Abstract
Despite the importance of the aberrant polymerization of Aβ in the early pathogenic cascade of Alzheimer's disease, little is known about the induction of Aβ aggregation in vivo. Here we show that induction of cerebral β-amyloidosis can be achieved in many different brain areas of APP23 transgenic mice through the injection of dilute Aβ-containing brain extracts. Once the amyloidogenic process has been exogenously induced, the nature of the induced Aβ-deposition is determined by the brain region of the host. Because these observations are reminiscent of a prion-like mechanism, we then investigated whether cerebral β-amyloidosis also can be induced by peripheral and systemic inoculations or by the intracerebral implantation of stainless steel wires previously coated with minute amounts of Aβ-containing brain extract. Results reveal that oral, intravenous, intraocular, and intranasal inoculations yielded no detectable induction of cerebral β-amyloidosis in APP23 transgenic mice. In contrast, transmission of cerebral β-amyloidosis through the Aβ-contaminated steel wires was demonstrated. Notably, plasma sterilization, but not boiling of the wires before implantation, prevented the induction of β-amyloidosis. Our results suggest that minute amounts of Aβ-containing brain material in direct contact with the CNS can induce cerebral β-amyloidosis, but that systemic cellular mechanisms of prion uptake and transport to the CNS may not apply to Aβ.
Keywords: Alzheimer's disease, amyloid, prion, sterilization, transmission
A number of degenerative neurological and systemic diseases are characterized by the aberrant polymerization and accumulation of specific proteins. In the CNS, these proteopathies include Alzheimer's disease (AD), Parkinson's disease, frontotemporal dementia, and the prion diseases, among others (1). It is now established beyond a reasonable doubt that prion diseases can be induced by exogenous, conformationally variant prion proteins (2–7). Other proteopathies have been considered nontransmissible, but there is convincing evidence indicating that systemic amyloid A and apolipoprotein AII amyloidoses can be induced in susceptible hosts (8, 9).
We have previously shown that intracerebral injection of Aβ-containing extract from AD brain or transgenic mouse brain can induce cerebral β-amyloidosis and associated pathology in the hippocampus of young APP transgenic mice (10, 11). β-amyloidosis could be effectively blocked when brain extracts were Aβ-immunodepleted or proteins were denatured. Because synthetic Aβ failed to act as an amyloid-inducing factor, an Aβ conformation different from that of synthetic Aβ and/or additional chaperones have been suggested to influence the amyloid-inducing activity (11). The exogenous induction of cerebral β-amyloidosis is not limited to transgenic mice, but also has been demonstrated in nonhuman primates (12, 13).
Thus, similar to prion diseases, cerebral β-amyloidosis may qualify as an infectious disorder mediated by protein structure and stipulated in the modified postulates of Koch (14, 15). However, a more widely used definition of infectivity is the occurrence of lateral transmission, as observed for prion disease (4, 16). It has been shown that an infectious prion particle can infect the host by various peripheral application routes. This observation suggests a transport process of the infectious particles (or at least of prion infectivity) from the periphery to the brain (4, 6, 17).
In the present work, we demonstrate that cerebral β-amyloidosis can be induced by a single intracerebral injection of a β-amyloid-containing brain extract not only in the hippocampus, but also in other brain regions of susceptible host mice. In contrast, peripheral administration of the brain extract does not induce cerebral β-amyloidosis, despite the close procedural adherence to the inoculation routes used for the experimental transmission of prion disease. However, similar to previous findings regarding prion-contaminated stainless steel surfaces and the transmission of prion disease through contaminated surgical instruments (18–21), we demonstrate the ability of β-amyloid-contaminated stainless steel wires to induce cerebral β-amyloidosis when in direct contact with the brain of susceptible host mice.
Results
Induction of Aβ Deposition in Different Brain Regions.
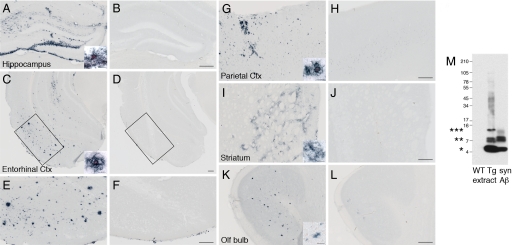
Aβ-containing brain extract from aged APP23 transgenic mice (Tg extract) was injected into different brain regions of young, predepositing 2- to 5-month-old APP23 mice. Analyses performed 3 or 6 months later revealed that Tg extract, but not extract from nontransgenic (wild-type) control mice (WT extract) or PBS injections, induced β-amyloidosis in the young APP23 mice (Fig. 1).
Fig. 1.
