A growing number of RNA viruses are now known to depend on virus-encoded ion channels for efficient production of infectious virions and, in some cases, for the subsequent infection of naïve cells. So-called viroporins are small hydrophobic proteins, usually less than 100 aa in length, and typically contain 1 or 2 transmembrane domains (TMDs); oligomerization is therefore necessary for the formation of ion channel complexes. By far the best-characterized viroporin is the M2 proton channel of influenza A virus, which is the target for the antiviral drugs amantadine and rimantadine (1). M2 sets a precedent for viroporins as therapeutic targets that has driven research into the ion channels of other clinically important viruses. In the light of rapid RNA virus evolution generating drug resistance, new compounds targeting viroporins could be a valuable addition to future combinatorial antiviral strategies. Difficulties associated with working with membrane proteins in high-throughput systems lend support to a rational approach for drug development based on the availability of high-resolution molecular structures.
In this issue of PNAS, Luik et al. (2), describe the first structure of a complete viroporin complex, the p7 ion channel of hepatitis C virus (HCV), at 16-Å resolution by using single-particle electron microscopy. The hexameric p7 complex (42 kDa) is one of the smallest objects to be visualized by these methods to date which, combined with the hydrophobic nature of p7, renders this work an impressive technical achievement. Whereas high-resolution structural information is available for M2 (3, 4), where a short amino-terminal peptide is sufficient to form the tetrameric channel complex, p7 channels comprise both TMDs from each protomer and so have thus far proved elusive in crystallographic or NMR-based studies. This first p7 structure is therefore of great importance to the HCV field and provides vital information on channel size, stoichiometry, and the orientation of p7 protomers within the channel complex. The authors include an energy-minimized model for the channel complex that fits within the observed density and adopts a “flower petal” conformation visible in the EM reconstructions. Combining molecular modeling with observed 3D structures not only provides clues to the gating of p7 channels, but could pave the way to future rational drug design, as a basis for deriving high-resolution structures in the future, but also in the short term by validating these molecular models for use in virtual compound screening.
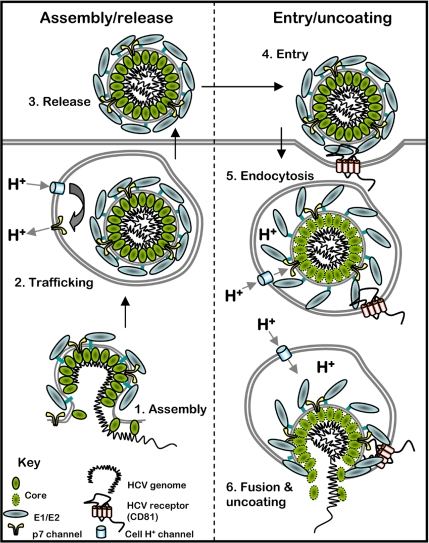
Like other viroporins, p7 has been shown by several groups to display cation channel activity in vitro (5–7) and is now known to play a vital role during virion secretion in culture (8, 9). Critically, p7 is required for the virus to replicate in chimpanzees (10), and compounds that block its activity in vitro also inhibit particle production in culture, validating it as a therapeutic target (11, 12). The precise way in which p7 channel activity enhances particle production, however, is unknown. Growing evidence suggests that p7 behaves similarly to M2 (13–15), which functions during both particle assembly and cell entry: protecting viral glycoproteins from low pH during secretion, then, as a minor virion component, promoting uncoating of the viral core after exposure to low pH in the endosome during infection. Unlike influenza virus, however, secreted HCV virions are relatively acid stable, and it is not known whether p7 resides in virions, although p7 inhibitors partially block virus entry (11). Much work is needed to define precisely how p7 functions and so elucidate the stage of the virus life cycle where inhibitory compounds act (see Fig. 1).
Fig. 1.
Potential sites of action for p7 inhibitors in the HCV life cycle. p7 could function during multiple stages of HCV particle production and may also play a role during infection. As such, inhibitors could block HCV at multiple stages of the life cycle. At early stages (stage 1) p7 may interact with NS2 to promote efficient virion assembly. p7 channels in secretory vesicles can prevent their acidification (stage 2), which could protect viral glycoproteins from premature fusogenic change or alter vesicle trafficking during exocytosis. The effect of p7 inhibitors on virus entry suggests that the ion channel is incorporated into secreted virions (stage 3). After virus entry (stage 4; for simplicity, only the CD81 receptor is shown), endosome acidification (stage 5) induces fusogenic change in the viral glycoproteins and p7 potentially permits the passage of protons into the viral core, destabilizing the structure and promoting efficient uncoating (stage 6).
