Abstract
Toxoplasma gondii, as an obligate intracellular and promiscuous pathogen of mammalian cells, utilizes host sugars for energy and to generate glycoconjugates that are important to its survival and virulence. Here, we report that T. gondii glucose transporter (TgGT1) is proficient in transporting mannose, galactose, and fructose besides glucose, and serves as a major hexose transporter at its plasma membrane. Toxoplasma harbors 3 additional putative sugar transporters (TgST1–3), of which TgST2 is expressed at its surface, whereas TgST1 and TgST3 are intracellular. Surprisingly, TgGT1 and TgST2 are nonessential to the parasite as their ablations inflict only a 30% or no defect in its intracellular growth, respectively. Indeed, Toxoplasma can also tolerate the deletion of both genes while incurring no further growth phenotype. Unlike Δtgst2, the modest impairment in Δtggt1 and Δtggt1/Δtgst2 mutants is because of a minor delay in their intracellular replication, which is a direct consequence of the abolished import of glucose. The Δtggt1 displays an attenuated motility in defined minimal media that is rescued by glutamine. TgGT1-complemented parasites show an entirely restored growth, motility, and sugar import. The lack of exogenous glucose in Δtggt1 culture fails to accentuate its intrinsic growth defect and prompts it to procure glutamine to sustain its metabolism. Unexpectedly, in vivo virulence of Δtggt1 in mice remains unaffected. Taken together, our data demonstrate that glucose is nonessential for T. gondii tachyzoites, underscore glutamine is a complement substrate, and provide a basis for understanding the adaptation of T. gondii to diverse host cells.
Keywords: glucose transport, glutamine metabolism, genetic manipulation
Toxoplasma gondii is an obligate intracellular parasite of the phylum Apicomplexa that causes infections in humans and in animals. Infection is usually asymptomatic in immunocompetent individuals, but it may cause severe complications or even be fatal in immunocompromised people. Unlike other pathogens, Toxoplasma has adapted to replicate in most nucleated cells of vertebrates, regardless of their cellular metabolism (1), and thus displays an exceptional metabolic robustness.
Most apicomplexan parasites reside and replicate in a nonfusogenic parasitophorous vacuole, which protects them from lysosomal degradation and confers a membrane interface with the nutrient-rich host cytosol. Its membrane acts as a molecular sieve, allowing the permeation of soluble host metabolites below 1.4 kDa, such as amino acids, sugars, and nucleotides (2). The hexoses, including glucose, being central to the cellular metabolism, are deemed to be vital for the replication of intracellular parasites. To facilitate the import of glucose, these parasites express one or more sugar permeases (www.membranetransport.org). Plasmodium species harbor sugar transporters mediating the uptake of glucose, and one of these has been validated as a drug target (3, 4). Glucose and its permeases are also indispensable for the survival of kinetoplastid parasites, Leishmania and Trypanosoma species (5).
The tachyzoite stage of T. gondii, responsible for an acute infection, rapidly metabolizes glucose via glycolysis (6). Glycolysis serves as a carbon source for the fatty acid synthesis (7), and has been suggested to be essential for driving parasite motility and host cell invasion (8). The glycosylation of proteins and the biogenesis of lipids in T. gondii are among the other vital processes that use sugars (9). To fulfill these cellular requirements, T. gondii harbors the necessary pathways (www.ToxoDB.org), and a glucose transporter (TgGT1) that has been considered indispensable (8). The prominent metabolic role of glucose and other hexoses, has motivated us to examine the significance of sugar transport in T. gondii.
This study demonstrates that TgGT1, the major hexose transporter at the parasite plasma membrane, is not essential for the in vitro survival and in vivo virulence of T. gondii tachyzoites. The parasite harbors a set of 3 additional putative sugar permeases, only 1 of which resides at its surface. Unexpectedly, T. gondii can tolerate the deletion of its surface transporters and thrives by catabolizing glutamine, despite its impaired access to host-derived glucose.
Results
TgGT1 Can Transport Major Sugars in L. mexicana Null Mutant.
Joët et al. (3) have shown that TgGT1 can facilitate the transport of glucose and fructose in Xenopus oocytes. However, it remains unknown whether TgGT1 can also mediate the import of galactose and mannose, required for glycolipid and glycoprotein synthesis. TgGT1 was tested in the L. mexicana sugar transporter null mutant, Δlmgt, which is defective in importing glucose, mannose, fructose, and galactose (10). TgGT1 restored the ability of Δlmgt cells to take up all 4 hexoses, whereas the control cells (pX63NeoRI) were unable to import sugars (Fig. 1A). All parasites incorporated [3H]adenosine, indicating that all transgenic lines were viable and competent in transporting other substrates. Substrate saturation curves revealed an apparent Km of ≈50 μM for glucose (Fig. 1B). To assess the affinity of TgGT1 toward other hexoses, we tested their ability to inhibit the glucose uptake (Fig. 1C). Glucose inhibited the transport of glucose with a Ki of ≈53 μM that is similar to its Km, and mannose exhibited a 5-times higher Ki of ≈250 μM. In contrast, fructose and galactose did not significantly prevent TgGT1-mediated glucose transport up to 5 mM, but it was reduced by ≈50–70% at 50 mM inhibitors. These results confirm that TgGT1 has high affinity for glucose and mannose, and fructose and galactose are low-affinity ligands.
