Abstract
Obesity is accompanied by an increase in both adipocyte number and size. The increase in adipocyte number is the result of recruitment to the adipocyte lineage of pluripotent stem cells present in the vascular stroma of adipose tissue. These pluripotent cells have the potential to undergo commitment and then differentiate into adipocytes, as well as myocytes, osteocytes, and chondrocytes. In this article, we show that both bone morphogenetic protein (BMP)2 and BMP4 can induce commitment of C3H10T1/2 pluripotent stem cells into adipocytes. After treatment of C3H10T1/2 stem cells with these BMPs during proliferation followed by exposure to differentiation inducers at growth arrest, nearly all cells enter the adipose development pathway, express specific adipocyte markers, and acquire the adipocyte phenotype. Overexpression of constitutively active BMP receptor (CA)-BMPr1A or CA-BMPr1B induces commitment in the absence of BMP2/4, whereas overexpression of a dominant-negative receptor dominant-negative-BMPr1A suppresses commitment induced by BMP. Also, knockdown of the expression of Smad4 (coregulator in the BMP/Smad signaling pathway) with RNAi disrupts commitment by the BMPs. However, knockdown of expression of p38 MAPK (an intermediary in the BMP/MAPK signaling pathway) with RNAi had little effect on BMP-induced commitment. Together, these findings indicate that the BMP/Smad signaling pathway has a dominant role in adipocyte lineage determination. Proteomic analysis identified lysyl oxidase (LOX), a bona fide downstream target gene of the BMP signaling pathway. Expression of LOX is induced by BMP2/4 during adipocyte lineage commitment, and knockdown of its expression disrupts the commitment process.
Keywords: lysyl oxidase, preadipocyte, Smad4, adipogenesis, differentiation
Obesity is accompanied by an increase in the size and number of adipocytes (1, 2). The rise in adipocyte number is the result of: (i) recruitment of new preadipocytes from the population of mesenchymal stem cells (MSCs) in the vascular stroma of adipose tissue (3), and (ii) “mitotic clonal expansion” of the preadipocyte population during differentiation (4). Pluripotent MSCs have the potential to undergo commitment into adipocyte, myocyte, osteocyte, or chondrocyte lineages (5). The developmental pathway that gives rise to mature adipocytes involves 2 distinct stages: commitment and terminal differentiation. Although the sequential steps of terminal adipocyte differentiation have been clearly defined (6–8), the steps in commitment of pluripotent stem cells to the adipocyte lineage have not. In the former case, the 3T3-L1 preadipocyte model system has been particularly useful, because the synchronous steps in the differentiation process can be resolved in cell culture (6). Likewise, the C3H10T1/2 cell line constitutes a reliable MSC model cell system for the study of stem cell commitment to the adipocyte lineage (9, 10). This cell line represents an earlier developmental stage than that of the 3T3-L1 line, but when appropriately induced or genetically manipulated, has the capacity to undergo commitment to cells with the characteristics of preadipocytes (11–15).
An independent line of investigation supports the role of bone morphogenetic proteins (BMPs) in the commitment of pluripotent stem cells to the adipocyte lineage (14). Thus, brief treatment of proliferating C3H10T1/2 stem cells with an inhibitor of DNA methylation (5-azacytidine) produced clonal lines with preadipocyte characteristics, i.e., capable of differentiating into adipocytes when subjected to differentiation inducers without exogenous BMP. Remarkably, such cell lines express/secrete BMP endogenously during the time window in which BMP4 must be exogenously supplied to “naive” C3H10T1/2 stem cells to induce adipocyte commitment. The requirement for secreted BMP4 in the commitment process was verified by the finding that exposure of “committed” C3H10T1/2 cells to noggin, a naturally occurring BMP-binding antagonist, during this critical time window blocked subsequent differentiation. The BMP4 gene locus in naive C3H10T1/2 stem cells possesses a highly methylated region of CpG islands, whereas this region in committed C3H10T1/2 cells possesses fewer methylated Cs (14). This difference correlates with a switch in BMP promoter usage (promoter 1B to promoter 1A) during commitment induced by the methylation inhibitor, suggesting that a change in methylation status renders promoter 1A more accessible for transcriptional activation (14).
BMPs signal through 2 receptor types, BMPr1 and BMPr2, which form cell-surface complexes with serine/threonine kinase activity (16, 17). Binding of BMP to the BMPr1:BMPr2 complex induces phosphorylation; thus, activating the BMPr1 kinase. Two types of BMP-induced signaling pathways are known, the Smad and p38 MAPK pathways. In the former case, BMPr1 phosphorylates Smad-1,-5,-8, which forms a complex with Smad4 that translocates into the nucleus and regulates gene expression. In contrast, the p38 MAPK pathway involves a phosphorylation cascade with MAPK kinase kinase (KK), MKK3 or MKK6, and p38 MAPK, phosphorylated/activated p38 MAPK activating the expression of BMP-target genes.
