Abstract
Staphylococcus aureus is a major cause of community-acquired and nosocomial infections including the life-threatening conditions endocarditis, necrotizing pneumonia, necrotizing fasciitis, and septicemia. Toll-like receptor (TLR)-2, a membrane-bound microbial sensor, detects staphylococcal components, but macrophages lacking TLR2 or both TLR2 and TLR4 remain S. aureus responsive, suggesting that an alternative microbial recognition receptor might be involved. The cytoplasmic sensor nucleotide-binding oligomerization domain containing (NOD) 2/caspase recruitment domain (CARD) 15 detects muramyl dipeptide from bacterial peptidoglycans and mediates cytokine responses to S. aureus in vitro, but the physiological significance of these observations is not well defined. Here we show that NOD2-deficient mice exhibit a delayed but ultimately exacerbated ulcerative response and impaired bacterial clearance after s.c. infection with S. aureus. NOD2-dependent recognition of S. aureus and muramyl dipeptide is facilitated by α-toxin (α-hemolysin), a pore-forming toxin and virulence factor of the pathogen. The action of NOD2 is dependent on IL-1β-amplified production of IL-6, which promotes rapid bacterial killing by neutrophils. These results significantly broaden the physiological importance of NOD2 in innate immunity from the recognition of bacteria that primarily enter the cytoplasm to the detection of bacteria that typically reside extracellularly and demonstrate that this microbial sensor contributes to the discrimination between commensal bacteria and bacterial pathogens that elaborate pore-forming toxins.
Keywords: Innate immunity, interleukin-1, interleukin-6, microbial pathogenesis
Staphylococcus aureus is the major cause of human skin and wound infections acquired in hospitals and in the community (1). The increasing prevalence of virulent strains of methicillin-resistant S. aureus is a paramount clinical challenge and underlines the importance of understanding the mechanisms of invasive disease and normal host defense. After S. aureus penetration of the skin and entry into the s.c. tissues, often facilitated by cutaneous injury, surgery, or medical devices, local innate immune responses are crucial in limiting the establishment of an infectious focus and reducing disease severity. Microbial sensing by the innate immune system is mediated by pattern recognition receptors, such as Toll-like receptors (TLRs) and the more recently described group of nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) (2, 3). TLRs recognize diverse bacterial ligands at the cell surface or within endosomes (2). By contrast, many NLRs detect their microbial ligands in the cytoplasm. For example, the prototypic NLR family member NOD2/caspase recruitment domain (CARD) 15 senses muramyl dipeptide (MDP), a ubiquitous building block of the cell wall peptidoglycan in most gram-positive and gram-negative bacteria, in the cytosol. Activation of NOD2 initiates an inflammatory response through NF-κB and MAPK signaling pathways. NOD2 has a role in host defense against the invasive intracellular enteric pathogen Listeria monocytogenes (4), but little is known about the physiological functions of the phylogenetically conserved NOD2 in host defense against a broader range of bacteria.
Although TLR2 is involved in the detection of staphylococcal cell wall components (5, 6), macrophages of TLR2/TLR4 double-deficient mice continue to respond to S. aureus with proinflammatory cytokine secretion, suggesting that an alternative microbial receptor might be involved in bacterial recognition (7–9). NOD2 mediates cytokine responses to S. aureus in transfected cells and seems to contribute to host defense against systemic infection with the pathogen (10–12), but the underlying recognition mechanisms and their physiological significance in the skin, the predominant infection site, are not understood. Here we report that NOD2 is critical for innate recognition of S. aureus and for effective antibacterial defense in a murine skin infection model. IL-1β and IL-6 production, as well the pore-forming α-hemolysin toxin of the pathogen, play key roles in the NOD2-mediated defense pathway.
Results
Delayed and Exacerbated Local Inflammation in NOD2-Deficient Mice.
