Abstract
Overexpression of the Gossypium hirsutum sucrose synthase (SuSy) gene under the control of 2 promoters was examined in hybrid poplar (Populus alba × grandidentata). Analysis of RNA transcript abundance, enzyme activity, cell wall composition, and soluble carbohydrates revealed significant changes in the transgenic lines. All lines showed significantly increased SuSy enzyme activity in developing xylem. This activity manifested in altered secondary cell wall cellulose content per dry weight in all lines, with increases of 2% to 6% over control levels, without influencing plant growth. The elevated concentration of cellulose was associated with an increase in cell wall crystallinity but did not alter secondary wall microfibril angle. This finding suggests that the observed increase in crystallinity is a function of altered carbon partitioning to cellulose biosynthesis rather than the result of tension wood formation. Furthermore, the augmented deposition of cellulose in the transgenic lines resulted in thicker xylem secondary cell wall and consequently improved wood density. These findings clearly implicate SuSy as a key regulator of sink strength in poplar trees and demonstrate the tight association of SuSy with cellulose synthesis and secondary wall formation.
Keywords: crystallinity, hybrid poplar, carbon allocation, wood density
Sucrose is the primary translocatable carbohydrate in the majority of plants. As such, its metabolism is vital to the regulation of photoassimilate in sink tissues (1). Sucrose hydrolysis can be catalyzed by 2 enzymes: invertase, which cleaves sucrose into glucose and fructose, and sucrose synthase (SuSy), which catalyzes the formation of UDP-glucose and fructose from sucrose. Both SuSy and invertase have been shown to be tightly associated with the processes of phloem unloading, and SuSy has been identified repeatedly as playing a central role in modulating sink strength in plants (2–4). Furthermore, SuSy can be found in very high levels in companion cells (5). SuSy also has been linked with the synthesis of both storage and structural carbohydrates, acting as the catalyst in the metabolism of sucrose that results in the liberation of the precursor for the generation of callose (6), cellulose (7), and mixed linkage β (1–3), (1–4)-glucans (8). Finally, SuSy expression has been highly correlated with the development and deposition of secondary xylem from the vascular cambium in trees (9, 10).
SuSy has dual functionality; providing the immediate precursor for cellulose biosynthesis and concomitantly recycling UDP, which has been identified as an inhibitor of cellulose biosynthesis (11). It has been suggested that SuSy exists in 2 forms, soluble (S-SuSy) and particulate (P-SuSy), with the latter being membrane bound and directly supplying UDP-glucose to the cellulose synthase complex for cellulose biosynthesis (7). As such, the high energy bond is retained for use in the synthesis of polysaccharides. In maize, phosphorylation of SuSy has been shown to alter the enzyme from a membrane-bound to a soluble form (12, 13), but this phosphorylation seems to be reversible (14).
A mutant form of the mung bean SuSy (S11E: replacement of Ser-11 by Glu-11), which was shown to have higher catalytic efficiency toward sucrose, was overexpressed in transgenic poplar (Populus alba) (15). Using a dual-labeling system, it was shown that only a fraction of the sucrose loaded into the phloem was used directly by the CesA complex associated with SuSy, thus conserving the high energy bond, inherent to the UDP-glucose derived from SuSy-mediated cleavage of sucrose, for use in polysaccharide synthesis. Altering the expression of SuSy also has been shown to cause changes in structural and storage carbohydrates in other species. For example, maize SuSy mutants displayed increased sucrose levels (16), whereas increased glucose and fructose levels were apparent in tobacco overexpressing SuSy (17). In wheat, natural variations in SuSy levels have demonstrated clear associations between SuSy activity and increased cell wall polysaccharide levels (18). Similar results have been observed in wheat roots, with high SuSy activity (caused by hypoxia) being associated with increased cellulose content (19). Studies investigating gene expression patterns in poplar also have identified SuSy as being associated with cellulose synthesis and with the formation of tension wood where increased cellulose deposition occurs (10, 20). As such, increasing the expression of SuSy appears to be a key target to accelerate or improve the production of cellulose within forest trees.
This article investigates the effect of misregulating SuSy in hybrid poplar, and the ensuing trees were assessed for changes in transcript abundance, enzyme activity, biomass production, storage, and structural polysaccharides, as well as cell wall ultrastructure. The results clearly show that SuSy activity indeed modulates the production of cellulose and its ultrastructural characteristics without deleterious effects on plant growth.
Results
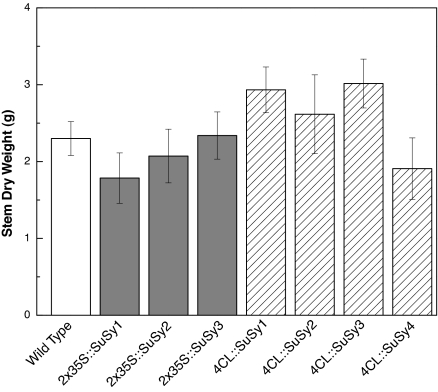
Transformed hybrid poplar trees regenerated from leaf tissue were propagated from single shoots originating from individual explants; therefore each line represents an individual transformation event. Of the transformed lines generated, 3 of the 2 × 35S::SuSy lines and 4 of the 4CL::SuSy lines were selected for greenhouse growth trials and in-depth cell wall characterization, based on transcript abundance and enzyme activity. Together with corresponding wild-type controls, 12 individual trees per line were transferred simultaneously to the greenhouse. As expected, biological variation common to growth parameters was apparent; however, there was no change in total biomass accumulation, as evident from leaf [supporting information (SI) Fig. S1) and stem dry weight, relative to controls (Fig. 1). Furthermore, at harvest, all transgenic lines appeared phenotypically similar to the wild-type trees.
Fig. 1.
Biomass measurements of SuSy transgenic and wild-type poplar trees. Mean (SE) values were calculated from 10 plants per line.
Transcript Level and Enzyme Activity.
