Abstract
The role of classical neurotransmitters in the transfer and processing of olfactory information is well established in many organisms. Neuropeptide action, however, is largely unexplored in any peripheral olfactory system. A subpopulation of local interneurons (LNs) in the Drosophila antannal lobe is peptidergic, expressing Drosophila tachykinins (DTKs). We show here that olfactory receptor neurons (ORNs) express the DTK receptor (DTKR). Using two-photon microscopy, we found that DTK applied to the antennal lobe suppresses presynaptic calcium and synaptic transmission in the ORNs. Furthermore, reduction of DTKR expression in ORNs by targeted RNA interference eliminates presynaptic suppression and alters olfactory behaviors. We detect opposite behavioral phenotypes after reduction and over expression of DTKR in ORNs. Our findings suggest a presynaptic inhibitory feedback to ORNs from peptidergic LNs in the antennal lobe.
Keywords: olfactory behavior, presynaptic inhibition, tachykinin, two-photon imaging
In Drosophila, odor detection begins when odor molecules activate olfactory receptor neurons (ORNs) in the antennae and maxillary palps. Each of the ORNs expresses only 1 or a few members of a large family of odorant receptor genes (1–4). These ORNs propagate activity to neurons with dendrites in the glomerular compartments of the antennal lobe; each glomerulus receives inputs from ORNs that express the same odorant receptor (1, 3, 5, 6). In the glomeruli, the activity is read by second-order neurons, designated projection neurons (PNs), which relay information to higher olfactory centers in the brain (7).
Inhibitory circuits in the glomeruli, mediated by local interneurons (LNs), play a key role in modulating glomerular signal activity. Presynaptic GABAergic inhibition of the ORNs has been shown in both Drosophila (8, 9) and in mammals (10–12). Conversely, cholinergic LNs in the Drosophila antennal lobe have been suggested to increase and redistribute odor-evoked activity at low odor concentrations (13,14).
In addition to GABA and acetylcholine it is likely that certain neuropeptides are used as neuromodulators in the antennal lobe circuitry of insects (15, 16), as also suggested in the olfactory bulb in mammals (17, 18). One neuropeptide gene that has been implicated in olfactory processing is dtk (19), a gene encoding 5 tachykinin-related peptides, DTKs (20). The DTKs are expressed in ≈150 neurons in the Drosophila brain, and in the antennal lobe glomeruli, there are extensive DTK-immunoreactive arborizations derived from a subset of antennal lobe LNs (21). Two DTK receptors, DTKR and NKD, have been identified in Drosophila (22, 23) and 1 of these, DTKR, is strongly expressed in antennal lobe glomeruli (24). Behavioral evidence for a role of DTKs in olfaction was obtained from analysis of flies where dtk expression was knocked down globally using RNA interference (RNAi); these flies displayed diminished odor sensitivity (19).
To gain insight into the neuromodulation provided by the DTK signaling system in the antennal lobe, we targeted RNAi to specific neurons. We demonstrated that ORNs express the DTKR. Furthermore, RNAi expression reduced receptor expression in ORNs, establishing a tool to investigate the physiological function and behavioral effect of DTK-mediated neuromodulation. Physiological and behavioral data together suggest that DTKs produced by certain LNs exert a presynaptic inhibitory action on ORNs.
Results
Distribution of DTK Peptide and Its Receptor DTKR in the Olfactory System.
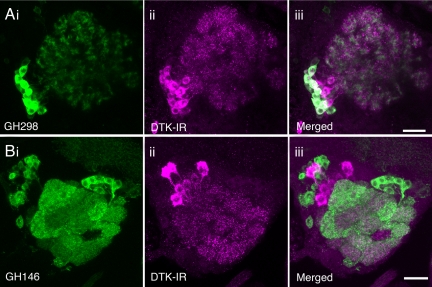
LNs in the antennal lobe have been suggested to play a role in the transformation of olfactory information, and thus the shaping of elaborate behavioral responses to odor cues, through synaptic interactions with the ORNs and PNs in the antennal lobe circuitry (13, 14, 25–29). Immunocytochemistry with a well characterized antiserum has revealed that DTKs are expressed in certain LNs that form a dense supply of neuronal processes to the antennal lobe glomeruli (21). To further analyze DTK expression in antennal lobe LNs we applied the same tachykinin antiserum to flies with GFP expression in a large population of LNs due to the transgenes UAS-CD8-GFP and GH298-Gal4. We found that ≈21 (21 ± 0.9; n = 5) LNs were tachykinin-immunoreactive. Of these DTK immunoreactive neurons, approximately 70% are also GH298-positive (15 ± 0.5; n = 5) (Fig. 1A), In contrast we found that PNs identified by the GH146-Gal4 do not express DTKs (Fig. 1B), confirming earlier work that suggested lack of DTK in all PNs (21).