Although it has been established that BMPs have a critical role in the processes by which pluripotent stem cells undergo commitment to the adipocyte lineage, little is known about the downstream signaling pathway(s) involved. Here, we provide compelling evidence that both BMP/Smad and BMP/p38MAPK pathways are involved in the transcriptional activation of target genes of the commitment process. Proteomic analysis in combination with RNAi interference has identified lysyl oxidase (LOX) as a target gene necessary for adipocyte lineage commitment by BMP2/4.
Results
BMP2 and BMP4 Induce Commitment of C3H10T1/2 Pluripotent Stem Cells to the Adipocyte Lineage.
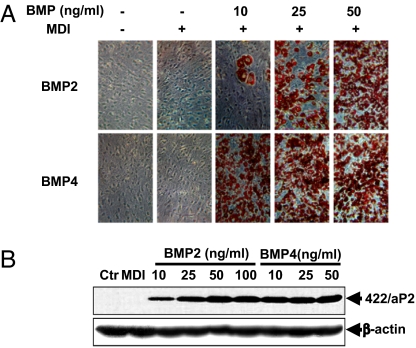
As previously reported, BMP4 induces commitment of C3H10T1/2 stem cells into preadipocytes (15). BMP2, a homologue of BMP4, has been implicated in the development/differentiation of osteoblasts, chondroblasts, and adipocytes. To compare their capacity to induce commitment of C3H10T1/2 cells into preadipocytes, cells plated at low density were treated with purified recombinant human BMP2 or BMP4 at levels of 10, 25, 50, or 100 ng/mL during proliferation. On reaching confluence, the standard adipocyte differentiation protocol (18) was applied. As illustrated in Fig. 1, <5% of the C3H10T1/2 cells differentiated spontaneously in the absence of BMP2/4 or the differentiation inducers (MDI), whereas treatment with BMP2/4 induced commitment/differentiation in >80% of the cells. The basis for the low level of spontaneous commitment/differentiation is discussed in Materials and Methods. The results indicate that BMP4 was somewhat more potent than BMP2 as an inducer of commitment at ≤25 ng/mL; however, at higher levels, they are equally effective.
Fig. 1.
Induction of adipocyte lineage commitment. Dependence on BMP2 and BMP4 concentration. C3H10T1/2 pluripotent stem cells at low density, were cultured without or with BMP2 or BMP4 at 10, 25, and 50 ng/mL until postconfluent, and then subjected to the adipocyte differentiation protocol. (A) On day 8, the accumulation of cytoplasmic triglyceride was assessed by Oil Red O staining. (B) On day 4, whole-cell extracts were subjected to SDS/PAGE and 422/aP2 was detected by immunoblotting.
BMP2/4 Induces Adipocyte Commitment Through Type 1A and 2 BMP Receptors.
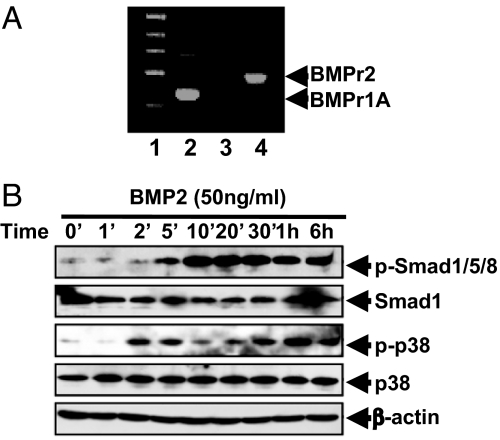
Signaling by BMPs is transduced via specific BMP receptors (r)1A or BMPr1B and BMPr2 (19, 20). To determine which of these receptors initiate BMP-induced commitment, their expression by C3H10T1/2 stem cells was assessed by RT-PCR. As illustrated in Fig. 2A, these cells express BMPr1A and BMPr2, but not BMPr1B. Also, BMP2 induces the phosphorylation of Smad1/5/8 of p38 (Fig. 2B), downstream targets in the BMP pathway.
Fig. 2.
C3H10T1/2 stem cells express BMP receptors 1A and 2 and respond to BMP signals. (A) C3H10T1/2 stem cells were cultured in DMEM with 10% calf serum. Total RNA was isolated and subjected to RT-PCR. DNA marker (lane 1); BMPr1A (lane 2); BMPr1B (lane 3); and BMPr2 (lane 4). (B) Two days after plating, the cells were treated with 50 ng of BMP2 per mL and equal levels of cell extract protein were subjected to SDS/PAGE and immunoblotted with the indicated antibodies.