Quantitative real-time PCR was used to measure the transcript abundance of the exogenous SuSy transgene relative to initiation factor 5A (TIF5A) (Table 1). All lines clearly showed the presence of the exogenous gene and the elevated level of expression. In general, transcript abundance of SuSy was higher in leaf tissue than in the developing xylem. Correspondingly, SuSy activity, as determined by the breakdown of sucrose into UDP-glucose and fructose, was greater in both tissues (developing xylem and leaf tissue) of all of the transgenic lines examined (Table 1). Enzyme activity ranged from 7.61 to 12.77 μg fructose mg−1 protein min−1, compared with 4.99 μg fructose mg−1 protein min−1 in the control trees, representing a 2.5-fold increase in transgene enzyme activity. To ensure that there was no co-suppression of native SuSy genes, transcript levels of 3 SuSy genes most closely co-coordinately expressed with cellulose synthase genes in poplar were measured also. None of the genes evaluated showed a decrease in transcript level, and in fact most lines experienced an increase in native SuSy transcript abundance (Table S1).
Table 1.
Mean transcript abundance and enzyme activity in leaf and developing xylem tissue for SuSy transgenic and wild-type poplar trees
| Transgenic line | SuSy transcript level ΔCt |
SuSy enzyme activity (μg fructose min−1 mg−1) |
||
|---|---|---|---|---|
| Leaf | Developing xylem | Leaf | Developing xylem | |
| Control | No data | No data | 406.71 (45.66) | 4.99 (0.54) |
| 2 × 35S::SuSy 1 | 2.50 (0.29)*† | 0.18 (0.05)*† | 1126.43 (178.08)*† | 8.18 (0.50)*† |
| 2 × 35S::SuSy 2 | 2.34 (0.71)*† | 0.49 (0.23)*† | 845.45 (59.84)*† | 9.22 (1.17)† |
| 2 × 35S::SuSy 3 | 1.32 (0.06)*† | 0.14 (0.13)*† | 555.90 (63.48) | 11.95 (2.29)† |
| 4CL::SuSy 1 | 0.59 (0.05)*† | 0.82 (0.53)*† | 788.67 (101.54)† | 9.10 (0.34)*† |
| 4CL::SuSy 2 | 1.17 (0.57)*† | 0.12 (0.01)*† | 541.56 (24.32) | 8.06 (0.84)† |
| 4CL::SuSy 3 | 0.99 (0.19)*† | 0.34 (0.15)*† | 680.56 (74.44) | 12.77 (1.34)*† |
| 4CL::SuSy 4 | 0.80 (0.00)*† | 0.12 (0.01)*† | 581.24 (105.83) | 7.61 (0.87)† |
Mean (SE) values were calculated from 3 plants per line.
*, significance at α = 0.05;
†, significant difference from control values at α = 0.10.
Cell Wall Chemistry.
Changes in total leaf soluble sugar content were variable among the transformed lines (Table 2). However, there was a clear increasing trend in soluble sugars in all transgenic lines, with 1 line showing statistical significance. In contrast, in the developing xylem the total soluble sugars of the transgenic lines were significantly elevated, with 4 of the 7 lines demonstrating significant increases, ranging from 61.8 to 136.8 mg g−1 total soluble carbohydrate, compared with the 44 mg g−1 common to the control trees. Starch content generally was unchanged in both the leaves and developing xylem of all but 1 of the single transgenic lines; 4CL::SuSy-4 had increased leaf starch content (1.28 mg g−1) relative to the control (0.76 mg g−1) (Table 2).
Table 2.
Total soluble carbohydrates and starch in leaf and developing xylem tissue of SuSy transgenic and wild-type trees
| Transgenic Line | Leaf (mg g−1) |
Developing Xylem (mg g−1) |
||
|---|---|---|---|---|
| Soluble carbohydrates | Starch | Soluble carbohydrates | Starch | |
| Control | 159.66 (12.68) | 0.76 (0.15) | 44.04 (7.36) | 0.30 (0.05) |
| 2 × 35S::SuSy 1 | 166.59 (11.75) | 0.83 (0.26) | 61.81 (3.36) | 0.28 (0.01) |
| 2 × 35S::SuSy 2 | 191.24 (16.60) | 0.88 (0.31) | 105.22 (30.85) | 0.22 (0.05) |
| 2 × 35S::SuSy 3 | 168.11 (16.87) | 0.68 (0.11) | 134.27 (8.71)*† | 0.26 (0.08) |
| 4CL::SuSy 1 | 185.51 (13.52) | 0.61 (0.13) | 136.73 (21.27)† | 0.24 (0.00) |
| 4CL::SuSy 2 | 175.79 (14.73) | 1.28 (0.13)† | 91.52 (9.43)*† | 0.39 (0.07) |
| 4CL::SuSy 3 | 193.50 (8.74)† | 0.94 (0.35) | 121.37 (9.48)*† | 0.30 (0.04) |
| 4CL::SuSy 4 | 169.83 (13.48) | 0.90 (0.43) | 87.91 (20.98) | 0.31 (0.06) |
Mean (SE) values were calculated from 3 plants per line.
*, significance at α = 0.05;
†, significant difference from control values at α = 0.10.
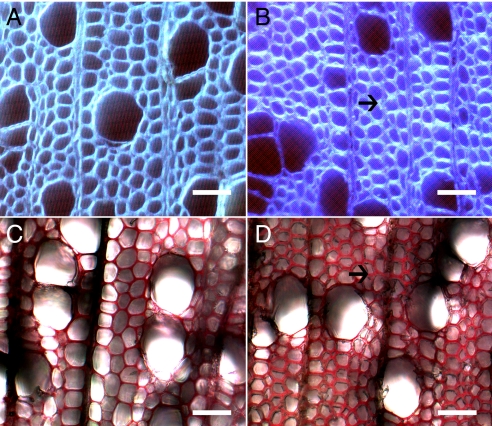
Cell wall carbohydrate content was altered substantially in the transgenic lines. All lines showed increased glucose content, with all 2 × 35S::SuSy lines and all but 1 of the 4CL::SuSy lines displaying statistically significant increases. The 2 × 35S::SuSy-3 and 4CL::SuSy-1 lines also had increased mannose levels, which probably could be ascribed to elevated levels of glucomannan. All lines also showed decreases in arabinose content, but in general galactose, rhamnose, and xylose remained relatively unchanged when compared with the wild-type trees (Table 3). Although a number of lines showed a decreasing trend in the acid-soluble lignin content when compared with the wild-type control trees, there were no statistically significant changes in overall lignin content. Phloroglucinol staining confirmed the wet chemical determination of total cell wall lignin content and suggests that lignin in the transgenics is comparable to that in wild-type trees (Fig. 2).
Table 3.
