Abstract
The p53 tumor suppressor protein is a key regulator of cellular proliferation and survival whose function is tightly regulated at the levels of transcription and protein stability. Here, we unveil the fine control of p53 on translationally active polysomes. We have previously reported that Ubc13, an E2 ubiquitin-conjugating enzyme, directly regulates p53 localization and transcriptional activity. We now demonstrate that the association of p53 and Ubc13 on polysomes requires ongoing translation and results in p53 ubiquitination that interferes with its tetramerization. JNK phosphorylation of p53 at Threonine 81 occurring on polysomes is required for the dissociation of Ubc13 from p53, leading to p53 multimerization and transcriptional activation. Inhibition of JNK activity or expression of a nonphosphorylatable mutant of p53 maintains an Ubc13-p53 complex that inhibits p53 multimerization. Our findings reveal a layer in the regulation of p53 multimerization that requires the concerted action of JNK and Ubc13 on polysome-bound p53.
Keywords: polysomes, ubiquitin
The p53 tumor suppressor gene is frequently mutated in human malignancies (1) and inherited mutations in this gene result in the profoundly cancer-predisposing Li-Fraumeni syndrome (2). At the cellular level, p53 protein plays a critical role in the cellular stress response, where it is implicated in the regulation of cell cycle progression, DNA repair, replicative senescence, and apoptosis (3, 4). Through these functions, p53 prevents the accumulation of cells with compromised genomic stability and/or aberrant cell cycle progression. Because of its essential role in the regulation of cell fate, p53 function is tightly controlled. In nonstressed cells, p53 levels are relatively low because of its short half-life regulated by ubiquitin ligases, including Hdm2 (5, 6). Different stress stimuli increase p53 stability and activity through a series of specific posttranslational modifications to enable its control of growth arrest, senescence or apoptosis (4).
We have recently shown that Ubc13, an E2 ubiquitin-conjugating enzyme, elicits K63-dependent ubiquitination of p53, which attenuates Hdm2-dependent polyubiquitination and subsequent degradation of p53 (7). Albeit increasing p53 levels, Ubc13 prevents its tetramerization and promotes its cytoplasmic localization, thereby rendering it transcriptionally inactive (7). Importantly, these alterations in the subcellular localization and oligomerization of p53 require the ubiquitin-conjugating activity of Ubc13 (7). Following DNA damage response, p53 activation induces the down-regulation of Ubc13 expression, suggesting the presence of a feedback loop mechanism between Ubc13 and p53 (7).
We show here that the formation of p53-Ubc13 complexes on polysomes requires active translation. Activation of c-Jun N-terminal kinase (JNK) by translational inhibitors or UV irradiation sufficiently disrupts these complexes, leading to multimerization of p53. Consistent with previous observations, JNK phosphorylation of p53 increases its stability and transcriptional activity (8). Our findings reveal a functional relationship between Ubc13 and JNK in the cotranslational regulation of p53 macromolecular structure and activity.
Results
Ubc13 Binds and Ubiquitinates p53 on Polysomes.
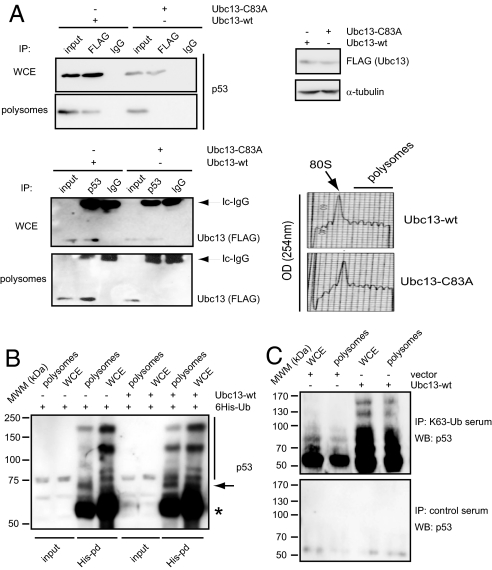
We previously reported that Ubc13 associates with polysomes and increases the polysomal abundance of p53 in a manner that requires its ubiquitin-conjugating activity (7). Here, we further explored whether Ubc13 and p53 reside in the same polysomal complexes. Immunoprecipitation of overexpressed Ubc13 (either wild-type or a catalytically inactive mutant) and endogenous p53 revealed that in polysomal fractions, only wild-type Ubc13 is able to interact with p53 (Fig. 1A). To ensure that the lack of association between mutant Ubc13 and p53 on polysomes was not because of the conditions under which the experiments were performed, we repeated the above immunoprecipitations in whole cell extracts. Both wild-type and mutant Ubc13 coimmunoprecipitated with endogenous p53 in whole cell extracts (Fig. 1A), indicating that although ubiquitin-conjugating activity of Ubc13 is not necessary for binding to p53, it plays an essential role in the formation of ribosomal Ubc13-p53 complexes. These data are consistent with our previous findings demonstrating that Ubc13-mediated increase in the polysomal association of p53 requires Ubc13 activity (7).