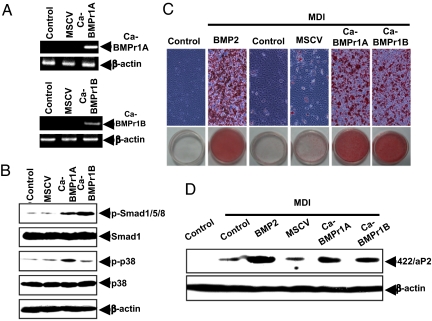
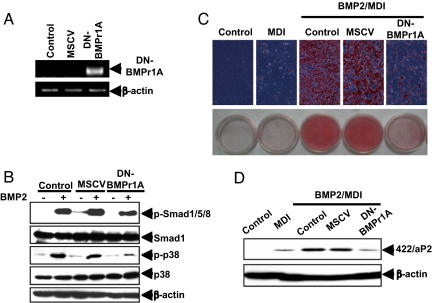
BMPr1A and BMPr2 are serine/threonine kinases that undergo phosphorylation on ligand binding and, thus, activation of BMP-induced signal transmission. To ascertain whether overexpression of BMPr1A can initiate adipocyte lineage commitment in the absence of its BMP ligand, constitutively active (CA)-BMPr1A and CA-BMPr1B were expressed in C3H10T1/2 stem cells using a mouse stem cell virus (MSCV) retroviral system (Fig. 3A). The expression of CA-BMPr1A and CA-BMPr1B mRNA was confirmed by RT-PCR using appropriate primers to distinguish expression of the constitutively active receptors from endogenous BMP receptors; specific antibodies for these receptors were not available. However, the functional effects of their expression, i.e., phosphorylation of Smad1/5/8 and p38 MAPK, verify overexpression of the constitutively active receptors (Fig. 3B). Thus, their overexpression provoked a substantial rise in the phosphorylation of Smad1/5/8 and p38 MAPK, known downstream phosphorylated intermediates in the BMP signaling pathway (Fig. 3B) (16, 17). Important in this context, overexpression of either BMPr1A or BMPr1B induced these changes in the absence of BMP2 or BMP4 treatment (Fig. 3B). Thus, receptor overexpression led to commitment/terminal adipocyte differentiation as assessed by the accumulation of cytoplasmic triglyceride and expression of the specific adipocyte marker 422/aP2 (Fig. 3 C and D). It should be noted that although C3H10T1/2 stem cells do not express BMPr1B, like BMPr1A, its overexpression induces adipocyte lineage commitment (Fig. 3 C and D). For further support for their role in the commitment process, we found that dominant-negative (DN)-BMPr1A, which lacks the kinase domain, reduces phosphorylation/activation of Smad1/5/8 and p38 MAPK induced by BMP2 (Fig. 4 B) and BMP4, as well as cytoplasmic triglyceride accumulation (Fig. 4C) and the expression of 422/aP2 (Fig. 4D).
Fig. 3.
Overexpression of constitutively active BMPr1 (CA-BMPr1A or CA-BMPr1B) induces adipocyte lineage commitment in the absence of BMP. C3H10T1/2 stem cells were infected with retroviral CA-BMPr1A or CA-BMPr1B. (A) One day after infection, total RNA was isolated and subjected to RT-PCR. (B) Cell lysates were prepared, and equal levels of extract protein were separated by SDS/PAGE and immunoblotted with the antibodies indicated. (C) After reaching postconfluence, the cells were induced to differentiate, and on day 12, cells were stained. (D) On day 4, cell extracts were prepared, and the expression of 422/aP2 was assessed by immunoblotting.
Fig. 4.
Overexpression of DN-BMPr1A prevents adipocyte lineage commitment induced by BMP. C3H10T1/2 stem cells were infected with retrovirus harboring DN-BMPr1A. (A) One day after infection, total RNA was isolated and subjected to RT-PCR to confirm the expression of DN-BMPr1A; β-actin was used as loading control. (B) Whole-cell lysates were prepared, and equal amounts of protein were separated by SDS/PAGE and immunoblotted with antibodies as indicated. (C) Upon reaching postconfluence, cells were induced to differentiate with standard adipocyte differentiation protocol, and on day 8, were stained with Oil Red O. (D) On day 4, cell extracts were prepared and the expression of 422/aP2 was assessed by immunoblotting.
Smad Is Required for BMP-Induced Adipocyte Lineage Commitment of C3H10T1/2 Stem Cells.
The Smads and p38 MAPK are intermediates in the BMP signaling pathways of various cell types (16, 17). To assess their roles in adipocyte lineage commitment, proliferating C3H10T1/2 cells were treated with BMP after which phosphorylation of Smad1/5/8, and p38MAPK was assessed by immunoblotting. As illustrated in Fig. 2B, treatment with BMP2 rapidly (≤5 min) induced phosphorylation of Smad1/5/8 and p38 MAPK. A previous study showed that BMP4 provoked a similar activation of phosphorylation (15). Consistent with these findings, retrovirus induced overexpression of either BMPr1A or BMPr1B in the absence of exogenous BMP promoted phosphorylation of Smad1/5/8 and p38 MAPK (Fig. 3B).
