Abstract
Evolution of planktic organisms from benthic ancestors is commonly thought to represent unidirectional expansion into new ecological domains, possibly only once per clade. For foraminifera, this evolutionary expansion occurred in the Early–Middle Jurassic, and all living and extinct planktic foraminifera have been placed within 1 clade, the Suborder Globigerinina. The subsequent radiation of planktic foraminifera in the Jurassic and Cretaceous resulted in highly diverse assemblages, which suffered mass extinction at the end of the Cretaceous, leaving an impoverished assemblage dominated by microperforate triserial and biserial forms. The few survivor species radiated to form diverse assemblages once again in the Cenozoic. There have, however, long been doubts regarding the monophyletic origin of planktic foraminifera. We present surprising but conclusive genetic evidence that the Recent biserial planktic Streptochilus globigerus belongs to the same biological species as the benthic Bolivina variabilis, and geochemical evidence that this ecologically flexible species actively grows within the open-ocean surface waters, thus occupying both planktic and benthic domains. Such a lifestyle (tychopelagic) had not been recognized as adapted by foraminifera. Tychopelagic are endowed with great ecological advantage, enabling rapid recolonization of the extinction-susceptible pelagic domain from the benthos. We argue that the existence of such forms must be considered in resolving foraminiferal phylogeny.
Keywords: end-Cretaceous, foraminifera, Mg/Ca, plankton evolution, SSU rRNA
The high-resolution stratigraphic record of the microscopic benthic and planktic organisms called foraminifera provides extraordinary insight into paleoenvironments (1). Their shells of calcium carbonate, imprinted with a trace element and isotopic record of the environment in which they grew, are preserved as abundant microfossils in oceanic sediments. Their fossils change and major events in Earth history, including the end-Cretaceous mass extinction after a bolide impact at 65.5 Ma (2–5). Planktic foraminifera suffered their most extreme extinction at this time, whereas benthic foraminifera in both shallow and deep-water environments survived relatively unscathed (6, 7). Microperforate biserial and triserial foraminifera constituted the majority of the planktic assemblage surviving this mass extinction (2, 3, 8). All survivor species have been described as living predominantly in coastal, relatively shallow waters (nerito-plankton) (4, 5, 8), and as being relatively rare in the more typical pelagic open ocean domain of planktic foraminifera (2, 4, 5). Biserial planktic foraminifera are intermittently abundant in fossil assemblages throughout the Upper Cretaceous and much of the Cenozoic (2–5, 8), with 2 biserial species, Streptochilus globigerus and Streptochilus globulosus living in the present oceans (9) [supporting information (SI) Text]. Their spatial and temporal distributions are poorly known, because of their sporadic stratigraphic and geographic occurrences and because they occur in the rarely studied small size fraction (63–125 μm). The planktic foraminiferal evolutionary tree is under considerable debate (8, 10).
Traditionally, all planktic foraminifera have been seen as monophyletic [Suborder Globigerinina (12)], descended from a single Early–Middle Jurassic ancestor (13), similar to the monophyletic origins of other planktic groups (14). It has, however, not been possible to derive a satisfactory cladogram on fossil data, and the current foraminiferal molecular phylogeny has limited resolution for the prediction of deep-time ancestral relationships. We investigated the ancestry of the living biserial planktic species S. globigerus from the Indian Ocean, where we observed a wide range of ontogenetic stages of this species in the planktic assemblage, and studied its relations to living planktic and benthic foraminifera through analysis of the SSU-rRNA gene. We used a previously undescribed approach for high-resolution geochemical analysis (secondary ionization mass spectrometry, SIMS) of their challengingly small tests to gain insight into its life history by estimating the temperature of calcification of ontogenetic stages.