Induction of Aβ deposition in different brain regions of young APP23 mice. β-amyloid-containing brain extract from aged APP23 transgenic mice (Tg extract) or extract from age-matched nontransgenic (wild-type) control mice (WT extract) was injected into the hippocampus, entorhinal cortex, parietal cortex, striatum, or olfactory bulb of 2- to 5-month-old APP23 mice. The mice were then analyzed 3 to 6 months postinjection. Aβ immunoreactivity was detected in all brain regions injected with the Tg brain extract at 3 months (data not shown) and 6 months (A, C, E, G, I, and K), but not in regions injected with the WT extract (B, D, F, H, J, and L). The postsurgery interval for the olfactory bulb was 4 months. The induction of Aβ deposits was most robust in proximity to the injection site, but was observed throughout the injected brain region. Some of the induced amyloid deposits in the hippocampus, neocortex, and entorhinal cortex were congophilic, while only diffuse Aβ deposits were found in the striatum (inserts in A, C, G, I, and K). Aβ deposition was not induced in the APP23 mice after PBS injections or when Tg extract was injected into nontransgenic littermate control mice (data not shown). The number of mice was n = 3 for each region and time point and type of extract. (M) Brain extracts were analyzed by Western blot. Aβ bands (monomer and oligomer bands denoted by asterisks) were detected in the Tg extract, but not in the WT extract, using the human Aβ-specific antibody 6E10. A mixture of synthetic Aβ1–40 and Aβ1–42 (syn Aβ) was used as a positive control. (Scale bar: 200 μm; inserts, 20 μm.)
Although all injected brain regions (i.e., olfactory bulb, parietal cortex, entorhinal cortex, striatum, and hippocampus) carry the potential for the exogenous induction of Aβ deposition, differences were noted in the amount and type of amyloid induction among the various brain areas (Fig. 1). For example, in the entorhinal cortex and hippocampus, amyloid induction was strong and significantly congophilic, while in the striatum, far less amyloid was induced and the deposits were mainly diffuse (Fig. 1). These regional differences closely resemble the normal age-related deposition of Aβ in the brain of APP23 mice (22, 23).
Induction of Aβ deposition was observed already 3 months after injection in the proximity of the injection site, and it increased and spread into adjacent brain areas 6 months after injection. For example, injections in the entorhinal cortex induced Aβ deposition in the molecular layer of the dentate gyrus as well, while injections into the striatum also induced Aβ deposition in the overlying neocortex (data not shown), suggesting that spreading can occur along fiber tracts.
The injected Tg extract also induced congophilic Aβ deposition in the vasculature, predominantly in the walls of pial and thalamic vessels. This induction in the vasculature was observed independent of the injection site. As in the case of parenchymal deposits, longer postinjection times further increased cerebrovascular Aβ deposition.
No Aβ deposits were found when APP23 host brains were analyzed 1 week after Tg extract injection (data not shown), suggesting that the immunoreactive Aβ deposits are not the injected material itself (11).
In agreement with previous studies (11), analysis of the Tg brain extract revealed monomeric, dimeric, trimeric, and some larger oligomeric species (Fig. 1M). Quantitative Aβ analysis of 2 representative Tg extracts revealed total Aβ concentrations of 4.4 and 8.0 ng/μL using immunoassay measurements and 8.8 and 9.2 ng/μL using densitometry of Western blots. These values again are very similar to those reported previously (11).
Peripheral Administration of Tg Extract Does Not Induce Cerebral Aβ Deposits.
Because the induction of Aβ deposits by intracerebral injection is reminiscent of intracerebral prion transmission, the possibility that cerebral β-amyloidosis could be induced by peripheral exposure, similar to prion diseases, was investigated. Predepositing 2- to 5-month-old APP23 transgenic mice were given Tg or WT extracts through the oral, i.v., intraocular, or intranasal route (Table 1). These peripheral applications closely resembled the inoculation routes and procedures used for the experimental transmission of prionoses. Again, similar to the findings in prion transmission studies, the total amount of extract was several-fold higher (up to 2E4-fold for oral intake) compared with that used in intracerebral administration. Analysis at 4–8 months postinoculation revealed that none of the peripheral application routes yielded detectable induction of cerebral β-amyloidosis in any brain region, including retina and optic nerve (Table 1). Special attention was given to the brain areas closest to and/or in synaptic contact with the injection site, that is, the olfactory bulb for intranasal application and the retino-recipient nuclei for intraocular application. For oral and i.v. administration, serum Aβ antibody titers were monitored; no appreciable Aβ antibody titers relative to nonexposed control mice were detected.
Table 1.
