Abstract
Background: The purpose of this study was to determine the incidence of and survival following brain metastases among women with triple receptor-negative breast cancer.
Patients and methods: In all, 679 patients with nonmetastatic triple receptor-negative breast cancer diagnosed from 1980 to 2006 were identified. Cumulative incidence of brain metastases was computed. Cox proportional hazards models were fitted to explore factors that predict for development of brain metastases. Survival was computed using the Kaplan–Meier product limit method.
Results: Median follow-up was 26.9 months. In all, 42 (6.2%) patients developed brain metastases with a cumulative incidence at 2 and 5 years of 5.6% [95% confidence interval (CI) 3.8% to 7.9%] and 9.6% (95% CI 6.8% to 13%), respectively. A total of 24 (3.5%) patients developed brain metastases as the first site of recurrence with cumulative incidence at 2 and 5 years of 2.0% (95% CI 2.6% to 6.0%) and 4.9% (95% CI 3.2% to 7.0%), respectively. In the multivariable model, no specific factor was observed to be significantly associated with time to brain metastases. Median survival for all patients who developed brain metastases and those who developed brain metastases as the first site of recurrence was 2.9 months (95% CI 2.0–7.6 months) and 5.8 months (95% CI 1.7–11.0 months), respectively.
Conclusion: In this single-institutional study, patients with nonmetastatic triple receptor-negative breast tumors have a high early incidence of brain metastases associated with poor survival and maybe an ideal cohort to target brain metastases preventive strategies.
Keywords: brain metastases, breast cancer, triple negative
introduction
Breast cancers are known to be heterogeneous and are characterized by a wide spectrum of clinical, pathological, and molecular characteristics that comprise a number of recognized biological subtypes [1–4]. Major subtypes identified by gene expression profiles classify breast cancers into basal, luminal (hormone receptor positive), and HER-2/neu-positive/estrogen receptor (ER)-negative subtypes which have differing prognostic profiles [1–4]. It is also recognized that the basal and HER-2/neu-positive/ER-negative subtypes are associated with particularly poor outcomes compared with the hormone receptor-positive luminal subtype [1–4]. The 10%–15% of breast carcinomas known to be ‘triple receptor negative’ (i.e. not expressing ERs and progesterone receptors (PRs) and not exhibiting overexpression and/or gene amplification of HER-2/neu) constitutes ∼85% of all basal-type tumors, which are known to have a poor overall outcome. A number of studies have focused on understanding the natural history and the effect of chemotherapy in this subtype [5, 6].
The risk of developing brain metastases at any time point during the course of metastatic disease has been reported to range from 10% to 16%, approaching 30% when autopsy diagnosis of brain metastases is included [7–9]. Brain metastases generally tend to occur late in the course of metastatic breast cancer and are typically associated with 1- and 2-year survival rates of only 20% and <2%, respectively [10, 11]. Factors that have been reported to be associated with a higher risk of developing brain metastases include young age, four or more positive lymph nodes, high tumor grade, and HER-2/neu over expression [7, 12–15]. In addition, several studies have reported a greater propensity of ER-negative tumors metastasizing to the brain compared with ER-positive tumors [12, 14, 16]. With the known overall poor outcome associated with triple receptor-negative breast cancer subtype, and the inference from retrospective studies that this subtype may have an increased propensity to develop brain metastases, which itself is associated with poor prognosis across all types of breast tumors, we embarked on this retrospective study. The purpose of this study was to determine the incidence of brain metastases among women with nonmetastatic triple receptor-negative breast cancer and to determine survival outcomes following a diagnosis of brain metastases in this cohort.
patients and methods
patients
Using an electronic database maintained at the Breast Medical Oncology Department of the University of Texas M.D. Anderson Cancer Center (MDACC), we retrospectively identified patients with histologically confirmed, hormone receptor-negative and HER-2/neu-negative invasive breast cancers. Exclusion criteria included patients who were male, those with more than one primary, and patients who presented with de novo stage IV disease. From 1980 to 2006, 877 patients who had triple receptor-negative disease were identified. One hundred and ninety-eight patients had stage IV disease at diagnosis and were subsequently excluded from the analyses. Variables recorded included patient demographics, primary tumor characteristics, initial clinical stage, and recurrence information. The medical records of all patients were reviewed to verify information recorded in the database. The MDACC institutional review board approved the retrospective chart review.
