At the interface of Earth science, chemistry, and biology, scientists are working to interpret the record of biological markers, or biomarkers, retained in natural environments. Although these molecular fossils take many forms, arguably the most useful and durable have lipid character and are amenable to gas-chromatographic separation. Many such compounds are related to extant natural products by distinctive molecular structures and thus can be related to specific taxonomic groups, functions, or processes. Moreover, biomarkers may contain information in their relative abundance of light and heavy isotopes. The isotopes of carbon have been most fully explored (1), but following a decade of advances in compound-specific deuterium analysis (2, 3), the relative abundance of the hydrogen isotopes protium (1H) and deuterium (2H or D) has received increasing attention. Most commonly these analyses are used to track past changes of climate and aridity, enabled by the strong correlation of deuterium content between plant-derived lipids and source water (4–8). In this issue of PNAS, Zhang et al. (9) move in a new direction and lay the groundwork for a proxy of cellular metabolism based on lipid deuterium. They present results from laboratory experiments with bacterial cultures demonstrating that the deuterium content of lipid biomarker compounds is strongly modulated by the central biochemical pathway a cell uses to generate reducing power for biosynthesis. These results suggest that the deuterium content of lipid biomarkers in the geomolecular record may provide a window into the central metabolic pathways active at a given time and place—isotopic remembrance of metabolism past.
The castof bacterial characters examined by Zhang et al. (9) includes four strains representing three lineages known as α, β, and γ Proteobacteria. The heterotroph Escherichia coli, a γ-proteobacterium, is the most (in)famous and was cultivated with oxygen on organic substrates. The phototroph Rhodopseudomonas palustris is an α-proteobacterium capable of both phototrophic and heterotrophic growth; it was cultivated both in the light, without oxygen and with or without organic compounds, and as a respiratory heterotroph, with oxygen and organic compounds. Finally, two species from the β-proteobacterial genus Cupriavidus—C. necator and C. oxalaticus—were cultivated under respiratory conditions as heterotrophs, as well as under an unusual condition where energy is derived from simple carbon-containing compounds, but biomass is derived from carbon dioxide autotrophy and water hydrotrophy. (I define hydrotrophy with respect to the sources of cellular hydrogen: in hydrotrophic metabolism, all carbon-bound cellular hydrogen derives from water.) The growth substrates for these different bacteria represent entry points into different metabolic cycles: the Embden–Meyerhof, Entner–Doudoroff, and pentose phosphate pathways, fed by sugars; the tricarboxylic acid (TCA) cycle, fed by organic acids; and autotrophic/hydrotrophic growth, fed both by small molecules such as formate and oxalate and by light energy. Zhang et al. systematically varied the deuterium content of the water in the growth medium while holding the deuterium content of organic substrates constant. They then measured the extent of deuterium fractionation in the cells' lipids. This experimental design mathematically constrains the sources providing hydrogen to lipids for each growth substrate, and it enables graphic representation of the isotopic relationships through fractionation curves (9, 10). With the aid of these curves the authors rigorously exclude lipid biosynthetic reactions as a major determinant of the observed deuterium fractionation.
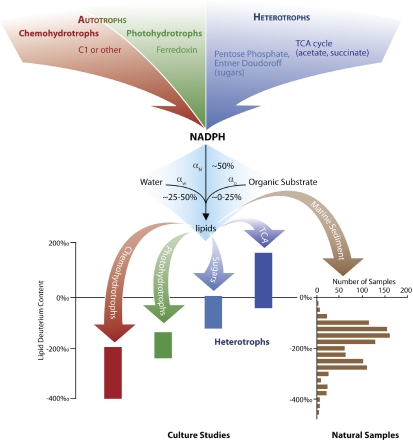
To explore the source of the observed fractionation the authors look to the enormous variation in lipid deuterium content within each single strain when grown on different substrates—as much as 50% (500‰ in the nomenclature used by geochemists; for perspective, the deuterium content of most plants varies by only ≈one-tenth this much). Given that lipid biosynthetic reactions are not responsible for this variation, the authors surmise that these dramatic differences must arise from differences in the deuterium content of the primary molecular precursor to lipid hydrogen, namely the reduced form of nicotinamide adenine dinucleotide phosphate, NADPH. The hydrogen of NADPH is derived from different reactions in the various metabolic pathways but is central to lipid biosynthesis, as shown schematically in Fig. 1. Hydride transferred from NADPH to lipids seemingly carries a deuterium signature set by its metabolic history.