Fig. 1.
Distribution of DTK peptide immunoreactivity (IR) in local antennal lobe interneurons. Using antiserum to LemTRP-1 (magenta) we localized DTK peptide distribution in relation to Gal4-driven GFP (image stacks from wholemounted specimens). (Ai–iii) Many LNs of the GH298-Gal4 line express DTK-IR. (Bi–iii). We did not detect DTK-IR in any of the projection neurons displayed in the GH146-Gal4 line. (Scale bars, 20 μm.)
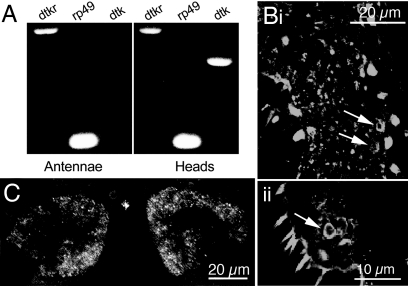
We next investigated the synaptic target of DTK neurons. Immunocytochemistry using an antiserum to the tachykinin receptor DTKR (24) showed that expression in the antennae is localized to cell bodies of ORNs (Fig. 2B). DTKR immunoreactivity was also detected in the glomeruli of the antennal lobes (Fig. 2C). To verify that dtkr is expressed in ORNs we performed reverse transcriptase PCR analysis of isolated antennae. We found that dtkr transcripts are in RNA extracts from antennae and whole heads (Fig. 2A). However, transcript of the peptide gene dtk was only detected in RNA from heads (Fig. 2A). This result suggests that cells in the antenna express DTK receptors. We next drove the expression of dtkr-RNAi with the ORN specific line Or83b-Gal4 to test whether ORNs are the antennal cells that express DTKR. The efficacy of the RNAi was tested by quantitative PCR in flies bearing the pan-neural elav-Gal4 and UAS-dtkr-RNAi transgenes (Fig. S1C). We found that expression of the RNAi in Or83b neurons dramatically reduced the immunoreactivity from the DTKR antiserum (Fig. S1 E and F). Thus, ORNs appear to be the main population of cells expressing DTKR in the antenna.
Fig. 2.
Tachykinin receptor (DTKR) expression in the olfactory receptor neurons (ORNs). (A) Molecular analysis of the dtkr expression in ORNs. Reverse transcriptase PCR products from isolated antennae and whole heads of w1118 flies. Expression of dtkr in antennae and in whole heads is observed. However, dtk expression (peptide precursor) is only detected in heads. Parallel reactions with rp49 as a template control were performed. (B and C) DTKR immunoreactivity was observed in the ORNs (arrows) of the antenna (Bi and Bii) and in most of the glomeruli of the antennal lobe neuropils (C) of w1118 flies. The 2 antennal lobes are shown in a frontal 9-μm thick section.
The Tachykinin Receptor DTKR Mediates Presynaptic Inhibition in ORNs.
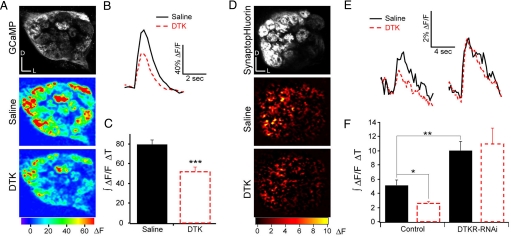
We next asked whether neurotransmission of ORNs is modulated by the DTK signaling system. First we measured calcium in ORN axon terminals using two-photon imaging in flies expressing the calcium sensor GCaMP in ORNs (30, 31). We expressed UAS-GCaMP in Or83b neurons. Electrical stimulation of the olfactory nerve elicits a calcium influx in ORN terminals, and this calcium response was reduced by the application of DTK (Fig. 3 A and B). When quantifying the effect of DTK across preparations we could determine an average 38% reduction in presynaptic calcium responses (Fig. 3C). Presynaptic calcium entry triggers the release of neurotransmitters from synaptic vesicles, hence DTK mediated reduction in presynaptic calcium should be accompanied by a decrease of synaptic vesicle release. To investigate this we used two-photon imaging of the antennal lobe of flies expressing synaptopHluorin (spH), an indicator of synaptic vesicle release (32), in Or83b neurons. Electrical stimulation of the olfactory nerve elicited an increase of spH fluorescence and applying DTK resulted in a reduction of this fluorescence signal by ≈50% (Fig. 3 D–F). We next examined whether the DTK mediated presynaptic inhibition requires DTKR expression in ORNs. When dtkr-RNAi was expressed in Or83b neurons the same stimulation of the olfactory nerve produced a larger increase in spH fluorescence intensity. Furthermore dtkr-RNAi abolished sensitivity to DTK application (Fig. 3F). These experiments indicate that DTKR in ORNs mediates presynaptic inhibition by reducing calcium influx into axon terminals and reducing neurotransmission.