staging and pathology review
Initial clinical stage of all patients was coded according to the staging criteria proposed by the 2003 sixth edition of the American Joint Committee on Cancer [17]. Histological type and grade of primary tumor specimens were classified according to the World Health Organization classification system [18] and modified Black's nuclear grading system [19], respectively. Pretreatment tumor biopsy specimens were used to define receptor status. For those specimens obtained before 1993, ER and PR status were determined using dextran-coated charcoal ligand-binding method. Thereafter, immunohistochemistry (IHC) on 4-μm paraffin-embedded tissue sections was carried out using mAbs. For determination of ER and PR status, 6F11 (Novacastra Laboratories Ltd, Burlingame, CA) and 1A6 (Novacastra Laboratories Ltd) mAbs were used, respectively. HER-2/neu status was determined using either IHC and/or a FISH. Tumor specimens that had no demonstrable staining by IHC method and/or no gene amplification by FISH method were considered to be HER-2/neu negative. Triple receptor-negative status was assigned to those specimens that were HER-2/neu negative and exhibited no staining for both ER and PR (hormone receptor-negative status).
statistical analyses
Characteristics of this patient cohort were tabulated or described by their median and range as appropriate. Median follow-up was calculated as the median observation time among all patients. Overall survival (OS) was computed from the date of diagnosis of breast cancer to date of death from any cause or last follow-up. Recurrence-free survival (RFS) was computed from the date of diagnosis of breast cancer to the date of first local or distant recurrence or last follow-up. Patients who died before experiencing a disease recurrence were considered censored at their date of death in the analysis of RFS. Survival subsequent to metastasis was measured from the date of metastasis to the date of death from any cause or last follow-up. Survival outcomes were estimated according to the Kaplan–Meier product limit method and compared across groups using the log-rank statistic. Time to brain metastases was computed from the date of breast cancer diagnosis to the date of diagnosis of brain metastases or last follow-up. We calculated the cumulative incidence of brain metastasis, considering death from any cause as a competing risk, and the cumulative incidence of brain as the first site of metastasis, considering both metastases at other sites and death as competing risks. Cox proportional hazards models were fitted to explore the simultaneous relationship of factors that could predict for the development of brain metastases in this cohort. All statistical tests were two sided and P values <0.05 were considered to be statistically significant. Analyses were carried out using SAS 9.1 (SAS Institute, Cary, NC), R 2.4.1 [20], and the contributed package cmprsk [21].
results
These analyses included 679 women with triple receptor-negative breast cancer diagnosed from 1980 to 2006. Table 1 summarizes characteristics of this cohort. Median age at disease diagnosis was 50 years (range 22–97 years). One hundred and forty-five (21.4%) patients initially presented with stage I disease, 339 (49.9%) patients presented with stage II disease, and 195 (28.7%) presented with stage III disease. The majority of patients had invasive ductal carcinoma (93.8%) and grade III disease (89.5%). Three hundred and thirty-two (49.4%) underwent mastectomy for their primary tumor, 326 (48.5%) underwent a segmental resection, five (0.7%) had only axillary lymph node dissection as no primary in the breast was found, and nine (1.3%) patients refused any form of surgery. Five hundred and ninety-three (87.3%) patients received either preoperative or adjuvant chemotherapy and 452 (66.6%) patients received adjuvant radiation therapy following surgery.
Table 1.