Fig. 1.
Ubc13 associates and ubiquitinates p53 on the polysomes. (A) Immunoprecipitation reactions were performed, in the whole cell extract (WCE) or the polysomal fraction of U2OS cells transfected with FLAG-tagged Ubc13 wild-type (wt) or catalytically inactive mutant (Cys83Ala, C83A), using anti-p53 and anti-FLAG antibodies, or control isotype-matched IgG. Immunoprecipitated material was analyzed by Western blot using the indicated antibodies. Inputs were 10% of the whole cell lysate or 5% of the resuspended ribosomal pellet, respectively. Upper-right inset shows the levels of the expression of Ubc13 variants in U2OS cells transfected with the indicated constructs as assessed by Western blot analysis. Lower-right inset represents the UV (254 nm) absorbance profile of the ribosomal complexes separated on the 10–40% sucrose gradient. 80S indicates the position of monosomes in the gradient. (B) U2OS cells were transfected as indicated and treated with MG132 (40 μM) for 6 h before WCE or pooled polysomal fractions were lysed under denaturing conditions using 6 M urea. The ubiquitinated protein was pulled down using Ni-NTA agarose beads and analyzed by Western blot using a mixture of 2 monoclonal anti-p53 antibodies (DO1 and pAb241). Asterisk and arrow indicate the position of mono- and di-ubiquitinated forms of p53, respectively. Vertical line indicates putative polyubiquitinated p53 species. (C) WCE and pooled polysomal fractions prepared from U2OS cells transfected with either an empty vector or wild-type Ubc13 were treated with MG132 (40 μM) for 6 h and then lysed under denaturing conditions using 6 M urea. Lysates were immunoprecipitated with 1 μg of anti-K63-Ub or control sera and analyzed by Western blot using a mixture of p53 antibodies (DO1 and pAb241).
Since p53 and active Ubc13 readily interact on polysomes, we tested the levels of p53 ubiquitination on polysomes. In vivo ubiquitination of endogenous p53 was monitored in U2OS cells in the presence of 6×His-ubiquitin and overexpressed Ubc13. These experiments revealed that the polysomal p53 is ubiquitinated, which was modestly augmented upon Ubc13 overexpression (Fig. 1B). In contrast, overexpression of Ubc13 did not induce a detectable change in the ubiquitination of p53 in whole cell extracts, consistent with our previous report (7). These data suggest that Ubc13 does not only promote polysomal association of p53, but that it also governs the ubiquitination of the fraction of p53 that is bound to polysomes. Finally, to prove that Ubc13 mediates K63 specific ubiquitin chain formation on p53, we took advantage of newly developed K63-linked ubiquitin-chain specific antibodies (9). Using this reagent, we confirmed that Ubc13 overexpression induces K63-mediated ubiquitination of p53 in polysomal fractions (Fig. 1C).
JNK Mediates Translation-Dependent Dissociation of Ubc13- p53 Complexes.
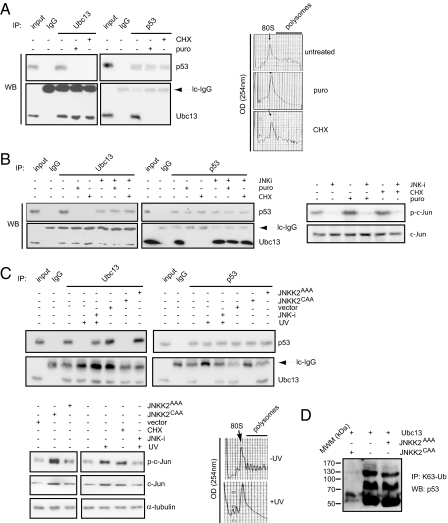
Since our data indicate that p53 and Ubc13 are part of the same polysomal complex, we next determined if ongoing translation is necessary for the formation of Ubc13-p53-containing complexes. To this end, the association of p53 and Ubc13 was determined in U2OS cells treated with either cycloheximide or puromycin (well-known inhibitors of protein synthesis) for 10 min (which did not affect the levels of either Ubc13 or p53). Remarkably, both drugs were able to abrogate the interaction between endogenous p53 and Ubc13 proteins on polysomes (Fig. 2A), indicating that the formation of Ubc13-p53 complexes critically depends on ongoing translation.
Fig. 2.