Results and Discussion
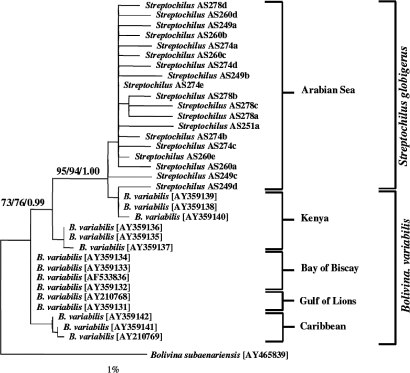
Our molecular data from a 3′ terminal fragment of the SSU rRNA gene of 5 individuals of S. globigerus (19 clones, SI Appendix) indicate that they are all of the same genotype. The clones exhibit a degree of intraspecific variation similar to that observed in many nonspinose planktic and benthic foraminiferal genotypes (11, 15). The same variable elements were common to all 5 specimens (SI Appendix). To investigate their ancestry, we compared their SSU rDNA clone sequences with those of other benthic and planktic foraminiferal taxa, with the highly surprising result that the S. globigerus sequences clustered among those of the morphologically similar (Figs. S1–S4) cosmopolitan benthic species Bolivina variabilis (Fig. 1). B. variabilis collected from the shelf margins of the Caribbean Sea, Gulf of Lions, Bay of Biscay, and Kenya (16) exhibit extensive regional and local intraspecific variation within the variable regions of their SSU rDNA sequences (SI Appendix). However, S. globigerus from the Arabian Sea has variable elements virtually identical to 1 of the 2 populations of B. variabilis sequenced from the Kenyan coastal region, southwest of the central Arabian Sea collection site. Our S. globigerus sequences cluster unambiguously with those of the Kenyan B. variabilis population in phylogenetic analyses (Fig. 1). This strongly supports its commonality with this regional population of B. variabilis. Our genetic evidence thus unequivocally shows that the planktic S. globigerus and the benthic B. variabilis are one and the same biological species.
Fig. 1.
Maximum likelihood (ML) phylogenetic tree showing the evolutionary relationships among B. variabilis isolates (15 isolates) (16) and the S. globigerus isolates (19 clones from 5 specimens) from this study. The phylogeny is rooted on Bolivina subaenariensis, and the scale bar corresponds to 1 change per 100 nt positions. The phylogeny is based on 803 unambiguously aligned nucleotide sites, and the ML tree was constructed using PHYML (v2.4.5) using a GTR+Γ model. Bootstrap values and Bayesian posterior probabilities indicating support for individual branches are shown on the tree (1,000 neighbor-joining bootstraps/1,000 maximum-likelihood bootstraps/Bayesian posterior probabilities).
B. variabilis/S. globigerus lives in quite disparate habitats. In the benthos it lives as a shallow-to-intermediate infaunal dweller, most commonly on the shelf and not present at great depths (SI Text). Yet we observed it in considerable numbers in the surface and thermocline of the open ocean, far offshore. Does B. variabilis/S. globigerus calcify and grow in the open ocean? To determine the temperature of calcification of ontogenetic stages of B. variabilis/S. globigerus, we carried out trace element analyses (Mg/Ca) on individual chambers of 8 B. variabilis/S. globigerus tests using secondary ion mass spectrometry (SIMS) (Fig. 2 and Materials and Methods). The ratio of Mg/Ca in planktic foraminiferal calcite is exponentially correlated to the temperature of seawater during calcification (17, 18) so that the calcification temperatures of individual chambers provide a record of foraminiferal ontogeny.
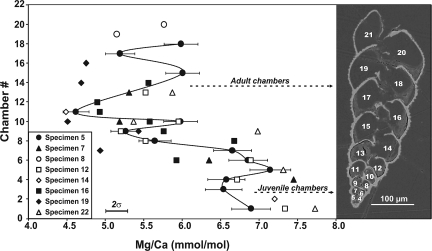
Fig. 2.
Mg/Ca values for sequential chambers of 8 different S. globigerus adult specimens analyzed by using secondary ionization mass spectrometry. The dashed line connects the data for specimen 5 and emphasizes the marked contrast in the element ratio between the juvenile chambers and the adult chambers. Note that the scale of the chamber numbering on the y axis does not correspond to the actual chamber position of the figured specimen.
Ontogenetic measurements of microdissected chambers of benthic foraminiferal tests in controlled culture conditions indicate that Mg may be enriched during middle development, independent of temperature, resulting in the oldest and youngest chambers having the lowest Mg/Ca signatures (19). Furthermore, analyses of multiple whole tests of various species of Bolivina from the benthos show no clear correlation between Mg/Ca and temperature (20). In contrast to the results of these studies, our Mg/Ca data from the B. variabilis/S. globigerus tests show consistent and clearly bimodal values, with higher ratios in the juvenile chambers and lower in the adult. The average Mg/Ca value of the first 7 (juvenile) chambers is 6.8 ± 0.3 mmol mol−1 (2σmean, n = 16), whereas the later chambers have a value of 5.4 ± 0.2 mmol mol−1 (2σmean, n = 29). This represents a relative temperature difference between juvenile and adult chambers of up to 3 °C, calculated from a planktic multispecies calibration (21, 22) (Fig. 3 and Materials and Methods). All analyzed specimens show the higher temperatures in the same early stage in ontogeny (Fig. 2).