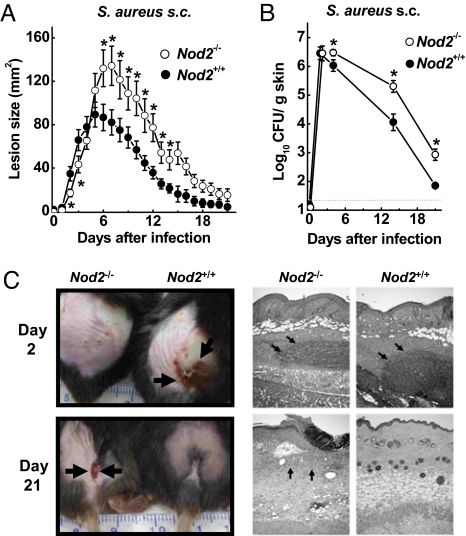
To determine the function of NOD2 in cutaneous host defense against S. aureus, we used WT (Nod2+/+) and NOD2-deficient (Nod2−/−) mice on the C57BL/6J genetic background and infected them s.c. with S. aureus (5 × 107 cfu). WT mice developed skin ulcerations by 2 days that peaked in size by 5 days and had largely healed by 21 days (Fig. 1 A and C). In contrast, Nod2−/− mice developed ulcerations more slowly during the first 3 days after infection but then experienced significantly larger and more persistent ulcerations starting at 6 days (Fig. 1 A and C). In parallel with the delayed but ultimately exacerbated ulcerative response, Nod2−/− mice had 5- to 10-fold higher bacterial cfu in the skin than the WT mice from day 4 onward, indicating that NOD2 is required for normal bacterial clearance (Fig. 1B). The defect in bacterial clearance was not related to an inability to recruit neutrophils, which are critical innate effector cells against S. aureus (13), because no differences were observed in neutrophil numbers at the site of infection in Nod2−/− compared with WT mice by histological assessment or measurement of myeloperoxidase in skin homogenates (Fig. 1C and supporting information (SI) Fig. S1). Similarly, serum IgG titers against S. aureus were not significantly different in WT and Nod2−/− mice (Fig. S2), suggesting that NOD2 is dispensable for normal induction of an adaptive immune response against the bacteria.
Fig. 1.
NOD2 is crucial for cutaneous host defense against S. aureus. (A and B) Nod2−/− mice (open circles) and WT mice (filled circles) were infected s.c. with 5 × 107 cfu of WT S. aureus. Skin lesions were measured daily for 21 days, and bacterial cfu were determined on the indicated days. Data are mean (SE), n ≥ 18/group for lesion sizes, and n ≥ 4 for cfu; *, P < 0.05. (C) Representative photographs of early and late skin lesions and representative H&E-stained paraffin sections. Both genotypes showed comparable early neutrophil infiltration (arrows) in dermis and panniculus carnosus, but Nod2−/− mice did not heal normally after infection, with continued epidermal ulcerations and dermal inflammation after 21 days (arrows).
Impaired Cytokine Response in NOD2-Deficient Mice.
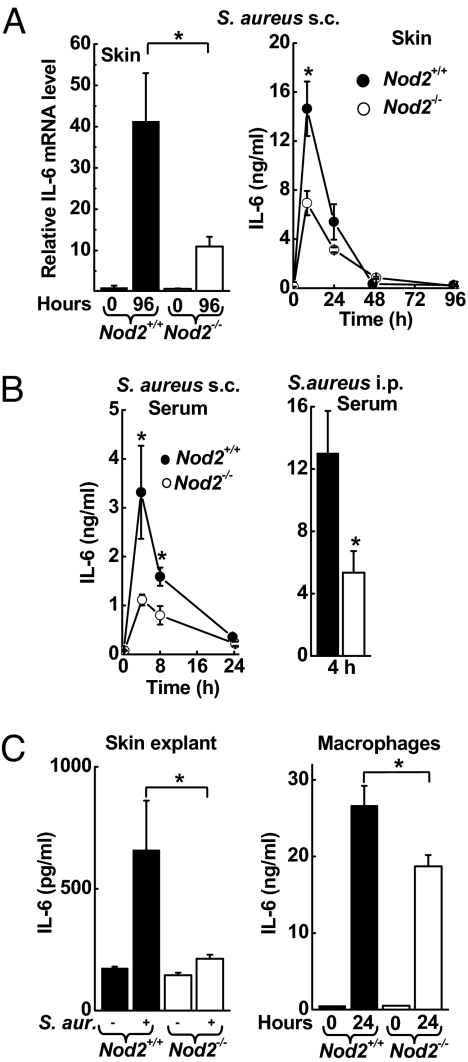
We next asked whether differences in cytokine responses to S. aureus infection may account for the different pattern of disease progression observed in the absence of NOD2. Real-time PCR analysis of several key cytokines implicated in innate immune defense revealed that mRNA levels for IL-6 (Fig. 2A), C-X-C Ligand (CXCL) 1, CXCL2, and TNFα (Fig. S3) were markedly (≈5-fold) reduced in the skin of Nod2−/− mice compared with WT mice 4 days after s.c. infection with S. aureus. Analysis of IL-6 in skin homogenates showed significantly decreased levels in Nod2−/− mice early after infection (Fig. 2A). In parallel, serum IL-6 levels were lower in Nod2−/− mice 8 h after s.c. infection and 4 h after a systemic challenge (Fig. 2B). Furthermore, ex vivo incubation of freshly isolated skin tissue with S. aureus revealed an almost complete loss of IL-6 induction in skin from Nod2−/− mice compared with WT mice (Fig. 2C), indicating that NOD2-dependent recognition by resident skin cells is critical for the innate IL-6 response to S. aureus. Keratinocytes can secrete IL-6 after S. aureus infection (14). Furthermore, bone marrow-derived macrophages from Nod2−/− mice released significantly less IL-6 after 24-h incubation with S. aureus than did cells from WT mice (Fig. 2C), indicating that macrophages also can be important IL-6 producers in a NOD2-dependent manner. In contrast, no significant differences in TNFα were found in skin homogenates of the different genotypes in the first 4 days after infection (Fig. S3).