Patient characteristics
| n | % | |
| N | 679 | – |
| Median age (range) | 50 (22–97) | – |
| Race | ||
| Black | 117 | 17.2 |
| Other | 115 | 16.9 |
| White | 447 | 65.8 |
| Menopausal status | ||
| Pre | 285 | 42.2 |
| Post | 391 | 57.8 |
| Histology | ||
| Other | 42 | 6.2 |
| Ductal | 637 | 93.8 |
| T | ||
| T0 | 2 | 0.3 |
| T1 | 224 | 33.3 |
| T2 | 295 | 43.9 |
| T3 | 62 | 9.2 |
| T4 | 89 | 13.2 |
| N | ||
| N0 | 300 | 44.5 |
| N1 | 249 | 36.9 |
| N2 | 47 | 7.0 |
| N3 | 78 | 11.6 |
| Stage | ||
| I | 145 | 21.4 |
| II | 339 | 49.9 |
| III | 195 | 28.7 |
| Grade | ||
| I | 3 | 0.4 |
| II | 67 | 10.0 |
| III | 598 | 89.5 |
| LVI | ||
| Negative | 474 | 71.9 |
| Positive | 185 | 28.1 |
| Surgery | ||
| ALND | 5 | 0.7 |
| Mastectomy | 332 | 49.4 |
| None | 9 | 1.3 |
| Segmental | 326 | 48.5 |
| Median nodes removed (range) | 13 (1–59) | – |
| Chemo | ||
| A alone | 131 | 19.3 |
| A + T | 444 | 65.4 |
| None | 86 | 12.7 |
| Other | 8 | 1.2 |
| T alone | 10 | 1.5 |
| Adjuvant XRT | ||
| No | 227 | 33.4 |
| Yes | 452 | 66.6 |
LVI, lymphovascular invasion; ALND, axillary lymph node dissection; XRT, radiation therapy; A, adriamycin; T, taxane.
RFS and OS estimates
Median follow-up among all patients was 26.9 months (range 1.1–321.3 months). Two hundred (29.5%) patients have experienced disease recurrence, and RFS at 2 and 5 years was 69.2% [95% confidence interval (CI) 65.0% to 73.0%] and 59.6% (95% CI 54.4% to 64.4%), respectively. At the time of the analyses, 153 (22.5%) patients had died and OS at 2 and 5 years was 85.9% (95% CI 82.6% to 88.7%) and 64.1% (95% CI 58.4% to 69.2%), respectively.
development of brain metastases
Table 2 summarizes the overall cumulative incidence of brain metastases at 2 and 5 years stratified by patient and tumor characteristics. Overall, 42 (6.2%) patients developed brain metastases. The cumulative incidence of brain metastases at 2 and 5 years was 5.6% (95% CI 3.8% to 7.9%) and 9.6% (95% CI 6.8% to 13%), respectively. Twenty-four (3.5%) patients developed brain metastases as the first site of recurrence. The cumulative incidence of brain metastases as the first site of recurrence at 2 and 5 years was 2.0% (95% CI 2.6% to 6.0%) and 4.9% (95% CI 3.2% to 7.0%), respectively. Factors such as race, age, menopausal status, and grade of disease were not significantly associated with the development of brain metastases overall or as the first site of recurrence. Higher stage of primary disease was associated with a significantly increased cumulative incidence of brain metastases as the first site of recurrence (P = 0.024).
Table 2.
Overall cumulative incidence of brain metastases at 2 and 5 years following a diagnosis of nonmetastatic triple receptor-negative breast cancer
| 2-Year cumulative incidence | 95% Confidence interval | 5-Year cumulative incidence | 95% Confidence interval | P value | |
| All | 5.6% | 3.8%–7.9% | 9.6% | 6.8%–13% | |
| Age | |||||
| <50 | 7.3% | 4.6%–10.8% | 11.9% | 7.9%–16.8% | |
| ≥50 | 3.4% | 1.6%–6.4% | 6.6% | 3.4%–11.4% | 0.097 |
| Race | |||||
| Black | 3.3% | 0.9%–8.6% | 6.2% | 2.2%–13% | |
| Other | 8.4% | 3.6%–15.6% | 13.8% | 6.7%–23.4% | |
| White | 5.5% | 3.4%–8.4% | 9.5% | 6%–13.9% | 0.359 |
| Menopausal status | |||||
| Pre | 7.0% | 4.1%–11.1% | 11.8% | 7.4%–17.2% | |