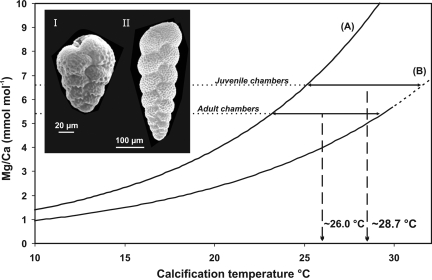
Fig. 3.
Estimation of calcification temperature. Calibration curves for Mg/Ca derived from multispecies datasets of planktic foraminifera based on core-top samples (A) (17) and sediment trap samples (B) (18). The curve (B) is extended to warmer temperatures with a dashed line. The 2 stippled horizontal lines represent the average Mg/Ca values for the juvenile and adult chambers as analyzed by SIMS. The horizontal solid arrows show the temperature offset between the 2 calibration curves, whereas the dashed vertical arrows indicate the derived average calcification temperatures. The Inset shows a picture of a juvenile (I) and an adult (II) S. globigerus specimen (SI Text).
The derived calcification temperature for the juvenile chambers is ≈28.7 °C (Fig. 3), a temperature equivalent to that of the central Arabian Sea mixed layer as seen in conductivity, temperature, depth (CTD) profiles (28.7 °C; see Fig. S5). The derived calcification temperature for the adult chambers is ≈26.0 °C (Fig. 3), corresponding to a calcification depth of 70–80 m in the thermocline, close to the chlorophyll maximum (Fig. S5). Juvenile and mature test chambers of B. variabilis/S. globigerus thus calcified at temperatures consistent with those found in the central Arabian Sea water column.
The Mg/Ca temperature signatures indicate that the juvenile chambers of our specimens could only have formed on the shelf, but only at intertidal or shallow subtidal depths, because these temperatures are too high to represent those of the deeper outer shelf benthos. The bimodal signatures also negate the possibility that they were expatriated from the deeper outer shelf benthos by upwelling: Here temperatures are lower than either the juvenile or adult chamber signatures. Further evidence also supports their growth in the open ocean. We consider it highly improbable that only specimens with exactly 7 chambers would have been transported from the inner-shelf regions to the open ocean: Turbulent transport would carry a mix of ontogenetic stages (23). If the whole population had been transported into the open ocean from the inner shelf, we would expect to see that different specimens formed different numbers of chambers at the higher temperature. Yet, we observed that all specimens moved into colder water at the same stage in ontogeny, as planktic foraminifera do. We therefore argue that B. variabilis/S. globigerus most likely behaves like many other nonspinose planktic species in the open ocean, where juvenile stages calcify near the surface and adult chambers lower in the water column (9, 24). More observations are clearly needed to fully understand the ecology, life cycle and geographic distribution of this distinctive species.
The bimodal calcification temperatures thus suggest that the B. variabilis/S. globigerus specimens collected 600 nautical miles offshore in the central Arabian Sea underwent almost their full ontogenetic development in the pelagic domain and are not quiescent shelf expatriates: They are actively growing and calcifying in the thermocline of the central Arabian Sea. The high degree of genetic identity within the Arabian Sea benthic and planktic biserial foraminifera indicates that they must represent a single, genetically connected population. There must either be 2-way regular gene flow between the benthos in coastal regions and the open ocean plankton, or the identity of the planktic population is maintained by continual reseeding from the benthos. Transport of dispersal stages is a widespread strategy in benthic foraminifera (25), especially in such cosmopolitan shelf species such a B. variabilis (SI Text). We have no information whether B. variabilis/S. globigerus reproduces in the open ocean, but we argue that the species occupies niches in both the benthos and the plankton and grows normally to adult size in both environments. In other words, they are benthic, but at times planktic, foraminifera. The generic name Streptochilus was coined specifically for Neogene planktic taxa that are morphologically similar to the benthic genus Bolivina (26). Our data document that one biological species can be both benthos and plankton and thus imply that a planktic lifestyle does not warrant assignment to a separate genus. Streptochilus is thus a junior synonym of Bolivina (SI Text).