Fig. 1.
TgGT1 can mediate the import of glucose, mannose, fructose, and galactose in L. mexicana null mutant (Δlmgt). (A) TgGT1-complemented promastigotes were assayed for their ability to transport 100 μM d-[3H]glucose, d-[3H]mannose, d-[14C]fructose, or d-[14C]galactose. The pX63NeoRI vector was used as the control. (B) Substrate saturation curve for d-[3H]glucose. Uptake was determined over a 30-s period for a range of substrate concentrations (0.01–2 mM). (C) Inhibition of 100 μM d-[3H]glucose uptake in the presence of sugar inhibitors (0–50 mM). The Km and Ki were determined by Michaelis–Menten and nonlinear regression analyses.
TgGT1 Is Not Essential to the Survival of T. gondii.
TgGT1 has been considered as the only glucose transporter in T. gondii, and presumed to be indispensable for parasite survival. Our data on the import of major hexoses by TgGT1 consolidate this conjecture. To test it, the TgGT1 gene was deleted by double homologous recombination using the knockout construct pTUB8CAT (Fig. S1A). Following selection of stable transformants, chloramphenicol-resistant clones were analyzed for disruption of the TgGT1 locus (Fig. S1B). The knockout construct-specific PCRs revealed the presence of the CAT resistance cassette adjacent to the 5′- and 3′-UTRs in the genome of the Δtggt1 parasites and in the control plasmid, but not in the parental cells. Similarly, PCRs with 5′ and 3′ recombination-specific primers yielded DNA bands of the expected sizes in TgGT1-ablated parasites, but not in the parental and plasmid samples. Sequencing of the obtained UTRs confirmed the occurrence of the recombination events exactly at the TgGT1 locus. The absence of the TgGT1 transcript in the Δtggt1 (Fig. S1C) indicated its specific deletion with no apparent influence on the mRNAs of TgST1–3. The successful disruption of TgGT1 gene proves its nonessential nature for T. gondii tachyzoites.
T. gondii Tachyzoites Express 3 Additional Novel Sugar Transporters.
To test whether the dispensability of TgGT1 is because of functional redundancy of sugar transport in T. gondii, we searched the Toxoplasma Gene Database (www.ToxoDB.org) for the presence of potential transporters. Three genes, named as TgST1, TgST2, and TgST3 were identified and their full-length ORFs were cloned from tachyzoite mRNAs (Fig. S2A). All of the novel sugar permeases harbor a sugar transport domain (Pfam 00083) of the conserved multifacilitator SLC2A family and typical transmembrane/hydrophobic regions (Fig. S3). TgGT1 is 37.2% and 25% identical to Plasmodium (PfHT1) and human transporters (HsGlut1) (Fig. S2B). TgST1–3 proteins are only ≈20% identical to TgGT1, PfHT1, and HsGlut1, but they show a higher degree of mutual resemblance. TgST1 and TgST2, with 34.4% identity, are most proximal. The sequence divergence of TgST1–3 proteins is also supported by phylogenetic analysis, which revealed their clustering distant from protozoan, plant and mammalian permeases (Fig. S2C). In addition, the N termini of TgST1–3 extend beyond TgGT1 (see Fig. S2A). Although similar to each other, none of these extensions is homologous to a protein with known function. Our attempts to find the substrates for TgST1–3 in Saccharomyces cerevisiae and L. mexicana have met with no success, so far.
TgGT1 and TgST2 Localize to the Surface of T. gondii.
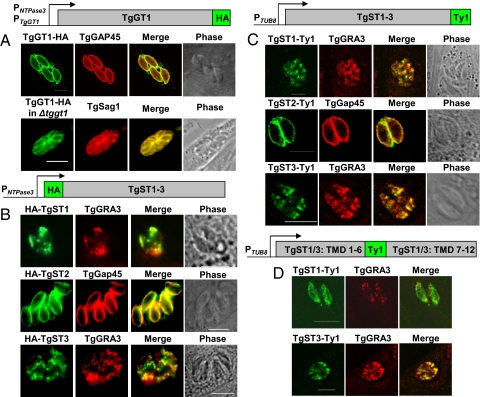
The transport of major hexoses by TgGT1 in L. mexicana mutant and unique features of TgST proteins prompted us to examine their subcellular localization in T. gondii to obtain an insight into their function. Therefore, we performed the ectopic over-expression of their epitope-fusion constructs in intracellular tachyzoites (Fig. 2). TgGT1-HA under the control of the NTPase3 gene promoter localizes in the plasma membrane as confirmed by its colocalization with TgGAP45, a protein adjacent to the inner membrane complex of T. gondii. Its surface targeting in the TgGT1-HA-expressing Δtggt1 strain that is governed by its endogenous promoter corroborates these data (see Fig. 2A). TgGT1 expression appears to be homogeneous, indicating a uniform uptake of hexoses by the parasite. The localization of the myc-TgGT1 resulted in punctuate intracellular mislocalization that suggests the prerequisite of its free N terminus for sorting to the parasite surface (Fig. S4A). Only permeabilized tachyzoites were stained for TgGT1-HA, confirming the cytosolic orientation of its C terminus (Fig. S4B).