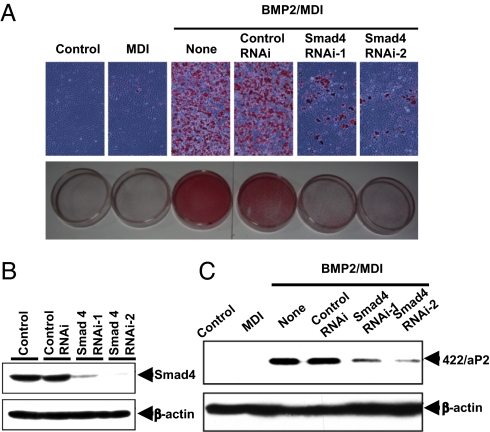
It has been established that on phosphorylation, Smad1/5/8 by BMPr1A or 1B form a complex with Smad4, which translocates to the nucleus to regulate target gene transcription. To ascertain whether this signaling pathway is required for the adipocyte commitment process, Smad4 expression was disrupted in proliferating C3H10T1/2 cells with Smad4 RNAi (Fig. 5A). After reaching confluence, the cells were subjected to standard adipocyte differentiation protocol. It was found that Smad4 RNAi blocked commitment/differentiation into adipocytes (Fig. 5 B and C) even at a BMP2 concentration that would normally induce adipocyte commitment of naive C3H10T1/2 cells (Fig. 1). These findings show that BMP/Smad signaling is required for BMP-induced adipocyte lineage commitment of C3H10T1/2 stem cells.
Fig. 5.
Dependence of stem cell commitment on Smad signaling. C3H10T1/2 stem cells were plated at 30% confluence and transfected with Smad4 Stealth RNAi, and 24 h later were treated with BMP2 (50 ng/mL) until postconfluent. Cells were then induced to differentiate with the adipocyte differentiation protocol. Knockdown of Smad4 expression was confirmed by immunoblotting (B), and its effect on adipocyte commitment and terminal differentiation was assessed both by staining of cytoplasmic triglyceride (A) and by expression of 422/aP2 (C).
The p38MAPK Is Involved in BMP-Induced Adipocyte Lineage Commitment of C3H10T1/2 Stem Cells.
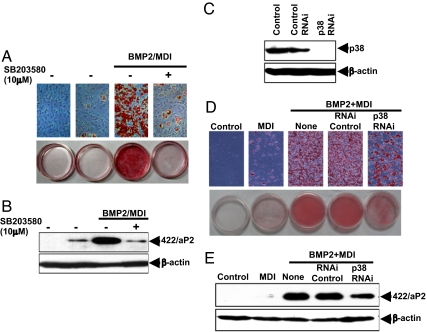
The p38MAPK signaling also appears to participate in BMP-induced commitment. To assess the role of p38MAPK, 2 approaches were used: C3H10T1/2 stem cells were either treated with the specific p38MAPK inhibitor SB203580 or with a p38MAPK RNAi to knockdown p38MAPK expression. The p38MAPK inhibitor SB203580 almost totally prevented commitment/differentiation as assessed by the accumulation of cytoplasmic triglyceride (Fig. 6A) and the expression of the adipocyte marker 422/aP2 (Fig. 6B). Likewise, knockdown p38MAPK expression with RNAi (Fig. 6C) partially disrupted cytoplasmic triglyceride accumulation (Fig. 6D) and expression of the adipocyte marker protein 422/aP2 (Fig. 6E). These findings indicate that p38MAPK is also involved in the BMP-induced signaling pathway leading to stem cell commitment, although the effect of its disruption is less than that of Smad.
Fig. 6.
Dependence of stem cell commitment on p38MAPK signaling. (A and B) C3H10T1/2 stem cells were plated at low density, treated with a specific p38MAPK inhibitor SB203580 (10 μM) before treatment with BMP2 (50 ng/mL). After reaching postconfluence, cells were induced to differentiate. Commitment and terminal differentiation were assessed by: staining of cytoplasmic triglyceride (A) and expression of an adipocyte marker protein (422/aP2) (B). (C–E) C3H10T1/2 stem cells were plated at 30% confluence and transfected with p38MAPK Stealth RNAi. After 24 h, cells were treated with BMP2 (50 ng/mL) until reaching postconfluence, and then were induced to differentiate using the adipocyte differentiation protocol. (C) Knockdown of expression of p38MAPK was verified by immunoblotting. (D and E) The effect on commitment and terminal differentiation was assessed both by staining of cytoplasmic triglyceride (D) and by expression of specific adipocyte marker protein (422/aP2) (E).
Identification of the LOX Gene as a Target of the BMP-Induced Pathway of Adipocyte Lineage Commitment.
