore will enable us to develop therapeutic strategies based on the manipulation of α-synuclein uptake in the stem cells to generate grafts with improved and long-lasting effects.
Materials and Methods
Materials.
The sources of all reagents can be found in the SI Materials and Methods.
Cell Culture and α-Synuclein Expression.
Maintenance and differentiation of the human neuroblastoma cell line SH-SY5Y, preparation of primary cortical neurons, and procedures for α-synuclein expression using recombinant adenoviral vectors are described in ref. 31 and in SI Materials and Methods. Procedures for rat B103 neuroblastoma culture and MCNSC culture also are described in SI Materials and Methods.
Coculture of Donor and Acceptor Cells.
For mixed culture of α-synuclein donor and acceptor cells, differentiated SH-SY5Y cells (acceptor cells) were added to SH-SY5Y cells infected with adeno/α-syn or adeno/lacZ (donor cells) on day 1 of infection and grown for 3 days. To distinguish between donor and acceptor cells in some experiments, acceptor cells were labeled with the Qtracker 585 cell labeling kit (Invitrogen), following the supplier's protocol, before being mixed with other cells. The protocols used for coculture of rat B103 neuroblastoma cells with MCNSCs are detailed in SI Materials and Methods.
Preparation of Conditioned Medium and Uptake of Extracellular α-Synuclein.
The procedures for conditioned media preparations and subsequent uptake experiments are described in SI Materials and Methods.
Injection of MCNSCs into Mouse Brains.
A total of 36 α-synuclein transgenic mice and 36 nontransgenic mice received 2 μL of cell suspension (MCNSCs infected with lentivirus-GFP at a multiplicity of infection of 30) injected unilaterally (≈1 × 105 cells per mouse) stereotaxically into the hippocampus or a sham control injection. Groups of 12 α-synuclein transgenic (n = 6 with cell suspension and n = 6 sham injection) and 12 nontransgenic mice (n = 6 with cell suspension and n = 6 sham injection) were killed 1, 2, and 4 weeks postinjection. Procedures for cell preparation and analyses are described in SI Materials and Methods.
Immunohistochemistry, Immunofluorescent Cell Staining, and Image Analysis.
The procedure for cell staining has been described elsewhere (32), and the details of tissue handling and image analysis are provided in SI Materials and Methods.
Western Blot Analysis.
Protein extraction and Western blot analysis were performed as described in ref. 32.
Electron Microscopy.
The procedures for sample handling and image analysis are provided in SI Materials and Methods.
Statistical Analysis.
All experiments were performed blind-coded and at least in triplicate. Values in the figures are expressed as means ± SEM. To determine the statistical significance, values were compared by running one-way ANOVA followed by Fisher's posthoc test comparing the MCNSCs to sham controls or by Tukey's posthoc test in the case of in vitro studies.
Supplementary Material
Acknowledgments.
We thank Ji-Eun Suk, Junghwa Suh, and Lei Cho for technical assistance. This work was supported by the Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology (Grant M103KV010021–06K2201–02110), the Diseases Network Research Program of the Ministry of Education, Science and Technology (Grant 2007–04303), the Korea Science and Engineering Foundation funded by the Korean government (Grant R01-2007-000-20200-0), and the U.S. National Institutes of Health (Grants AG5131, AG18440, AG02074, and AG10435).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 12571.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903691106/DCSupplemental.
References
- 1.Kim C, Lee S-J. Controlling the mass action of α-synuclein in Parkinson's disease. J Neurochem. 2008;107:303–316. doi: 10.1111/j.1471-4159.2008.05612.x. [DOI] [PubMed] [Google Scholar]
- 2.Lesage S, Brice A. Parkinson's disease: From monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18:R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 3.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K. The role of α-synuclein in Parkinson's disease: Insights from animal models. Nat Rev Neurosci. 2003;4:727–738. doi: 10.1038/nrn1199. [DOI] [PubMed] [Google Scholar]
- 5.Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 6.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 7.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 9.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 10.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 11.Brundin P, Li JY, Holton JL, Lindvall O, Revesz T. Research in motion: The enigma of Parkinson's disease pathology spread. Nat Rev Neurosci. 2008;9:741–745. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- 12.Lee S-J. Origins and effects of extracellular α-synuclein: Implications in Parkinson's disease. J Mol Neurosci. 2008;34:17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- 13.Lee H-J, Patel S, Lee S-J. Intravesicular localization and exocytosis of α-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H-J, et al. Assembly-dependent endocytosis and clearance of extracellular α-synuclein. Int J Biochem Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Lee H-J, Suk JE, Bae EJ, Lee S-J. Clearance and deposition of extracellular α-synuclein aggregates in microglia. Biochem Biophys Res Commun. 2008;372:423–428. doi: 10.1016/j.bbrc.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 16.Rockenstein E, et al. Differential neuropathological alterations in transgenic mice expressing α-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- 17.Pan T, Kondo S, Le W, Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain. 2008;131:1969–1978. doi: 10.1093/brain/awm318. [DOI] [PubMed] [Google Scholar]
- 18.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 19.Caughey B. Transmissible spongiform encephalopathies, amyloidoses and yeast prions: Common threads? Nat Med. 2000;6:751–754. doi: 10.1038/77476. [DOI] [PubMed] [Google Scholar]
- 20.Meyer-Luehmann M, et al. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 21.Ren PH, et al. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahr BA, Bendiske J. The neuropathogenic contributions of lysosomal dysfunction. J Neurochem. 2002;83:481–489. doi: 10.1046/j.1471-4159.2002.01192.x. [DOI] [PubMed] [Google Scholar]
- 24.Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004;5:554–565. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki K, et al. Neuronal accumulation of α- and β-synucleins in the brain of a GM2 gangliosidosis mouse model. NeuroReport. 2003;14:551–554. doi: 10.1097/00001756-200303240-00004. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, et al. Neuronal and glial accumulation of α- and β-synucleins in human lipidoses. Acta Neuropathol. 2007;114:481–489. doi: 10.1007/s00401-007-0264-z. [DOI] [PubMed] [Google Scholar]
- 27.Gitler AD, et al. α-Synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez A, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 29.Mendez I, et al. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: A second case report. Mov Disord. 2008;23:2303–2306. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- 31.Lee H-J, Khoshaghideh F, Patel S, Lee S-J. Clearance of α-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24:1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H-J, Shin SY, Choi C, Lee YH, Lee S-J. Formation and removal of α-synuclein aggregates in cells exposed to mitochondrial inhibitors. J Biol Chem. 2002;277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- 33.Lee H-J, et al. Neuron-to-glia transmission of α-synuclein causes glial inclusion formation and immune responses in synucleinopathies. J Exp Med. 2009 in press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.