Fig. 2.
TgGT1 and TgST2 localize in the plasma membrane of T. gondii, whereas TgST1 and TgST3 reside in intracellular vesicles. (A) The parental or Δtggt1 strains were transfected with TgGT1-HA-pNTP3 and TgGT1-HA-pGT1 constructs, respectively. (B) Transiently transfected parasites expressing N-terminally HA-tagged TgST1, TgST2, or TgST3 in pNTP3 vector. (C) C-terminally Ty1-fused and TUB8-regulated T. gondii sugar permeases in stably transfected parasites. (D) TgST1 and TgST3 were tagged with Ty1 between sixth and seventh transmembrane domains in pTUB8.
N-terminally HA-fused TgSTs were expressed transiently under the control of the NTPase3 promoter (see Fig. 2B). TgST2 co-localizes with TgGAP45 to the periphery of T. gondii. In contrast, TgST1 and TgST3 were only detected within the parasite, and shown to partially localize with a dense granule protein, TgGRA3. To rule out the risk of a mislocalization because of over-expression under a strong promoter, such as pNTPase3 or N-terminal tag as observed for TgGT1, we tested C-terminal Ty1-tagged permeases with the TUB8 promoter in stably transgenic parasites (see Fig. 2C). TgST2-Ty1 is targeted to the parasite surface, and TgST1 and TgST3 were again intracellular. To further assess the puzzling localization of TgST1 and TgST3, we introduced an internal Ty1 tag between their sixth and seventh transmembrane helices (see Fig. 2D). Yet again, TgST1 and TgST3 were present in intracellular compartments. Further microscopic analysis demonstrated that irrespective of the epitope fusion, the localization pattern is comparable in all 3 cases, suggesting that a mislocalization because of tag interference or over-expression is quite improbable. Definitive assessment of their subcellular location will await a confirmation with specific antibodies recognizing the endogenous proteins. These data also confirm that the sorting of TgST2 to the parasite surface does not require its free N and C termini. Both termini of all 4 permeases should project into the parasite cytosol with their putative substrate-binding site facing the parasitophorous vacuole (TgGT1, TgST2) or intracellular organelles (TgST1, TgST3) (Fig. S5).
TgST2 Is Dispensable and Nonredundant with TgGT1 in T. gondii.
To examine whether the surface-associated potential sugar permease TgST2 is vital for T. gondii, we deleted the TgST2 gene. Upon transfection of the RH hxgprt- tachyzoites with p2854 knockout construct, the pool of pyrimethamine-resistant parasites was screened to identify clones, in which the TgST2 had been replaced by the dihydrofolate reductase (DHFR) cassette (Fig. S6A). The presence of 5′- and 3′-UTRs was validated in the Δtgst2 parasites and in the plasmid, but not in the parental strain by using the construct-specific primers (Fig. S6B). Likewise, the 5′ and 3′ recombination-specific primers amplified the expected PCR bands in the Δtgst2 mutant but not in the parental strain and control plasmid. Sequencing of the UTRs and the absence of TgST2 but not of the TgST1, TgST3, and TgGT1 transcripts corroborated the successful ablation of the TgST2 locus (Fig. S6C). As with the TgGT1 gene, disruption of TgST2 confirms its dispensability for the survival and propagation of tachyzoites in tissue culture. However, the localization of both permeases at the parasite surface raised the obvious question of their functional redundancy. Hence, we generated a Δtggt1/Δtgst2 double-deletion mutant by ablating the TgST2 gene in the Δtggt1 strain. Further PCR screening yielded the mutants that had undergone double homologous recombination. The mRNAs of TgGT1 and TgST2 were not detectable in this mutant, whereas the TgST1 and TgST3 transcripts were present (Fig. S6D). The in vitro viability of the Δtggt1/Δtgst2 mutant confirms the collective nonessentiality as well as the nonredundant functions of TgGT1 and TgST2 in T. gondii tachyzoites.
Only TgGT1 Deletion Confers a Growth Defect in T. gondii.