Fig. 2.
NOD2 dependence of cytokine responses to S. aureus. (A and B) Nod2−/− mice (open bars, open circles) and WT mice (closed bars, filled circles) were infected s.c. or i.p. with 5 × 107 cfu of WT S. aureus. IL-6 mRNA levels (normalized to GAPDH) in the infected skin were determined by real-time PCR and are expressed relative to the levels in uninfected WT mice. n = 5 mice/group. IL-6 levels were assayed by ELISA in skin homogenates (n ≥ 4/group) or serum (n ≥ 10/group). All data are mean (SE); *, P < 0.05. (C) Freshly explanted skin of WT mice and Nod2−/− mice was inoculated with S. aureus, and IL-6 levels were determined by ELISA in tissue homogenates 4 h later. Data are mean (SE); n = 4; *, P < 0.05. Bone marrow-derived macrophages from WT mice and Nod2−/− mice were co-cultured with S. aureus (MOI 20) for 24 h. IL-6 levels were determined in supernatants by ELISA. Data are mean (SD) of 1 representative of 3 independent experiments; n = 3; *, P < 0.05.
Pore-Forming α-Toxin Facilitates NOD2-Dependent Recognition of S. aureus and Muramyl Dipeptide
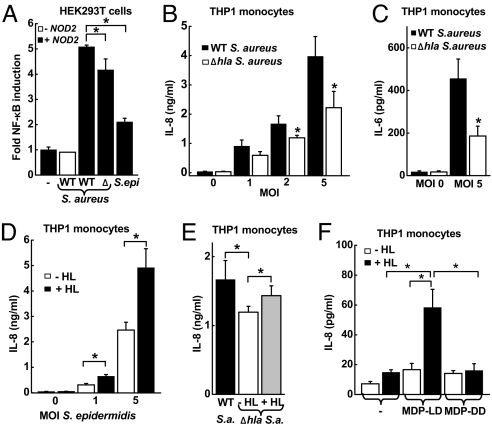
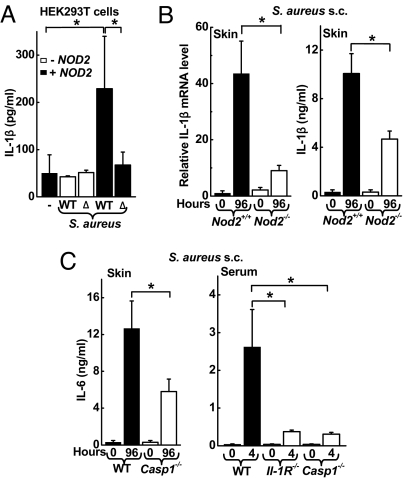
Based on the observation that IL-6 induction was a NOD2-dependent innate response to S. aureus, we next turned to the question of how the cytoplasmic sensor NOD2 can contribute to recognition of S. aureus. HEK293T cells were transfected with NOD2 cDNA and an NF-κB-dependent luciferase reporter, because IL-6 is a NF-κB target gene. Infection of the cell cultures with S. aureus stimulated NF-κB activity in a NOD2-dependent manner, whereas infection with a commensal member of the skin microflora, Staphylococcus epidermidis, had minimal impact on NF-κB activity (Fig. 3A). Consistent with the increase in NF-κB activity in transfected HEK293T cells, the expression of the 2 prototypic NF-κB target genes, IL-6 and IL-8, were increased after S. aureus infection of THP-1 monocytic cells (Fig. 3 B and C), which express NOD2 constitutively. S. aureus and S. epidermidis are distinguished, among other factors, by the production of specific toxins, including the pore-forming α-toxin (α-hemolysin) by S. aureus. To determine the importance of α-hemolysin in the activation of NF-κB and cytokine expression, we used an α-hemolysin-deficient isogenic mutant, DK 1090 (Δhla), derived from the WT parental S. aureus strain. The mutant exhibited a significantly reduced capacity to activate NF-κB and induce IL-8 and IL-6 secretion (Fig. 3 A–C), suggesting that the α-hemolysin contributes to cytokine induction in this system.
Fig. 3.