Chemical composition of stem segments (PI = 5 to PI = 15) of SuSy transgenic and wild-type poplar trees
| Transgenic Line | % Carbohydrates |
% Lignin |
||||||
|---|---|---|---|---|---|---|---|---|
| Arabinose | Galactose | Glucose | Xylose | Mannose | Rhamnose | Acid soluble | Acid insoluble | |
| Control | 0.53 (0.03) | 1.00 (0.08) | 36.56 (1.05) | 15.49 (0.22) | 1.65 (0.05) | 0.50 (0.01) | 3.94 (0.26) | 20.15 (0.46) |
| 2 × 35S::SuSy 1 | 0.36 (0.02)*† | 0.93 (0.03) | 40.97 (1.06)*† | 15.33 (0.34) | 2.34 (0.36) | 0.55 (0.02) | 2.72 (0.02)*† | 20.68 (0.55) |
| 2 × 35S::SuSy 2 | 0.43 (0.03)† | 0.99 (0.02) | 40.71 (0.87)*† | 15.82 (0.74) | 2.38 (0.38) | 0.51 (0.03) | 3.16 (0.32) | 20.92 (0.12) |
| 2 × 35S::SuSy 3 | 0.41 (0.01)*† | 1.03 (0.01) | 41.01 (1.09)*† | 16.03 (0.95) | 2.41 (0.25)† | 0.51 (0.01) | 3.56 (0.43) | 22.50 (1.78) |
| 4CL::SuSy 1 | 0.47 (0.01) | 1.04 (0.05) | 39.82 (0.86)† | 16.32 (0.89) | 2.13 (0.12)*† | 0.54 (0.02) | 3.26 (0.16)† | 19.65 (0.33) |
| 4CL::SuSy 2 | 0.37 (0.0)*† | 1.03 (0.11) | 41.27 (1.49)† | 15.16 (0.03) | 2.25 (0.42) | 0.49 (0.00) | 3.23 (0.30) | 19.55 (0.51) |
| 4CL::SuSy 3 | 0.42 (0.05) | 0.99 (0.04) | 39.32 (0.98) | 15.86 (0.22) | 2.22 (0.33) | 0.47 (0.03) | 3.48 (0.10) | 20.41 (1.20) |
| 4CL::SuSy 4 | 0.40 (0.04)† | 1.03 (0.07) | 39.75 (0.55)† | 15.78 (0.43) | 1.91 (0.12) | 0.51 (0.06) | 3.36 (0.13) | 20.74 (0.47) |
Mean (SE) values were calculated from 3 plants per line.
*, significance at α = 0.05;
†, significant difference from control values at α = 0.10.
Fig. 2.
Calcofluor (A, B) and phloroglucinol (C, D) staining of wild-type (A, C) and 2 × 35S::SuSy-1 transformed (B, D) poplar trees. Arrows indicate the observed increased cell wall thickening in transgenic lines. (Scale bars: 90 μm.)
The observed increases in glucose content from the Klason analysis resulted not only from glucose-associated increases in hemicelluloses but also from increases in α-cellulose content (Table 4). All lines were confirmed to have increased α-cellulose content, ranging from 2% to 6% by weight, as compared with the corresponding wild-type trees. To investigate whether the changes in cellulose were related to a change in cellulose production and not to a formation of tension wood (generally known to have more cellulose of higher crystallinity and lower microfibril angle), the crystallinity and microfibril angle of all transgenic and wild-type stems were determined. Cell wall crystallinity was increased in all but 1 of the transgenic lines with a maximal change of 5% (of the total cell wall material) in 1 line. None of the transgenic lines showed major changes in microfibril angle (Table 4). Microscopy and histochemical staining of stem cross-sections also clearly demonstrates an increase in cellulose content, as shown by calcofluor staining, in all transgenic lines (Fig. 2) and no indication of a G-layer commonly associated with tension wood formation. Furthermore, the cell walls appear to be thicker in the transgenic lines than in the wild-type trees; this increased thickness also may contribute to the high stem weights observed in the transgenic lines (Fig. 1). The increased secondary cell wall thickness, as observed visually, was confirmed by x-ray densitometry determination of wood density, showing that all transgenic lines had significantly greater wood density (Table 4).
Table 4.
α-Cellulose content, cell wall crystallinity, microfibril angle (MFA), and wood density of stem segments (PI = 5 to PI = 15) of SuSy transgenic and wild-type poplar trees
| Transgenic Line | α-Cellulose (% dry weight) | Cell wall crystallinity (%) | MFA (°) | Wood density (kg/m3) |
|---|---|---|---|---|
| Control | 31.96 (0.07) | 51.40 (0.51) | 16.70 (0.35) | 288.8 (14.1) |
| 2 × 35S::SuSy 1 | 38.29 (1.22)*† | 56.60 (1.75)*† | 15.66 (0.81) | 345.6 (29.6)*† |
| 2 × 35S::SuSy 2 | 36.61 (0.54)† | 54.00 (1.00)† | 16.63 (0.87) | 334.4 (14.8)*† |
| 2 × 35S::SuSy 3 | 35.76 (1.20)† | 56.40 (1.44)*† | 16.63 (0.55) | 310.6 (13.4)† |
| 4CL::SuSy 1 | 37.13 (0.04)*† | 53.40 (0.75)*† | 16.43 (1.31) | 315.8 (23.4)*† |
| 4CL::SuSy 2 | 37.35 (0.87)† | 53.25 (0.48)*† | 19.08 (1.22) | 328.1 (18.7)*† |
| 4CL::SuSy 3 | 35.90 (0.47)† | 53.00 (1.00) | 18.21 (0.88) | 324.7 (32.2)*† |
| 4CL::SuSy 4 | 35.08 (0.46)*† | 54.00 (1.00)*† | 17.30 (1.04) | 365.5 (31.2)*† |
Mean (SE) values were calculated from 3 plants per line.
*, significance at α = 0.05;
†, significant difference from control values at α = 0.10.
Discussion
This study investigated the effects of overexpressing exogenous SuSy on tree growth and secondary cell wall chemistry and ultrastructure in hybrid poplar. Quantification of transcript abundance of the transgenes showed variable expression among lines and tissues. In general, the 2 × 35S::SuSy transgenic lines had higher expression in leaf tissue than in developing xylem and similarly, had higher transcript abundance than the 4CL::SuSy transgenic lines. Despite the tissue specificity of the 4CL promoter, there was no discernable difference in transgene expression in the developing xylem when the transcript abundance of 4CL::SuSy and 2 × 35S::SuSy transgenic lines were compared.