To establish the impact of TgGT1 and TgST2 gene deletions, we evaluated the phenotype of all mutants by plaque assays that recapitulate all of the events of several parasite lytic cycles. Their representative images in Fig. 3A confirm that the plaques formed by the Δtgst2 were similar in size to those of the parental strain. The Δtggt1 and Δtggt1/Δtgst2 mutants, by contrast, showed a reduction in their plaque sizes. The quantitative measurements, shown in Fig. 3B, corroborated these data and established a 30% growth defect in the Δtggt1. Notably, the growth of the Δtggt1/Δtgst2 strain was similar to the Δtggt1, and no apparent accentuated defect was observed. This impairment was entirely restored in the TgGT1-HA-harboring Δtggt1 strain. To examine whether the reduced plaques of the Δtggt1 were because of a decrease in its motility, we performed motility assays in buffers mimicking the culture conditions. No reduction was observed in motility of the mutant when compared to the parental strain (Fig. S7).
Fig. 3.
The Δtggt1 but not Δtgst2 strain of T. gondii demonstrates a protracted in vitro growth. (A) Representative images of the parental, Δtggt1, Δtgst2, and Δtggt1/Δtgst2 strains as generated by plaque assays. (B) The images were digitized and plaques were manually encircled to calculate the area of 80 plaques formed by the individual strains by using the ImageJ program. (C) Replication rates of T. gondii tachyzoites in human foreskin fibroblasts
Although the plaque assay is a good indicator of the overall fitness of T. gondii, it is not suitable to assess selective defects during its intracellular replication. Therefore, in vitro replication assays were performed to estimate the intracellular replication rate (see Figs. 3C and 4B). The Δtgst2 mutant exhibited a doubling time of ≈8.8 h, and thus demonstrated no significant delay of replication when compared to its parental strain (≈8.6 h). The Δtggt1 and Δtggt1/Δtgst2 mutants with comparable rates of ≈9.8 h and ≈9.5 h, however, divide ≈10 to 12% slower than the control parasites. This defect was entirely restored in the TgGT1-complemented Δtggt1 strain, exhibiting a doubling rate of ≈8.4 h that is similar to the parental strain. Taken together, whereas TgGT1 contributes to—but is not essential for—the in vitro growth of tachyzoites, TgST2 is absolutely expendable. It can also be concluded that the collective deletion of both surface transporters does not exert a synthetic lethal or cumulative growth phenotype.
Fig. 4.
The Δtggt1 but not Δtgst2 strain of T. gondii is compromised in using host-derived glucose and exhibits a delayed replication. (A) Glucose utilization assays were performed with parasite-infected human foreskin fibroblasts (HFF) monolayers (multiplicity of infection = 4) at 24-h postinfection. (B) HFFs were infected for 40 h in glucose-free or normal media, and the inoculum-normalized parasite yield was calculated.
The Δtggt1 but Not Δtgst2 Mutants Display Attenuated Glucose Metabolism.
To investigate whether the replication defect in the Δtggt1 and Δtggt1/Δtgst2 strains is a result of attenuated import of host-derived glucose, we measured the utilization of sugar by intracellular parasites. These results (Fig. 4A) demonstrated ≈80% reduction in labeling of the Δtggt1 when incubated with [14C]glucose, which was restored in the TgGT1-HA-complemented Δtggt1 strain, confirming the specificity of the phenomenon. The ablation of TgST2 did not exert any measurable decrease in glucose import, whereas the Δtggt1/Δtgst2 cells exhibited a comparable diminution in its labeling as observed for Δtggt1. To deduce the origin of residual labeling in Δtggt1, we used a 20-fold excess of 2-deoxy-d-glucose (2-DOG) to inhibit the glucose metabolism in host cells. 2-DOG is also known to affect the glucose transport by TgGT1; however, more than 3,000-fold excess of inhibitor over glucose has been applied to abolish the process (3). These assays resulted in a complete loss of metabolic counts in the Δtggt1 parasites that is consistent with the import of other glucose-derived metabolites from the host cell. Collectively, these data reveal that the hampered growth of Δtggt1 strain is a direct consequence of attenuated uptake of host glucose, and TgST2 does not participate in glucose transport.
In Vitro Survival of T. gondii Does Not Require Host-Derived Glucose.
The modest contribution of host glucose for the replication of Δtggt1 mutants motivated us to test whether the parasite can tolerate a lack of glucose in culture media. All strains were able to replicate under the conditions where glucose was omitted in the medium a day before infection. In glucose-free culture, Δtgst2 replicates at rates that are similar to the Δtggt1 and Δtggt1/Δtgst2 strains in glucose media and parental strain in nonglucose media (see Fig. 3C and 4B). Together with our data on parasite glucose import, these results imply that tachyzoites can tolerate a substantial depletion of glucose in the host cytosol. More importantly, the absence of host-derived glucose failed to further delay the replication of the Δtggt1 and Δtggt1/Δtgst2, confirming the aforesaid notion. In accordance, akin to the parental tachyzoites, the Δtggt1-TgGT1 strain regained its modest dependence on exogenous glucose. The dispensable nature of glucose for these mutants implies the dependence of tachyzoites on alternative host-derived nutrients.