Role of α-hemolysin in NOD2-mediated cell responses to S. aureus and MDP. (A) NOD2-transfected HEK293T cells were incubated for 8 h with WT or α-hemolysin-deficient (Δ) S. aureus or with S. epidermidis (S. epi) at a MOI of 5. NF-κB activity was determined by luciferase reporter assay and is expressed as ratio relative to uninfected controls. Data from 1 representative of 4 independent experiments are shown as mean (SD); n = 3. (B and C) Human THP1 monocytic cells were incubated for 24 h with WT (closed bars) or Δhla (open bars) S. aureus, and IL-8 and IL-6 levels in the supernatants were assayed by ELISA. Data shown are mean (SE), n ≥ 6, of 3 independent experiments. (D) THP1 cells were incubated for 24 h with S. epidermidis at the indicated MOI in the presence (+ HL, closed bar) or absence (− HL, open bar) of staphylococcal α-hemolysin (100 ng/mL). Data are mean (SE), n ≥ 6, of 3 independent experiments. (E) THP1 cells were incubated for 24 h with WT or Δhla S. aureus (S.a.) at a MOI of 2 in the presence or absence of α-hemolysin (HL) (100 ng/mL). Data are mean (SD) of 1 representative of 4 independent experiments; n = 3. (F) THP1 cells were treated for 8 h with the indicated combinations of active MDP isomer (MDP-LD, 1 μg/mL), inactive MDP isomer (MDP-DD, 10 μg/mL), and α-hemolysin (HL; 100 ng/mL). IL-8 levels in the supernatants were determined by ELISA. Data are mean (SE) of 3 independent experiments. *, P < 0.05.
To explore further the role of α-hemolysin in facilitating innate immune recognition of the bacteria in vitro, we used recombinant α-hemolysin in the cell culture models. Addition of α-hemolysin significantly enhanced IL-8 secretion of THP-1 monocytes in response to infection with the S. aureus Δhla strain or with S. epidermidis (Fig. 3 D and E). Because the S. aureus α-hemolysin is a membrane-active pore-forming toxin, we reasoned that such pores might facilitate access of bacterial cell wall components to cytoplasmic NOD2. To examine this possibility, we stimulated THP-1 monocytic cells with the bacterial cell wall component and known NOD2 activator, MDP, in the presence or absence of α-hemolysin. MDP alone had little impact on IL-8 secretion, a finding that is consistent with its inability to cross cell membranes effectively, nor did α-hemolysin alone affect IL-8 secretion (Fig. 3F). However, treatment of the cells with the combination of MDP and α-hemolysin strongly activated IL-8 secretion. The inactive DD isoform of MDP did not stimulate IL-8 secretion with or without α-hemolysin, indicating that the IL-8 response to the combined MDP/α-hemolysin had the specific characteristics of NOD2-dependent recognition (Fig. 3F). These data suggest that S. aureus α-hemolysin promotes the entry of MDP into the cytoplasm and thereby facilitates recognition by the intracellular sensor NOD2.
Interdependence of NOD2 and α-Toxin for Cutaneous Defense Against S. aureus.
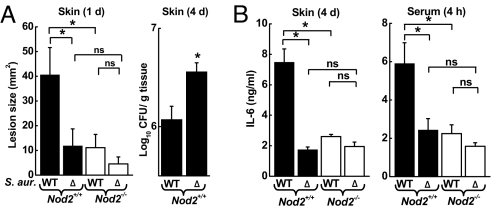
Given the importance of α-hemolysin in facilitating cytokine induction in response to S. aureus in vitro, we next determined its physiologic role in early innate defense against the bacteria in vivo. Infection of WT mice with the Δhla mutant caused smaller acute lesions (day 1), resembling those seen after infection of Nod2−/− mice with WT S. aureus (Fig. 4A). Furthermore, on day 4 bacterial numbers in the skin were significantly higher in WT mice infected with Δhla than in mice infected with WT S. aureus (Fig. 4A), again reminiscent of the findings in Nod2−/− mice infected with WT bacteria (Fig. 1B). Consistent with the in vitro data, the Δhla mutant also induced significantly lower IL-6 levels in the serum and in the skin of WT mice after s.c. infection (Fig. 4B). The attenuation in IL-6 induction after infection of WT mice with the mutant was comparable to that observed after infection of Nod2−/− mice with the WT and Δhla mutant of S. aureus, underlining the interdependence of NOD2 and α-hemolysin in innate activation of host defense in vivo. Together, these results indicate that α-hemolysin-dependent recognition by NOD2 is important for inducing an early innate inflammatory response to cutaneous infection with S. aureus.
Fig. 4.