| Post | 4.2% | 2.3%–7% | 7.8% | 4.4%–12.4% | 0.191 |
| Stage | |||||
| I | 2.0% | 0.4%–6.6% | 7.5% | 1.8%–18.8% | |
| II | 5.1% | 2.8%–8.4% | 10.1% | 6.2%–15.1% | |
| III | 8.8% | 4.9%–14.2% | 10.7% | 6.2%–16.6% | 0.340 |
| Grade | |||||
| I/II | 0.0% | – | 11.4% | 2.6%–27.4% | |
| III | 6.4% | 4.3%–9% | 8.8% | 6.3%–11.9% | 0.419 |
| LVI | |||||
| Negative | 4.9% | 3%–7.6% | 7.6% | 4.9%–11.2% | |
| Positive | 7.6% | 4%–12.7% | 14.6% | 8.5%–22.3% | 0.042 |
| Adjuvant XRT | |||||
| No | 5.2% | 2.4%–9.5% | 12.8% | 7%–20.5% | |
| Yes | 5.8% | 3.6%–8.6% | 8.2% | 5.3%–11.8% | 0.277 |
| Surgery | |||||
| Mastectomy | 7.7% | 4.7%–11.5% | 13.3% | 8.7%–18.9% | |
| Segmental | 3.7% | 1.8%–6.7% | 5.9% | 3.2%–9.6% | 0.045 |
| Nodes removed | |||||
| <10 | 4.1% | 1.9%–7.6% | 4.7% | 2.3%–8.5% | |
| ≥10 | 6.2% | 3.8%–9.4% | 12.0% | 8.1%–16.7% | 0.040 |
| Chemo | |||||
| A | 4.0% | 1.3%–9.3% | 6.4% | 2.6%–12.6% | |
| A + T | 7.0% | 4.5%–10.1% | 12.1% | 8.2%–16.9% | |
| None | 1.5% | 0.1%–7.2% | 4.3% | 0.7%–13.8% | 0.39 |
LVI, lymphovascular invasion; XRT, radiation therapy; A, adriamycin; T, taxane.
The results of the multivariable model for time to development of brain metastases, as either the first or subsequent site of metastases, are summarized in Table 3. The variables lymphovascular invasion and number of nodes examined (>10 versus <10) were included due to their statistical significance in the univariate analysis. The variables age, grade, and stage of primary disease were included in the model due to their clinical significance regardless of statistical significance. None of the variables included in the final model were associated significantly with time to development of brain metastases.
Table 3.
Multivariable model for time to brain metastasis
| Variable | Hazard ratio | LCI | UCI | P value |
| Age at diagnosis (continuous) | 0.98 | 0.95 | 1.01 | 0.236 |
| Grade (III versus I/II) | 1.22 | 0.37 | 4.05 | 0.745 |
| LVI (positive versus negative) | 1.92 | 0.99 | 3.72 | 0.053 |
| Nodes removed (> 10 versus <10) | 1.54 | 0.68 | 3.50 | 0.302 |
| Stage (II versus I) | 1.28 | 0.41 | 3.99 | 0.677 |
| Stage (III versus I) | 1.61 | 0.47 | 5.58 | 0.452 |
LCI, lower confidence interval; UCI, upper confidence interval; LVI, lymphovascular invasion.
survival following brain metastases
Sixteen (38.1%) patients had three or fewer lesions in the brain, 19 (45.2%) had more than three brain lesions, and the number of brain lesions was unknown in seven (16.7%) patients. Three (7.1%) patients underwent surgery as treatment for first-line brain metastases; 24 (57.1%) underwent whole-brain radiation therapy, three (7.1%) received surgery and radiation therapy, 10 (23.8%) did not receive any treatment, and the type of treatment was unknown in two (4.8%) patients.
Table 4 summarizes the survival estimates following a diagnosis of brain metastases by patient characteristics. At the time of this analysis, 34 of the 42 patients (80.95%) with brain metastases have died. Median survival in this group was 2.9 months (95% CI 2.0–7.6 months) (Figure 1). On univariate analysis, increasing initial stage of disease and menopausal status were associated with a significant reduction in median survival following a diagnosis of brain metastases.
Table 4.