The difference in exogenous transcript abundance between leaf and xylem tissue also was reflected in the enzyme activity: SuSy was much more active in the leaf tissue than in the developing xylem. These observations are consistent with those in the wild-type trees, which inherently have an approximately 100-fold difference in SuSy activity between leaf and developing xylem tissue (Table 1). These findings concur with Le Hir et al. (21), who observed high levels of SuSy in leaf tissue of Quercus robur, with undetectable levels in the stem. An obvious trend toward increased SuSy enzyme activity was apparent in all transgenic lines, with increases greater than 2.75-fold being observed in the leaf tissue in some lines. Similar increases were observed in developing xylem, and in 1 line the increase was greater than 2.5-fold. Furthermore, transcript levels of native SuSy genes were not adversely affected by the addition of the exogenous genes.
An assessment of plant growth and architecture suggested that the overexpression of the SuSy transgene had no pleiotropic effect on tree growth, because the trees appeared phenotypically normal. Although a few lines appeared slightly shorter or had minor changes in caliper measurement relative to corresponding wild-type trees, the total stem biomass at harvest fell within the biological variation observed in the control trees. Konishi et al. (15) recently reported an increase in height growth in some lines of Populus alba transformed with a modified mung bean SuSy under the control of the 35S promoter. In tobacco, the overexpression of the G. hirsutum SuSy also resulted in increased height growth (17). Similarly, the downregulation of SuSy has been shown to influence height growth in other plants. For example, using antisense suppression, Tang and Sturm (22) generated carrot plants that were smaller and had fewer leaves, and D'Aoust et al. (23) generated tomatoes with reduced fruit size and compromised sucrose unloading capacity. Because SuSy generally is thought to exert control over sink strength, biomass accumulation is consistent with its overexpression, whereas suppression of SuSy can be associated with decreased growth.
Previous studies evaluating SuSy transgenic plants have demonstrated significant changes in soluble carbohydrate contents manifested by the misregulation of these genes. For example, a maize endosperm SuSy mutant had a 2- to 4-fold increase in localized sucrose levels (16). Consistently, the overexpression of SuSy in tobacco resulted in an increase in stem glucose and fructose concentrations (17). Similar findings are apparent in the present study, suggesting that plants generally attempt to maintain a basal concentration of sucrose in the sink tissue (stem) despite the substantially increased catabolism of sucrose into glucose and fructose, which consequently accumulate. With increased SuSy activity in the sink tissue, there is an associated increase in sucrose catabolism, and therefore more sucrose can be translocated to the sink tissue. A comparison of wheat variants (Triticum aestivum) with differing water-soluble carbohydrate concentrations showed that SuSy transcript abundance (and enzyme activity) was inversely correlated with water-soluble carbohydrate accumulation (18). The same pattern was apparent with the transcript abundance of 3 cellulose biosynthesis subunits (TaCesA1, TaCesA4-like, and TaCesA10). It was concluded that these gene families, along with others, when present in lower abundance, play a role in the accumulation of water-soluble carbohydrates, and that this accumulation is associated with a decrease in sucrose hydrolysis and decreased polysaccharide accumulation. No associated change in height growth or stem strength was observed with a lower level of cell wall polysaccharides (18).
Like SuSy, UDP glucose pyrophosphorylase (UGPase) affects sink strength in plants. Most studies have focused on the downregulation of UGPase because it is thought to exist in abundance in plants (24, 25). UGPase antisense reduction in potato tubers has resulted in conflicting results, which range from no change in soluble sugars, despite a 96% reduction in activity (26), to significant reductions in soluble carbohydrates (27). In Arabidopsis, plants with decreased UGPase activity had lower soluble carbohydrate and starch content (28). Tobacco plants overexpressing UGPase, on the other hand, showed increased glucose and fructose content but only small changes in sucrose concentration and no change in cellulose production (17). Poplar overexpressing UGPase under the control of the 2 × 35S promoter had significant increases in all soluble carbohydrates in leaf tissue. In the developing xylem, glucose and sucrose were increased, although less so than in leaf tissue (29). Studies of UGPase expression in native poplar clearly show the coordinate upregulation of UGPase with late cell expansion and secondary cell wall formation (10) as well as during the formation of tension wood (20), which is known to be closely linked to cellulose production. These findings suggest that the generation of altered levels of UDP-glucose, either by UGPase or SuSy, can facilitate the re-allocation of carbon skeletons derived from photosynthate toward cellulose deposition, because UDP-glucose is the immediate precursor to cellulose polymer biosynthesis (29).
Contrary to the current results, Konishi et al. (15) did not observe altered carbon allocation in poplar, because the xylem cellulose content of trees expressing the modified mung bean SuSy was unchanged. This discrepancy may be a consequence of the origin of the exogenous gene and resulting differences in expression. Konishi et al. (15) attributed the lack of changes in structural carbohydrates to the relatively higher expression of SuSy in leaf tissue as compared with stem tissue. Furthermore, the expression of the mung bean SuSy was higher in the soluble fraction than in the microsomal membrane fraction and as such probably influenced the recycling of fructose. Subsequently, in feeder studies with these transgenic lines, H3-labeled fructose was shown to be incorporated into both cellulosic and non-cellulosic polymers at a greater rate than in control plants. In the current study, even after the inherently higher levels of SuSy activity in leaves were taken into account, SuSy activity in the leaves of the transgenic overexpressing poplar was increased by up to 1.7-fold as compared with the wild-type control trees. Similarly, SuSy activity in the developing xylem was significantly increased, and this increase undoubtedly accounts for the increased cellulose production in the secondary xylem of the woody stems. Furthermore, the SuSy transgene used in the current study has been shown previously to be strongly associated with cellulose formation in cotton and may associated more closely with the activity of the cellulose synthase complex in the cell wall (7). This hypothesis is supported by evidence in pea, clearly showing that different isoforms of SuSy are associated with the different metabolic fates of sucrose (30).