Induction of cerebral β-amyloidosis in APP23 mice
| Inoculation | Total amount (μL) of Tg extract | Relative amount of Tg extract | Number of mice with Aβ deposits/total mice* |
|---|---|---|---|
| Intracerebral | 0.125 | 1 | 11/11† |
| Oral | 2,500 | 2E4 | 0/10 |
| Intravenous | 10 | 80 | 0/14 |
| Intraocular | 1 | 8 | 0/6 |
| Intranasal | 40 | 3.2E2 | 0/6 |
APP23 host mice were inoculated with either Aβ -containing extract from aged transgenic APP23 mice (Tg extract) or age-matched nontransgenic wild-type control mice (WT extract). The total amount of 10% extract given to each animal is indicated. Intracerebral injections were done by bilateral injection of 2.5 μL of 0.5% extract, yielding 0.125 μL of 10% extract into the hippocampus. Oral intake was 500 μL for 5 consecutive days. For intravenous injections, extract was diluted 1:100 in sterile PBS before administration. Five 200-μL doses were given within 10 days. Intraocular injections were done unilaterally with a single dose of 1 μL. For intranasal administration, each mouse received 20 μL of extract per nostril in a single session. The number of animals with induced Aβ deposits out of the total number of animals inoculated per group is indicated for the Tg extract. A similar number of mice were inoculated with the WT extract that induced no cerebral β -amyloid deposits in any of the conditions.
*Half of the mice were sacrificed after 4 months; the other half, after 6–8 months.
†n includes the 5 mice published in Meyer-Lühmann et al. (11).
Stainless Steel Wire Coated With Tg Extract Induces Cerebral Aβ Deposition.
Prion diseases can be transmitted via steel surgical instruments contaminated with minute amounts of prionotic tissue (18, 19). To determine whether Aβ deposition might be similarly inducible, stainless steel wires were immersed in Aβ-rich brain extract, dried, and permanently implanted unilaterally into the hippocampus and overlying neocortex of predepositing 4- to 5-month-old APP23 transgenic mice. Analysis 4 months later revealed strong induction of Aβ deposits in brains implanted with Tg extract-coated wire, but no amyloid induction in brains implanted with WT extract-coated wire (Fig. 2; Table 2). Although Aβ deposits were seen primarily in the vicinity of the implanted wire, smaller Aβ deposits were found throughout the hippocampus, as well as in thalamic and pial vessels of the hemisphere containing the wire. Most of the induced Aβ deposits near the wire were Congo red-positive (Fig. 2C), while more distant Aβ deposits were Congo red-negative.
Fig. 2.
Stainless steel wire coated with Aβ rich brain extract induced cerebral β-amyloidosis. Stainless steel wires were immersed for 16 h at room temperature with brain extract from aged APP23 transgenic mice (Tg extract) (A and C) or aged wild-type control mice (WT extract) (B). After air-drying, wires were implanted unilaterally into the hippocampus and overlying cortex of APP23 hosts (stereotaxic coordinates: AP, −2.5 mm; L, +2.0 mm; DV, −1.8 mm). Immunohistochemical analysis with anti-Aβ antibody revealed strong local induction of Aβ deposits in the vicinity of the Tg extract-coated wire (arrowhead in A). The number of mice used was n = 8. Higher magnification of the dentate gyrus region of adjacent sections double-stained with anti-Aβ antibody and Congo red (C) revealed spreading of Aβ deposition along the hippocampal fissure and dentate gyrus molecular layers (distance between sections shown, 600 μm). No Aβ deposits were observed when the wire was coated with WT extract (B). The number of mice used was n = 4. Bicine-tris urea SDS/PAGE of coated wires and subsequent Western blot detection with 6E10 antibody (D) revealed detectable amounts of Aβ on wire (WTg; lane 2), corresponding to approximately 0.02–0.05 μL of 10% seeding extract (reflecting 0.2–0.5 × 10−5 g of total brain) (lane 1). Similar amounts of Aβ were detected after the wires were heated in PBS for 10 min at 95 °C (WTg 95 °C; lane 3). After plasma sterilization (WTg pl; lane 4), no Aβ40 or Aβ42 was left on the wire, but a weak higher-molecular-weight band appeared that likely represents Aβ-specific signal, because plasma-sterilized wires coated with WT extract exhibited no bands (WWT pl; lane 5). (Scale bar: 1,000 μm in A and B; 200 μm in C.)
Table 2.
Induction of cerebral β-amyloidosis by implantation of a stainless steel wire
| Inoculation | Number of mice with Aβ deposits/total mice |
|---|---|
| WT-extract-coated wire | 0/4 |
| Tg-extract-coated wire | 8/8 |
| Tg-extract-coated wire after 10 min at 95 °C | 4/4 |
| Tg-extract-coated wire after plasma sterilization | 0/4 |
Stainless steel wires were incubated in Aβ -containing extract from aged transgenic APP23 mice (Tg extract) or age-matched nontransgenic wild-type control mice (WT extract) for 16 h at room temperature and then air-dried. For heat-denaturation, wires were boiled in PBS for 10 min at 95 °C. Plasma sterilization was done in a STERRAD sterilization system. Wires were implanted unilaterally into the hippocampus and overlaying cortex. Four months after implantation, mouse brains were analyzed immunohistologically using an antibody against human Aβ. The number of animals with induced Aβ deposits out of the total number of animals inoculated per group is indicated.