The word “tychopelagic” is used to describe organisms that usually live as benthos but can survive and grow in fairly large numbers as plankton and may be advected well offshore into open ocean assemblages (27). Such a lifestyle is known from diatoms, but until now has never been documented for foraminifera. Tychopelagic forms may well evolve into true pelagic forms: The fossil record shows convincingly that biserials are particularly abundant in the planktic foraminiferal assemblage at some time intervals, with isotopic as well as distributional evidence suggesting that they were truly pelagic forms, reproducing in situ (8, 9, 28–31). The tychopelagic mode of life differs fundamentally from that of the few documented meroplanktic benthics such as Tretomphalus (32), a generic name used for individuals of the genus Rosalina, which at a specific, late stage in their life cycle form a floating chamber containing gametes (32, 33). Such forms do not grow and calcify in the plankton, where they survive only a short time (≈6 h) while reproducing sexually (33). A tychopelagic mode of life is likewise quite distinct from the suggestion that some pelagic planktic foraminifers may exhibit pseudobenthic behavior in artificial culture experiments (34): There is no evidence that these planktic forms actually live and calcify on the sea floor in nature. In strong contrast to these earlier described forms, tychopelagics are able to not only survive but also grow and calcify in the plankton, and thus must be able to maintain buoyancy. Interestingly, buoyancy is generally assumed to be one of the major constraining evolutionary traits on the passage from benthos to plankton (14). Species of the genus Bolivina might be more buoyant than other benthic taxa: They have been observed in plankton nets over and just off the shelf more commonly than species of other genera (23).
The ability to survive in both planktic and benthic habitats should be seen as an extraordinary ecological adaptation for long-term survival. After mass extinctions in the plankton, e.g., as caused by bolide impacts (8) and oceanic anoxic events (35), tychopelagic species are able to repopulate the pelagic realm and evolve into purely planktic forms. Some early planktic foraminifera have been described as living in inner shelf as well as more open ocean regions (24, 35, 36), but the possibility that a tychopelagic lifestyle exists (as we demonstrate for B. variabilis/S. globigerus) has not been taken into account in phylogenetic studies based on the planktic foraminiferal fossil record, including the discussion of the first evolution of planktic foraminifera in the Jurassic as well as radiations in the Early Cretaceous (13, 24, 35–39)
Our view of biserial foraminifera as potentially tychopelagic species requires another look at the planktic environment and pattern of survival after the Cretaceous mass extinction, as well as the Mesozoic and Cenozoic evolution of microperforate foraminifera (8, 35). All species surviving the end-Cretaceous mass extinction have been described as nerito-plankton, living predominantly in coastal, relatively shallow waters, although no evidence has been presented that these species were actually living as plankton, i.e., floating in the water column, rather than as bottom-dwellers in the neritic environment (SI Text). We argue that at least some of the assumedly nerito-planktic species may very well have been tychopelagic. A wide variety of Paleogene and Cretaceous species (including biserial, triserial, and spiral morphologies) have been considered nerito-plankton based on their abundant occurrences in slope and continental shelf environments, specifically in upwelling areas (2, 34, 36–38, 40), a distribution similar to that of Recent Streptochilus (39). Oxygen isotopic evidence indicates that at least some of these species were benthic (40).
We thus argue that radiation and repopulation of the empty niche in the plankton after the end Cretaceous mass extinction may at least in part have occurred from benthic tychopelagic species rather than from nerito-planktic ones. The triserial survivor Guembelitria cretacea and the biserial survivor Zeauvigerina waiparaensis have species lives of many millions of years, typical of benthic rather than planktic species (41). Both species have been described as shallow-water dwellers, typical of disturbed environments (42), and the latter has a nondistinctive isotopic signature, so that its planktic nature has commonly been questioned (8). Many Cretaceous biserial planktic taxa (genus Heterohelix) likewise have long species lives, were common in epicontinental seas, and are seen as opportunistic taxa surviving in disturbed environments and under high-productivity conditions (28, 36). The available isotopic evidence on some Heterohelix species is either ambiguous or indicates a benthic existence (8, 43, 44).