In the hybrid poplar system evaluated in this study, the elevated levels of cellulose deposition in the secondary xylem resulting from the overexpression of cotton SuSy also was mirrored by a significant change in the ultrastructural characteristic of the cellulose, because the trees had increased cell wall crystallinity. The higher crystalline nature of the cell wall was not associated with a commensurate increase in microfibril angle, as would be expected in the formation of tension wood (31). Calcofluor staining also clearly shows an increased fluorescence associated with increased cellulose, and there is no evidence of the G-layer that would appear in tension wood (Fig. 2). Furthermore, the individual cell walls appear to be thicker than those in wild-type plants. Measures of wood cell wall density confirmed the increased cell wall thickness, as all transgenic lines displayed increased wood density. In a previous study (20), an evaluation of the gene expression patterns associated with the formation of tension wood identified many genes, including both SuSy and UGPase, associated with its formation and the inherent higher degree of cellulose production. SuSy was identified as being among the most highly expressed genes in tension wood, with ratios of 1.57 (PttSuS1) and 1.39 (PttSuS2) relative to their expression in normal wood. In an independent study, UDP-glucose pyrophosphorylase (PHUGP2) was shown to be present at a ratio of 2.31 relative to normal wood (20).
The inconsistencies in biomass results among investigations evaluating SuSy expression may be related directly to the observed differences in cell wall constituents (cellulose content). Increasing cellulose deposition, augmented by the misregulation of genes influencing key pathway steps, could manifest in non-accelerated tree growth as more energy and carbon skeletons are directed or committed to cell wall deposition rather than to cell initiation or elongation. To this effect, varying results have been observed in trees with increased cellulose content. For example, we previously have shown large increases in cellulose content (2.8% to 6.5%) in UGPase-transformed poplar; these increases were at the expense of biomass accumulation (29). Furthermore, these trees were severely stunted, an effect that was attributed in part to synthesis and accumulation of a salicylic acid glucoside, which has been shown in tobacco to be synthesized by a salicylic acid glucosyltransferase that employs UDP-glucose as the sole source of glucose (32). The measurements of height growth in the current study do not mirror the findings of Konishi et al. (15) or those observed in UGPase-overexpressing transgenics, perhaps because the closer connection between SuSy (over UGPase) and the cellulose synthase complex proteins (CesA subunits) facilitates a direct metabolic channel for the biosynthesis of UDP-glucose to cellulose. Other studies, however, have shown marginal increases in cellulose content associated with an increase in height (33, 34).
Similarly, in other plant species, the level of SuSy expression has been shown to be associated strongly with cellulose synthesis. Expression of the modified mung bean SuSy (S11E) in Acetobacter xylinum caused enhanced cellulose production by preventing the accumulation of UDP, which is known to inhibit cellulose formation in A. xylinum (35, 11). In addition, carrot plants with suppressed SuSy showed decreased cellulose content (22), whereas suppression of SuSy in cotton resulted in an almost fiberless phenotype (36). Given that both SuSy and UGPase independently increased cellulose accumulation in poplar, an attempt to create double transgenics harboring both genes, by pyramiding genes under the regulation of the vascular specific promoter, was attempted. To this end, gene stacking did not offer an advantage in diverting carbon skeletons from photosynthate to cellulose deposition, because additive effects were not apparent (data not shown). Thus, it appears that, in poplar, SuSy alone provides the largest effect in maintaining height growth and biomass accumulation and in improving the extent of cellulose deposition.
In summary, the overexpression of SuSy in hybrid poplar resulted in increased cellulose synthesis, providing evidence for a direct connection between sucrose supply (sink strength), its breakdown, and cellulose deposition through the activity of SuSy. In addition to the increased deposition of cellulose in the secondary cell walls, the crystalline nature of the wall was increased, as was xylem cell wall thickness and, consequently, wood density. These improved cell wall traits have significant economic and productivity implications in the industrial utility of this wood fiber, both in the traditional wood sectors and as a substrate for future bioenergy crops.
Methods
Plasmid Construction.
SuSy was cloned from Gossypium hirsutum (Perez-Grau, GenBank U73588) as previously described (17) and was inserted into pBIN under the regulation of 1 of 2 promoters: the enhanced tandem cauliflower mosaic virus 35S constitutive promoter (2 × 35S) (37, 38) or the vascular-specific 4CL (Petroselinum crispum 4-coumarate:CoA ligase) promoter (39).
Plant Transformation and Maintenance.
Hybrid poplar (Populus alba × grandidentata) was transformed using Agrobacterium tumefaciens EHA105 (40), according to Coleman et al. (17). Plants were confirmed as transgenic by PCR screening of genomic DNA using gene-specific oligonucleotides, SUS-F (5′-ctcaacatcacccctcgaat-3′) and SUS-R (5′-accaggggaaacaatgttga-3′). All shoot cultures, including transgenic and wild-type lines, were maintained on solid woody plant medium (41) with 0.01 μM 1-naphthaleneacetic acid in GA-7 vessels (Fisher Scientific) at 22 °C under a 16-h photoperiod with an average photon flux of 50 μmol m−2 s−1 until out-planting to the greenhouse.
Growth Conditions and Biomass Measurements.
Wild-type control trees and each transgenic line, represented by a minimum of 12 individual trees, were transferred into 7.5-L pots (containing 50% peat, 25% fine bark, and 25% pumice soil mixture) in the greenhouse. The trees were grown under 16-h days supplemented with overhead lighting with a radiant flux density of 300 W m−2. After 4 months of growth, the trees were harvested, and the total height and the stem diameter (10 cm above root collar) were determined for each tree.
Tissue developmental stage was standardized using a plastichron index in which PI = 0 was defined as the first leaf greater than 5 cm in length and PI = 1 was defined as the leaf immediately below PI = 0. Stem segments spanning PI = 15 to PI = 16 were retained for wood cell wall and chemical analysis. Leaves from PI = 3 to PI = 5 were frozen in liquid nitrogen and were retained for RNA, enzyme, and soluble carbohydrate analysis. Developing xylem spanning PI = 3 to PI = 5 was scraped and flash frozen in liquid nitrogen for analysis of enzyme activity, RNA transcript abundance, and soluble carbohydrate content. Each of the following analyses was conducted on a minimum of 3 independent ramets of each transgenic line and the control plants (the selected ramet was used for all analyses).
Transcription Levels.