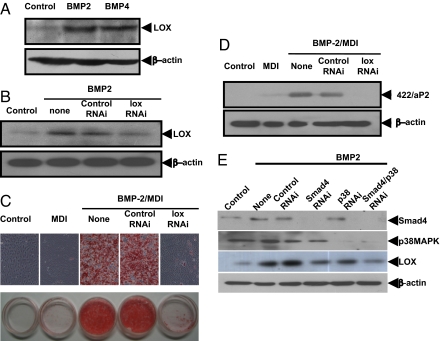
Proteomic analysis was performed to identify target genes whose expression is induced during BMP-induced commitment of C3H10T1/2 stem cells. Proteins in extracts of cells, induced or not with BMP, were separated by 2-D gel chromatography, stained, differentially expressed proteins selected by computer-assisted analysis, and their identities determined by MS (see Materials and Methods). The most highly up-regulated (≈3-fold) protein was identified as LOX. Verification that expression of LOX is increased during BMP-induced commitment of C3H10T1/2 stem cells was confirmed in an independent experiment (Fig. 7A). Also, knockdown of expression of LOX with RNAi abolished acquisition of the adipocyte phenotype as evidenced by the accumulation of cytoplasmic triglyceride, as evidenced by Oil Red O staining (Fig. 7C) and adipocyte marker (422/aP2) expression (Fig. 7D). Knockdown of expression of LOX with RNAi also prevented the commitment and terminal adipocyte differentiation of mouse embryonic fibroblasts (MEFs) as indicated by the suppressed expression of the adipocyte marker 422/aP2 (Fig. S1) and accumulation of cytoplasmic triglyceride. Further evidence that LOX is a targeted gene of BMP signaling pathway for adipocyte lineage commitment is shown by the fact that overexpression of CA-BMPr1A induced the expression of LOX, whereas overexpression of DN-BMPr1A prevented its induction (Fig. S2). Also, blocking the BMP signaling pathway by knockdown of Smad4 (Fig. 5A) totally prevented the induction of LOX by BMP2 (Fig. 7E), whereas a blockade of p38MAPK signaling pathway by knocking down expression of p38MAPK had only a small effect on LOX expression induced by BMP2 (Fig. 7E). This result is consistent with the finding that BMP/Smad signaling has a dominant role in adipocyte lineage determination (Figs. 5 and 6).
Fig. 7.
Role of LOX in the BMP-induced commitment to the adipocyte lineage. (A) Effect of BMP2 and BMP4 on the induction of LOX expression. C3H10T1/2 stem cells were plated at 30% confluence and after 24 h were treated with 50 ng/mL of BMP2 or BMP4 until postconfluence. (B) Expression of LOX was assessed by immunoblotting. C3H10T1/2 cells were plated as above and transfected with LOX Stealth RNAi. (C and D) After 24 h, cells were treated with BMP2 (50 ng/mL) until postconfluent, at which time the expression of LOX was measured and the effect on adipocyte commitment and terminal differentiation was assessed both by staining of cytoplasmic triglyceride (C) and expression of an adipocyte-specific marker (422/aP2) (D). (E) Evidence that LOX is a downstream target gene of BMP signaling pathway. C3H10T1/2 stem cells were plated at 30% confluence, transfected with Smad4 or p38MAPK RNAi 24 h later, cultured without or with BMP until postconfluent, cell extracts were prepared, and the effect on Smad4, p38MAPK and LOX expression was assessed by immunoblotting.
Discussion
Adipocyte size and number increase with adiposity. The rise in adipocyte number results from recruitment of undifferentiated MSCs in the vascular stroma of adipose tissue. In other tissue contexts, these pluripotent MSCs have the potential to develop into osteocytes, myocytes, or chondrocytes (5, 21–23). During adipogenesis, development appears to occur in 2 stages, i.e., commitment of MSC stem cells to produce preadipocytes followed by differentiation of preadipocytes to produce adipocytes (6). Although both processes are complex, many steps in the differentiation phase have been characterized using 3T3-L1 preadipocytes in cell culture as a model system (6). When induced to differentiate growth-arrested 3T3-L1, preadipocytes synchronously undergo multistep mitotic clonal expansion and DNA replication, cell cycle arrest, and finally, expression of proteins/enzymes that produce the adipocyte phenotype (6). Far less is known about the steps by which MSC stem cells become preadipocytes. Our previous investigations indicate that the C3H10T1/2 cell line is a faithful model of MSC stem cell commitment both ex vivo and in vivo (14, 15). These cells can be induced to undergo commitment in cell culture (15) and when implanted s.c. into athymic mice, conditions under which they give rise to tissue that is indistinguishable from endogenous adipose tissue (15).
BMPs are members of the TGF-β superfamily that exert pleiotropic effects on cells during development including embryogenesis, organogenesis, and morphogenesis (24). Previous reports from this (14, 15) and other laboratories (13, 25, 26) have provided compelling evidence for the participation of BMPs, notably BMP4, in the commitment to the adipocyte lineage. Exposure of proliferating C3H10T1/2 stem cells to BMP4 induces commitment into preadipocytes, which acquire adipocyte characteristics when treated with differentiation inducers (15). Although BMP2, a homolog of BMP4, has been reported to promote commitment into osteoblasts, chondroblasts, and preadipocytes, its effects on adipocyte lineage commitment are controversial (25–31). One report indicates that BMP2 can induce stem cells to commit to chondroblast, osteoblast, or adipogenic fates dependent on supplementation with cofactors (31). Thus, in combination with TGF-β1, insulin, and ascorbic acid, BMP2 can produce the chondrocytic phenotype (31), or with rosiglitazone, a PPARγ agonist, BMP2 can induce stimulate adipogenic commitment/differentiation of MSCs (30). In contrast, BMP2 and retinoic acid repress adipogenesis and promote osteoblast differentiation (29). In the present report, we show evidence that BMP2, like BMP4, can induce commitment of C3H10T1/2 stem cells to the adipocyte lineage, although at a slightly higher concentration (Fig. 1).