When Tg extract-coated wires were subjected to plasma sterilization before implantation, induction of Aβ deposition was completely blocked (Table 2). In contrast, heating the wires in PBS for 10 min at 95 °C did not diminish the amyloid-inducing activity of the implanted wires (Table 2). Consistent with these observations, measurement of the concentrations of Aβ bound to the wires before implantation (by boiling the extract-coated wires in sample buffer with 1.32% SDS and 3.33% 2-mercaptoethanol, with subsequent Western blot analysis) revealed ≈0.2–0.5 ng Aβ (corresponding to 0.2–0.5 × 10−5 g total brain or 0.02–0.05 μL of 10% Tg brain extract). Similar values were found for the wires that had been boiled in PBS. Very little Aβ was detected on the surface of wires after plasma sterilization (Fig. 2D).
Discussion
Despite the importance of the aberrant polymerization of Aβ in the early pathogenic cascade of AD (24), surprisingly little is known about the induction of Aβ aggregation in vivo. In the present study, we have extended our previous findings of β-amyloid induction in the hippocampus (10, 11) and demonstrated that the injection of minute amounts of an Aβ-rich brain extract can induce Aβ amyloidosis in other brain areas as well, including the entorhinal and parietal cortices, striatum, and olfactory bulb. In each of the injected brain areas, the amyloid induction was time-dependent; that is, the amount of induced amyloid was greater at 6 months postinjection than at 3 months postinjection. Although the amyloid induction in all examined brain areas appeared to spread from the site of injection to more distal regions, it is not clear whether “true” spreading of the amyloid induction occurred (i.e., the induced amyloid formed novel seeds), or whether the apparent spreading simply reflects the concentration gradient of the injected material away from the injection site.
By injecting brain extract into different APP transgenic mouse lines, we previously found that the exogenous induction of cerebral β-amyloidosis is governed in part by intrinsic properties of the host (11). This conclusion can now be refined by the observation that the nature and efficiency of amyloid induction is also brain region-dependent and closely resembles the normal endogenous age-related deposition of Aβ in the APP23 host (22, 23). For example, exogenously induced Aβ deposits in the striatum are largely diffuse and sparse compared with those in the other injected brain regions, despite the use of the same brain extract for seeding. The endogenously generated amyloid deposits in the striatum of aged APP23 mice also are diffuse and less frequent compared with those in the neocortex and hippocampus (22, 23). The regional heterogeneity of lesions might reflect the lower transgene expression levels in the striatum (22) compared with the neocortex and hippocampus (and thus less available endogenous Aβ to build on the injected “seeds”), but also might result from region-specific intrinsic factors, because the striatum also is not a primary site of β-amyloid deposition in AD (25).
Interestingly, the induction of Aβ deposition in the pial and thalamic vasculature appears to be relatively independent of the site of extract injection. This finding suggests that vascular amyloid can be induced in regions far away from the injection site, possibly by transport or passive diffusion of the amyloid-inducing activity/amyloid seeds over significant distances along perivascular drainage pathways (26). Moreover, the observation that the induced Aβ deposition in the vasculature is significantly congophilic reinforces our conclusion that the brain compartment of the host determines the nature of the Aβ deposition once the amyloidogenic process is exogenously activated.
In contrast to the intracerebral inoculations, none of the peripheral applications of the β-amyloid-containing brain extract yielded detectable induction of cerebral Aβ deposition. This was so despite the administration of 8 to 2E4 more brain extract by these routes compared with the amount used in intracerebral injections. Moreover, the postinoculation period for these peripheral application routes was up to 8 months, whereas amyloid induction in APP23 mice after intracerebral extract injection was noted starting at 8 weeks postinoculation (11). Because the amyloid-inducing brain extracts were prepared from the same inbred mouse line that also served as the host, putative transmission barriers due to amyloid strains were avoided (4, 11). Nevertheless, we cannot rule out the possibility that longer incubation times and/or more extract may be required to induce cerebral Aβ deposition after peripheral inoculations.
Systemic or peripheral inoculations of prion disease is rare in humans, but has been documented in cases of ritualistic cannibalism, contaminated human growth hormone, blood transfusion, and prion-contaminated food consumption (27–30). Such lateral transmission appears to occur more often in deer and sheep with chronic wasting disease and scrapie, respectively, although the mechanism of transmission often is not clear (31). Under experimental conditions, prion transmission has been demonstrated by systemic and peripheral inoculation routes, including oral, i.p., i.v., intranasal, intraocular, intranerval, and via scarified skin and tongue infection (6, 32–34). By far the most potent inoculation route is intracerebral, followed, in order, by intranerval, i.v., intranasal, and i.p. In rodents, oral transmission of prion infectivity is estimated to be 10E5–10E9 less efficient than intracerebral inoculation, although these values are highly variable and depend on the susceptibility of the host, as well as on the tropism and type of the inoculated prion strain (6, 34).