Real-time PCR was used to determine transcript abundance of each transgene. Leaf and developing xylem samples (1 g fresh weight) were ground in liquid nitrogen, and RNA was extracted according to the method of Kolosova et al. (42) Then 10 μg of RNA was treated with TURBO DNase™ (Ambion) to remove residual DNA. Subsequently, 1 μg of DNase-treated RNA was used for the synthesis of cDNA using SuperScript II Reverse Transcriptase (Invitrogen) and dT16 primers according to the manufacturer's instructions. Samples were run in triplicate with Brilliant SYBR Green QPCR Master Mix (Stratagene) on an Mx3000P Real-Time PCR System (Stratagene) to determine critical thresholds (Ct). The primers used for RT-PCR analysis of SuSy were GS-RTF (5′-ccgtgagcgtttggatgagac-3′) and GS-RTR (5′-ggccaaaatctcgttcctgtg-3′). As a house-keeping control, the transcript abundance of TIF5A was used for normalization (43). The primers for TIF5A transcript quantification were TIF5A-RTF (5′-gacggtattttagctatggaattg-3′) and TIF5A-RTR (5′-ctgataacacaagttccctgc-3′). Conditions for the RT-PCR reactions were as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 seconds, 62 °C (64 °C for SuSy, 55 °C for TIF5A) for 1 min, and 72 °C for 30 seconds. Relative expression was determined according to Levy et al. (44) using the equation: Δct = 2−(ctUGPase or SuSy− ctTIF5A).
Concurrently, we examined transcript levels of native poplar SuSy genes. We selected 3 SuSy genes based on their coordinate upregulation with the cellulose synthase complex genes, as shown by Hertzberg et al. (10). Of the 3 genes, 2 (AI162073 and AI163208) had an extremely high level of homology and were measured using the same primer set PtSuS1-F (5′-accagcacattccaagagattgct-3′) and PtSuS1-R (5′-tgtagaggccagggagagtga aag-3′). The third gene (AI165194) was quantified with the primers PtSuS2-F (5′-gataagaatcgaaacagacccgga-3′) and PtSuS2-R (5′-taattgttctttgatgaactcc-3′).
Enzyme Activity.
Leaf and developing xylem samples (1 g fresh weight) were ground in liquid nitrogen with 1 mg of insoluble polyvinylpolypyrrolidone (PVPP) and 4 volumes of extraction buffer (50 mM Hepes-KOH, pH 7.5, 10 mM MgCl2, 1 mM EDTA, 2 mM DTT, 1 mM PMSF, 5 mM ε-Amino-n-caproic acid, 0.1% vol/vol Triton X-100, and 10% vol/vol glycerol). The samples were centrifuged subsequently at 15,000 × g for 20 min at 4 °C. The extract was passed through a desalting column (DG 10; BioRad) pre-equilibrated with ice-cold extraction buffer without Triton X-100 and PVPP. Extracts were collected in prechilled vials and assayed immediately. SuSy activity was assayed in the direction of sucrose breakdown (45), using 50 μL of plant extract. The liberated fructose content was determined using a tetrazolium blue assay (46). This SuSy assay employs the appropriate controls without the supplementation of UDP to quantify inherent invertase activity and therefore represents only the catalysis of sucrose by sucrose synthase. Total protein content of the extracts was determined using a Bio-Rad protein assay.
Soluble Carbohydrate and Starch Analysis.
Soluble carbohydrates (glucose, fructose, and sucrose) were extracted from ground freeze-dried tissue overnight at −20 °C using methanol:chloroform:water (12:5:3) as previously described (17). Soluble carbohydrates then were analyzed by anion exchange HPLC using a Dionex DX-600 equipped with a Carbopac PA1 column and an electrochemical detector. The residual pellet of plant residue then was hydrolyzed in 4% sulfuric acid at 121 °C for 4 min. The liberation of glucose, representing starch content, was quantified directly by HPLC under similar conditions.
Cell Wall Chemistry.
Oven-dried stem material was ground using a Wiley mill to pass through a 40-mesh screen and was Soxhlet extracted with hot acetone for 24 h. Lignin and carbohydrate content were determined on 0.2 g dry-weight extract-free tissue using a modified Klason method (29), on wood material isolated 5 cm above the root collar, adjacent to samples used for the determination of wood ultrastructure. Carbohydrate content was determined by HPLC using a Dionex DX-600 equipped with an anion exchange PA1 column, a pulsed amperometric detector with a gold electrode, and post-column detection. Acid-insoluble lignin was determined gravimetrically; acid-soluble lignin was determined using spectrophotometric analysis at 205 nm according to Technical Association of the Pulp and Paper Industry Useful Method 250 (TAPPI UM-250) (47).
Determination of α-Cellulose and Holocellulose Content.
Holocellulose and α-cellulose contents were determined according to the method of Yokoyama et al. (48). After gravimetric determination, the resultant α-cellulose fraction was acid hydrolyzed to ensure purity of the cellulosic fraction (complete removal of hemicellulose or lignin moieties).
Crystallinity and Microfibril Angle.
Microfibril angle and cell wall crystallinity were determined by x-ray diffraction using a Bruker D8 Discover x-ray diffraction unit equipped with a general area detector diffraction system (GADDS) on the radial face of the wood section precision cut (1.69 mm) from the growing stem isolated 5 cm above the root collar. Wide-angle diffraction was used in transmission mode, and the measurements were performed with CuKα1 radiation (λ = 1.54 Å), the x-ray source fit with a 0.5-mm collimator, and the scattered photon collected by a GADDS detector. Both the x-ray source and the detector were set to theta = 0° for microfibril angle determination; the 2 theta (source) was set to 17° for wood crystallinity determination. The average T-value of the two 002 diffraction arc peaks was used for microfibril angle calculations, as per the method of Megraw et al. (49). Crystallinity was determined by mathematically fitting the data using the method of Vonk (50). Crystallinity measures were precalibrated by capturing diffractograms of pure A. xylinum bacterial cellulose (known to be 87% crystalline). The 2 radii were taken from samples isolated 5 cm above ground on each tree, and these values were averaged for each tree.
Wood Density.
Wood density was measured by x-ray densitometry (Quintek Measurement Systems).on the same precision-cut samples used for crystallinity and microfibril angle determination. Pith-to-bark sections of each tree were scanned at a resolution of 0.0254 mm, and the data are reported as relative density on an oven-dry weight basis.
Microscopy.
Stem cross-section specimens (40 μm thick) were cut using a microtome from poplar stems isolated 5 cm above the root collar (the same section used for ultrastructure determination). Sections were mounted on glass slides and visualized using a Leica microscope under UV fluorescence.