Is there a need for p7 inhibitors to be developed as new HCV therapies? Certainly there is great demand for new virus-specific HCV drugs to treat the 170 million individuals chronically infected with the virus. A high level of innate resistance and the rapid evolution of viral quasispecies within individuals render the current standard of care therapy comprising interferon α (IFNα) and ribavirin (Rib) ineffective in around 50% of cases. This lack of efficacy is largely because of the highly prevalent genotype 1 viruses, which are associated with poor treatment outcome and more aggressive disease. The immune-enhancing effect of IFN and the uncertain mode of action for Rib have therefore led researchers to pursue specific HCV antivirals as a new generation of therapy. Drugs targeting the viral protease and polymerase are forthcoming, yet evidence already suggests that the virus rapidly becomes resistant where these drugs are used alone, just as seen for the first HIV patients receiving AZT (3′-azido-3′-deoxythymidine). Combination of IFN/Rib with specific HCV antivirals is effective, but is unlikely to raise the genetic barrier to viral escape as effectively as combining virus-specific compounds. This eventuality is well illustrated in HIV patients by the success of HAART (highly active antiretroviral therapy) and in vitro by the suppression of resistance in influenza by using combinations of amantadine with oseltamivir (16). Future HCV treatment regimes, therefore, will no doubt entail cocktails of several specific inhibitors, making the expansion of available drug targets of paramount importance.
To date, p7 inhibitors identified in vitro or in culture have been co-opted from other virus systems, and no tailor-made compounds have been developed. Adamantanes (amantadine and rimantadine) hail from influenza, whereas both alkylated iminosugars and amilorides were originally targeted for HIV therapy. Of these, amantadine has been the subject of numerous clinical trials alongside IFN/Rib, although many of these were instigated before identifying p7 as a target. Inclusion of amantadine over a 48-week treatment has been shown to have little, if any, impact on patient end-of-treatment response, although retreating previous IFN/Rib nonresponders with IFN/Rib/amantadine triple therapy can improve results (17). This observation has led to controversy over the usefulness of p7 inhibitors as effective HCV treatments, yet it is highly likely that this lack of efficacy is due to the evolution or preexistence of amantadine-resistant HCV quasispecies early during treatment (18). Supportive of this notion, triple therapy nonresponders have been shown to acquire specific mutations in the p7 sequence not seen in IFN/Rib dual therapy (19), and amantadine monotherapy can transiently reduce viral load (20). Accordingly, the effects of p7 inhibitors on particle production in culture are strain specific (11), suggesting that a relatively small number of amino acid changes might be necessary for HCV to escape this class of inhibitors. The genetic barrier to such changes, however, may be raised compared with enzymatic targets, given the requirement to retain interactions between protomers involved in the formation of the intricate, flower-shaped, hexameric channel complex observed by Luik et al. (2).
What then, does the future hold for the development of p7 inhibitors as HCV antivirals? The lessons learned from HIV and from influenza must be followed if the limited number of available HCV-specific compounds is to provide ample coverage; use of these compounds in isolation would no doubt lead to their becoming clinically inexpedient, as is the situation for rimantadine and influenza. Testing documented p7-specific drugs in combination with HCV protease or polymerase inhibitors rather than IFN/Rib may provide the required proof of principle of their clinical efficacy and a means of rapidly adding drugs already validated in other systems to the anti-HCV repertoire. The Australian company Biotron has a new p7 inhibitor, BIT225, in early stage Ib/IIa trials, but other examples of such compounds are not forthcoming. The expansion of newly available p7 inhibitors will require either a convenient means of measuring ion channel activity in high-throughput systems or a rational approach achieved through an understanding of the channel complex structure. The work of Luik et al. (2) represents the first major advance toward this goal and heightens optimism that p7 inhibitors could become part of the standard of care HCV therapy.
Acknowledgments.
Work on p7 in my laboratory is funded by the United Kingdom Medical Research Council (G0700124), the Wellcome Trust (082812), and the Royal Society (RG081138).
Footnotes
The author declares no conflict of interest.
See companion article on page 12712.