Fig. 3.
Tachykinin receptors mediate presynaptic inhibition in Drosophila olfactory receptor neurons. (A) Two-photon images of the antennal lobe of a fly expressing GCaMP in Or83b neurons (Top). Pseudocolored images reveal the response to electrical stimulation of the olfactory nerve in saline (Middle) and after addition of 10 μM DTK (Bottom). (B) Fluorescence change over time. Black and red traces show representative responses before and after drug application, respectively. (C) Effect of DTK on presynaptic calcium response quantified as the integrated fluorescence change over time (area under the curve in B) across preparations. (D) Two-photon images of the antennal lobe of a fly expressing synaptopHluorin in Or83b neurons (Top). Pseudocolored images reveal the response to electrical stimulation of the olfactory nerve in saline (Middle) and after addition of 10 μM DTK (Bottom). (E) Fluorescence change over time; traces are the average of 3 trials. (F) Effect of DTK on presynaptic calcium response quantified as the integrated fluorescence change over time across preparations. Electrical stimulations were 1 ms in duration and 10 V in amplitude, 45 pulses (A–C) or 80 pulses (D–F) at 100 Hz. (n) 16 (C) and 5 (F). t test: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
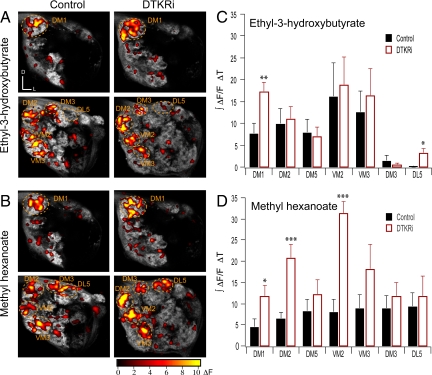
Next we investigated the role of DTKR in the modulation of neurotransmission from the ORNs in responses to high (10−1) and low (10−4) concentrations of the food related odors: ethyl-3-hydroxybutyrate and methyl hexanoate. To monitor the activity in the ORN axon terminals we imaged flies expressing spH in Or83b neurons in control flies and flies that also express dtkr-RNAi. High concentration of ethyl-3-hydroxybutyrate mainly activated 5 glomeruli (DM1, DM2, DM5, VM2, and VM3) (Fig. 4 A and C). Reduction of DTKR levels in Or83b neurons, significantly increased olfactory responses in the DM1 and DL5 glomeruli (Fig. 4 A and C). High concentration of methyl hexanoate activated 7 glomeruli (DM1, DM2, DM5, VM2, VM3, DM3, and DL5) in control flies and knocking down DTKR expression in Or83b neurons significantly increased the response in the DM1, DM2, and VM2 glomeruli (Fig. 4 B and D). Stimulating the flies with the lower odor concentration (10−4) did not result in any significant change in response between control and dtkr-RNAi expressing flies (Fig. S2). Thus, olfactory response in antennal lobe glomeruli is modulated by the DTK signaling pathway in some but not all glomeruli.
Fig. 4.
Presynaptic tachykinin receptors modulate odor-evoked olfactory receptor neuron transmission. Two-photon imaging of ORN synaptic transmission elicited by odor stimulation at high odor concentration (10−1) in control flies and flies expressing dtkr-RNAi in Or83b neurons. (A and B) Two-photon images of the antennal lobe from flies expressing spH in Or83b neurons in control flies (Left) and flies that also express dtkr-RNAi in ORNs (Right). Pseudocolor overlays reveal the change in fluorescence in response in response to (A) ethyl-3-hydroxybutyrate and (B) methyl hexanoate at 2 different optical planes. (C) Ethyl-3-hydroxybutyrate and (D) methyl hexanoate evoked responses quantified as the integrated fluorescence change over time for each glomerulus. Odors were delivered for 2 seconds. (n) 7–8 preparations. t test: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
DTKR Signaling Modulates Sensitivity in Odor-Guided Behavior.