Histochemical examination of cellulose was carried out using calcofluor staining. The samples were mounted in 10% KOH (wt/vol) and 0.1% calcofluor white (wt/vol) and were viewed after 5 min of staining under bright-field illumination with a Leica DMR microscope equipped with a QICAM CCD camera (QImaging). In contrast, histochemical examination of lignin was analyzed using phloroglucinol staining, achieved by mounting similar stem sections in a saturated solution of phloroglucinol in 20% HCl. Samples were viewed under dark-field illumination on the Leica DMR microscope.
Supplementary Material
Acknowledgments.
This project was supported by the Natural Science and Engineering Research Council of Canada (S.D.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900188106/DCSupplemental.
References
- 1.Asano T, et al. Rice SPK, a calmodulin-like domain protein kinase, is required for storage product accumulation during seed development: Phosphorylation of sucrose synthase is a possible factor. Plant Cell. 2002;14(3):619–628. doi: 10.1105/tpc.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun J, Loboda T, Sung S-JS, Black CCJ. Sucrose synthase in wild tomato, Lycopersicon chmielewskii, and tomato fruit sink strength. Plant Physiol. 1992;98:1163–1169. doi: 10.1104/pp.98.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zrenner R, Salanoubat M, Willmitzer L, Sonnewald U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.) Plant J. 1995;7(1):97–107. doi: 10.1046/j.1365-313x.1995.07010097.x. [DOI] [PubMed] [Google Scholar]
- 4.Dejardin A, Sokolov LN, Kleczkowski LA. Sugar/osmoticum levels modulate differential abscisic acid-independent expression of two stress-responsive sucrose synthase genes in Arabidopsis. Biochem J. 1999;344:503–509. [PMC free article] [PubMed] [Google Scholar]
- 5.Nolte KD, Koch KE. Companion-cell specific localization of sucrose synthase in zones of phloem loading and unloading. Plant Physiol. 1993;101:899–905. doi: 10.1104/pp.101.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subbaiah CC, Sachs MM. Altered patterns of sucrose synthase phosphorylation and localization precede callose induction and root tip death in anoxic maize seedling. Plant Physiol. 2001;125:585–594. doi: 10.1104/pp.125.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amor Y, Haigler C, Johnson S, Wainscott M, Delmer DP. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA. 1995;92:9353–9357. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckeridge MS, Vergara CE, Carpita NC. The mechanism of synthesis of a mixed-linkage (1–3), (1–4) β-D-glucan in maize. Evidence for multiple sites of glucosyl transfer in the synthase complex. Plant Physiol. 1999;120:1105–1116. doi: 10.1104/pp.120.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauch S, Magel E. Extractable activities and protein content of sucrose-phosphate synthase, sucrose synthase and neutral invertase in trunk tissues of Robinia pseudoacacia L. are related to cambial wood production and heartwood formation. Planta. 1998;207:266–274. [Google Scholar]
- 10.Hertzberg M, et al. A transcriptional roadmap to wood formation. Proc Natl Acad Sci USA. 2001;98:14732–14737. doi: 10.1073/pnas.261293398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benziman M, Aloni Y, Delmer DP. Unique regulatory properties of the UDP-glucose: β-1,4-glucan synthetase of Acetobacter xylinum. Journal of Applied Polymer Science. 1983;(37):131–143. [Google Scholar]
- 12.Winter H, Huber JL, Huber SC. Membrane association of sucrose synthase: Changes during the graviresponse and possible control by protein phosphorylation. FEBS Lett. 1997;420:151–155. doi: 10.1016/s0014-5793(97)01506-8. [DOI] [PubMed] [Google Scholar]
- 13.Komina O, Zhou Y, Sarath G, Chollet R. In vivo and in vitro phosphorylation of membrane and soluble forms of soybean nodule sucrose synthase. Plant Physiol. 2002;129:1664–1673. doi: 10.1104/pp.002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter H, Huber SC. Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Crit Rev Biochem Mol Biol. 2000;35(4):253–289. doi: 10.1080/10409230008984165. [DOI] [PubMed] [Google Scholar]
- 15.Konishi T, Ohmiya Y, Hayashi T. Evidence that sucrose loaded into the phloem of a poplar leaf is used directly by sucrose synthase associated with various β-glucan synthases in the stem. Plant Physiol. 2004;134:1146–1152. doi: 10.1104/pp.103.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chourey PS, Nelson SE. The enzymatic deficiency conditioned by the shrunken-1 mutation in maize. Biochem Genet. 1976;14:1041–1055. doi: 10.1007/BF00485135. [DOI] [PubMed] [Google Scholar]
- 17.Coleman HD, Ellis DD, Gilbert M, Mansfield SD. Up-regulation of sucrose synthase and UDP-glucose pyrophosphorylase impacts plant growth and metabolism. Plant Biotechnology Journal. 2006;4:87–101. doi: 10.1111/j.1467-7652.2005.00160.x. [DOI] [PubMed] [Google Scholar]
- 18.Xue G-P, et al. Molecular dissection of variation in carbohydrate metabolism related to water soluble carbohydrate accumulation in stems of wheat (Triticum aestivum L.) Plant Physiol. 2007;146:441–454. doi: 10.1104/pp.107.113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albrecht G, Mustroph A. Localization of sucrose synthase in wheat roots: Increased in situ activity of sucrose synthase correlates with cell wall thickening by cellulose deposition under hypoxia. Planta. 2003;217:252–260. doi: 10.1007/s00425-003-0995-6. [DOI] [PubMed] [Google Scholar]
- 20.Andersson-Gunneras S, et al. Biosynthesis of cellulose-enriched tension wood in Populus: Global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary wall biosynthesis. Plant J. 2006;45:144–165. doi: 10.1111/j.1365-313X.2005.02584.x. [DOI] [PubMed] [Google Scholar]
- 21.Le Hir R, Pelleschi-Travier S, Viemont J, Leduc N. Sucrose synthase expression pattern in the rhythmically growing shoot of common oak (Quercus robur L.) Annals of Forest Science. 2005;62:585–591. [Google Scholar]