Importance of α-hemolysin in the early host response to S. aureus infection. Nod2−/− mice (open bars) and Nod2+/+ mice (closed bars) were infected s.c. with 5 × 107 cfu of WT or isogenic α-hemolysin-deficient (Δ) S. aureus (S. aur.). (A) Skin lesions were measured after 1 day. Cfu in skin homogenates were determined on day 4. (B) IL-6 levels were determined by ELISA in skin homogenates after 4 days or in serum after 4 h. All data are mean (SE) of 3 or 4 independent experiments. *, P < 0.05; ns, not significant.
NOD2-Dependent Release of IL-1β Promotes IL-6 Response to S. aureus.
To understand better the host factors that govern NOD2-dependent IL-6 induction, we focused on the role of IL-1β, a potent proinflammatory mediator that can be released upon NOD2 activation (15). Incubation of transfected HEK293T cells with WT S. aureus resulted in the release of mature, bioactive IL-1β in a NOD2-dependent manner (Fig. 5A and Fig. S4). The α-hemolysin-deficient S. aureus mutant (Δhla) did not stimulate IL-1β secretion, further supporting the conclusion that the α-hemolysin of S. aureus is required for NOD2 activation upon S. aureus infection.
Fig. 5.
IL-1β mediates induction of IL-6 upon infection with S. aureus. (A) HEK293T cells were transfected with expression plasmids for NOD2, pro-IL-1β, and caspase-1 and were incubated for 8 h with WT or α-hemolysin-deficient (Δ) S. aureus at a MOI of 5. Secretion of IL-1β into the supernatants was determined by ELISA. Data are mean (SD) from 1 representative of 3 independent experiments. (B and C) Nod2−/−, Il-1R−/−, Caspase-1−/−, and WT mice were infected s.c. with 5 × 107 cfu of WT S. aureus. IL-1β mRNA levels (normalized to GAPDH) in the infected skin were determined by real-time PCR and are expressed relative to the levels in uninfected WT mice (n = 5 mice/group). IL-1β and IL-6 levels were assayed by ELISA in skin homogenates (n ≥ 5/group) or serum (n ≥ 5/group). All data are mean (SE); *, P < 0.05.
In confirmation of the in vitro findings, Nod2−/− mice displayed decreased IL-1β mRNA expression and IL-1β levels in skin homogenates after s.c. infection with WT S. aureus (Fig. 5B). Importantly, IL-1β was a strong amplifier of the IL-6 response to S. aureus, because IL-6 levels were markedly attenuated in skin homogenates of S. aureus-infected mice deficient for the critical IL-1β processing enzyme, caspase-1 (Fig. 5C). Furthermore, decreased serum IL-6 levels were observed in mice deficient for caspase-1 or IL-1 receptor 4 h after s.c. infection with S. aureus (Fig. 5C). In contrast, IL-1β levels in skin homogenates were not affected significantly by IL-6 deficiency [mean (SD), 18.2 (4.2) ng in IL-6-deficient mice vs. 10.2 (4.8) ng in control mice; n = 3, P = not significant.]. Together, these results indicate that IL-1β plays a critical role in promoting the IL-6 response to S. aureus infection.
IL-6-Deficient Mice Mimic the Cutaneous Phenotype of NOD2-Deficient Mice upon S. aureus Infection.
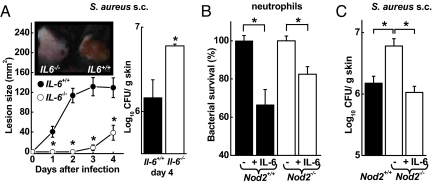
Because IL-6 was markedly attenuated in Nod2−/− mice after S. aureus infection, we next determined the function of this cytokine in host defense against the bacteria. Mice deficient for IL-6 (Il6−/−) were infected s.c. with S. aureus and examined for lesion development and bacterial load. Similar to Nod2−/− mice, Il6−/− mice showed delayed appearance of ulcerated skin lesions relative to WT mice (Fig. 6A). In parallel, Il6−/− mice had significantly increased bacterial numbers in the skin, indicating that they could not control the infection normally (Fig. 6A). Thus, loss of NOD2 or IL-6 caused a similar phenotype in acute skin defense against S. aureus, suggesting that IL-6 may, at least in part, mediate the functions of NOD2 in this model.
Fig. 6.
Contribution of IL-6 to antibacterial defense. (A) Il6−/− mice (open circles) and WT mice (filled circles) were infected s.c. with 5 × 107 cfu of WT S. aureus and were examined daily for lesion sizes and for bacterial load on day 4. Data are mean (SD); n ≥ 3/group. A representative photograph of the lesions on day 2 is shown in the inset. (B) Freshly prepared bone marrow neutrophils from Nod2−/− mice (open bars) and WT mice (closed bars) were incubated with S. aureus (MOI = 1) with and without IL-6 (50 ng/mL) for 30 min, and surviving bacteria were determined by cfu assay. Data are mean (SE) of 3 independent experiments. (C) Nod2−/− mice (open bar) and WT mice (closed bar) were infected s.c. with 5 × 107 cfu of WT S. aureus. Data are mean (SE); n ≥ 4/group. The indicated group of Nod2−/− mice was treated once daily s.c. with 400 ng of recombinant IL-6. *, P < 0.05.