Dissociation of Ubc13-p53 complexes is mediated by JNK. (A) U2OS cells were treated with puromycin (puro), cycloheximide (CHX), or incubated with vehicle. Immunoprecipitation reactions were carried out on polysomal fractions using anti-p53, anti-Ubc13, or control isotype-matched IgG antibodies, and further analyzed by Western blot using the indicated antibodies. Right inset represents the UV (254 nm) absorbance profiles of the ribosomal complexes separated on the 10–40% sucrose gradient. 80S indicates the position of monosomes. (B) Left: U2OS cells were treated with puromycin (puro), cycloheximide (CHX), or incubated with a vehicle. Where indicated, the cells were preincubated with a JNK inhibitor (JNK-i). Immunoprecipitations were carried out using anti-p53 and anti-Ubc13 antibodies, or control isotype-matched IgG and the amount of p53 and Ubc13 in the immunoprecipitated material was assessed by Western blot analysis. Inputs were 7.5% of the ribosomal material used in the immunoprecipitation reactions. Right: Levels and phosphorylation of JNK target, c-Jun, upon the indicated treatments were determined by Western blot analysis using c-Jun and anti-phospho-c-Jun (p-c-Jun) antibodies. (C) U2OS cells were untreated, exposed to UV-C light (UV), treated with JNK inhibitor (JNK-i) before the exposure to UV-C light, or transfected with an empty vector (vector), JNKK2CAA or JNKK2AAA constructs. Immunoprecipitations were carried out with anti-p53 and anti-Ubc13 antibodies, or control isotype-matched IgG, and further analyzed by Western blot using the indicated antibodies. Inputs were 10% of the material used for immunoprecipitation. The levels of phosphorylated c-Jun (p-c-Jun), c-Jun, and α-tubulin in the extracts used in the immunoprecipitations reactions were determined by Western blot analysis (lower-left inset). Lower-right inset shows the UV absorbance profiles at 254 nm of the ribosomal gradients from UV treated (+UV) and untreated cells (−UV). (D) U2OS cells were transfected as indicated, and immunoprecipitations from whole cell extracts were carried as described in Methods.
We sought to identify the signaling pathway responsible for dissociation of p53 from Ubc13 upon the inhibition of protein synthesis. JNK is a member of the MAPK family that has been previously implicated in the control of protein translation and that also plays a key role in the regulation of p53 stability and function (10–12). To test the possible role of JNK in this process, we assessed whether the effects of puromycin and cycloheximide on Ubc13-p53 interaction could be reversed by inhibition of JNK activity. U2OS cells were pretreated with 2 different specific JNK inhibitors followed by addition of either puromycin or cycloheximide. While treatment with either the translational inhibitors readily led to JNK activation, as illustrated by c-Jun phosphorylation, inhibition of JNK, efficiently attenuated JNK activation (Fig. 2B, Right, and Fig. S1, Bottom).
We next tested if the inhibition of JNK activity would affect cycloheximide- or puromycin-induced dissociation of p53 from Ubc13. Immunoprecipitations carried out using endogenous proteins revealed that while cycloheximide and puromycin treatment reduced the association between p53 and Ubc13, preincubation with JNK inhibitors efficiently preserved the Ubc13-p53 complexes (Fig. 2B, Left, and Fig. S1, Top). Further, we show that inhibition of JNK1 and JNK2 expression, achieved with the use of siRNAs, is sufficient to abolish the translational stress-mediated Ubc13-p53 complex disruption (Fig. S2). Altogether, these findings indicate that disruption of Ubc13-p53 complexes by puromycin or cycloheximide requires JNK activity.
Activation of JNK Signaling Disrupts the Binding of p53 to Ubc13.
So far, our data indicate that the association of p53 with Ubc13 can be efficiently disrupted by the inhibition of protein synthesis, which is mediated by activation of the JNK pathway. Since JNK is a major stress-activated protein kinase, we have assessed whether JNK effect on Ubc13-p53 complexes could be also seen in response to DNA damage, such as UV-irradiation—a stimulus that is known to strongly activate JNK. Interestingly, in UV-treated U2OS cells, we were unable to detect interaction between p53 and Ubc13 in whole cell extracts (Fig. 2C and Fig. S1). More importantly, pretreatment of cells with either JNK inhibitors or JNK siRNA, before UV exposure, was sufficient to reverse the latter effect (Fig. 2C and Fig. S1). Taken together, these data indicate that UV irradiation-induced activation of JNK efficiently disrupts Ubc13-p53 complexes, which recapitulates the ability of translational inhibitors to induce dissociation of p53 from Ubc13. Of note, we found that UV treatment leads to polysome dissociation in U2OS cells (Fig. 2C, Right bottom), further strengthening the link between protein synthesis and Ubc13-p53 association.
To establish the role of JNK in the regulation of Ubc13-p53 interaction, we activated JNK in U2OS cells through the expression of a constitutively active mutant of a JNK-kinase, the JNKK2CAA mutant protein. We found that whereas in cells expressing a control mutant (JNKK2AAA), Ubc13 and p53 efficiently coimmunoprecipitated; in cells overexpressing JNKK2CAA, the interaction between Ubc13 and p53 was abolished (Fig. 2C). Remarkably, we also found that expression of JNKK2CAA (but not of JNKK2AAA) abolished the Ubc13-dependent K63-specific ubiquitination of p53 (Fig. 2D). Altogether, these data provide independent support for the negative role of JNK in the regulation of p53 and Ubc13 association, especially under conditions in which polysomes are not disrupted compared with UV irradiation, thereby corroborating the importance of JNK signaling in the control of Ubc13-mediated regulation of p53 function.