Survival following brain metastasis
| n | No. of events | Median survival (months) | Lower 95% CI | Upper 95% CI | P value | |
| All | 42 | 34 | 2.9 | 2.0 | 7.6 | |
| Age | ||||||
| <50 | 29 | 25 | 3.7 | 2.5 | 8.9 | |
| ≥50 | 13 | 9 | 1.7 | 0.9 | 5.8 | 0.060 |
| Race | ||||||
| Black | 5 | 5 | 1.9 | 1.0 | 6.3 | |
| Other | 10 | 6 | 3.7 | 2.5 | 29.5 | |
| White | 27 | 23 | 3.6 | 1.7 | 8.5 | 0.115 |
| Menopausal status | ||||||
| Pre | 22 | 18 | 6.3 | 2.9 | 11.3 | |
| Post | 19 | 15 | 1.8 | 1.5 | 5.0 | 0.009 |
| Stage | ||||||
| I | 5 | 5 | 2.5 | 0.9 | 8.5 | |
| II | 21 | 18 | 7.6 | 2.9 | 11.3 | |
| III | 16 | 11 | 1.9 | 1.6 | 5.8 | 0.021 |
| Grade | ||||||
| I/II | 3 | 2 | 29.5 | 0.9 | 29.5 | |
| III | 37 | 30 | 2.9 | 1.9 | 7.6 | 0.073 |
| LVI | ||||||
| Negative | 23 | 17 | 2.9 | 2.0 | 11.3 | |
| Positive | 18 | 16 | 3.6 | 1.7 | 7.6 | 0.081 |
| Adjuvant XRT | ||||||
| No | 17 | 14 | 2.9 | 1.8 | 8.5 | |
| Yes | 25 | 20 | 3.6 | 1.5 | 8.9 | 0.794 |
| Surgery | ||||||
| Mastectomy | 29 | 22 | 2.6 | 1.7 | 8.5 | |
| Segmental | 13 | 12 | 3.7 | 2.5 | 8.9 | 0.931 |
| Nodes removed | ||||||
| <10 | 9 | 9 | 2.4 | 1.5 | 2.5 | |
| ≥10 | 31 | 23 | 5.8 | 1.9 | 8.9 | 0.074 |
| Chemotherapy | ||||||
| A | 7 | 7 | 2.9 | 2.5 | 7.6 | |
| A + T | 32 | 24 | 3.6 | 1.8 | 9.0 | |
| None | 3 | 3 | 1.5 | 0.9 | 8.5 | 0.405 |
| No. of brain metastases | ||||||
| ≤3 | 16 | 10 | 3.7 | 1.7 | 11.3 | |
| >3 | 19 | 18 | 5.0 | 2.4 | 8.5 | 0.901 |
CI, confidence interval; LVI, lymphovascular invasion; XRT, radiation therapy; A, adriamycin; T, taxane.
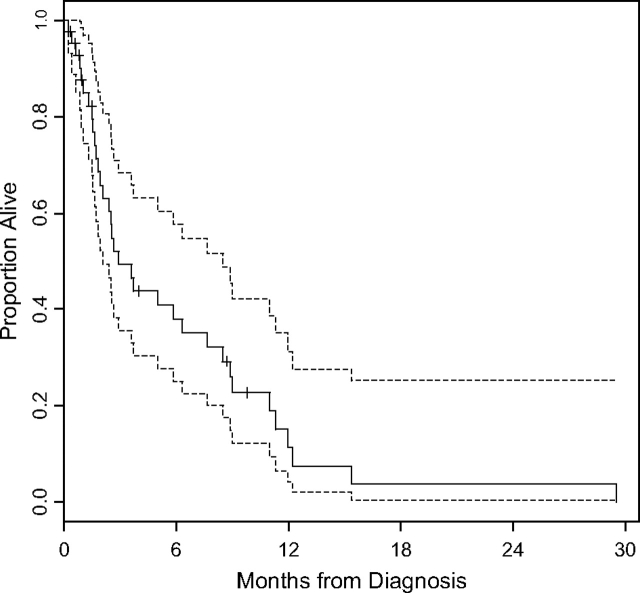
Figure 1.
Kaplan–Meier plots illustrating survival and 95% confidence intervals among all 42 patients who developed brain metastases. Median survival for this group following a diagnosis of brain metastases was 2.9 months (95% confidence interval 2.0–7.6 months).
Among the 24 patients who developed brain metastasis as their first site of metastasis, 17 have died. Among the 176 patients whose first metastasis was at other sites, 114 have died. Median survival among those who did and did not develop brain metastases as the first site of recurrence was 5.8 months (95% CI 1.7–11 months) and 13.0 months (95% CI 11.4–17.6 months), respectively (P < 0.0001) (Figure 2).
Figure 2.