Next, we asked whether the observed DTK mediated presynaptic inhibition could be translated into a modulation of odor-guided behavior, and whether this could correlate to the imaging data that showed activity in select glomeruli. To address this we knocked down or over-expressed DTKR by crossing UAS-dtkr-RNAi or UAS-dtkr with Or83b-Gal4 and scored the behavioral responses to specific odors. The ectopic expression of DTKR in ORNs was confirmed by reverse transcriptase PCR of isolated antennae, by microscopical detection of GFP tagged DTKR and by increased DTKR immunolabeling (Fig. S1 A, B, and D and Fig. S3A). The GFP-tagged DTKR has been shown to be functional when expressed in HEK-296 cells (24). Odor-guided behavior was measured by a trap assay with free walking flies that were given a choice between a specific odor and water in a small arena (33, 34). A response index was calculated where a negative index indicates that flies are repelled by the odor and a positive index that they are attracted.
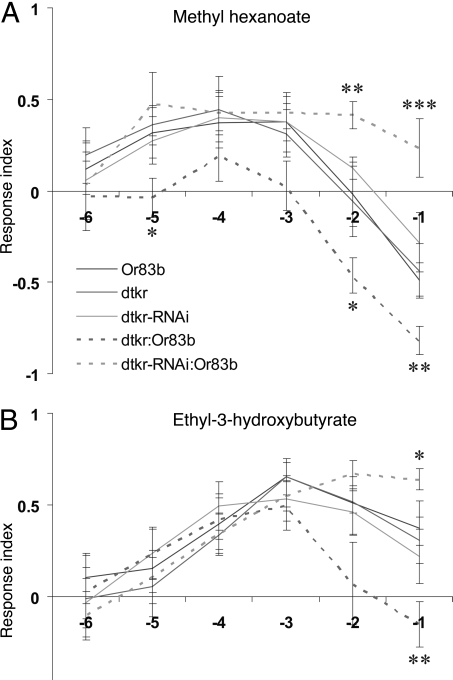
Many odorants, including methyl hexanoate and ethyl-3-hydroxybutyrate, are attractive at low concentrations and aversive at high concentrations (35–37), and this was found also in our assay. DTKR knock down in Or83b-ORNs, resulted in an increase in response index at high concentrations of the tested odorants (Fig. 5). This result suggests that the DTKR mediated presynaptic inhibition is linked to the inhibition of behavioral responses to high concentrations of food-related odors.
Fig. 5.
Interference with tachykinin receptor (DTKR) expression in the ORNs alters odor-guided behavior. Olfactory behavior of adult Drosophila exhibiting altered expression of DTKR in the ORNs. Behavior is scored as a response index (RI) where positive values represent attraction and negative RIs indicate repulsion. Each of the average RI values is based on 15–25 replicates of 20 flies each. (A and B) Down regulation of DTKR in ORNs (dtkr-RNAi:Or83b) leads to an increased RI for tested odors at high concentrations compared with control flies (Or83 and dtkr-RNAi). Ectopic expression of DTKR in the ORNs (dtkr:Or83b), however, leads to a decrease in RI at high concentrations for the odorants tested: methyl hexanoate (A) and ethyl-3-hydroxy butyrate (B) compared with control flies (Or83b and dtkr). ANOVA, Tukey's posttest: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We then asked whether over-expression of DTKR in ORNs leads to behavioral phenotypes opposite to those of the receptor knock-downs. Indeed, this is the case for both methyl hexanoate and ethyl-3-hydroxybutyrate (Fig. 5). For these odors we observed a significant decrease in the response index with increasing odor concentrations for flies with DTKR ectopically expressed in ORNs (Fig. 5). Thus, ectopic expression of DTKR produces a behavioral response that is opposite to that of RNAi knock down.
Another possible synaptic target of DTK producing LNs could be the PNs. Thus, we investigated whether DTKR plays a role in PNs by driving dtkr-RNAi expression with GH146-Gal4. The behavioral responses of the experimental flies did not significantly differ from the controls over the entire range of odor concentrations tested (Fig. S4). Ectopic expression of DTKR in the PNs, confirmed by detection of the GFP fusion protein (Fig. S3B), also did not alter the behavioral responses to the tested odorants (Fig. S4). Because the DTKR knockdown in GH146 neurons did not alter odor choice, it is likely that these PNs do not express DTKR. Likewise, because ectopic expression of DTKR in GH146 neurons did not affect odor choice, it is likely that these PNs lack DTK signaling capabilities.
Discussion
The present study shows that Drosophila ORNs express a presynaptic neuropeptide receptor, DTKR, which appears to serve in a feedback circuit from local peptidergic interneurons, LNs, of the antennal lobe. These LNs express the peptide products, DTK1–5, of the dtk gene (see ref. 21). Our two-photon imaging and behavioral data, using RNAi and over-expression of the DTK receptor, provide evidence that ORNs are modulated presynaptically by DTKs. This peptidergic presynaptic inhibition of ORNs is detected behaviorally only at high odorant concentrations, and may thus serve to modulate the dynamic range in sensitivity to relevant odors.