JNK-Mediated Phosphorylation of p53 at Threonine 81 in Polysomes Is Necessary for Its Dissociation from Ubc13.
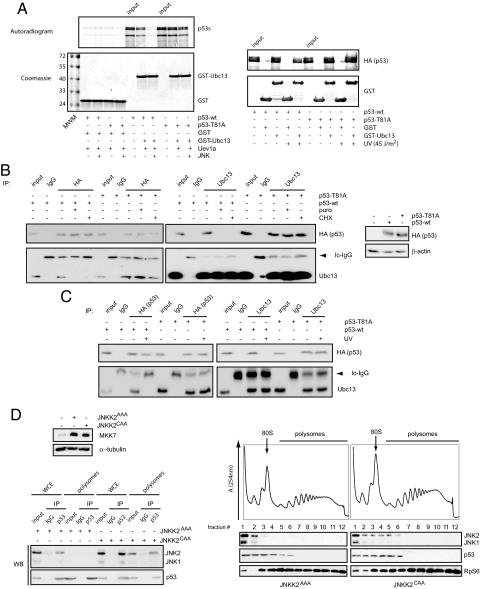
As a response to various DNA-damaging and stress-inducing agents, JNK phosphorylates p53 on residue Thr-81, which leads to its stabilization and transcriptional activation (8). It is therefore plausible that Thr-81 acts as a molecular determinant that mediates the effect of JNK on Ubc13-p53 complexes. To test our hypothesis, we set up an in vitro system recapitulating the interaction between p53 and Ubc13 using p53 produced in reticulocyte lysates and Ubc13 and its adaptor Uev1a produced in bacteria [as reported in ref. 7]. Our experiments show that p53 protein phosphorylated by JNK is no longer able to interact with Ubc13. Remarkably, the Thr81Ala mutant of p53 is insensitive to JNK regulation (Fig. 3A, Left). Moreover, pull-down assays using a GST-tagged Ubc13 protein and cell extracts, containing either wild-type or Thr81Ala mutant of p53 obtained from cells that were or were not exposed to UV, confirmed that an intact Thr81A JNK phosphoacceptor site in p53 is required to mediate the negative regulation of JNK on the Ubc13-p53 association (Fig. 3A, Right). Further, experiments using U2OS cells expressing HA-tagged either wild-type or Thr81Ala p53 and subsequently either treated with translation inhibitors (Fig. 3B) or UV-irradiated (Fig. 3C), established that dissociation of Ubc13 from p53 is triggered by the phosphorylation of p53 at the residue Thr-81 by JNK.
Fig. 3.
Phosphorylation of Thr-81 residue of p53 by JNK is required for its dissociation from Ubc13. (A) Left: In vitro binding assay between p53 either wild-type (wt) or Thr81Ala mutant (produced in reticulocyte lysates and radiolabeled) and GST-Ubc13 (produced in bacteria) in the presence of Uev1a (produced “cold” in reticulocyte lysates). Unphosphorylated or JNK-phosphorylated p53 wt or Thr81Ala mutant (obtained as described in SI Methods) were incubated with 50 μL Uev1a (when indicated) and 250 ng of either GST (control) or GST-Ubc13 and subjected to GSH-pull-down assays followed by PhosphorImaging analysis. Right: 1 μg of either GST or GST-Ubc13 was used to pull-down associated p53 (either wild-type or Thr81Ala mutant) from 2-mg extracts produced from 293 cells overexpressing p53 and that were subjected to UV-irradiation (45 J/m2) and left recover for 45 min. (B) U2OS cells transfected with HA-tagged wild-type p53 (p53-wt) or Thr81Ala p53 construct (p53-T81A) were treated with puromycin (puro), cycloheximide (CHX), or incubated with a vehicle. Immunoprecipitations were carried out in total cell extracts using anti-HA and anti-Ubc13 antibodies, or control isotype-matched IgG. Immunoprecipitated material was analyzed by Western blot using the indicated antibodies. Right inset shows the expression of wild-type and Thr81Ala p53 mutant in these cells as assessed by Western blot analysis. β-actin served as a loading control. (C) U2OS cells were transfected as in B, exposed to UV-C light (UV) or left untreated. Immunoprecipitations and Western blots were carried out as mentioned above. In both images, inputs were 10% of the material used in the immunoprecipitation reactions. (D) Left upper: U2OS cells were transfected as indicated and the expression of JNKK2 (MKK7) was analyzed by Western blot analysis 48 h after transfection. β-tubulin served as a loading control. Right: Ribosomal extracts of the same cells were fractionated on 10–40% sucrose gradient. Distribution of JNK and p53 across the gradient was detected by Western blot analysis. Ribosomal protein S6 (RpS6) served as a loading control. Corresponding UV (254 nm) absorbance profiles with the indicated fraction number are presented above. 80S indicates the position of the monosome. Left lower: Immunoprecipitation reactions were carried out in WCE and pooled ribosomal fractions (i.e., fractions 4–12) using anti-p53 or control IgG antibodies. Inputs were 10% of the material used for immunoprecipitation. The amount of p53 and JNK in the immunoprecipitated material was determined by Western blot analysis.