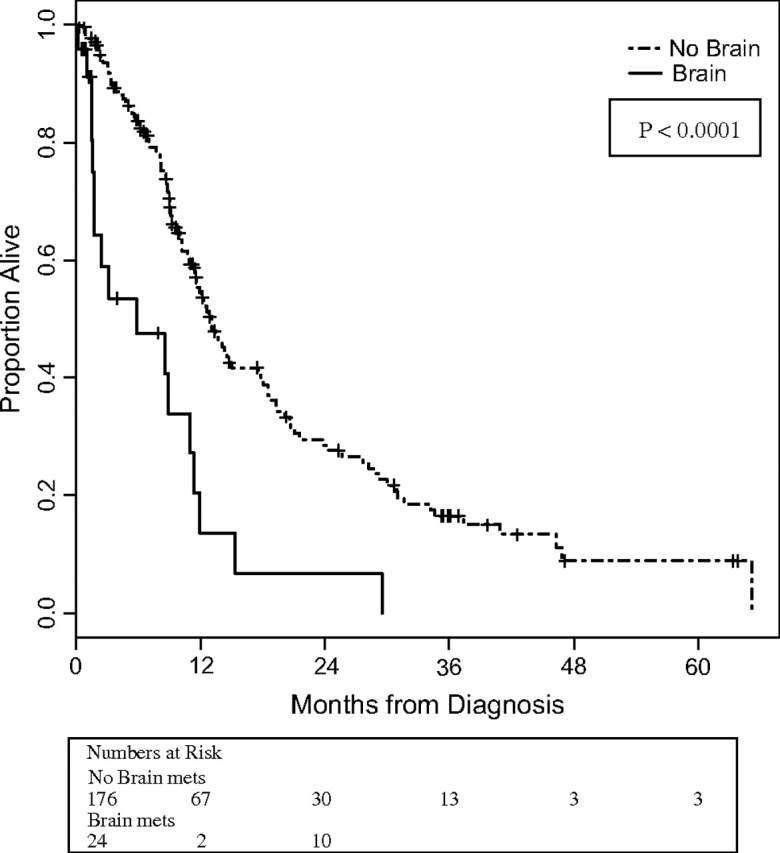
Kaplan–Meier plots illustrating survival among patients who developed brain metastases as the first site of recurrence and those who did not. Median survival among those who did and did not develop brain metastases as a first site of recurrence was 5.8 months [95% confidence interval (CI) 1.7–11 months] and 13.0 months (95% CI 11.4–17.6 months), respectively.
discussion
Several studies have shown that the women with triple receptor-negative breast cancer are at a higher likelihood of relapse and have an associated poorer prognosis compared with women with other subtypes of breast cancer [5, 6]. However, the incidence of and survival from brain metastases in this cohort has not been well defined. In this study, we evaluated 679 women with nonmetastatic triple receptor-negative breast cancer and determined the incidence of and survival from brain metastases. We observed that among women with triple receptor-negative-breast cancer, brain metastases occurs relatively early in the natural course of the disease and is associated with poor survival outcomes.
A higher incidence of brain metastases in women with hormone receptor-negative breast cancer compared with those with hormone receptor-positive disease has been reported by several investigators. In one of the earlier studies to look at this issue, Samaan et al. [16] reported on a cohort of 217 women with primary breast cancer and found that women whose tumors were ER negative were more likely to metastasize to the brain compared with women whose tumors were ER positive (10% versus 4%). Using a cohort of >10 000 women, Tham et al. [14] noted similar results with women whose tumors were ER negative having higher odds of developing brain metastases (odds ratio = 2.8, 95% CI 2.1–3.7, P < 0.001). Such retrospective studies were unable to define the incidence of developing brain metastases specifically within the cohort of patients who have triple receptor-negative disease due to the large amount of missing data on HER-2/neu status. Our cohort provided us with a unique opportunity of defining the incidence of brain metastases in the triple receptor-negative cohort. Overall, we observed a cumulative incidence of 5.6% and 9.6% at 2 and 5 years, respectively, an incidence that is much higher than those reported by other investigators for women with ER-negative disease.