The transport of prion infectivity from peripheral sites to the brain and its spread within the CNS are dependent on the endogenous generation of PrPc by the host (17, 35, 36). By analogy, the lack of human APP transgene expression in the periphery in APP23 mice may be responsible for the failure of β-amyloid induction after peripheral inoculations. Although this possibility cannot be ruled out, the absence of amyloid induction after intraocular and intranasal administration argues against it, because both the retina and the olfactory bulb, as components of the CNS, express the human APP protein in APP transgenic mice using neuronal promoters (37, 38).
Thus, our results provide no evidence that cerebral Aβ deposition can be induced by any route other than the direct inoculation of the extract into the brain. However, in theory, any particle that can mimic the β-amyloid-inducing activity of Aβ from brain extracts could trigger the process of amyloid induction. It has been shown that the induction of amyloid A amyloidosis can be triggered in Tg mice by heteronucleants such as silk (39, 40), and heteronucleation of apolipoprotein AII amyloidosis also has been demonstrated in mice (41). In light of the fact that the vast majority of AD cases are idiopathic in nature, a brain-penetrant environmental factor with Aβ-nucleating properties remains a potential candidate for the initiation of the Aβ cascade (8).
Of immediate clinical interest is our finding that stainless steel binds the amyloid-inducing agent and can induce cerebral β-amyloidosis when in direct contact with the CNS. From the present work, it is not possible to conclude whether the amyloid-inducing activity desorbs from the wire or initiates induction and spreading amyloid from the bound state. The finding of less extract on the wire than what appears to be necessary for similar amyloid induction using intracerebral inoculation may suggest that the immobilization of the extract on the wire prolongs the presence of the “seed.” Prions also bind to stainless steel, and prion-contaminated stainless steel wires or surgical instruments can transmit prionosis to mice (20, 21, 42). In addition, prion infectivity through prion-contaminated surgical instruments has been demonstrated in both nonhuman primates and humans (18, 19). Thus, transmission of β-amyloid-inducing activity to patients through the use of neurosurgical instruments contaminated with β-amyloid-containing brain extract is a theoretical possibility, particularly if the instruments are improperly cleaned and sterilized. Such transmission in humans has not been documented, but it may be masked by a very long incubation time. The exogenous induction of cerebral β-amyloidosis has been found to take several years in nonhuman primates (i.e., marmosets) (12, 13), and thus it may take decades in humans, obscuring causal links between exposure and illness. Moreover, although our data indicate that cerebral β-amyloidosis can be actuated in transgenic mice by contaminated steel, there remains no evidence indicating that the complete clinicopathologic manifestations of AD, which occur only in humans, can be transmitted in this way.
We have previously reported that heating of the amyloid-containing brain extract to 95°C does not entirely eliminate the inductive potential of the extract; this is also true for the amyloid-inducing agent bound to stainless steel. Plasma sterilization of stainless steel wires has been shown to be an effective method of prion inactivation (42), and we used this sterilization technique in the present study to prove conceptually the possibility of inactivation of β-amyloid-inducing activity on the wire. Further identification of the conditions required to eliminate the amyloid-inducing activity of contaminated surgical instruments appears to be necessary.
Materials and Methods
Mice.
APP23 transgenic mice (22) were backcrossed with B6 mice for >10 generations [C57BL/6J-Tg(Thy1-APPK670N;M671L)] (23). In all experiments, 2- to 5-month-old male or female mice were used. At this age, APP23 mice do not yet deposit Aβ in the brain (11, 22). All mice were kept under specific pathogen-free conditions. All experimental procedures were conducted in accordance with the veterinary office regulations of Baden-Württemberg (Germany) and Basel (Switzerland) and approved by the local Animal Care and Use Committees.
Brain Tissue Extracts.
Unless stated otherwise, brain extracts were derived from aged (22- to 28-month-old) amyloid-depositing male or female APP23 transgenic mice and age-matched nontransgenic, control mice. The cerebellum and lower brainstem were removed, and the forebrains were frozen in dry ice and stored at −80 °C. Tissue was then homogenized at 10% (wt/vol) in PBS, vortexed, sonicated for 3 × 5 s, and centrifuged at 3,000 × g for 5 min (11). The supernatant was then aliquoted and frozen. For all experiments, the 10% extract was used unless stated otherwise.
Aβ Quantification in the Extract by Electrochemiluminescence-Linked Immunoassay.