Our data suggest that a fraction of p53 is cotranslationally regulated by Ubc13, and this regulation is negatively controlled by JNK. However, it is not clear whether JNK spatially targets p53 also in the ribosome (as Ubc13) or in the cytoplasm. To address this important question, we decided to test if upon activation, JNK associates with polysomes. To avoid the disruption of ongoing translation by cycloheximide, puromycin, or UV irradiation, JNK was activated using JNKK2CAA in U2OS cells, and the distribution of p53 and JNK and their association were assessed across a ribosomal gradient. These experiments revealed that upon its activation, JNK associates with 40S, 60S, 80S, and light polysomal fractions (Fig. 3D). In contrast, in cells expressing JNKK2AAA, JNK exclusively resided in the preribosomal mRNP fraction. Remarkably, we also found that upon its activation, JNK selectively interacts with the ribosome-associated p53 (Fig. 3D, Bottom), strongly suggesting that JNK activation induces its relocalization to the vicinity of the ribosome, where it targets p53, blocking its interaction with Ubc13.
JNK-Mediated Dissociation of Ubc13-p53 Complexes Correlates with Increased Multimerization of p53 and Leads to Activation of p53.
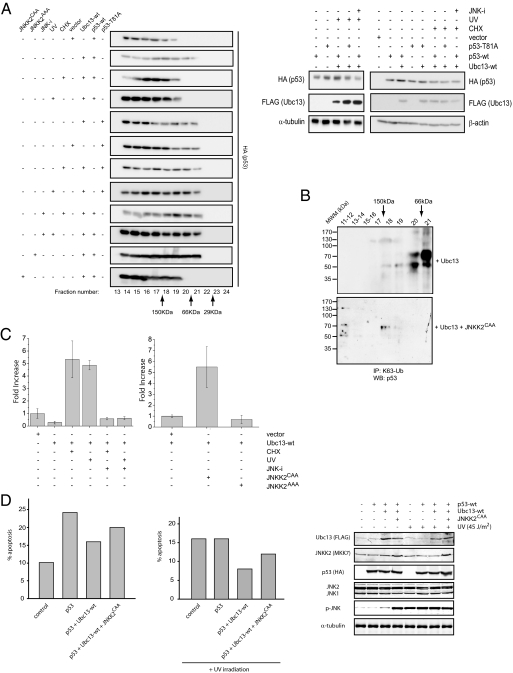
We have previously reported that Ubc13 increased the cellular concentration of monomeric p53, resulting in the suppression of its transcriptional capabilities (7). However, it is established that the activation of JNK leads to p53 stabilization and up-regulation of p53-dependent transcription (8–12). Thus, we tested whether the activation of JNK attenuates the effects of Ubc13 on tetramerization of p53. First, we determined the molecular weight of p53-containing complexes in the whole cell lysates of U2OS cells expressing either HA-tagged wild-type p53 or Thr81Ala mutant, together with Ubc13, using size exclusion chromatography followed by Western blot analysis (Fig. 4A). Consistently with our previous study (7), wild-type p53 predominantly localized within the higher molecular weight fractions, whereas the overexpression of Ubc13 led to its accumulation in the fractions corresponding to molecular weights closer to a monomeric form (i.e., fractions corresponding to 50 to 66 kDa) (Fig. 4A). In contrast, the Thr81Ala p53 mutant was enriched in low molecular weight fractions corresponding to monomeric p53, even in the cells that were not overexpressing Ubc13 (Fig. 4A). These results suggest that the endogenous Ubc13 levels are sufficient to keep the Thr81Ala mutant monomeric. Accordingly, association between Ubc13 and the Thr81Ala p53 mutant both in vitro and in vivo appeared stronger than with wild-type p53 (see Fig. 3 A and B). Further, we also noticed that cosedimentation of Thr81Ala p53 mutant with heavier polysomal fractions did not require Ubc13 overexpression (Fig. S2). It is possible that Thr81Ala mutant p53 could affect p53 oligomerization by another unknown mechanism.
Fig. 4.