Recent studies of peripheral olfactory signal processing in Drosophila have shown that there are afferent and local excitatory cholinergic circuits, combined with presynaptic and postsynaptic GABAergic inhibition of ORNs and interneurons (8, 9, 13, 14, 28). We now demonstrate a second presynaptic inhibitory pathway mediated by DTK-expressing LNs, providing an additional modulation mechanism in peripheral olfactory processing. Given the importance of presynaptic inhibition future experiments will be necessary to demonstrate a synaptic connection between LNs and ORNs. However, in studies of the cockroach Periplaneta americana both GABAergic and other LNs were found presynaptic to ORNs (38, 39).
There is ample physiological evidence to suggest that vertebrate and invertebrate ORN axon terminals can be presynaptically modulated by GABA and inhibitory LNs (9–12, 40), but our study is unique in that we demonstrate peptidergic presynaptic inhibition of ORNs by local interneurons. In animals other than Drosophila there is only immunocytochemical data to suggest such circuitry. For example, in the rat, periglomerular cells, interneurons that modulate the first synaptic relay in olfactory processing, have been shown to express 2 neuropeptides: somatostatin and cholecystokinin (18). Moreover, somatostatin receptor immunoreactivity in axons of the rat olfactory nerve has been demonstrated (41). These morphological studies may suggest that presynaptic peptidergic modulation of ORNs is not exclusive to Drosophila. In addition, there are examples of peptidergic modulation in the olfactory system by efferent neurons. Centrifugal peptidergic modulation has been demonstrated in the olfactory epithelia of a salamander, where neuropeptide Y was shown to enhance responses evoked by a food-related odor in hungry animals (42). The peptide FMRFamide has been shown to modulate the activity of ORNs in the olfactory epithelium of the mouse and the salamander, but the circuitry is not clear (17, 43).
Recently, a study of how specific glomeruli mediate olfactory-guided behavior showed that the activation of the DM1 glomerulus is necessary and sufficient to mediate fly attraction to vinegar odor (44). Here, we tested, physiologically and behaviorally, the response to 2 other food related odors. Both odors elicited activation of DM1 and in flies with down regulated DTKR levels we detected an increased activation of this specific glomerulus. In line with this, we observed that DTKR knock down flies were more attracted to the 2 odors, suggesting that the behavioral responses to these odors may be linked to DTK mediated inhibition of DM1.
Neuropeptides often colocalize with classical neurotransmitters in neurons and may act as cotransmitters at synapses (15, 45, 46). We found here that DTK is expressed in a population partly overlapping with GAD1-expressing LNs, likely to be GABAergic (Fig. S5). Therefore, we conducted experiments to determine whether GABA and DTK act synergistically on ORNs (Fig. S5). Our preliminary data, however, suggest that the 2 compounds may act independently. This is in contrast to findings in the crayfish visual system where GABA hyperpolarizes photoreceptors and a tachykinin-like peptide potentiates this response (47). In the crayfish, it is not clear which GABA receptor type that mediates the response, although a Cl− conductance appears to be activated (47), whereas in Drosophila ORNs the presynaptic inhibition is mediated by metabotropic GABAB receptors (9).
In summary, our findings suggest a presynaptic inhibitory feedback to ORNs from a population of peptidergic LNs in the antennal lobe. These LNs express DTKs, and the ORNs of the antennae express the DTK receptor. We provide evidence for peptidergic modulation in the antennal lobe before the olfactory signals are relayed via projection neurons to higher brain centers, possibly acting as a mechanism to control olfactory sensitivity.
Materials and Methods
Fly Strains.
To ectopically express the DTKR receptor we designed a UAS-dtkr-GFP fusion construct according to Birse et al. (24). This dtkr fusion construct has been shown to be functional when expressed in HEK-296 cells (24). A Bglll/Notl fragment containing the dtkr (CG7887) sequence and the GFP fusion was excised from the pEGFP1 vector. The sequences were then subcloned into a pUAST vector. We used immunocytochemistry with an antiserum to DTKR (see ref. 24 and SI Text) to confirm that the construct was able to produce ectopic expression of the receptor in flies bearing the UAS-dtkr-GFP construct and Or83b-Gal4 (Fig. S1 A and B).