Aβ levels were determined using the MSD 96-well MULTI-SPOT Human (6E10) Aβ Triplex Assay (Meso Scale Discovery). Extracts were treated with formic acid (minimum 95%), sonicated for 30 s on ice, and centrifuged at 25,000 × g for 1 h at 4 °C. Supernatants were neutralized (1 M Tris base, 0.5 M Na2HPO4, 0.05% NaN3). Aβ detection was performed according to the manufacturer's instructions. The plates were read on a Sector Imager 6000.
Aβ Quantification by Western Blot Analysis.
Total Aβ levels were determined on 10–20% Novex Tricine Gels using Novex Tricine sample buffer including reducing agent (Invitrogen). To determine Aβ40 and Aβ42 levels, 10% bicine-Tris, 8 M urea SDS/PAGE was used as described previously (43). To measure Aβ concentrations on wires, the wires were heated in 100 μL of sample buffer in a 1.5-mL microfuge tube at 95 °C for 5 min. Synthetic Aβ1–40 and Aβ1–42 (American Peptide) were used as controls. Proteins were transferred onto a nitrocellulose membrane, probed with 6E10 antibody (Signet Pathology Systems), and visualized with chemiluminescence using SuperSignal West Pico (Thermo Scientific). Densitometric values of band intensities were analyzed using the public domain software ImageJ, version 1.34 (http://www.rbs.info.nih.gov/ij).
Stereotaxic Injection of Extract Into Different Brain Regions.
APP23 host mice were anesthetized with a mixture of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight) in saline solution. Stereotaxic injections of 2.5 μL of brain homogenate were placed with a Hamilton syringe into the hippocampus (AP, −2.5 mm; L, ±2. 0 mm; DV, −1.8 mm), parietal cortex (AP, +1.0 mm; L, ±2.0 mm; DV, −1.2 mm), striatum (AP, +1.0 mm; L, ±2.0 mm; DV −3.3 mm), entorhinal cortex (AP, −3.6 mm; L, ±3.8 mm; DV, −4.5 mm), or olfactory bulb (AP, +4.6 mm; L, ±1.3 mm; DV, −1.0 mm). For all regions except the olfactory bulb, the injections were bilateral. The rate of injection was 1.0 μL/min, and the needle was kept in place for 2 min after injection before being slowly withdrawn. The surgical area was cleaned with sterile saline solution, the incision was sutured, and the mice were monitored until recovery from anesthesia.
Oral Feeding of Extract.
APP23 transgenic mice were given 500 μL of brain extract orally daily for 5 days. The extract was delivered via a flexible polypropylene catheter placed over the tongue about 1–2 cm into the esophagus. Half of the mice were given human brain tissue extracts derived from an autopsy sample of the superior frontal gyrus from an 85-year-old female AD patient, and the other half were given the aforementioned APP23 mouse brain extract.
Intravenous Inoculation.
For i.v. injections, APP23 mouse brain extract was further diluted 1:100 in sterile PBS, yielding a 0.1% extract. APP23 transgenic mice were given five 200-μL injections of the diluted extract within a maximum of 10 days, corresponding to a total of 10 μL of 10% mouse brain extract.
Intranasal Inoculation.
Host mice were anesthetized with a mixture of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight) in saline solution. With the mouse held upright, mouse brain homogenate was pipetted dropwise directly onto one nostril, and inhalation was monitored. A total of 10 μL of homogenate was given within 5 min, followed by a break of 10 min and then inoculation of the other nostril. This procedure was then repeated, so that each mouse received a total of 40 μL (20 μL per nostril). Between applications, the mice were kept under an infrared warming lamp.
Intraocular Inoculation.
Host mice were anesthetized with a mixture of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight) in saline solution. Each mouse received a unilateral injection of 1 μL of mouse brain homogenate into the vitreous cavity using a 32-gauge needle attached to a 10-μL Hamilton syringe. After injection, the needle was left in the vitreal chamber for 1 min to minimize leakage of the inoculum.
Intracerebral Stainless Steel Wire Inoculation.
Stainless steel wire segments (0.25 mm diameter, 2 cm long) were autoclaved before coating. The sterile stainless steel wires were placed in 0.5-mL microfuge tubes containing 50 μL of brain homogenate (10%) for 16 h at room temperature and then air-dried. The wires were either heated in PBS at 95 °C for 10 min or plasma-sterilized before implantation. To do this, the wires were sealed in a STERRAD Tyvek pouch and sterilized in a STERRAD 100 S hydrogen peroxide gas plasma sterilizer (Advanced Sterilization Products) using the routine long cycle as programmed by the manufacturer (43). The wires were then implanted unilaterally into the hippocampus using a stereotaxic apparatus (AP, −2.5 mm; L, +2.0 mm; DV, −1.8 mm). The wire was inserted through a hole in the skull, and the dorsally projecting end was adhesively bound to the skull and sealed using GLUMA Comfort Bond light-curing, one-component adhesive (Heraeus).