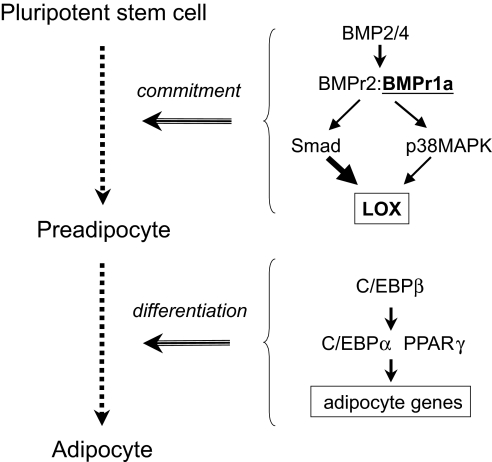
We show that BMP2/4 activates the expression and phosphorylation of downstream signaling intermediates of the BMP signaling pathway to initiate adipogenic commitment. Our findings demonstrate that: (i) BMPr1A and BMPr2 are expressed by naive C3H10T1/2 stem cells (Fig. 2); (ii) overexpression of CA-BMPr1A induces the adipocyte lineage commitment in the absence of exogenous BMP (Fig. 3), whereas overexpression of DN-BMPr1A disrupts commitment (Fig. 4); (iii) blocking Smad signaling by knockdown of expression of Smad4 prevented commitment of C3H10T1/2 stem cells to the adipocyte lineage induced by BMP2/4 (Fig. 5), whereas knocking down expression of p38MAPK only partially prevented commitment (Fig. 6); and (iv) proteomic analysis identified LOX as a downstream protein target whose expression is induced by BMP (Fig. 7). LOX is a copper-containing amine oxidase that catalyzes covalent cross-linking of fibrillar collagens and elastin at peptidyl lysine residues in extracellular matrix formation. Our studies verify that expression of LOX is induced in stem cells treated by BMP2/4 (Fig. 7A), Also, knockdown of LOX expression prevents adipocyte commitment and differentiation of MEFs (Fig. S1), as well as C3H10T1/2 stem cells treated with BMP2/4. A model is proposed illustrating how BMP and LOX are involved in adipocyte lineage commitment and terminal differentiation (Scheme 1). The induction of LOX expression is consistent with microarray analysis by Urs et al. (32), who showed that expression of LOX is substantially higher in human preadipocytes compared with adipocytes. These findings implicate LOX as an early marker of the adipogenic commitment process.
Scheme 1.
Adipocyte lineage commitment and terminal differentiation.
Materials and Methods
Cell Culture, Induction of Commitment/Differentiation, and Oil Red O Staining.
Commitment was induced with BMP2 or BMP4 and cells stained as described (15). There are small discrepancies in the levels expression of aP2 and autodifferentiation between control 10T1/2 cells treated with MDI (as illustrated in Figs. 1D, 3D, and 4D). Our experience over many years indicates that these small discrepancies result from epigenetic drift with increasing passage number; as passage number increases, a small fraction (2–5%) of the cells acquire the capacity to autodifferentiate spontaneously. This phenomenon also depends on the serum source. Although we carefully test and select our serum sources to minimize this effect, some variability persists.
Differentially Expressed Genes Induced by BMP During Commitment.
C3H10T1/2 cells were cultured in DMEM containing 10% calf serum without or with purified recombinant BMP2 (50 ng/mL) or BMP4 (10 ng/mL). At postconfluency, cell extracts in lysis buffer [9 M urea/2% CHAPS/0.5% DTT/014% PMSF and 2% IPG buffer (pH3–10)], 2-DE analysis was performed (33), and gels stained with CBB. Stained gels were scanned and analyzed with ImageMaster software (Amersham Pharmacia). By comparing the BMP treated and untreated samples, protein spots were selected, excised, trypsin digested, and measured with ABI 4700 MALDI TOF MS. Data were searched with MASCOT (Matrix Science) against National Center for Biotechnology Information nonredundant protein sequence database, and the automatic data analysis and data searching were performed by the GPS Explore software. Eight genes were found to be up-regulated by BMP2 and 28 genes by BMP4, whereas 5 genes were up-regulated by both BMP2/4. LOX was the most prominent gene required during the adipocyte lineage commitment.
Construction of Expression Plasmids and Generation of Retrovirus.