Fig. 1.
At the top: NADPH is the primary immediate biosynthetic precursor of hydrogen for lipids and is generated by diverse mechanisms associated with different central metabolic pathways as indicated. In the middle: The deuterium content of lipids is set by the balance of hydrogen precursors, namely water, NADPH, and organic material. The approximate percentage contribution of each source is indicated. Each reaction is accompanied by a fractionation, designated by α. The results of Zhang et al. (9) indicate that fractionation associated with lipid biosynthesis is secondary to the upstream reactions that set the deuterium content of NADPH. At the bottom: The deuterium content of lipid biomarkers is displayed, all originating from waters with similar deuterium content. At left are the fatty acids from the cultivation studies of Zhang et al., distinguished by substrates that feed into the identified metabolic pathways. At right is a histogram summarizing the distribution of deuterium content for ≈1,000 analytes extracted from the anoxic marine sediments of the Santa Barbara Basin (11). Included in the histogram are several classes of compounds, including fatty acids, alcohols, alkanes, and isoprenoids, the latter of which tend to be depleted in deuterium. Fatty acids from this sediment range in deuterium content from −32‰ to −280‰, consistent with distinct contributions from different metabolic pathways.
These results fundamentally change our understanding as to how lipid deuterium content is set. It is well established that lipid deuterium content is controlled at the first order by deuterium levels in potential sources—namely water and/or organic molecules (10). In the absence of evidence to the contrary, the paradigm has been that isotopic fractionation during lipid biosynthesis is superimposed on these sources to set the ultimate deuterium content of the lipid. Such fractionation, commonly designated by the factor α, seemingly discriminates against deuterium. (This aspect of lipid biosynthesis is highlighted within the shaded region in the center of Fig. 1.) Zhang et al. (9) overturn this paradigm by demonstrating that the upstream metabolic pathways generating reducing power for lipid biosynthesis exert greater control on ultimate lipid deuterium content than does net fractionation during biosynthesis. Water and organic material do supply the deuterium in lipids, but the pathway that generates biosynthetic reducing power in the form of NADPH serves as an important second level of control. Fractionation during lipid biosynthesis likely impacts lipid deuterium content too, but it now appears relegated to a tertiary role.
Through a comparative analysis of the four strains identified above and previous studies with other organisms, Zhang et al. (9) provide further correlative evidence that lipids derived through the same pathways, but in different organisms, share similar deuterium content. Perhaps more fortuitously, different pathways yield different (although slightly overlapping) deuterium contents. These trends are highlighted in Fig. 1, with four metabolic types being differentiated by the resulting lipid deuterium content. When corrected for the deuterium content of source water, cultivation studies yield lipid deuterium contents ranging over ≈600‰. These differences point toward the potential application of lipid deuterium as an indicator of central metabolic pathways. But is such variability mirrored in the environment?
A recent study by Li et al. (11) helps to address this question through analysis of deuterium content in more than 1,100 lipids of putative marine origin from the sediments of the Santa Barbara Basin. A histogram summarizing their results is presented in Fig. 1, demonstrating an overall range of ≈500‰ for lipid deuterium, with a range of ≈250‰ for fatty acids. This broad range and the underlying patterns are promising and are consistent with the geomolecular record of lipid deuterium containing information about metabolism. But how might we extract and interpret this information?
Numerous questions need to be more fully addressed before the results of Zhang et al. (9) can be broadly applied to nature and the geologic record: Do all organisms follow similar metabolic patterns of deuterium abundance, or more realistically, are the inevitable exceptions acceptable? Where will the lipids of fermentative organisms lie on the deuterium scale? How does natural variability in growth, environmental conditions, and lipid biosynthesis impact lipid deuterium content? For how long will the information be retained in the geologic record? What specific molecules serve as the most promising targets? Can contributions from multiple sources be deconvoluted to estimate deuterium content of individual organisms? Do patterns observed for fatty acids apply to other biomarkers? Clearly there is much work still to be done, but the results of Zhang et al. point in an exciting direction. Support and interest willing, an approach that simultaneously investigates deuterium systematics in both modern and geologic systems holds promise. Three basic avenues of inquiry are necessary, and indeed underway. First, measurement of deuterium content and fractionation for lipid biomarkers is needed for a variety of other organisms and metabolic types to determine the applicability and limitations of the present results. Second is investigating modern environmental systems in which the deuterium content and distribution of lipid biomarkers can be compared with extant metabolism. Third, the geologic record should be mined for patterns and variations in deuterium content of lipid bio-markers, with an eye toward assessing preservation beyond a few million years (12) and extracting primary signals. After such validation activities, then maybe a new paleometabolic proxy will be born.