The Cenozoic planktic foraminiferal phylogeny of microperforates, the group containing biserial and triserial forms, has generally presented taxonomists with problems (8, 45). Many of these genera and species show discontinuous stratigraphic records, making ancestor–descendant patterns difficult to reconstruct. This could be the result of a lack of observation of the small forms, in a size fraction that commonly is not included in study. In our view, however, such ancestor–descendant relations simply do not exist. This is supported by recent evidence that the living triserial planktic foraminifer Gallitellia vivans had a Miocene benthic ancestor (46) and thus did not evolve from the Cretaceous–Paleocene triserial Guembelitria cretacea. Appearances of biserial and triserial planktic forms in the geological record should therefore not be considered as necessarily discrete punctuated evolutionary events but as a series of excursions of expatriated tychopelagic microperforates into the planktic domain.
Materials and Methods
Collection.
Specimens of the biserial planktic foraminifera S. globigerus were collected along a cruise transect, ≈600 nautical miles offshore in the central Arabian Sea (20°22.81 N/64°29.36E–02°36.03S/56°54.75E) during the summer monsoon of 2003 (cruise Charles Darwin CD148, NERC; see Fig. S6). Samples were collected either by pumping (5 m depth) from the nontoxic water supply through a plankton screen (83-μm mesh) or by vertical net tow (0–100 and 0–200 m depth, 83-μm mesh) in waters with an average depth of 3,500 m. Fully grown individual specimens of Streptochilus with bright orange cytoplasm were selected and processed for DNA analysis as described previously (47). For SEM imaging and geochemical analysis, specimens were either dried on slides or collected as bulk samples in ethanol.
Isolation and Sequencing of SSU rRNA Gene Sequences.
DNA extraction and amplification by PCR of an ≈1,000-bp region of the terminal 3′ end of the foraminiferal SSU rRNA gene were as described previously (47). Although specimens were initially directly sequenced, a low level of ambiguity in the highly variable regions was observed. PCR products (obtained by using primers N5/N8) were therefore cloned by using a PCR 2.1 TOPO TA cloning kit (Invitrogen) before sequencing. Partial SSU rDNA sequences were aligned manually within version 2.2 of the Genetic Data Environment (GDE) package (48). Phylogenetic trees were constructed using neighbor joining (NJ) (PAUP v4.0b10), maximum likelihood (ML) (PHYML v2.4.5), and Bayesian inference (BI) (MrBayes v3.1.2) using a GTR+Γ model.
Secondary Ionization Mass Spectrometry (SIMS).
For the SIMS analyses, whole specimens were mounted in epoxy resin, polished down to expose a cross-section of the test, cleaned, and gold-coated. To assess the quality of the sample preparation, exposure, and test thickness, the samples were imaged by using scanning electron microscopy before analyses. The specimens chosen for analysis showed a typical test length of 400 μm and a test wall thickness of maximum of 10 μm down to 2 μm for the internal septa that separate individual chambers. To achieve the high spatial resolution required to detect geochemical differences between individual chambers in such small foraminifers, it was necessary to develop a specific analytical protocol.
Trace element analyses were performed on 8 specimens by using the Cameca ims 4f SIMS at the NERC Ion Microprobe Facility, University of Edinburgh. Positive secondary ions of 26Mg+ and 44Ca+ were produced by a-4 nA, 15-kV, 16O− primary beam. Secondary ions were analyzed with a 25-μm image field and zero energy offset by using the 150-μm contrast and the 100-μm field apertures. This mode of operation significantly reduces overall count rates but ensures an effective spatial resolution of ≈1.8 μm. Potential near-surface contamination was ablated by presputtering each sample area for 5 min before analysis. Secondary ions were counted on an electron multiplier in single collector mode operation and measured for 20 cycles, each cycle consisting of 10- and 2-s count times on Mg and Ca, respectively. The Mg to Ca values were converted to μg g−1 in calcite and then finally the Mg, Ca ratio expressed as mmol mol−1. The OKA calcite (carbonatite complex in the Monteregian Hills, Quebec, Canada: Mg = 688 μg g−1 and Ca = 400439 μg g−1) was used to calculate the elemental result for Mg with an analytical uncertainty of ≈10% (2σr).