The Δtggt1 Mutant Utilizes Glutamine as a Major Alternative Nutrient.
Glycolysis has been shown to drive parasite motility (8); hence, we implemented this assay in a defined media to deduce the source of alternative nutrients for the Δtggt1 mutant (Fig. 5). As shown in Fig. 5A, the number of mutants forming a secretory trail as well as its length (detected by anti-Sag1 antibody) in the absence of glucose is much lower compared to the parental strain. The fraction of motile Δtggt1 parasites is below 10% in salt buffers and was not influenced by external glucose, indicating Δtggt1 is unable to use the sugar (see Fig. 5B). As predicted, the TgGT1-complemented mutant displayed normal motility and its dependence on external glucose. Next to the sugar, glutamine and pyruvate are the most abundant carbon sources in culture media, and the former has been shown to serve as the major nutrient in transformed or cancer cells (11, 12). Notably, the motility of the mutant is completely rescued in minimal media containing glutamine but not when the media is supplemented with pyruvate. Intracellular Δtggt1 parasites incorporated 60% more glutamine than control strain, a phenomenon that was reversed in the complemented strain (see Fig. 5C), indicating glutamine acting as an alternative nutrient. Collectively, these results confirm that glutamine acts as a supplement bioenergetic substrate in T. gondii, and reveal that glucose contributes to, but is not essential, to empower the parasite motility.
Fig. 5.
Glutamine fulfills the metabolic needs of the Δtggt1 mutant. Freshly harvested tachyzoites were used to perform the motility assays. (A) Representative images of the parental and Δtggt1 strains in Hanks's balanced salt solution supplemented with the indicated reagents. (B) Quantitative diagram of the motile fraction in three independent experiments. (C) Glutamine (0.5 μCi/mmol of [3H]glutamine) incorporation assays were executed with parasite-infected HFF monolayers (multipliciy of infection = 4) at 24 h postinfection.
In Vivo Virulence of T. gondii Does Not Depend on Glucose Import.
Because the Δtggt1 mutant cannot use external glucose and display a 30% growth defect together with a markedly reduced motility in minimal media, we conducted in vivo assays to test its virulence in BALB/c mice (Fig. S8). All mice infected with 50 wild-type or mutant tachyzoites exhibited severe defect at day 8 and were killed. From the group infected with 500 parasites of the mutant, 1 mouse had to be killed at day 7, whereas all of the other animals were killed at day 8 because of their physical impairments. Briefly, no difference in virulence of the Δtggt1 mutant was observed in comparison to the RH strain. These data are rather unexpected in light of the aforementioned facts, and that sugars are used to produce key virulence molecules in T. gondii.
Discussion
Toxoplasma as an obligate intracellular pathogen procures vital sugars from the host cell to satisfy its metabolic needs. In this article, we demonstrate TgGT1 as the only surface-resident glucose transporter in T. gondii, and its deletion abolishes the sugar import to the parasite interior. Impaired utilization of host-derived glucose by Δtggt1 mutants in standard media slows T. gondii replication to a degree comparable to the effect inflicted by the absence of external glucose on parental and the Δtgst2 strains. If sugar is indeed essential for T. gondii, then the depletion of exogenous glucose should severely compromise or abolish the growth of parental and mutant parasites. Our results do reveal the contribution of glucose to the parasite growth, albeit modest. Minor influence of the lack of glucose on the parental and Δtgst2 strains can be anticipated, as these parasites may fulfill their metabolic needs by TgGT1-mediated uptake of residual sugar from glucose-depleted host cytosol that might sustain their growth. However, the Δtggt1 strain is already impaired in glucose import, and subsequent glucose exclusion virtually abolishes the parasite's access to host glucose. The sustained existence of the Δtggt1 mutant, despite this sugar deficiency, strongly argues for the expendable nature of glucose for its in vitro culture. The limited requirement for glucose in T. gondii may confer it the ability to replicate in most cell types, regardless of their glucose levels.
The Δtgst2 strain still acquires the normal amounts of host-derived glucose, indicating that TgST2 does not partake in glucose import and cannot functionally replace TgGT1 in intracellular tachyzoites. Its nonredundant nature is validated further by collective deletion of TgST2 and TgGT1. Our futile attempts to find any apparent growth defect in the Δtgst2 also reflect its absolute nonessential function in tachyzoites. The presence of TgST1 and TgST3 isoforms in distinct intracellular compartments implies their functional specialization. It is noteworthy that in some organisms, such as in S. cerevisiae, glucose transporter-like proteins have been shown to participate in environmental sensing (13). Hence, one plausible explanation is that TgST1 and TgST3 function as intracellular sugar sensors. Whether the deletions of their genes will influence the parasite phenotype is being investigated.