NOD2 Mediates Its Effects Through IL-6 Secretion and Neutrophil Activation.
To explain how IL-6 can contribute to antibacterial defense, we tested its effect on neutrophil killing of S. aureus. The addition of IL-6 to neutrophils freshly obtained from the bone marrow of either WT or Nod2−/− mice significantly enhanced bacterial killing within 30 min (Fig. 6B). This activity may be related to the ability of IL-6 to stimulate intracellular calcium fluxes in neutrophils, leading to the release of bactericidal oxygen radicals (16), and to induce inducible NOS expression in phagocytes (17). No difference in S. aureus killing was observed by unstimulated Nod2−/− vs. WT neutrophils. Thus, although neutrophils express NOD2 constitutively, these data suggest that NOD2 activity is not simply intrinsic to the sensing neutrophil but rather triggers a paracrine mechanism of IL-6 secretion and activation of accumulating neutrophils at the site of infection.
Finally, to validate pharmacologically the role of IL-6 in the host defense defect observed in Nod2−/− mice, we infected the mice s.c. with S. aureus and treated them daily with recombinant IL-6 by local s.c. injection. IL-6 treatment of Nod2−/− mice completely reversed the bacterial clearance defect in the mice, with bacterial numbers that were comparable to those in untreated WT mice (Fig. 6C). Thus, IL-6 complementation could functionally overcome the loss of NOD2, strongly suggesting that IL-6 is responsible for mediating the activity of NOD2 in cutaneous defense against S. aureus.
Discussion
The data we report significantly broaden the physiological importance of the cytoplasmic microbial sensor NOD2, from the limited recognition of bacteria, such as L. monocytogenes, that enter and reside in the host cell cytoplasm (4), to the detection of bacteria that usually reside outside the cytoplasm and often extracellularly, as represented by the prototypic bacterial pathogen S. aureus. The expanded surveillance capacity of NOD2 for microbial signature molecules in extracytoplasmic domains is facilitated by the pore-forming α-hemolysin of S. aureus. Furthermore, α-hemolysin may have an additional role in promoting cytoplasmic access of whole bacteria, because it is required for S. aureus escape from the endocytic vesicle to the cytosol in certain epithelial cell lines (18). To date, α-hemolysin has been most appreciated for its cytotoxic activities, which can harm host epithelial cells and promote S. aureus resistance to macrophage killing (19–21). However, our data and those for another major NLR, NOD1 (22), suggest that the mammalian immune system is not only a passive target of this virulence factor but has evolved to exploit its pore-forming activity for accelerated and enhanced bacterial recognition and as a means to discriminate between commensal and pathogenic bacteria. Although all bacteria have MDP in the cell wall, pore-forming toxins are produced only by important subsets of bacterial pathogens, such as S. aureus. Thus the distinction between commensals and pathogens, both of which can activate TLRs, may depend mainly on their differential ability to activate cytosolic NLRs such as NOD2.
NOD2 stimulation leads to direct activation of NF-κB and increased expression of its target genes (12), and it activates caspase-1 and thereby promotes the release of mature IL-1β (15). The relative importance of these 2 pathways for mediating the physiologic functions of NOD2 is poorly understood. Our results show that caspase-1 and IL-1 are important physiologic amplifiers of the NOD2-initiated innate immune response. In the absence of this amplification pathway, the NOD2-dependent IL-6 response to S. aureus was diminished, and host defense was compromised. Furthermore, our data suggest a new mechanism by which IL-1β exerts its acute host defense function against S. aureus (23), identifying IL-6 as an important downstream mediator of IL-1 signaling. IL-6, in turn, can activate the killing capacity of neutrophils as critical early effectors against the bacteria (24), a function that may be further augmented by IL-1 (25).
Mutations of NOD2 are associated with the development of several clinically important chronic inflammatory diseases, most notably intestinal Crohn's disease (26, 27). The respective NOD2 mutants are widely believed to exhibit loss-of-function (28, 29), but a general explanation for how such loss promotes inflammatory responses has not yet emerged. Our results suggest a model mechanism by which loss of NOD2 function exacerbates local inflammation. Delayed and ineffective recognition of a localized bacterial infection in the absence of NOD2 can lead to an increase in bacterial load and secondarily to a delayed but more severe local inflammatory response. In this model, NOD2 acts indirectly through local production of factors, such as IL-6, which stimulate innate immune defense, particularly the ability of neutrophils to kill bacteria effectively. These concepts may have implications for understanding the mechanisms by which mutant NOD2 promotes Crohn's disease, where intestinal bacteria are considered to be a key driving force in disease pathogenesis, and increased survival of S. aureus has been reported in neutrophils of patients with the disease (30, 31).