Recent studies have also reported on survival outcomes following the development of brain metastases among women with various subtypes of breast cancer. Using a cohort of 126 patients with breast cancer and brain metastases, Nam et al. [22] reported that 37.3% had triple receptor-negative disease associated with the a median survival of 3.4 months. Using a cohort of patients from the Memorial Sloan Kettering Cancer Center, Hines et al. [23] reported on 91 patients with breast cancer and brain metastases whose biological markers were available for assessment. In this cohort, the authors reported that 22% of patients had triple receptor-negative disease associated with a median survival of 7 months (range 0–20 months) following brain metastases. In our cohort of women with nonmetastatic triple receptor-negative disease, OS regardless of site of recurrence was similar to those reported in other retrospective studies [5, 6]. One hundred and seventy-eight patients developed a distant recurrence, accounting for 89% of the total recurrences. This is also consistent with the fact that women with triple receptor-negative disease are more likely to develop distant recurrences. Forty-two patients developed brain metastases, and in this subgroup, median survival subsequent to brain metastases was 2.9 months (95% CI 2.0–7.6 months). This is much lower than the median survival of 11.6 months we reported for women with HER-2/neu-positive disease who received trastuzumab either before or at the time of diagnosis of brain metastases [24]. Furthermore, 6-month survival among women with nonmetastatic triple receptor-negative breast cancer who developed brain metastases was only 38.0% (95% CI 22.6% to 53.3%). The fact that brain metastases accounted for ∼24% of all distant recurrences and was associated with a poor median survival provides evidence that the development of brain metastases maybe a major cause of the generally observed poor survival in women with triple receptor-negative breast cancers.
The large body of work done in identifying risk factors associated with the development of brain metastases has been primarily undertaken in an effort to identify subgroups of patients at high risk of developing brain metastases who would benefit from early detection and/or prevention measures. Preventive measures would hypothetically be especially useful in women who are at high risk of developing brain metastases as a first site of recurrence. In a large retrospective study of >9000 women with early-stage breast cancer treated on the International Breast Cancer Study Group clinical trials, Pestalozzi et al. [12] reported that the incidence of central nervous system (CNS) disease as the first site of metastatic event was significantly higher among women with ER-negative tumors (5-year cumulative incidence 1.9%) compared with those with ER-positive tumors (5-year cumulative incidence = 0.7%). Although HER-2/neu information was available, the investigators did not report on the incidence of CNS metastases among women with triple receptor-negative breast cancer. Based on such evidence, we explored the cumulative incidence of brain metastases as the first site of metastatic event in our study population. In our cohort, 5-year cumulative incidence of brain metastases as the first site of recurrence was 4.9% (95% CI 3.2% to 7.0%), a much higher incidence than that observed among women with ER-negative disease in the cohort reported by Pestalozzi et al. [12]. Furthermore, we observed that the median survival of women who developed brain metastases as a first site of recurrence was significantly lower compared with those who did not develop brain metastases as a first event (5.8 months versus 13.0 months, P < 0.0001). Interestingly, the incidence of brain metastases in our cohort appears to be higher than those observed among women with HER-2/neu-positive breast cancer who have not received trastuzumab. In the study by Pestalozzi et al. [12], women with HER-2/neu-positive disease had a 2- and 5-year cumulative incidence of brain metastases of 1.2% and 2.3%, respectively. These numbers are likely to be much higher among women with HER-2/neu-positive breast cancer who receive trastuzumab as part of their adjuvant treatment as a result of improved systemic control resulting in brain metastases occurring much later in the course of the disease [24].
We acknowledge that our study has a number of limitations inherent to most retrospective studies. First, in our analyses, we only included patients who had complete information on ER, PR, and HER-2/neu status. Thus, selection bias is a concern. Secondly, triple receptor-negative breast tumors account for ∼85% of basal-like tumors. Thus, in order to extend our results to all basal-like tumors detailed molecular profiling and hierarchical cluster analysis would be required. However, despite these limitations, our study stands on the strength of its large sample size and the fact that all patients received similar treatment and follow-up.