It has been suggested that glycolysis powers the parasite motility (8), and that impairment in gliding motility is associated with the absence of plaque formation (14). Our results on the Δtggt1 mutant are in agreement with the former report as the fraction of motile tachyzoites is substantially reduced in defined minimal media irrespective of the presence of glucose. However, motility of the Δtggt1 is not affected in nutrient-rich media, and it is rescued in the buffers containing glutamine. These results advocate the existence of compensatory mechanisms in T. gondii, such as glutaminolysis and gluconeogenesis that can maintain the energy and carbon requirements in the absence of TgGT1. In fact, glutamine has been shown to be the key nutrient that fulfills the most biosynthetic requirements in transformed and cancer cells (11, 12). The rapid multiplication of T. gondii and tumor cells imposes a high metabolic burden, indicating their analogous biosynthetic needs that are apparently not dictated by glucose. Indeed, the absence of glutamine, but not of glucose, in the Δtggt1 culture is detrimental to its growth (data not shown). Furthermore, the genomic annotations confirm the presence of glutamine catabolism and of gluconeogenesis in T. gondii that should result in utilization of substrates, such as amino acids, pyruvate, lactate, and glycerol (15). In fact, the Δtggt1 mutant displayed a 2- to 4-fold induction of its glutamine aminotransferase and glutamate dehydrogenase transcripts (data not shown). Based on our preliminary data, we assume that glutamine can feed into the tricarboxylic acid cycle and gluconeogenesis and acts as a bioenergetic substrate to T. gondii. In addition, there are also annotations for the syntheses and import of sugar-phosphates that should sustain lipid and protein glycosylation. Glycosylated proteins and lipids contribute to the virulence in T. gondii and to the host immunity (16, 17). It is rather unanticipated and salient that the in vivo virulence of the Δtggt1 mutant in mice remains unaltered, and it is probable that as observed in cancer cells, glutamine can compensate for all parasitic requirements. It remains to be seen if other nutrients can also contribute to the cellular needs of T. gondii. The deployment of motility assays using the Δtggt1 and its derivative mutants will facilitate the search of auxiliary nutrients. Whether the Δtgst2 and/or Δtggt1/Δtgst2 mutants display an altered in vivo immunogenic potential would also be a topic of future research. All experiments undertaken in the present work were performed on the tachyzoite stage. It will be interesting to determine whether glucose is essential for other stages of T. gondii, including bradyzoites.
Taken together, our data confirm that TgGT1 protein is expendable in T. gondii, despite it being the major hexose transporter at the parasite plasma membrane. TgGT1 is also not functionally redundant with other surface-localized sugar permease present in T. gondii. The genetic disruption of TgGT1 abolishes the import of host-derived glucose by T. gondii, which resorts to glutamine catabolism to sustain its cellular requirements. The Δtggt1 strain, with its restricted access to external glucose, underlines the metabolic robustness of T. gondii and provides an excellent model to dissect the intricate redundancies of nutrient acquisition by T. gondii as an obligate intracellular pathogen of diverse host cells.
Materials and Methods
Chemical and Biological Resources.
Cell culture chemicals and radioactive sugars were procured from Biotherm and Hartmann Analytic. Other reagents, including secondary antibodies, and mouse anti-HA antibody were purchased from Invitrogen and Sigma, respectively. Anti-TgGRA3, DG52 anti-TgSag1, and anti-Ty1 antibodies were kindly provided by J.-F. Dubremetz (University of Montpellier, France), J. Boothroyd (Stanford University School of Medicine), and K. Gull (University of Oxford, U.K.), respectively.
Cell Culture and Cloning of Sugar Transporters.
All strains of T. gondii tachyzoites and the derived mutants were maintained in confluent monolayer cultures of the HFF, as described previously (18). Host cells were cultured and infected in DMEM supplemented with 10% FCS, 2 mM glutamine, 1× MEM nonessential amino acids, 1 mM sodium pyruvate, 100 units/mL penicillin, and 100 μg/mL streptomycin (37 °C, 10% CO2). Tachyzoites (108) were subjected to mRNA extraction or first-strand cDNA synthesis using μMACS mRNA isolation and cDNA synthesis kits (Miltenyi Biotec). The mRNA or cDNA pool was used to generate the cDNAs of TgST1–3 and TgGT1 by Pfu-Ultra FusionII Polymerase (Stratagene) and cDNA-specific primers (Table S1).
Generation of Immunolocalization Constructs.
The N- or C-terminus epitope-tagging of all sugar permeases was performed using the indicated primers (see Table S1). The N terminus HA-fusion of TgST1–3 in pNTP3 and of TgGT1 in pTetO7Sag1 vector was executed with their respective F2/R2 primers. Likewise, the COOH-fusion of Ty1-epitope in TgST1–3 in pTUB8 and of TgGT1 in pNTP3 plasmid was performed using F3/R3 primers. Internal tagging of Ty1 epitope between the sixth and seventh transmembrane helices of TgST1 and TgST3 in pTUB8 was done by 2 independent cloning for each cDNA by consecutive use of F3/R3.1 and F4/R4 primer sets.