- 22.Tang GQ, Sturm A. Antisense repression of sucrose synthase in carrot (Daucus carota L.) affects growth rather than sucrose partitioning. Plant Mol Biol. 1999;41:465–479. doi: 10.1023/a:1006327606696. [DOI] [PubMed] [Google Scholar]
- 23.D'Aoust M-A, Yelle S, Nguyen-Quoc B. Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit. Plant Cell. 1999;11:2407–2418. doi: 10.1105/tpc.11.12.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appeldoorn NJG, et al. Developmental changes of enzymes involved in conversion of sucrose to hexose-phosphate during early tuberisation of potato. Planta. 1997;202:220–226. [Google Scholar]
- 25.Magel E, Abdel-Latif A, Hampp R. Non-structural carbohydrates and catalytic activities of sucrose metabolizing enzymes in trunks of two Juglans species and their role in heartwood formation. Holzforschung. 2001;55:135–145. [Google Scholar]
- 26.Zrenner R, Willmitzer L, Sonnewald U. Analysis of the expression of potato uredinediphosphate-glucose pyrophosphorylase and its inhibition by antisense RNA. Planta. 1993;190:247–252. doi: 10.1007/BF00196618. [DOI] [PubMed] [Google Scholar]
- 27.Spychalla JP, Scheffler BE, Sowokinos JR, Bevan MW. Cloning, antisense RNA inhibition, and the coordinated expression of UDP-glucose pyrophosphorylase with starch biosynthetic genes in potato tubers. J Plant Physiol. 1994;144:444–453. [Google Scholar]
- 28.Kleczkowski LA, Geisler M, Ciereszko I, Johansson H. UDP-glucose pyrophosphorylase. An old protein with new tricks. Plant Physiol. 2004;134:912–918. doi: 10.1104/pp.103.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman HD, Canam T, Kang KY, Ellis DD, Mansfield SD. Over-expression of UDP-glucose pyrophosphorylase in hybrid poplar affects carbon allocation. J Exp Bot. 2007;58(15/16):4257–4268. doi: 10.1093/jxb/erm287. [DOI] [PubMed] [Google Scholar]
- 30.Barratt DHP, et al. Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol. 2001;127:655–664. [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi CP. Xylem-specific and tension stress-responsive expression of cellulose synthase genes from aspen trees. Appl Biochem Biotechnol 105- 2003;108:17–25. doi: 10.1385/abab:105:1-3:17. [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Raskin I. Glucosylation of salicylic acid in Nicotiana tabacum Cv. Xanthi-nc. Phytopathology. 1998;88(7):692–697. doi: 10.1094/PHYTO.1998.88.7.692. [DOI] [PubMed] [Google Scholar]
- 33.Park YW, et al. Enhancement of growth and cellulose accumulation by overexpression of xyloglucanase in poplar. FEBS Lett. 2004;564:183–187. doi: 10.1016/S0014-5793(04)00346-1. [DOI] [PubMed] [Google Scholar]
- 34.Hu W-J, et al. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nature. 1999;17:808–812. doi: 10.1038/11758. [DOI] [PubMed] [Google Scholar]
- 35.Nakai T, et al. Enhancement of cellulose production by expression of sucrose synthase in Acetobacter xylinum. Proc Natl Acad Sci USA. 1999;96:14–18. doi: 10.1073/pnas.96.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruan YL, Llewellyn DJ, Furbank RT. Suppression of sucrose synthase gene expression represses cotton fibre cell initiation, elongation, and seed development. Plant Cell. 2003;15:952–964. doi: 10.1105/tpc.010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datla RSS, et al. Improved high-level constitutive foreign gene translation using an AMV RNA4 untranslated leader sequence. Plant Sci. 1993;94:139–149. [Google Scholar]
- 38.Kay R, Chan A, Daly M, McPherson J. Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science. 1987;236(4806):1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- 39.Hauffe KD, et al. A parsley 4CL-1 promoter fragment specifies complex expression patterns in transgenic tobacco. Plant Cell. 1991;3:435–443. doi: 10.1105/tpc.3.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hood EE, Gelvin SB, Melcher LS, Hoekema A. New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 1993;2(4):208–218. [Google Scholar]
- 41.McCown BH, Lloyd G. Woody Plant Medium (WPM)—a mineral nutrient formulation for microculture for woody plant species. Hortic Sci. 1981;16:453. [Google Scholar]
- 42.Kolosova N, et al. Isolation of high-quality RNA from gymnosperm and angiosperm trees. BioTechniques. 2004;36(5):821–824. doi: 10.2144/04365ST06. [DOI] [PubMed] [Google Scholar]
- 43.Ralph S, et al. Genomics of hybrid poplar (Populus trichocarpa × deltoides) interacting with forest tent caterpillars (Malacosoma disstria): Normalized and full-length cDNA libraries, expressed sequence tags (ESTs), and a cDNA microarray for the study of insect-induced defenses in poplar. Molecular Ecology. 2006;15:1275–1297. doi: 10.1111/j.1365-294X.2006.02824.x. [DOI] [PubMed] [Google Scholar]
- 44.Levy M, Edelbaum O, Sela I. Tobacco mosaic virus regulates the expression of its own resistance gene N1. Plant Physiol. 2004;135:2392–2397. doi: 10.1104/pp.104.044859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chourey PS. Genetic control of sucrose synthetase in maize endosperm. Mol Gen Genet. 1981;184(3):372–376. [Google Scholar]
- 46.Kennedy JF, White CA. Bioactive Carbohydrates in Chemistry, Biochemistry and Biology. New York: Halstead Press; 1983. [Google Scholar]
- 47.Technical Association of the Pulp and Paper Industry. Atlanta: Tappi Press; 1991. Tappi Useful Method UM-250. Acid-soluble lignin in wood and pulp. [Google Scholar]
- 48.Yokoyama T, Kadla JF, Chang HM. Microanalytical method for the characterization of fiber components and morphology of woody plants. J Agric Food Chem. 2002;50:1040–1044. doi: 10.1021/jf011173q. [DOI] [PubMed] [Google Scholar]
- 49.Megraw RA, Leaf G, Bremmer D. Longitudinal shrinkage and microfibril angle in loblolly pine. In: Butterfield BA, editor. Microfibril Angle in Wood. Christchurch, New Zealand: University of Canterbury Press; 1998. pp. 27–61. [Google Scholar]
- 50.Vonk CC. Investigation of non-ideal two-phase polymer structures by small-angle x-ray scattering. J Appl Crystallogr. 1973;6:81–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.