Plasmids containing CA-BMPr1A (CA-BMPr1A-pCMV5, ALK3, Q233D), CA-BMPr1B (CA-BMPr1B-pCMV5, ALK6, Q203D), or DN-BMPr1A (DN-BMPr1A-pCDNA3, CFK-23a) were provided by Linzhao Cheng (Johns Hopkins University). The cDNAs were generated by PCR using the following primers.
Ca-BMPr1A: (i) 5′- CGAAGATCTATGCCTCAGCTATACATTTACAT-3′ and (ii) 5′- CGACTCGAGTCAGATTTTTACATCTTGGGAT-3′; Ca-BMPr1B: (i) 5′- CGAAGATCTATGCTCTTACGAAGCTCTGGA-3′ and (ii) 5′-CGACTCGAGTCAGAGTTTAATGTCCTGGGA-3′; DN-BMPr1A: (i) 5′-CGAAGATCTACGTGCGAATTGGACAATGA-3′ and (ii) 5′-CGACTCGAGTTAAGCGTAGTCTGGGACGTCG-3′.
The PCR products were cloned into MSCV retroviral vectors with BglII and XhoI and added to the C3H10T1/2 stem cells with polybrene at a final concentration of 8 μg/mL as described (34).
Immunoblotting and RT-PCR.
Immunoblotting and RT-PCR were performed as described (34). Primers for detecting endogenous types 1A, 1B, and 2 BMP receptors were as follows.
BMPr1A: (i) 5′-GCGAACTATTGCCAAACAG-3′ and (ii) 5′-GAGGTGGCACAGACCACAAG-3′; BMPr1B: (i) 5′-GACACTCCCATTCCTCATC-3′ and (ii) 5′-GCTATTGTCCTTTGGACCAG-3′; BMPr2: (i) 5′-AATCAAGAACGGCTGTGTGCA-3′ and (ii) 5′-CATGCTGTGAAGACCCTGTTT-3′.
Primers for CA type 1A and 1B BMP and DN BMP receptor (downstream primer of CA-BMPr1A and DN-BMPr1A contained part of vector sequence) were as follows.
CA-BMPr1A: (i) 5′-ATCGGTGGAACAGTGATG-3′ and (ii) 5′-GGCTTCGGCCAGTAA-3′; CA-BMPr1B: (i) 5′-TCTGGAATGCCTGTTGTC-3′ and (ii) 5′-TGGGGTGGAGGTCTTTA-3′; DN-BMPr1A : (i) 5′-GCAAGGATTCACCAAAAGC-3′ and (ii) 5′-TTAAGCGTAGTCTGGGACG-3′; β-actin: (i) 5′-TTCGCGGGCGACGATGCT-3′ and (ii) 5′-CGGTTGGCCTTAGGGTTCA-3′.
RNA Interference.
Synthetic siRNA oligonucleotides specific for regions in the Smad4 and p38 MAPK mRNA were designed and synthesized by Invitrogen Stealth RNAi. The silencing effects of several siRNA oligonucleotides were screened and tested initially for their ability to knockdown expression of Smad4 or p38 MAPK by immunoblotting. The sequences for successful RNAi knockdown were: RNAi-1: CAUACACACCUAAUUUGCCUCACCA and RNAi-2: CACCUGGAAUUGAUCUCUCAGGAUU for Smad4; CCUUUGAAAGCAGGGACCUUCUCAU for p38 MAPK; GCGGAUGUCAGAGACUAUGACCACA for LOX RNAi. Stealth siRNA Negative Control Duplexes with a similar GC content are used as a controls. C3H10T1/2 stem cells were transfected at 30–50% confluence with siRNA oligonucleotides by using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions.
Supplementary Material
Acknowledgments.
Q.-Q.T. was supported by National Key Basic Research Project Grant 2006CB943704, National Natural Science Foundation for Distinguished Scholars Grant 30625015, Program for Outstanding Medical Academic Leader Grant B-LJ06032, and Shanghai Key Science and Technology Research Project 08dj1400603; H.H. was supported by National Natural Science Foundation Grant 30700403; Shanghai Leading Academic Discipline Project B110; and M.D.L. was supported by National Institute of Diabetes and Digestive and Kidney Diseases Research Grant DK38418.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906266106/DCSupplemental.