Mg/Ca Temperature Calibration.
There is no species-specific temperature calibration for S. globigerus, so we used a combination of a core-top and a sediment trap multispecies temperature calibration (17, 18). These studies assume that the temperature dependence of a multispecies calibration can be applied to all planktic species and describe interspecific differences via a preexponential constant. We consider that the 2 different temperature calibrations represent the temperature extremes and took the average Mg/Ca estimates for reconstructing calcification temperatures as they were the most equivalent to the Arabian Sea water column temperatures. In benthic Bolivina species, no temperature correlation is observed in Mg/Ca, and values are thought to reflect pore water carbonate content rather than temperature (20).
Supplementary Material
Acknowledgments.
We thank the crew and scientists of RRS Charles Darwin (cruise CD148) for their assistance, Blair Steel for sampling on cruise CD148, Dick Kroon (School of GeoSciences, University of Edinburgh, Edinburgh) for specimens of Streptochilus globulosus and Mike Hall for sample preparation. Molecular work was undertaken with support from the Institute of Genetics, University of Nottingham. Analytical work relied on the expertise of the Edinburgh Ion Microprobe Facility and the Edinburgh School of GeoSciences Scanning Electron Microscope Facility. This work was supported by Natural Environment Research Council of the United Kingdom Grants NER/J/S2000/00860 and NE/D009707/1 (to K.F.D), National Science Foundation Grant NSF-OCE 720049 (to E.T.), and a Biotechnology and Biological Sciences Research Council of the United Kingdom PhD studentship (to H.A.S).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. GQ265787–GQ265805).
This article contains supporting information online at www.pnas.org/cgi/content/full/0902827106/DCSupplemental.
References
- 1.Kucera M. Planktonic foraminifera as tracers of past oceanic environments. In: Hillaire-Marcel C, de Vernal A, editors. Proxies in Late Cenozoic Paleoceanography. Amsterdam: Elsevier; 2007. pp. 213–262. [Google Scholar]
- 2.Olsson RK, Hemleben C, Berggren WA, Huber BT. Smithsonian Contributions to Paleobiology. No. 85. Washington, DC: Smithsonian Institution Press; 1999. Atlas of Paleocene Planktonic Foraminifera. [Google Scholar]
- 3.Berggren WA, Pearson PN. A revised tropical to subtropical Paleogene planktonic foraminiferal zonation. J Foram Res. 2005;35:279–298. [Google Scholar]
- 4.d'Hondt S. Phylogenetic and stratigraphic analysis of earliest Paleocene biserial and triserial planktonic foraminifera. J Foram Res. 1991;21:168–181. [Google Scholar]
- 5.Liu C, Olsson RK. Evolutionary radiation of microperforate planktonic foraminifera following the K/T mass extinction event. J Foram Res. 1992;22:328–346. [Google Scholar]
- 6.Culver SJ. Benthic foraminifera across the Cretaceous–Tertiary (K–T) boundary: A review. Mar Micropal. 2003;47:177–226. [Google Scholar]
- 7.Thomas E. Cenozoic mass extinctions in the deep sea; what disturbs the largest habitat on Earth? GSA Spec Paper. 2007;424:1–24. [Google Scholar]
- 8.Huber BT, Olsson RK, Pearson PN. Taxonomy, biostratigraphy, and phylogeny of Eocene microperforate planktonic foraminifera (Jenkinsina, Cassigerinelloita, Chiloguembelina, Streptochilus, Zeauvigerina, Tenuitella and Cassigerinella) and problematic (Dipsidripella) Cushman Found Foram Res Spec Publ. 2006;41:461–508. [Google Scholar]
- 9.Schiebel R, Hemleben Ch. Modern planktic foraminifera. Paläontolog Z. 2005;79:135–148. [Google Scholar]
- 10.Darling KF, Wade CM, Kroon D, Leigh Brown AJ. Planktic foraminiferal molecular evolution and their polyphyletic origins from benthic taxa. Mar Micropal. 1997;30:251–266. [Google Scholar]