Knockout Constructs and Mutant Isolation.
The TgGT1 knockout construct contained 2.7 kb of the 5′-UTR and 3′-UTR of TgGT1 gene flanking the CAT ORF in pTUB8CAT vector (Table S2). To prepare the TgST2 deletion construct in p2854 vector harboring the DHFR expression cassette, 2.8 kb of its 5′- and 3′-UTRs were cloned (Table S2). The RH hxgprt− tachyzoites (107) were transfected with 50 μg of the plasmid and selected for the chloramphenicol (10 μg/mL) or pyrimethamine (1 μM)-resistant parasites as described elsewhere (19, 20). Individual clones were subjected to knockout screening.
Functional Expression of Parasite Transporters in L. mexicana.
L. mexicana null mutant, Δlmgt (10), was transformed with all ORFs in pX63NeoRI plasmid, and maintained in RPMI medium 1640 (pH 7.2) containing the drug G418 (100 μg/mL). The transfectants were examined for the import of 100 μM [3H]glucose, [3H]mannose, [14C]fructose, and [14C]galactose by an oil-stop method (21). To determine the Ki, inhibition curves were generated with 100 μM glucose by using 0–50 mM of each sugar as the inhibitor.
Glucose and Glutamine Uptake Assays.
Glucose and glutamine uptake assays were performed to examine the ability of the parasite mutants to import host-derived glucose or glutamine during their intracellular propagation in HFF (multipliciy of infection = 4, 24 h postinfection). All samples were washed 2 times with 4 mL of glucose-free DMEM before their labeling with 1 mL of [14C]glucose (0.2 μCi/mmol, 5 mM) or [3H]glutamine (0.5 μCi/mmol 2 mM) in DMEM for 2 h at 37 °C. To isolate the labeled tachyzoites, the samples were washed 4 times with DMEM-glucose (25 mM) and treated (10 min, 37 °C) with 1.5 mL of the egression buffer (10 μM A23187, 25 mM glucose in Hanks's balanced salt solution). Ionophore-egressed tachyzoites were collected (450 × g for15 min), washed once, and subjected to scintillation and cell counting.
Plaque and Replication Assays.
The parasite plaque assay recapitulates all of the events of parasite lytic cycle, including host cell invasion, intracellular growth, and egression followed by spreading by gliding motility (22). The HFF cells in 6-well plates were infected with 2,000 tachyzoites, cultured for 7 days, fixed with −80 °C methanol, stained with crystal violet, and scanned. The encircled area of each plaque was measured and the means of 80 plaques was calculated for subsequent data plots. For replication assays, infected HFFs (multipliciy of infection = 1.5) were cultured for 40 h, and then the parasites were egressed for 10 min by 10 μM A23187 in HBSS. The number of egressed parasites was normalized to the number of invasive parasites, deduced by complementary plaque assays.
Indirect Immunofluorescence Assay.
The ORF-transfected tachyzoites were subjected to immunofluorescence assay as reported previously (23). Briefly, the infected HFF cells were fixed with 4% paraformaldehyde (PFA) or 4% PFA plus 0.05% glutaraldehyde for 10 min or with −80 °C methanol for 2 min followed by neutralization in 100 mM glycine/PBS. The samples were permeabilized for 20 min in 0.2% Triton-X100/PBS, preblocked with 2% BSA in 0.2% Triton X-100/PBS, and stained with antibodies (anti-TgGAP45 at 1:3,000 dilution; anti-TgGra3 at 1:500; anti-HA at 1:1,500; anti-Ty1 at 1:50). After 3 washes with 0.2% Triton X-100/PBS, Alexa488, or Alexa568 antibodies (1:3,000 dilutions) were applied. The samples were washed 3 times before mounting with Fluoromount G.
Motility Assay.
Tachyzoites were syringe-released from the HFF monolayers (30–36 h postinfection) and preincubated on a coverslip for 10 min at room temperature in serum-free Hanks's balanced salt solution supplemented with indicated reagents. The samples were incubated (15 min, 37 °C) and fixed with 4% paraformaldehyde/0.05% glutaraldehyde and stained with mouse monoclonal anti-TgSag1 (1:1,500) and Alexa488 (1:3,000) antibodies. Images were recorded, and at least, 800 well-isolated parasites with visible trails (>3 μm) were counted in 5 random fields to calculate the motile fraction.
Supplementary Material
Acknowledgments.
We thank Paco Pino for animal experiments and Frank Seeber for his scientific suggestions and manuscript appraisal. This work was supported by GRK1121 and SFB618 grants from the German Research Foundation (to R.L. and N.G.), the Helmholtz Foundation PhD fellowship (to M.B.) in the laboratory of N.G., and the European Molecular Biology Organization funding (to M.B.) for a 3-month research in the laboratory of D.S.-F. (COST 857-Project C05.0142). D.S.-F. and T.F.'s contributions were supported by SystemsX (LipidX).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The protein sequences reported in this paper have been deposited in the NCBI GenBank database, www.ncbi.nim.nih.gov (accession nos. TgST1, EF198053; TgST2, EF427938; TgST3, EF427939).