Our results have a number of important implications. First, we show that it is important to report results stratified by breast tumor subtype rather than based on individual biological markers as other investigators have done in the past. Such uniform stratification will not only make for better comparisons across studies but also will allow for improved identification of individuals at highest risk of developing brain metastases. Secondly, our results indicate that among women with triple receptor-negative disease, brain metastases occurs with higher incidence, develops earlier in the course of the disease, and is associated with a poorer prognosis compared with historical controls of women with other subtypes of breast cancer. These results not only provide a reasonable explanation for the overall poorer prognostic outcomes known to be associated with women with this subtype of breast cancer but also indicates that women with triple receptor-negative breast tumors maybe an ideal cohort to target in clinical trials studying preventive measures against the development of brain metastases. Whole-brain radiation in the preventive setting is associated with long-term toxicity that maybe unacceptable in a cohort where 5-year OS is >60%. Before trials can investigate preventive and treatment strategies, much more needs to be learnt about the pathophysiology of brain metastases at a molecular level. Evidence already exist that immunohistochemical profiles of primary breast tumors and corresponding brain metastases in the same patients may in fact be different [25], a fact that maybe important in developing targeted agents for both the prevention and treatment of brain metastases. Specific pathways such as WNT/β-catenin signaling pathway have been shown to be active in basal tumors relapsing in the brain [26] and this maybe an important avenue for future research in this cohort.
funding
Susan G. Komen Foundation and the Nellie B. Connally Fund for Breast Cancer Research; K23CA121994-01 and ASCO Career Development Award to Dr AMG-A.
References
- 1.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumors. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T. Molecular portraits of breast cancer: tumour subtypes as distinct disease entities. Eur J Cancer. 2004;40:2667–2675. doi: 10.1016/j.ejca.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 5.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 6.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24(36):5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 7.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 8.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52:2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Patanaphan V, Salazar OM, Risco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1988;81:1109–1112. [PubMed] [Google Scholar]
- 10.DiStefano A, Yap HY, Hortobagyi GN, et al. The natural history of breast cancer patients with brain metastases. Cancer. 1979;44:1913–1918. doi: 10.1002/1097-0142(197911)44:5<1913::aid-cncr2820440554>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Engel J, EcKel R, Aydemir U, et al. Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys. 2003;55:1186–1195. doi: 10.1016/s0360-3016(02)04476-0. [DOI] [PubMed] [Google Scholar]
- 12.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 13.Hicks DG, Short SM, Prescott NL, et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK 5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 14.Tham YL, Sexton K, Kramer R, et al. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer. 2006;107:696–704. doi: 10.1002/cncr.22041. [DOI] [PubMed] [Google Scholar]
- 15.Stemmler HJ, Kahlert S, Siekiera W, et al. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006;15:219–225. doi: 10.1016/j.breast.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Samaan NA, Buzdar AU, Aldinger KA, et al. Estrogen receptor: a prognostic factor in breast cancer. Cancer. 1981;47:554–560. doi: 10.1002/1097-0142(19810201)47:3<554::aid-cncr2820470322>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 17.Singletary SE, Allred C, Ashley P, et al. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 18.The World Health Organization Histological Typing of Breast Tumors—Second Edition. The World Organization. Am J Clin Pathol. 1982;78:806–816. doi: 10.1093/ajcp/78.6.806. [DOI] [PubMed] [Google Scholar]
- 19.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105:97–102. [PubMed] [Google Scholar]
- 20.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2006; http://www.R-project.org (April 2008, date last accessed) [Google Scholar]
- 21.Gray B. cmprsk: Subdistribution Analysis of Competing Risks. R package version 2.1-5. 2004. http://www.r-project.org, http://biowww.dfci.harvard.edu/∼gray(April 2008, date last accessed) [Google Scholar]
- 22.Nam B-H, Kim SY, Han HS, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10:R20. doi: 10.1186/bcr1870. (April 2008, dater last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hines SL, Vallow LA, Tan WW, et al. Clinical outcomes after a diagnosis of brain metastases in patients with estrogen- and/or human epidermal growth factor receptor 2-positive versus triple-negative breast cancer. Ann Oncol. 2008;19(5):1561–1565. doi: 10.1093/annonc/mdn283. [DOI] [PubMed] [Google Scholar]
- 24.Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19(7):1242–1248. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 25.Yonemori K, Tsuta K, Shimizu C, et al. Immunohistochemical profiles of brain metastases from breast cancer. J Neurooncol. 2008;70(20):223–228. doi: 10.1007/s11060-008-9654-x. [DOI] [PubMed] [Google Scholar]
- 26.Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]