JNK-mediated disruption of Ubc13-p53 complexes controls p53 oligomerization status and its transcriptional activation. (A) U2OS cells transfected with HA-tagged wild-type p53 (p53-wt) or Thr81Ala p53 construct (p53-T81A) were cotransfected with either Ubc13 construct or with an empty vector. Where indicated, cells were treated with cycloheximide (CHX) or exposed to UV-C light (UV) with or without the pretreatment with a JNK inhibitor (JNK-i). U2OS cells were additionally cotransfected with wild-type p53, wild-type Ubc13, and JNKK2AAA or JNKK2CAA. Total cellular extracts obtained from these cells were fractionated by gel filtration, and the distribution of wild-type or mutant form of p53 protein across the fractions was monitored by Western blot analysis using anti-HA antibody. Right show the levels of p53 and Ubc13 in the extracts used for the gel filtration as determined by Western blot analysis. β-actin and α-tubulin served as loading controls. (B) U2OS cells were transfected as indicated. Forty-eight hours after transfection, cells were lysed, and whole cell extracts were fractionated by gel filtration. Resulting fractions were incubated in 6 M urea, immunoprecipitated with anti-K63-Ub antibodies, and analyzed by Western blot using a mixture of p53 antibodies. MWM denotes molecular weight markers as indicated. (C) Left: U2OS cells were cotransfected with p21-luciferase, β-galactosidase, and with either Ubc13 or an empty vector. Former cells were further treated with cycloheximide (CHX), UV-C light (UV) with or without the pretreatment with a JNK inhibitor (JNK-i). For each sample, luciferase activity was normalized to β-galactosidase activity, and the value obtained for the control, empty vector transfected cells was set to 1. Bars represent mean values ± SD (n = 3). Right: U2OS cells were cotransfected with p21-luciferase, β-galactosidase, and with either JNKK2AAA, JNKK2CAA, or an empty vector. Luciferase assays analysis and presentation of the data are as described above. (D) Saos-2 cells were transfected with the indicated plasmids. Forty-eight hours after transfection, cells were harvested. Cell cycle profiles were determined by staining DNA with PI followed by FACS analysis, and percentages of the subG1 populations, under the different experimental conditions indicated, were calculated and shown for representative experiments.
We then tested the effects of JNK activation on the multimerization status of p53 in Ubc13 overexpressing cells. Both cycloheximide treatment and exposure to UV led to increased multimerization of wild-type p53, but not of Thr81Ala p53 mutant that remained mostly monomeric (Fig. 4A). Furthermore, pretreatment with JNK inhibitor before UV or cycloheximide stimulation inhibited the shift of p53 toward higher molecular weight fractions, indicating that UV- and cycloheximide-mediated oligomerization of p53 in the Ubc13 overexpressing cells requires activation of JNK (Fig. 4A). Finally we tested the impact of JNK activation, by expression of the JNKK2CAA protein, on the multimerization status of p53 in the Ubc13 overexpressing cells. In these conditions, p53 prominently shifted toward the higher molecular weight fractions. As expected, expression of the inactive JNKK2 mutant (JNKK2AAA) did not affect the oligomerization status of p53 (Fig. 4A).
Additionally, the role of JNK in the multimerization of p53 was assessed by monitoring the localization of p53 phosphorylated on Thr-81. Antibodies that recognize the Thr-81 phosphorylated p53 identified wild-type but not the Thr-81 mutant form following UV-irradiation or JNKK2CAA expression (Fig. S3A). Notably, the phosphorylated form of p53 was identified within the larger MW complex following coexpression of JNKK2CAA and UBC13, but not in the Ubc13 expressing cells (Fig. S3B). Coincided with Ubc13 dissociation following JNK activation, Thr-81 phosphorylated p53 was no longer found within the polysome fraction (Fig. S3C). These data substantiate Thr-81 phosphorylation in multimerization of p53, following its dissociation from Ubc13.
We also found independent evidence—using K63-Ub specific antibodies—that the Ubc13-dependent modified p53 through K63-specific ubiquitination is unable to multimerize after analysis of fractions generated from Ubc13-transfected U2OS cells. Remarkably, this experimental setting also substantiated that JNK activation abolishes the formation of K63-ubiquitinated p53 species (Fig. 4B).
Tetramerization of p53 has been reported to lead to its transcriptional activation (13). Our data indicates that the activation of JNK pathway effectively attenuates Ubc13-induced monomerization of p53. Hence, we tested if the activation of JNK signaling would efficiently antagonize Ubc13 suppression of p53's transcriptional activity. Using a luciferase construct controlled by the promoter sequences of the p53 target gene p21, we monitored p53 transcriptional activities in U2OS cells treated with cycloheximide or UV irradiation with or without pretreatment with a chemical JNK inhibitor (Fig. 4C, Left). As expected, overexpression of Ubc13 induced a decrease in luciferase activity driven by the p21 promoter (≈4-fold) (Fig. 4C, Left). This suppression of the transcription driven by the p21 promoter was efficiently reversed by UV or cycloheximide treatment, which even led to a 4- to 5-fold increase in luciferase activity when compared with the appropriate control. These effects were efficiently inhibited by the preincubation with the JNK inhibitor (Fig. 4C, Left). Finally, we tested the effects of JNK activation by JNKK2CAA on p53 transcriptional activity in Ubc13-overexpressing cells. We found that expression of JNKK2CAA led to a 5-fold increase in the luciferase activity driven by the p21 promoter (Fig. 4C, right panel).