References
- 1.Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4:3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luik P, et al. The 3-dimensional structure of a hepatitis C virus p7 ion channel by electron microscopy. Proc Natl Acad Sci USA. 2009;106:12712–12716. doi: 10.1073/pnas.0905966106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnell JR, Chou JJ. Structure and mechanism of the M2 proton channel of influenza A virus. Nature. 2008;451:591–595. doi: 10.1038/nature06531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stouffer AL, et al. Structural basis for the function and inhibition of an influenza virus proton channel. Nature. 2008;451:596–599. doi: 10.1038/nature06528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin SD, et al. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug, Amantadine. FEBS Lett. 2003;535:34–38. doi: 10.1016/s0014-5793(02)03851-6. [DOI] [PubMed] [Google Scholar]
- 6.Pavlovic D, et al. The hepatitis C virus p7 protein forms an ion channel that is inhibited by long-alkyl-chain iminosugar derivatives. Proc Natl Acad Sci USA. 2003;100:6104–6108. doi: 10.1073/pnas.1031527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Premkumar A, Wilson L, Ewart GD, Gage PW. Cation-selective ion channels formed by p7 of hepatitis C virus are blocked by hexamethylene amiloride. FEBS Lett. 2004;557:99–103. doi: 10.1016/s0014-5793(03)01453-4. [DOI] [PubMed] [Google Scholar]
- 8.Jones CT, Murray CL, Eastman DK, Tassello J, Rice CM. Hepatitis C virus p7 and NS2 proteins are essential for production of infectious virus. J Virol. 2007;81:8374–8383. doi: 10.1128/JVI.00690-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinmann E, et al. Hepatitis C virus p7 protein is crucial for assembly and release of infectious virions. PLoS Pathog. 2007;3:e103. doi: 10.1371/journal.ppat.0030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai A, et al. The p7 polypeptide of hepatitis C virus is critical for infectivity and contains functionally important genotype-specific sequences. Proc Natl Acad Sci USA. 2003;100:11646–11651. doi: 10.1073/pnas.1834545100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin S, et al. Genotype-dependent sensitivity of hepatitis C virus to inhibitors of the p7 ion channel. Hepatology. 2008;48:1779–1790. doi: 10.1002/hep.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinmann E, et al. Antiviral effects of amantadine and iminosugar derivatives against hepatitis C virus. Hepatology. 2007;46:330–338. doi: 10.1002/hep.21686. [DOI] [PubMed] [Google Scholar]
- 13.Chew CF, Vijayan R, Chang J, Zitzmann N, Biggin PC. Determination of pore-lining residues in the hepatitis C virus p7 protein. Biophys J. 2009;96:L10–L12. doi: 10.1016/j.bpj.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin SD, et al. A conserved basic loop in hepatitis C virus p7 protein is required for amantadine-sensitive ion channel activity in mammalian cells but is dispensable for localization to mitochondria. J Gen Virol. 2004;85:451–461. doi: 10.1099/vir.0.19634-0. [DOI] [PubMed] [Google Scholar]
- 15.Meshkat Z, Audsley M, Beyer C, Gowans EJ, Haqshenas G. Reverse genetic analysis of a putative, influenza virus M2 HXXXW-like motif in the p7 protein of hepatitis C virus. J Viral Hepat. 2009;16(3):187–194. doi: 10.1111/j.1365-2893.2008.01064.x. [DOI] [PubMed] [Google Scholar]
- 16.Ilyushina NA, Bovin NV, Webster RG, Govorkova EA. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antiviral Res. 2006;70:121–131. doi: 10.1016/j.antiviral.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Deltenre P, et al. Evaluation of amantadine in chronic hepatitis C: a meta-analysis. J Hepatol. 2004;41:462–473. doi: 10.1016/j.jhep.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Maynard M, et al. Amantadine triple therapy for non-responder hepatitis C patients. Clues for controversies (ANRS HC 03 BITRI) J Hepatol. 2006;44:484–490. doi: 10.1016/j.jhep.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 19.Mihm U, et al. Amino acid variations in hepatitis C virus p7 and sensitivity to antiviral combination therapy with amantadine in chronic hepatitis C. Antivir Ther. 2006;11:507–519. [PubMed] [Google Scholar]
- 20.Chan J, O'Riordan K, Wiley TE. Amantadine's viral kinetics in chronic hepatitis C infection. Dig Dis Sci. 2002;47:438–442. doi: 10.1023/a:1013703013053. [DOI] [PubMed] [Google Scholar]