- 11.Darling KF, Wade CM. The genetic diversity of planktic foraminifera and the global distribution of ribosomal RNA genotype. Mar Micropal. 2008;67:216–238. [Google Scholar]
- 12.Sen Gupta BK. Systematics of modern foraminifera. In: Sen Gupta BK, editor. Modern Foraminifera. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 7–36. [Google Scholar]
- 13.Hart MB, et al . The search for the origin of the planktic foraminifera. J Geol Soc. 2003;160:341–343. [Google Scholar]
- 14.Rigby S, Milsom C. Benthic origins of zooplankton: An environmentally determined macroevolutionary effect. Geology. 1996;24:52–54. [Google Scholar]
- 15.Schweizer M, Pawlowski J, Kouwenhoven T, Guiard J, van der Zwaan GJ. Molecular phylogeny of Rotaliida (foraminifera) based on complete small subunit rDNA sequences. Mar Micropal. 2007;66:233–246. [Google Scholar]
- 16.Ertan KT, Hemleben V, Hemleben C. Molecular evolution of some selected benthic foraminifera as inferred from sequences of the small subunit ribosomal DNA. Mar Micropal. 2004;53:367–388. [Google Scholar]
- 17.Nürnberg D, Bijma J, Hemleben C. Assessing the reliability of magnesium in foraminiferal calcite as a proxy for water mass temperatures. Geochim Cosmochim Acta. 1996;60:803–814. [Google Scholar]
- 18.Lea DW, Mashiotta TA, Spero HJ. Controls on magnesium and strontium uptake in planktonic foraminifera determined by live culturing. Geochim Cosmochim Acta. 1999;63:2369–2379. [Google Scholar]
- 19.Hintz CJ, et al. Trace/minor element:calcium ratios in cultured benthic foraminifera. Part II. Ontogenetic variability. Geochim Cosmochim Acta. 2006;70:1964–1976. [Google Scholar]
- 20.Douglas R, Staines-Urias F. Dimorphism, shell Mg/Ca ratios and stable isotope content in species of Bolivina (benthic foraminifera) in the Gulf of California, Mexico. J Foram Res. 2007;37:189–203. [Google Scholar]
- 21.Elderfield H, Ganssen G. Past temperature and delta18O of surface ocean waters inferred from foraminiferal Mg/Ca ratios. Nature. 2000;405:442–445. doi: 10.1038/35013033. [DOI] [PubMed] [Google Scholar]
- 22.Anand P, Elderfield H, Conte MH. Calibration of Mg/Ca thermometry in planktonic foraminifera from a sediment trap time series. Paleoceanography. 2003;18:28–31. [Google Scholar]
- 23.Hueni C, Monroe JA, Gevirtz J, Casey R. Distribution of living benthonic foraminifera as indicators of oceanographic processes on the South Texas outer continental shelf. Trans Gulf Coast Assoc Geol Soc. 1978;28:193–200. [Google Scholar]
- 24.Caron M, Homewood P. Evolution of planktic foraminifers. Mar Micropal. 1982;7:453–462. [Google Scholar]
- 25.Alve E, Goldstein ST. Propagule transport as a key method of dispersal in benthic foraminifera (Protista) Limnol Oceanogr. 2003;48:2163–2170. [Google Scholar]
- 26.Brönnimann P, Resig JA. Neogene globigerinacean biochronologic time-scale of the southwestern Pacific. In: Winterer E, et al., editors. Initial Reports of the Deep Sea Drilling Project. No. 7. Washington, DC: US Government Printing Office; 1971. pp. 1235–1469. [Google Scholar]
- 27.McQuoid MR, Nordberg K. The diatom, Paralia sulcata, as an environmental indicator species in coastal sediments. Estuarine Coastal Shelf Sci. 2003;56:339–354. [Google Scholar]
- 28.Nederbragt AJ. Maastrichtian Heterohelicidae (Planktonic Foraminifera) from the North West Atlantic. J Micropaleontol. 1989;8:183–206. [Google Scholar]
- 29.Leckie RM. Paleoecology of mid-Cretaceous planktonic foraminifera: A comparison of open ocean and epicontinental sea assemblages. Micropaleontology. 1987;33:164–176. [Google Scholar]
- 30.Smart CW, Thomas E. The enigma of early Miocene biserial planktic foraminifera. Geology. 2007;34:1041–1044. [Google Scholar]