Additionally, the effect of the JNK-Ubc13-p53 axis was tested on the p53-mediated apoptotic pathway as previously described (7). Expression of p53 in Saos-2 cells induced a marked increase in the subG1 population undergoing cell death, which was attenuated in the presence of Ubc13 and restored upon JNK activation (Figs. 4D, Left graph, and Fig. S4). Interestingly, similar results were obtained under conditions that enhance the apoptotic activity of p53, such as UV irradiation (Figs. 4D, Right graph, and Fig. S4).
Taken together, our data indicate that the activation of JNK efficiently antagonizes the effects of Ubc13 on the multimerization status and transcriptional activity of p53. These findings further support our hypothesis that JNK-dependent activation of p53 is mediated by its dissociation from Ubc13. It has been reported that the transcriptional activation of p53 by JNK is attenuated by Thr81Ala mutation (8). Accordingly, we found that the Thr81Ala p53 mutant remained in the monomeric inactive form and failed to tetramerize upon activation of JNK signaling (Fig. 4A).
Discussion
Our findings reveal a link between JNK signaling and Ubc13-mediated regulation of p53. The association of p53 and Ubc13 requires ongoing translation and is disrupted by the activation of JNK through translational inhibitors, UV irradiation or expression of constitutively active JNK-kinase (JNKK2CAA). At the molecular level, JNK-mediated disruption of Ubc13-p53 complexes requires the integrity of the JNK phosphoacceptor site on p53 (residue Thr-81) in vivo and in vitro. Finally, JNK-induced dissociation of Ubc13 and p53 results in p53 multimerization and activation.
These findings provide mechanistic insight into the role of JNK in the stabilization and transcriptional activation of p53 (8–11). The finding that JNK promotes the degradation of p53 under nonstressed conditions (10) raises the possibility that such targeting engages Ubc13-marked monomeric forms of p53. The activation of JNK following stress and DNA damage stabilizes and activates p53 (8, 11), which we now show occurs on polysome-bound p53, by attenuating the inhibitory effect of Ubc13 on p53 multimerization.
Several recent studies link ribosomal function with the regulation of p53 levels and/or its activity. In particular, the ribosomal protein L26, whose stability is regulated by the p53 ubiquitin ligases Hdm2, has been shown to bind and regulate the translation of p53 mRNA (13, 14). These emerging regulatory mechanisms not only suggest the important role of the translational machinery in p53 regulation, but also indicate that the disruption of protein synthesis under stress conditions could be relayed to growth suppression, replicative senescence and apoptosis through translational activation of p53. Our data support a model in which under nonstressed conditions, p53 function is inhibited by Ubc13. Concomitantly, Ubc13 increases the stability of p53, suggesting that Ubc13-p53 complexes may serve as a reservoir of inactive p53 on polysomes. It is also plausible that K63-modified p53 has other roles, such as association with specific mRNA on polysomes. Upon stress, JNK phosphorylates p53 at Thr-81, which in turn releases p53 from Ubc13-containing complexes, leading to p53 multimerization and activation. Since p53 tetramers are no longer marked with K63 polyubiquitin chains, a deubiquitinating enzyme is expected to be part of this polysomal complex, acting to remove the noncanonical chains following JNK activation. Of note, whereas the modest fraction of p53 that underwent multimerization following JNK activation may suffice to activate p53 transcriptional activity to the degree seen, we cannot exclude the possibility that JNK may also activate other pools of p53 that could contribute to this activity. Remarkably, this mode of p53 activation does not require de novo transcription or translation and can be rapidly triggered by stress responses before the activation of transcriptional and/or translational pathways.
Our report also hints at the presence of a hitherto unidentified ubiquitin ligase on polysomes that in conjunction with Ubc13 would be responsible for p53 ubiquitination and monomerization. The likely candidates are Hdm2, which has been directly implicated in the regulation of p53 mRNA translation (13), PARC, which regulates p53 stability within large molecular weight complexes in the cytosol (15), and MSL2 and WWP1, which were shown to promote p53 ubiquitination without affecting its stability (16, 17).