Serum Titers of Aβ Antibodies.
Antibody titers were monitored in blood samples collected by cardiac puncture at the time of sacrifice using standard ELISA procedures (11).
Histology and Immunohistochemistry.
Brains were immersion-fixed for 48 h in 4% paraformaldehyde (PFA) in PBS, then cryoprotected in 30% sucrose. The i.v.-injected mice and mice with stainless steel wire implants were killed by perfusion with PBS, followed by 4% PFA under deep ketamine/xylazine anesthesia (ketamine 400 mg/kg, xylazine 40 mg/kg). The perfused brains were immersion-fixed for an additional 24 h in 4% PFA, then cryoprotected in 30% sucrose. Serial 25-μm-thick coronal sections were cut using a freezing-sliding Microtome and immunohistochemically stained as described previously (44). Polyclonal antibodies NT12 [similar to NT11 (22)] or DW6 (courtesy of D. Walsh) to Aβ were used. Congo red staining was done according to standard protocols.
Acknowledgments.
We thank L. Behrends, J. Coomaraswamy, I. Guhl, S. Käser, J. Odenthal, U. Obermüller, T. Rasse, C. Schäfer (all of Hertie Institute, Tübingen), P. Mauz (Tübingen), M.-J. Runser (Basel), M. Meyer-Lühmann (Boston, MA), D. Walsh (Dublin), and F. Heppner (Berlin) for their experimental help. This work was supported by grants from the Alzheimer Association (ZEN-06–27341, to M.J.), the German National Genome Network (BMBF-NGFNPlus, to M.J.), the German Competence Network on Degenerative Dementias (BMBF-01GI0705, to M.J.), the Prof. Max Cloëtta and Bonizzi-Theler Foundation (to M.H.), and the National Institutes of Health (RR-00165, to L.C.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- 2.Castilla J, et al. Crossing the species barrier by PrP(Sc) replication in vitro generates unique infectious prions. Cell. 2008;134:757–768. doi: 10.1016/j.cell.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legname G, et al. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 4.Aguzzi A, Sigurdson C, Heikenwalder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- 5.Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]
- 6.Prusiner SB. Prion Biology and Diseases. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2004. [Google Scholar]
- 7.Legname G, et al. Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc Natl Acad Sci USA. 2006;103:19105–19110. doi: 10.1073/pnas.0608970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker LC, Levine H, 3rd, Mattson MP, Jucker M. Inducible proteopathies. Trends Neurosci. 2006;29:438–443. doi: 10.1016/j.tins.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westermark P, Westermark GT. Review: Reflections on amyloidosis in Papua New Guinea. Philos Trans R Soc London B. 2008;363:3701–3705. doi: 10.1098/rstb.2008.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kane MD, et al. Evidence for seeding of beta-amyloid by intracerebral infusion of Alzheimer brain extracts in beta-amyloid precursor protein-transgenic mice. J Neurosci. 2000;20:3606–3611. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer-Lühmann M, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 12.Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Induction of beta (A4)-amyloid in primates by injection of Alzheimer's disease brain homogenate: Comparison with transmission of spongiform encephalopathy. Mol Neurobiol. 1994;8:25–39. doi: 10.1007/BF02778005. [DOI] [PubMed] [Google Scholar]
- 13.Ridley RM, Baker HF, Windle CP, Cummings RM. Very-long-term studies of the seeding of beta-amyloidosis in primates. J Neural Transm. 2006;113:1243–1251. doi: 10.1007/s00702-005-0385-2. [DOI] [PubMed] [Google Scholar]
- 14.Riek R. Cell biology: Infectious Alzheimer's disease? Nature. 2006;444:429–431. doi: 10.1038/444429a. [DOI] [PubMed] [Google Scholar]
- 15.Walker L, Levine H, Jucker M. Koch's postulates and infectious proteins. Acta Neuropathol. 2006;112:1–4. doi: 10.1007/s00401-006-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mabbott NA, MacPherson GG. Prions and their lethal journey to the brain. Nat Rev Microbiol. 2006;4:201–211. doi: 10.1038/nrmicro1346. [DOI] [PubMed] [Google Scholar]
- 17.Blattler T, et al. PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature. 1997;389:69–73. doi: 10.1038/37981. [DOI] [PubMed] [Google Scholar]
- 18.Bernoulli C, et al. Danger of accidental person-to-person transmission of Creutzfeldt-Jakob disease by surgery. Lancet. 1977;i(8009):478–479. doi: 10.1016/s0140-6736(77)91958-4. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs CJ, Jr, et al. Transmission of Creutzfeldt-Jakob disease to a chimpanzee by electrodes contaminated during neurosurgery. J Neurol Neurosurg Psychiatry. 1994;57:757–758. doi: 10.1136/jnnp.57.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zobeley E, Flechsig E, Cozzio A, Enari M, Weissmann C. Infectivity of scrapie prions bound to a stainless steel surface. Mol Med. 1999;5:240–243. [PMC free article] [PubMed] [Google Scholar]