Materials and Methods
Bacterial Strains.
S. aureus (ATCC 29213) and S. epidermidis (ATCC 12228) were obtained from the American Type Culture Collection (ATCC). S. aureus 8325 K and the Δhla mutant DK 1090 were kindly provided by A. Cheung (Dartmouth Medical School) and A. Bayer (UCLA).
Mouse Infection Model.
The murine model of necrotizing skin infection has been described elsewhere (32, 33). Briefly, log-phase S. aureus were resuspended in PBS, mixed 1:1 with sterile Cytodex beads (Sigma), and an inoculum of 5 × 107 cfu of S. aureus was injected s.c. into the flank of 8- to 12-week-old mice. Nod2−/− mice, Il6−/− mice, Caspase-1−/−mice, and Il-1R−/− mice have been described previously (34–37). C57BL/6J mice were used as WT controls. For IL-6 treatment, 400 ng of human recombinant IL-6 per mouse was injected s.c. daily for 4 days. Lesion size was monitored daily. Mice were killed at different times after infection, and the affected skin regions were fixed in Bouin's solution and examined on H&E-stained paraffin sections. In parallel, skin homogenates were prepared and used to determine bacterial numbers by cfu assay, cytokine levels by ELISA, and mRNA expression by real-time PCR. Real-time PCR was performed as previously described (38). Primer sequences are available upon request. Myeloperoxidase activity was determined by enzymatic assay as previously described (38).
Neutrophil-Killing Assays.
Neutrophils were isolated by density gradient centrifugation from single-cell suspensions of the bone marrow of Nod2−/− and Nod2+/+ mice. Log-phase bacteria were mixed with neutrophils at a 1:1 ratio in the absence or presence of 50 ng/mL human recombinant IL-6. Cultures were incubated for 30 min at 37 °C, and surviving bacteria were determined by cfu assay.
Dual Luciferase Assay.
HEK293T cells were grown in 12-well plates in low-glucose DMEM supplemented with 10% FBS and 10 mM Hepes. Subconfluent cells were transfected with the aid of FUGENE 6 reagent (Roche) with 1 ng NOD2 expression plasmid (WT hNOD2 cDNA in a pBKCMV backbone), 10 ng NF-κB-dependent firefly luciferase reporter plasmid (pNF-κB-luc, Stratagene), 10 ng pRL SV40 Renilla luciferase reporter plasmid (Promega), 50 ng each of expression plasmids for caspase-1 and pro-IL-1β, and pBluescript II KS (Stratagene) to adjust for DNA content (250 ng total DNA/well). Luciferase activity was assayed with a Dual Luciferase Reporter assay (Promega). Results are expressed as relative induction of stimulated compared with untreated cells, normalized for Renilla luminescence.
Infection Studies.
THP-1 cells, grown in RPMI medium (Cellgro) supplemented with 10% FBS, 2 mM glutamine, 10 mM Hepes, 1 mM sodium pyruvate, and 50 μM β-mercaptoethanol, were co-cultured with live bacteria at different multiplicities of infection (MOIs) as well as combinations of MDP [either active MDP-LD or inactive MDP-DD (InvivoGen)] and α-hemolysin from S. aureus (Sigma). Bone marrow-derived macrophages and skin explants were obtained from Nod2−/− and Nod2+/+ mice and co-cultured with live bacteria at the indicated MOIs.
Statistical Analysis.
Cfu counts were log10-transformed, and means and SEM were calculated. Differences between groups were evaluated by Mann-Whitney rank sum test or Student's t- test as appropriate, with P < 0.05 considered as significant.
Supplementary Material
Acknowledgments.
We thank all members of the Eckmann and Nizet laboratories for helpful discussions and Y. Andersen, J. Brandley, E. Hanson, R. Thomas, and He Chen for technical assistance. We thank Dr. A. Cheung, Dartmouth Medical School, and Dr. A. Bayer, UCLA, for providing S. aureus 8325 K and the Δhla mutant DK 1090, respectively. P.H. and A.S.Z. were supported by fellowship grants from the Swiss National Foundation, and G.J.B. was supported by a student research fellowship award from the American Gastroenterological Association. M.K. is an American Cancer Society Research Professor. The work was supported by National Institutes of Health grants to M.K., V.N., and L.E and by the UCSD Digestive Diseases Research Development Center.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904958106/DCSupplemental.