- 31.Smart CW, Thomas E. Emendation of the genus Streptochilus Brönnimann and Resig, 1971 (Foraminifera) and new species from the lower Miocene of the Atlantic and Indian Oceans. Micropaleontology. 2007;53:73–104. [Google Scholar]
- 32.Sliter WV. Laboratory experiments on the life cycle and ecologic controls of Rosalina globularis d'Orbigny. J Eukar Microbiol. 1965;12:210–215. [Google Scholar]
- 33.Myers EH. The present state of our knowledge concerning the life cycle of foraminifera. Proc Natl Acad Sci USA. 1938;24:10–17. doi: 10.1073/pnas.24.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilbrecht H, Thierstein HR. Benthic behavior of planktic foraminifera. Geology. 1996;24:200–202. [Google Scholar]
- 35.Leckie RM, Bralower TJ, Cashman R. Oceanic anoxic events and plankton evolution: Biotic response to tectonic forcing during the mid Cretaceous. Paleoceanography. 2002;17:PA000623. [Google Scholar]
- 36.Eicher DL, Worstell P. Cenomanian and Turonian foraminifera from the Great Plains, United States. Micropaleontology. 1970;16:269–324. [Google Scholar]
- 37.Boudagher-Fadel MK, Banner FT, Whittaker JE. British Micropalaeontological Society Publication Series. Berlin: Springer; 1997. The early evolutionary history of planktic foraminifera. [Google Scholar]
- 38.Koutsoukos AM, Leary PN, Hart MB. Favusella Michael; evidence of ecophenotypic adaptation of a planktonic foraminifer to shallow-water carbonate environments during the Mid-Cretaceous. (1989) J Foram Res. 1972;4:324–336. [Google Scholar]
- 39.Kroon D, Nederbragt AJ. Ecology and paleoecology of triserial planktic foraminifera. Mar Micropal. 1990;16:25–38. [Google Scholar]
- 40.Liu C, Olsson RK, Huber BT. A benthic paleohabitat for Praepararotalia gen. nov. and Antarcticella Loeblich and Tappan. J Foram Res. 1998;28:3–18. [Google Scholar]
- 41.Kucera M, Schoenfeld J. The origin of modern oceanic foraminiferal faunas and Neogene climate change. In: M Williams M, Haywood AM, Gregory FJ, Schmidt DN, editors. The Micropalaeontological Society Spec Publ 2. London: The Micropalaeontological Society; 2007. pp. 409–426. [Google Scholar]
- 42.Coccioni R, Luciani V. Guembelitria irregularis bloom at the K/T boundary: Morphological abnormalities induced by impact-related extreme environmental stress. In: Cockell C, Koeberl C, Gilmour I, editors. Biological Processes Associated with Impact Events. Berlin: Springer; 2006. pp. 179–196. [Google Scholar]
- 43.Huber BT, Hodell DA, Hamilton CP. Mid to Late Cretaceous climate of the southern high latitudes: Stable isotopic evidence for minimal equator-to-pole thermal gradients. Geol Soc Am Bull. 1995;107:1164–1191. [Google Scholar]
- 44.MacLeod KG, Huber BT, Pletsch T, Röhl U, Kucera M. Maastrichtian foraminiferal and paleoceanographic changes on Milankovitch time scales. Paleoceanography. 2001;16:133–154. [Google Scholar]
- 45.Stewart IA, Darling KF, Kroon D, Wade CM, Troelstra SR. Genotypic variability in subarctic Atlantic planktic foraminifera. Mar Micropal. 2001;43:143–153. [Google Scholar]
- 46.Ujiié Y, Kimoto K, Pawlowski J. Molecular evidence for an independent origin of modern triserial planktonic foraminifera from benthic ancestors. Mar Micropal. 2008;69:334–340. [Google Scholar]
- 47.Darling KF, et al. Molecular evidence for genetic mixing of Arctic and Antarctic subpolar populations of planktonic foraminifers. Nature. 2000;405:43–47. doi: 10.1038/35011002. [DOI] [PubMed] [Google Scholar]
- 48.Smith SW, Overbeek R, Woese R, Gilbert W, Gillievet PM. The genetic data environment: An expandable GUI for multiple sequence analysis. Comput Appl Biosci. 1994;10:671–675. doi: 10.1093/bioinformatics/10.6.671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.