Beyond the development of a new paleometabolic proxy, the present results may find application for interpreting paleoclimatic and environmental lipid deuterium results, as well as in studies of metabolic dynamics. Environmental changes such as temperature, salinity, or incident radiation may subtly impact the dynamics of NADPH in the cell and thereby affect resulting lipid deuterium content. Such a mechanism should be considered as a possible explanation for the minor variations observed in numerous studies. The use of deuterium variations in other metabolic products might even allow us to track the flow of reducing power; simple measurements might yield insight into the underlying metabolic dynamics, with application toward fermentations, natural product biosynthesis, inhibition studies, and other metabolic flux investigations.
Footnotes
The author declares no conflict of interest.
See companion article on page 12580.
References
- 1.Hayes JM. Stable Isotope Geochemistry. Vol 43. Washington, DC: Mineralogical Soc America; 2001. Fractionation of carbon and hydrogen isotopes in biosynthetic processes; pp. 225–277. Reviews in Mineralogy and Geochemistry. [Google Scholar]
- 2.Sessions AL. Isotope-ratio detection for gas chromatography. J Sep Sci. 2006;29:1946–1961. doi: 10.1002/jssc.200600002. [DOI] [PubMed] [Google Scholar]
- 3.Sessions AL, Burgoyne TW, Schimmelmann A, Hayes JM. Fractionation of hydrogen isotopes in lipid biosynthesis. Org Geochem. 1999;30:1193–1200. [Google Scholar]
- 4.Chikaraishi Y, Naraoka H. Compound-specific delta D-delta C-13 analyses of n-alkanes extracted from terrestrial and aquatic plants. Phytochemistry. 2003;63:361–371. doi: 10.1016/s0031-9422(02)00749-5. [DOI] [PubMed] [Google Scholar]
- 5.Huang YS, Shuman B, Wang Y, Webb T. Hydrogen isotope ratios of palmitic acid in lacustrine sediments record late Quaternary climate variations. Geology. 2002;30:1103–1106. [Google Scholar]
- 6.Sachse D, Radke J, Gleixner G. Hydrogen isotope ratios of recent lacustrine sedimentary n-alkanes record modern climate variability. Geochim Cosmochim Acta. 2004;68:4877–4889. [Google Scholar]
- 7.Sauer PE, Eglinton TI, Hayes JM, Schimmelmann A, Sessions AL. Compound-specific D/H ratios of lipid biomarkers from sediments as a proxy for environmental and climatic conditions. Geochim Cosmochim Acta. 2001;65:213–222. [Google Scholar]
- 8.Sternberg LDL. D/H ratios of environmental water recorded by D/H ratios of plant lipids. Nature. 1988;333:59–61. [Google Scholar]
- 9.Zhang X, Gillespie A, Sessions AL. Large D/H variations in bacterial lipids reflect central metabolic pathways. Proc Natl Acad Sci USA. 2009;106:12580–12586. doi: 10.1073/pnas.0903030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sessions AL, Hayes JM. Calculation of hydrogen isotopic fractionations in biogeochemical systems. Geochim Cosmochim Acta. 2005;69:593–597. [Google Scholar]
- 11.Li C, Sessions AL, Kinnaman FS, Valentine DL. Hydrogen-isotopic variability in lipids from Santa Barbara Basin sediments. Geochim Cosmochim Acta. 2009 doi: 10.1016/j.gca.2009.05.056. [DOI] [Google Scholar]
- 12.Sessions AL, Sylva SP, Summons RE, Hayes JM. Isotopic exchange of carbon-bound hydrogen over geologic time scales. Geochim Cosmochim Acta. 2004;68:1545–1559. [Google Scholar]