- 21.Flechsig E, et al. Transmission of scrapie by steel-surface-bound prions. Mol Med. 2001;7:679–684. [PMC free article] [PubMed] [Google Scholar]
- 22.Sturchler-Pierrat C, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calhoun ME, et al. Neuron loss in APP transgenic mice. Nature. 1998;395:755–756. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- 24.Selkoe DJ, Schenk D. Alzheimer's disease: Molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 25.Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H. The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowledge Environ. 2006;2006:re1. doi: 10.1126/sageke.2006.6.re1. [DOI] [PubMed] [Google Scholar]
- 26.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 27.McKintosh E, Tabrizi SJ, Collinge J. Prion diseases. J Neurovirol. 2003;9:183–193. doi: 10.1080/13550280390194082. [DOI] [PubMed] [Google Scholar]
- 28.Belay ED, Schonberger LB. The public health impact of prion diseases. Annu Rev Public Health. 2005;26:191–212. doi: 10.1146/annurev.publhealth.26.021304.144536. [DOI] [PubMed] [Google Scholar]
- 29.Turner ML, Ludlam CA. An update on the assessment and management of the risk of transmission of variant Creutzfeldt-Jakob disease by blood and plasma products. Br J Haematol. 2009;144:14–23. doi: 10.1111/j.1365-2141.2008.07376.x. [DOI] [PubMed] [Google Scholar]
- 30.Wadsworth JD, Collinge J. Update on human prion disease. Biochim Biophys Acta. 2007;1772:598–609. doi: 10.1016/j.bbadis.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Aguzzi A, Sigurdson CJ. Antiprion immunotherapy: To suppress or to stimulate? Nat Rev Immunol. 2004;4:725–736. doi: 10.1038/nri1437. [DOI] [PubMed] [Google Scholar]
- 32.Sbriccoli M, et al. Neuroinvasion of the 263K scrapie strain after intranasal administration occurs through olfactory-unrelated pathways. Acta Neuropathol. 2009;117:175–184. doi: 10.1007/s00401-008-0474-z. [DOI] [PubMed] [Google Scholar]
- 33.Bartz JC, Kincaid AE, Bessen RA. Rapid prion neuroinvasion following tongue infection. J Virol. 2003;77:583–591. doi: 10.1128/JVI.77.1.583-591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kincaid AE, Bartz JC. The nasal cavity is a route for prion infection in hamsters. J Virol. 2007;81:4482–4491. doi: 10.1128/JVI.02649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandner S, et al. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 36.Brandner S, et al. Normal host prion protein (PrPC) is required for scrapie spread within the central nervous system. Proc Natl Acad Sci USA. 1996;93:13148–13151. doi: 10.1073/pnas.93.23.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimazawa M, et al. Reduced retinal function in amyloid precursor protein-overexpressing transgenic mice via attenuating glutamate-N-methyl-d-aspartate receptor signaling. J Neurochem. 2008;107:279–290. doi: 10.1111/j.1471-4159.2008.05606.x. [DOI] [PubMed] [Google Scholar]
- 38.Ning A, Cui J, To E, Ashe KH, Matsubara J. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci. 2008;49:5136–5143. doi: 10.1167/iovs.08-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johan K, et al. Acceleration of amyloid protein A amyloidosis by amyloid-like synthetic fibrils. Proc Natl Acad Sci USA. 1998;95:2558–2563. doi: 10.1073/pnas.95.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundmark K, Westermark GT, Olsen A, Westermark P. Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: Cross-seeding as a disease mechanism. Proc Natl Acad Sci USA. 2005;102:6098–6102. doi: 10.1073/pnas.0501814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu X, et al. Induction of AApoAII amyloidosis by various heterogeneous amyloid fibrils. FEBS Lett. 2004;563:179–184. doi: 10.1016/S0014-5793(04)00295-9. [DOI] [PubMed] [Google Scholar]
- 42.Yan ZX, Stitz L, Heeg P, Pfaff E, Roth K. Infectivity of prion protein bound to stainless steel wires: A model for testing decontamination procedures for transmissible spongiform encephalopathies. Infect Control Hosp Epidemiol. 2004;25:280–283. doi: 10.1086/502392. [DOI] [PubMed] [Google Scholar]
- 43.Herzig MC, et al. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci. 2004;7:954–960. doi: 10.1038/nn1302. [DOI] [PubMed] [Google Scholar]
- 44.Stalder AK, et al. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J Neurosci. 2005;25:11125–11132. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]