References
- 1.Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5:299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd PR, et al. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- 3.Yu ZK, Wright JT, Hausman GJ. Preadipocyte recruitment in stromal vascular cultures after depletion of committed preadipocytes by immunocytotoxicity. Obes Res. 1997;5:9–15. doi: 10.1002/j.1550-8528.1997.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 4.Tang Q-Q, Otto TC, Lane MD. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc Natl Acad Sci USA. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young He, et al. Mesenchymal stem cells reside within the connective tissues of many organs. Dev Dynam. 1995;202:137–144. doi: 10.1002/aja.1002020205. [DOI] [PubMed] [Google Scholar]
- 6.Otto TC, Lane MD. Adipose development: From stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005;40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- 7.Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev. 1995;5:571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 8.Tschop M, Statnick MA, Suter TM, Heiman ML. GH-releasing peptide-2 increases fat mass in mice lacking NPY: Indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology. 2002;143:558–568. doi: 10.1210/endo.143.2.8633. [DOI] [PubMed] [Google Scholar]
- 9.Reznikoff KA, Brankow DW, Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973;33:3231–3238. [PubMed] [Google Scholar]
- 10.Pinney D, Emerson C., Jr 10T12/cells: An in vitro model for molecular genetic analysis of mesodermal determination and differentiation. Environ Health Persp. 1989;80:221–227. doi: 10.1289/ehp.8980221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor SM, Jones PA. Multiple new phentypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979;17:771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- 12.Konieczny SF, Emerson CP. 5-Azacytidine induction of stable mesodermal stem cell lineages from 10T1/2 cells: Evidence for regulatory genes controlling determination. Cell. 1984;38:791–800. doi: 10.1016/0092-8674(84)90274-5. [DOI] [PubMed] [Google Scholar]
- 13.Butterwith SC, Wilkie RS, Clinton M. Treatment of pluripotential C3H 10T1/2 fibroblasts with bone morphogenetic protein-4 induces adipocyte commitment. Biochem Soc Trans. 1996;24:163S. doi: 10.1042/bst024163s. [DOI] [PubMed] [Google Scholar]
- 14.Bowers RR, Kim JW, Otto TC, Lane MD. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: Role of the BMP-4 gene. Proc Natl Acad Sci USA. 2006;103:13022–13027. doi: 10.1073/pnas.0605789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA. 2004;101:9607–9611. doi: 10.1073/pnas.0403100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldin CH, Miyazono K, ten-Dijke P. TFG-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 17.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang QQ, Zhang JW, Daniel Lane M. Sequential gene promoter interactions of C/EBPbeta, C/EBPalpha, and PPARgamma during adipogenesis. Biochem Biophys Res Commun. 2004;319:235–239. doi: 10.1016/j.bbrc.2004.04.176. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita H, ten Dijke P, Franzen P, Miyazono K, Heldin CH. Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-beta. J Biol Chem. 1994;269:20172–20178. [PubMed] [Google Scholar]
- 20.Ruberte E, Marty T, Nellen D, Affolter M, Basler K. An absolute requirement for both the type II and type I receptors, punt and thick veins, for dpp signaling in vivo. Cell. 1995;80:889–897. doi: 10.1016/0092-8674(95)90292-9. [DOI] [PubMed] [Google Scholar]
- 21.Caplan A. Mesenchymal Stem Cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 22.Caplan A. The mesengenic process. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 23.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 24.Hogan B. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- 25.Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9:57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- 26.Ahrens M, et al. Expression of human bone morphogenetic proteins-2 or −4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993;12:871–880. doi: 10.1089/dna.1993.12.871. [DOI] [PubMed] [Google Scholar]
- 27.Date T, Doiguchi Y, Nobuta M, Shindo H. Bone morphogenetic protein-2 induces differentiation of multipotent C3H10T1/2 cells into osteoblasts, chondrocytes, and adipocytes in vivo and in vitro. J Orthop Sci. 2004;9:503–508. doi: 10.1007/s00776-004-0815-2. [DOI] [PubMed] [Google Scholar]
- 28.Chan GK, et al. Parathyroid hormone-related peptide interacts with bone morphogenetic protein 2 to increase osteoblastogenesis and decrease adipogenesis in pluripotent C3H10T 1/2 mesenchymal cells. Endocrinology. 2003;144:5511–5520. doi: 10.1210/en.2003-0273. [DOI] [PubMed] [Google Scholar]
- 29.Skillington J, Choy L, Derynck R. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol. 2002;159:135–146. doi: 10.1083/jcb.200204060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hata K, et al. Differential Roles of Smad1 and p38 Kinase in Regulation of Peroxisome Proliferator-activating Receptor gamma during Bone Morphogenetic Protein 2-induced Adipogenesis. Mol Biol Cell. 2003;14:545–555. doi: 10.1091/mbc.E02-06-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.zur Nieden NI, Kempka G, Rancourt DE, Ahr HJ. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: Effect of cofactors on differentiating lineages. BMC Dev Biol. 2005;5:1. doi: 10.1186/1471-213X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urs S, et al. Gene expression profiling in human preadipocytes and adipocytes by microarray analysis. J Nutr. 2004;134:762–770. doi: 10.1093/jn/134.4.762. [DOI] [PubMed] [Google Scholar]
- 33.Shen H, et al. Expressed proteome analysis of human hepatocellular carcinoma in nude mice (LCI-D20) with high metastasis potential. Proteomics. 2006;6:528–537. doi: 10.1002/pmic.200500232. [DOI] [PubMed] [Google Scholar]
- 34.Wolfgang MJ, et al. Brain-specific carnitine palmitoyl-transferase-1c: Role in CNS fatty acid metabolism, food intake, and body weight. J Neurochem. 2008;105:1550–1559. doi: 10.1111/j.1471-4159.2008.05255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.