References
- 1.Lowy FD. Staphylococcus aureus infections. New England Journal of Medicine. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 3.Ting JP, Davis BK. CATERPILLER: A novel gene family important in immunity, cell death, and diseases. Annu Rev Immunol. 2005;23:387–414. doi: 10.1146/annurev.immunol.23.021704.115616. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 5.Lien E, et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi O, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 7.Kapetanovic R, et al. Contribution of phagocytosis and intracellular sensing for cytokine production by Staphylococcus aureus-activated macrophages. Infect Immun. 2007;75:830–837. doi: 10.1128/IAI.01199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoebe K, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 10.Deshmukh HS, et al. The critical role of NOD2 in regulating the immune response to Staphylococcus aureus. Infect Immun. 2009;77:1376–1382. doi: 10.1128/IAI.00940-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girardin SE, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 12.Ogura Y, et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 13.Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun. 1997;65:2517–2521. doi: 10.1128/iai.65.7.2517-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki T, et al. Effects of staphylococci on cytokine production from human keratinocytes. British Journal of Dermatology. 2003;148:46–50. doi: 10.1046/j.1365-2133.2003.05017.x. [DOI] [PubMed] [Google Scholar]
- 15.Hsu LC, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci USA. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sitaraman SV, et al. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. 2001;107:861–869. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawada T, et al. IL-6 induction of protein-DNA complexes via a novel regulatory region of the inducible nitric oxide synthase gene promoter: Role of octamer binding proteins. J Immunol. 1997;158:5267–5276. [PubMed] [Google Scholar]
- 18.Jarry TM, Memmi G, Cheung AL. The expression of alpha-haemolysin is required for Staphylococcus aureus phagosomal escape after internalization in CFT-1 cells. Cellular Microbiology. 2008;10:1801–1814. doi: 10.1111/j.1462-5822.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 19.Kubica M, et al. A potential new pathway for Staphylococcus aureus dissemination: The silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS ONE. 2008;3:e1409. doi: 10.1371/journal.pone.0001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bubeck Wardenburg J, et al. Poring over pores: Alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 21.Liang X, Ji Y. Involvement of alpha5beta1-integrin and TNF-alpha in Staphylococcus aureus alpha-toxin-induced death of epithelial cells. Cellular Microbiology. 2007;9:1809–1821. doi: 10.1111/j.1462-5822.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 22.Ratner AJ, et al. Nod1 mediates cytoplasmic sensing of combinations of extracellular bacteria. Cellular Microbiology. 2007;9:1343–1351. doi: 10.1111/j.1462-5822.2006.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marina-Garcia N, et al. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 24.von Kockritz-Blickwede M, et al. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. Am J Pathol. 2008;173:1657–1668. doi: 10.2353/ajpath.2008.080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pechkovsky DV, Potapnev MP, Zalutskaya OM. Different patterns of cytokine regulation of phagocytosis and bacterial killing by human neutrophils. International Journal of Antimicrobial Agents. 1996;7:33–40. doi: 10.1016/0924-8579(96)00007-6. [DOI] [PubMed] [Google Scholar]
- 26.Cuthbert AP, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology. 2002;122:867–874. doi: 10.1053/gast.2002.32415. [DOI] [PubMed] [Google Scholar]
- 27.Abreu MT, et al. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn's disease. Gastroenterology. 2002;123:679–688. doi: 10.1053/gast.2002.35393. [DOI] [PubMed] [Google Scholar]
- 28.Tanabe T, et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 2004;23:1587–1597. doi: 10.1038/sj.emboj.7600175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonen DK, et al. Crohn's disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology. 2003;124:140–146. doi: 10.1053/gast.2003.50019. [DOI] [PubMed] [Google Scholar]
- 30.Worsaae N, Staehr Johansen K, Christensen KC. Impaired in vitro function of neutrophils in Crohn's disease. Scandinavian Journal of Gastroenterology. 1982;17:91–96. doi: 10.3109/00365528209181050. [DOI] [PubMed] [Google Scholar]
- 31.Couper R, Kapelushnik J, Griffiths AM. Neutrophil dysfunction in glycogen storage disease Ib: Association with Crohn's-like colitis. Gastroenterology. 1991;100:549–554. doi: 10.1016/0016-5085(91)90229-e. [DOI] [PubMed] [Google Scholar]
- 32.Nizet V, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 33.Buchanan JT, et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 34.Barreau F, et al. CARD15/NOD2 is required for Peyer's patches homeostasis in mice. PLoS ONE. 2007;2:e523. doi: 10.1371/journal.pone.0000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaccum MB, et al. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 36.Kopf M, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 37.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 38.Dann SM, et al. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J Immunol. 2008;180:6816–6826. doi: 10.4049/jimmunol.180.10.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.