To down-regulate DTKR a dtkr-RNAi construct was designed according to the method of Giordano et al. (48). For further information on the generation of dtkr-RNAi flies and the efficacy of the dtkr-RNAi see SI Text. To reduce expression of the GABAB receptor in ORNs we used a UAS-GABABR2-RNAi described in Root et al. (9).
We also used the following driver lines: Or83b-Gal4 (gift of Leslie Vosshall, Rockefeller University, New York), which is active in ≈70–80% of the ORNs (34), GH146-Gal4 (gift of Reinhard Stocker, University of Freiburg, Switzerland), which is active in ≈60% of the PNs (7,49), GH298-Gal4 (gift of Reinhard Stocker), and Gad1-Gal4 (gift of Gero Miesenböck, University of Oxford, U.K.), both expressed in LNs. Gad1-Gal4 is active in most GABAergic neurons in the brain because it is based on promoter/enhancer elements of Gad1, the gene encoding glutamic acid decarboxylase 1 (GAD1), the key enzyme in GABA biosynthesis (50). GH298-Gal4 is active in a subset (≈36 LNs) of the LNs (7, 28). In the behavioral experiments, for the silencing of dtkr, we used UAS-dtkr-RNAi1a,Or83b-Gal4 and for ectopic expression of dtkr we used UAS-dtkr-GFP10b/Or83b-Gal4. We used corresponding parental strains as controls.
Immunocytochemistry.
Dissected fly brains were fixed in 4% paraformaldehyde and 1.0% Triton X-100. To test for the coexpression of DTK in populations of GFP-expressing neurons we incubated the brains with an antiserum to a generic sequence of insect tachykinin-related peptides (anti-LemTRP-1; code K 9836) (21) and a mouse monoclonal antibody against GFP (1:100, Molecular Probes). DTKR immunocytochemistry was performed as described by Birse et al. (24). For each experiment at least 10 specimens were analyzed. For further details see SI Text.
Reverse Transcriptase PCR.
To verify DTKR expression in olfactory receptor neurons we performed reverse transcriptase PCR (rt-PCR) analysis of antennae of w1118, Or83b-Gal4/UAS-dtkr-GFP, and UAS-dtkr-GFP/+ flies. Individual flies were frozen in liquid nitrogen, and for each genotype 100 pairs of antennae were used for RNA preparation. As a positive control, RNA from 20 heads was extracted. Total RNA was prepared using TRIzol (Invitrogen) according to the instruction of the manufacturer. RT-PCR with sequence specific primer pairs for dtkr, dtk, and rp49 was performed using the QIAGEN One-Step RT-PCR Kit. Ribosomal protein rp49 was used as a transcriptional standard (51). For primer sequences see SI Text.
Two-photon Imaging.
Isolated brain preparations (31) were obtained by micro dissection of decapitated flies to remove head cuticle and connective tissues. Neural activity of the fly brain was reduced by dissecting in chilled calcium free AHL saline. The antenna and brain preparation was pinned in a Sylgard dish and the olfactory nerves were carefully severed near the base of the antenna with fine forceps for nerve stimulation experiments. After dissection the preparations were rinsed and kept in AHL saline (108 mM NaCl, 5 mM KCL, 2 mM CaCl2, 8.2 mM MgCl2, 4 mM NaHCO3, 1 mM NaH2PO4, 5 mM trehalose, 10 mM sucrose, and 5 mM Hepes, pH 7.5, 265 mOsm). For odor stimulation experiments, the preparation was mounted on a slide and the brain was embedded in 2% agarose in AHL saline leaving the antennae exposed to air, as originally described (31). Synthetic DTK-1 (produced by Vulpes LTD), was dissolved as 1,000× stock in AHL. CGP54626 (Tocris) was dissolved as 2,000× stocks in DMSO. SKF97541 (Tocris) was dissolved as a 500× stock in 100 mM NaCl. The appropriate volume (1–4 μL) was first diluted with 100 μL AHL saline and then added to the preparation to achieve the final concentration of 10 μM, 25 μM, and 20 μM for DTK, CGP54626, and SKF97541, respectively.
Calcium and synaptopHluorin imaging was performed with a custom-built two-photon microscope (see SI Text) similar to the one described (31). Images were captured at 4 frames per second with a resolution of 128 × 128 pixels. At the end of the experiment, a high resolution Z-stack of images (512 × 512 pixels) was collected for glomerular identification. Electrical stimulation of the olfactory nerve was delivered with a glass suction electrode that was made by pulling capillary glass to a fine tip, broken with forceps, and then fire polished to achieve a diameter that is ≈1.5× the diameter of the nerve. The nerve was sucked as a loop into the electrode. A Grass stimulator was used to stimulate the nerve with pulses at 100 Hz, 1 ms in duration, and 10 V in amplitude for a duration of 450 or 800 ms. In odor experiments, a constant carrier airflow of 1 L/min was applied to the antennae in a pipe of 12 mm in diameter. Odor onset was controlled by solenoid valves that mixed 50% of the carrier air with air redirected through 100-mL bottles containing 20 μL odorant on a piece of filter paper. Ethyl-3-hydroxybutyrate and methyl hexanoate were diluted in water.