This article contains supporting information online at www.pnas.org/cgi/content/full/0903831106/DCSupplemental.
References
- 1.Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607–623. doi: 10.1128/mmbr.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin AM, Liu T, Lynn BC, Sinai AP. The Toxoplasma gondii parasitophorous vacuole membrane: transactions across the border. J Eukaryot Microbiol. 2007;54:25–28. doi: 10.1111/j.1550-7408.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- 3.Joët T, et al. Comparative characterization of hexose transporters of Plasmodium knowlesi, Plasmodium yoelii and Toxoplasma gondii highlights functional differences within the apicomplexan family. Biochem J. 2002;368(Pt 3):923–929. doi: 10.1042/BJ20021189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joët T, et al. Validation of the hexose transporter of Plasmodium falciparum as a novel drug target. Proc Natl Acad Sci USA. 2003;100:7476–7479. doi: 10.1073/pnas.1330865100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landfear SM. Drugs and transporters in kinetoplastid protozoa. Adv Exp Med Biol. 2008;625:22–32. doi: 10.1007/978-0-387-77570-8_3. [DOI] [PubMed] [Google Scholar]
- 6.Ohsaka A, Yoshikawa K, Hagiwara T. 1H-NMR spectroscopic study of aerobic glucose metabolism in Toxoplasma gondii harvested from the peritoneal exudate of experimentally infected mice. Physiol Chem Phys. 1982;14:381–384. [PubMed] [Google Scholar]
- 7.Fleige T, Fischer K, Ferguson DJP, Gross U, Bohne W. Carbohydrate metabolism in the Toxoplasma gondii apicoplast: localization of three glycolytic isoenzymes, the single pyruvate dehydrogenase complex, and a plastid phosphate translocator. Eukaryot Cell. 2007;6:984–996. doi: 10.1128/EC.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomel S, Luk FC, Beckers CJM. Host cell egress and invasion induce marked relocations of glycolytic enzymes in Toxoplasma gondii tachyzoites. PLoS Pathog. 2008;4(10):e1000188. doi: 10.1371/journal.ppat.1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonda S, Hehl AB. Lipid biology of Apicomplexa: perspectives for new drug targets, particularly for Toxoplasma gondii. Trends Parasitol. 2006;22:41–47. doi: 10.1016/j.pt.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Burchmore RJS, et al. Genetic characterization of glucose transporter function in Leishmania mexicana. Proc Natl Acad Sci USA. 2003;100:3901–3906. doi: 10.1073/pnas.0630165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- 12.DeBerardinis RJ, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Özcan S, Dover J, Rosenwald AG, Wölfl S, Johnston M. Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc Natl Acad Sci USA. 1996;93:12428–12432. doi: 10.1073/pnas.93.22.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meissner M, Schluter D, Soldati-Favre D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–840. doi: 10.1126/science.1074553. [DOI] [PubMed] [Google Scholar]
- 15.Fleige T, Pfaff N, Gross U, Bohne W. Localisation of gluconeogenesis and tricarboxylic acid (TCA)-cycle enzymes and first functional analysis of the TCA cycle in Toxoplasma gondii. Int J Parasitol. 2008;38:1121–1132. doi: 10.1016/j.ijpara.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Fauquenoy S, et al. Proteomics and glycomics analyses of N-glycosylated structures involved in Toxoplasma gondii-host cell interactions. Mol Cell Proteomics. 2008;7:891–910. doi: 10.1074/mcp.M700391-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Debierre-Grockiego F, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Zahn MM, Coppens I, Joiner KA, Voelker DR. Selective disruption of phosphatidylcholine metabolism of the intracellular parasite Toxoplasma gondii arrests its growth. J Biol Chem. 2005;280:16345–16353. doi: 10.1074/jbc.M501523200. [DOI] [PubMed] [Google Scholar]
- 19.Kim K, Soldati D, Boothroyd JC. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262:911–914. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- 20.Donald RGK, Roos DS. Stable molecular transformation of Toxoplasma gondii: A selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc Natl Acad Sci USA. 1993;90:11703–11707. doi: 10.1073/pnas.90.24.11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seyfang A, Landfear SM. Four conserved cytoplasmic sequence motifs are important for transport function of the Leishmania inositol/H(+) symporter. J Biol Chem. 2000;275:5687–5693. doi: 10.1074/jbc.275.8.5687. [DOI] [PubMed] [Google Scholar]
- 22.Roos DS, Donald RG, Morrissette NS, Moulton AL. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 23.Gubbels MJ, Wieffer M, Striepen B. Fluorescent protein tagging in Toxoplasma gondii: identification of a novel inner membrane complex component conserved among Apicomplexa. Mol Biochem Parasitol. 2004;137:99–110. doi: 10.1016/j.molbiopara.2004.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.