In conclusion, the present study provides insights into the regulation of p53 before and after stress and DNA damage. Association of Ubc13 with newly translated polysome-bound p53 limits its tetramerization and is attenuated following stress and DNA damage by JNK phosphorylation of p53. p53 phosphorylation causes its dissociation from Ubc13, enabling its tetramerization, with concomitant effect on stability and transcriptional activity. While pointing to the mechanism underlying the regulation of p53 on polysomes, our findings also highlight an undisclosed function for Ubc13. Ubc13 association with p53 on polysomes may serve as a paradigm for Ubc13-dependent regulation of protein multimerization and function. Consistent with this possibility is the notion that many of the reported substrates of Ubc13 are proteins that require multimerization for their activity, including TRAF2, TRAF6, and PCNA.
Materials and Methods
Plasmids and Transfections.
The 6×His-tagged ubiquitin, hemagglutinin (HA)-tagged wild-type and Thr81Ala mutant p53, FLAG-tagged wild-type Ubc13, and C83A mutant Ubc13 constructs were previously described (7, 8). The pET15b-JNK2α2 vector was generated by PCR amplification of the human JNK2α2 cDNA (18) and cloning into the XhoI-BamHI sites of the pET15b backbone (Novagene). For transfections, cells were seeded in 100-mm dishes and transfected with 1 μg of each plasmid with Fugene 6 (Roche) according to the manufacturer's instructions. In cotransfection experiments, the total DNA in the transfection mixtures was adjusted with sonicated salmon sperm DNA (Sigma). Forty-eight hours after transfection, cells were lysed in the appropriate buffer.
Supplementary Material
Acknowledgments.
We thank Ryan Dowling for experimental assistance and members of the Ronai and Borden laboratories for advice; Benoit Grondin and Mathieu Tremblay for help with the luciferase assays; Craig Hauser and Alexy Eroshkin for profiling array and bioinformatic analyses; and Michael Karin for JNK constructs. This work was supported by National Cancer Institute (NCI) Grants CA138143, CA078419 (to Z.R.), and Training Grant CA077109 (to M.C.); National Institutes of Health (NIH) Grant RO1–80728 (to K.B.); and a fellowship from the Sass Foundation (G.J.G.). I.T. is a Special Fellow of the Leukemia and Lymphoma Society, USA. M.C. is a member of the Molecular Pathology Graduate Program at the University of California, San Diego (UCSD).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900596106/DCSupplemental.
References
- 1.Hainaut P, et al. IARC Database of p53 gene mutations in human tumors and cell lines: Updated compilation, revised formats and new visualisation tools. Nucleic Acids Res. 1998;26:205–213. doi: 10.1093/nar/26.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malkin D, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 3.Aylon Y, Oren M. Living with p53, dying of p53. Cell. 2007;130:597–600. doi: 10.1016/j.cell.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Harris SL, Levine AJ. The p53 pathway: Positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 5.Marine JC, et al. Keeping p53 in check: Essential and synergistic functions of Mdm2 and Mdm4. Cell Death Differ. 2006;13:927–934. doi: 10.1038/sj.cdd.4401912. [DOI] [PubMed] [Google Scholar]
- 6.Michael D, Oren M. The p53-Mdm2 module and the ubiquitin system. Semin Cancer Biol. 2003;13:49–58. doi: 10.1016/s1044-579x(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 7.Laine A, et al. Regulation of p53 localization and activity by Ubc13. Mol Cell Biol. 2006;26:8901–8913. doi: 10.1128/MCB.01156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buschmann T, et al. Jun NH2-terminal kinase phosphorylation of p53 on Thr-81 is important for p53 stabilization and transcriptional activities in response to stress. Mol Cell Biol. 2001;21:2743–2754. doi: 10.1128/MCB.21.8.2743-2754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton K, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs SY, et al. JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev. 1998;12:2658–2663. doi: 10.1101/gad.12.17.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs SY, Adler V, Pincus MR, Ronai Z. MEKK1/JNK signaling stabilizes and activates p53. Proc Natl Acad Sci USA. 1998;95:10541–10546. doi: 10.1073/pnas.95.18.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson G, et al. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 13.Takagi M, Absalon MJ, McLure KG, Kastan MB. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Ofir-Rosenfeld Y, et al. Mdm2 regulates p53 mRNA translation through inhibitory interactions with ribosomal protein L26. Mol Cell. 2008;32:180–189. doi: 10.1016/j.molcel.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolaev AY, et al. Parc: A cytoplasmic anchor for p53. Cell. 2003;112:29–40. doi: 10.1016/s0092-8674(02)01255-2. [DOI] [PubMed] [Google Scholar]
- 16.Kruse JP, Gu W. MSL2 promotes MDM2 independent cytoplasmic localization of p53. J Biol Chem. 2008;284:3250–3263. doi: 10.1074/jbc.M805658200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laine A, Ronai Z. Regulation of p53 localization and transcription by the HECT domain E3 ligase WWP1. Oncogene. 2007;26:1477–1483. doi: 10.1038/sj.onc.1209924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallunki T, et al. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994;15:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.