Olfactory Choice Assay.
We used a free-walking behavior assay adapted from the protocols described by Dekker et al. (33) and Larsson et al. (34). In this bioassay a group of 20 flies is introduced in a small chamber were they are allowed to choose between odor and control. After 22 h, flies trapped in the different vials (odor or control) or remaining in the arena were counted. A response index was calculated as (T − C)/(T + C + NR − D), where T is the number of flies in test vial, C number in the control vial, NR number of flies remaining in arena, and D number of dead flies in the arena. For more information on the olfactory choice assay and odors used see SI Text.
Supplementary Material
Acknowledgments.
We thank Kadijeh Rezaei for performing the quantitative PCR experiments; Paul H. Taghert (Washington University School of Medicine, St Louis) for donating pUAST and SympUAST vectors; and Leslie Vosshall (Rockefeller University, New York), Reinhard Stocker (University of Freiburg, Switzerland), Gero Miesenböck (University of Oxford, UK), and the Bloomington Drosophila Stock Center for providing fly lines. This work was supported Swedish Research Council Grants VR621–2002-3923 (to R.I.) and VR621–2004-2715 (to D.R.N.), National Institute on Deafness and Other Communication Disorders Grants R01DC009597 (to J.W.W.) and 1F31DC009511 (to C.M.R.), the Carl Tryggers Foundation, and the Royal Swedish Academy of Sciences Å.M.E.W.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813004106/DCSupplemental.
References
- 1.Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–785. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- 2.Clyne PJ, et al. A novel family of divergent seven-transmembrane proteins: Candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- 3.Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 4.Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- 5.Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 6.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Root CM, et al. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy GJ, Darcy DP, Isaacson JS. Intraglomerular inhibition: Signaling mechanisms of an olfactory microcircuit. Nat Neurosci. 2005;8:354–364. doi: 10.1038/nn1403. [DOI] [PubMed] [Google Scholar]
- 11.McGann JP, et al. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48:1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol. 2000;84:1194–1203. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- 13.Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenböck G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nässel DR, Homberg U. Neuropeptides in interneurons of the insect brain. Cell Tissue Res. 2006;326:1–24. doi: 10.1007/s00441-006-0210-8. [DOI] [PubMed] [Google Scholar]
- 16.Utz S, et al. Direct peptide profiling of lateral cell groups of the antennal lobes of Manduca sexta reveals specific composition and changes in neuropeptide expression during development. Dev Neurobiol. 2007;67:764–777. doi: 10.1002/dneu.20381. [DOI] [PubMed] [Google Scholar]
- 17.Ni MM, Luo Y, Liu J, Liao DQ, Tang YD. FMRFamide modulates outward potassium currents in mouse olfactory sensory neurons. Clin Exp Pharmacol Physiol. 2008;35:563–567. doi: 10.1111/j.1440-1681.2007.04840.x. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez-Mecinas M, et al. Characterization of somatostatin- and cholecystokinin-immunoreactive periglomerular cells in the rat olfactory bulb. J Comp Neurol. 2005;489:467–479. doi: 10.1002/cne.20649. [DOI] [PubMed] [Google Scholar]
- 19.Winther ÅM, Acebes A, Ferrus A. Tachykinin-related peptides modulate odor perception and locomotor activity in Drosophila. Mol Cell Neurosci. 2006;31:399–406. doi: 10.1016/j.mcn.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Siviter RJ, et al. Expression and functional characterization of a Drosophila neuropeptide precursor with homology to mammalian preprotachykinin A. J Biol Chem. 2000;275:23273–23280. doi: 10.1074/jbc.M002875200. [DOI] [PubMed] [Google Scholar]
- 21.Winther ÅM, Siviter RJ, Isaac RE, Predel R, Nässel DR. Neuronal expression of tachykinin-related peptides and gene transcript during postembryonic development of Drosophila. J Comp Neurol. 2003;464:180–196. doi: 10.1002/cne.10790. [DOI] [PubMed] [Google Scholar]
- 22.Li XJ, Wolfgang W, Wu YN, North RA, Forte M. Cloning, heterologous expression and developmental regulation of a Drosophila receptor for tachykinin-like peptides. EMBO J. 1991;10:3221–3229. doi: 10.1002/j.1460-2075.1991.tb04885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monnier D, et al. NKD, a developmentally regulated tachykinin receptor in Drosophila. J Biol Chem. 1992;267:1298–1302. [PubMed] [Google Scholar]
- 24.Birse RT, Johnson EC, Taghert PH, Nässel DR. Widely distributed Drosophila G-protein-coupled receptor (CG7887) is activated by endogenous tachykinin-related peptides. J Neurobiol. 2006;66:33–46. doi: 10.1002/neu.20189. [DOI] [PubMed] [Google Scholar]
- 25.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 26.Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silbering AF, Galizia CG. Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J Neurosci. 2007;27:11966–11977. doi: 10.1523/JNEUROSCI.3099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW. Propagation of olfactory information in Drosophila. Proc Natl Acad Sci USA. 2007;104:11826–11831. doi: 10.1073/pnas.0704523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 31.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 32.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 33.Dekker T, Ibba I, Siju KP, Stensmyr MC, Hansson BS. Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D sechellia. Curr Biol. 2006;16:101–109. doi: 10.1016/j.cub.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 34.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Devaud JM, Keane J, Ferrus A. Blocking sensory inputs to identified antennal glomeruli selectively modifies odorant perception in Drosophila. J Neurobiol. 2003;56:1–12. doi: 10.1002/neu.10216. [DOI] [PubMed] [Google Scholar]
- 36.Stensmyr MC, Giordano E, Balloi A, Angioy AM, Hansson BS. Novel natural ligands for Drosophila olfactory receptor neurones. J Exp Biol. 2003;206:715–724. doi: 10.1242/jeb.00143. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, et al. Blockade of neurotransmission in Drosophila mushroom bodies impairs odor attraction, but not repulsion. Curr Biol. 2003;13:1900–1904. doi: 10.1016/j.cub.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Malun D. Synaptic relationships between GABA-immunoreactive neurons and an identified uniglomerular projection neuron in the antennal lobe of Periplaneta americana: A double-labeling electron microscopic study. Histochemistry. 1991;96:197–207. doi: 10.1007/BF00271538. [DOI] [PubMed] [Google Scholar]
- 39.Distler PG, Boeckh J. Synaptic connections between identified neuron types in the antennal lobe glomeruli of the cockroach, Periplaneta americana: II. Local multiglomerular interneurons. J Comp Neurol. 1997;383:529–540. doi: 10.1002/(sici)1096-9861(19970714)383:4<529::aid-cne9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Wachowiak M, Cohen LB. Presynaptic inhibition of primary olfactory afferents mediated by different mechanisms in lobster and turtle. J Neurosci. 1999;19:8808–8817. doi: 10.1523/JNEUROSCI.19-20-08808.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindler M, Humphrey PP, Lohrke S, Friauf E. Immunohistochemical localization of the somatostatin sst2 (b) receptor splice variant in the rat central nervous system. Neuroscience. 1999;90:859–874. doi: 10.1016/s0306-4522(98)00483-7. [DOI] [PubMed] [Google Scholar]
- 42.Mousley A, Polese G, Marks NJ, Eisthen HL. Terminal nerve-derived neuropeptide y modulates physiological responses in the olfactory epithelium of hungry axolotls (Ambystoma mexicanum) J Neurosci. 2006;26:7707–7717. doi: 10.1523/JNEUROSCI.1977-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park D, Zawacki SR, Eisthen HL. Olfactory signal modulation by molluscan cardioexcitatory tetrapeptide (FMRFamide) in axolotls (Ambystoma mexicanum) Chem Senses. 2003;28:339–348. doi: 10.1093/chemse/28.4.339. [DOI] [PubMed] [Google Scholar]
- 44.Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hökfelt T, et al. Neuropeptides–an overview. Neuropharmacology. 2000;39:1337–1356. doi: 10.1016/s0028-3908(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 46.Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Glantz RM, Miller CS, Nässel DR. Tachykinin-related peptide and GABA-mediated presynaptic inhibition of crayfish photoreceptors. J Neurosci. 2000;20:1780–1790. doi: 10.1523/JNEUROSCI.20-05-01780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giordano E, Rendina R, Peluso I, Furia M. RNAi triggered by symmetrically transcribed transgenes in Drosophila melanogaster. Genetics. 2002;160:637–648. doi: 10.1093/genetics/160.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- 50.Ng M, et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 51.Merrill CE, et al. Visual arrestins in olfactory pathways of Drosophila and the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2002;99:1633–1638. doi: 10.1073/pnas.022505499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.