Abstract
Plants resist attack by haustorium-forming biotrophic and hemi-biotrophic fungi through fortification of the cell wall to prevent penetration through the wall and the subsequent establishment of haustorial feeding structures by the fungus. While the existence of cell wall-based defences has been known for many years, only recently have the molecular components contributing to such defences been identified. Forward genetic screens identified Arabidopsis mutants impaired in penetration resistance to powdery mildew fungi that were normally halted at the cell wall. Several loci contributing to penetration resistance have been identified and a common feature is the striking focal accumulation of proteins associated with penetration resistance at sites of interaction with fungal appressoria and penetration pegs. The focal accumulation of defence-related proteins and the deposition of cell wall reinforcements at sites of attempted fungal penetration represent an example of cell polarization and raise many questions of relevance, not only to plant pathology but also to general cell biology.
Keywords: Actin, disease resistance, immunity, MLO, papilla, PEN1, PEN2, PEN3, powdery mildew
Introduction
Plants exhibit what is referred to as non-host resistance to the majority of potential pathogens (Heath, 2000). Non-host resistance is defined as resistance in which an entire plant species is resistant to all genotypes of a given pathogen species and recent evidence suggests that this temporally durable resistance is conditioned by at least two layers of defence response (Thordal-Christensen, 2003; Lipka et al., 2005). The layers that account for non-host resistance can be broadly categorized as pre- and post-penetration defences, based on their engagement and efficacy at different stages of the infection sequence. After landing on a leaf of a prospective host plant, fungal spores must germinate and, for those fungi that penetrate directly into the epidermis, subsequently penetrate the cuticle and cell wall of the underlying epidermal cell to gain access to plant resources such as nutrients and water. Biotrophic fungi that do not directly penetrate the plant epidermis typically germinate on plant surfaces, enter via stomates or other natural openings, and subsequently penetrate mesophyll cell walls to gain access to nutrients. The waxy cuticle and thick cell wall surrounding plant epidermal cells serve as preformed and passive barriers to invasion by pathogens such as fungi. However, many fungi have evolved specialized infection structures known as appressoria to facilitate the breach of the cuticle and cell wall through mechanical and/or enzymatic means (Howard, 1997; Pryce-Jones et al., 1999). It is at this critical juncture, attempted penetration of the cell wall, that the first line of inducible plant defences is called into action. Non-adapted fungi, those that cannot cause disease on any member of a selected plant species, generally exhibit some ability occasionally to overcome this first layer of defence, albeit at a very low frequency compared to adapted pathogens. The conidiospores that overcome penetration resistance and successfully develop haustoria, feeding structures in close contact with a plant-derived extrahaustorial membrane, are subjected to a second layer of defence characterized by the hypersensitive response (HR)-like programmed cell death (PCD) of invaded host cells (Thordal-Christensen, 2003; Lipka et al., 2005). This article will focus primarily on penetration resistance against biotrophic powdery mildew fungi and, particularly, on newly discovered molecular components of this first layer of defence against fungal pathogens.
Molecular components of penetration resistance
It has been known for many years that plants assemble cell wall appositions, known as papillae, in the paramural space subtending the cell wall at sites of attempted fungal penetration. Papillae have been found to contain callose, phenolic compounds, lignin, reactive oxygen species, and proteins and are thought to act as a physical barrier to halt penetration by the fungal penetration pegs (Aist, 1976; McLusky et al., 1999). Until recently, studies of penetration resistance associated with papilla formation have been primarily descriptive in nature. Recently, however, forward genetic screens have identified a number of molecular components that contribute to the ability of Arabidopsis to resist penetration by the non-adapted powdery mildew fungus Blumeria graminis f. sp. hordei (Bgh), a pathogen of barley (Collins et al., 2003; Lipka et al., 2005; Stein et al., 2006). Ethyl methane sulphonate (EMS)-mutagenized Arabidopsis populations were screened for mutants that allowed increased frequencies of penetration by Bgh. Successful penetration by Bgh resulted in whole-cell autofluorescence and encasement of the nascent haustorium in callose, which could be viewed microscopically after staining inoculated tissue with the fluorescent dye aniline blue (Stein et al., 2006) or by visualizing whole cell autofluorescence (Collins et al., 2003). From these mutant screens, several penetration (pen) mutants allowing higher frequencies of Bgh entry into epidermal cells were recovered. Three of these mutants, designated pen1, pen2, and pen3, have been characterized and the corresponding genes identified.
PEN1 defines a defence pathway associated with vesicle-mediated secretion
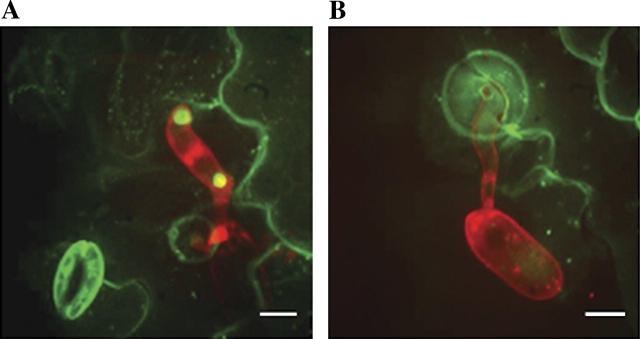
Map-based cloning revealed that PEN1 encodes one of 24 Arabidopsis syntaxins, designated SYP121 (Sanderfoot et al., 2000; Collins et al., 2003). Syntaxins, also known as tSNARES (target SNAP receptor), participate in vesicle fusion events through the formation of ternary SNARE complexes with a corresponding VAMP (vesicle associated membrane protein) and a SNAP25 homologue. ROR2 (REQUIRED FOR mlo RESISTANCE 2), a barley (Hordeum vulgare) homologue of PEN1, was found to contribute to broad spectrum powdery mildew resistance conferred by loss of function at the MLO locus and to basal penetration resistance to Bgh in susceptible barley encoding a functional MLO (Freialdenhoven et al., 1996; Collins et al., 2003). Functional fusions of PEN1 and ROR2 to GFP and YFP, respectively, were found to localize at the plasma membrane and to accumulate at sites of attempted penetration by appressoria of both adapted and non-adapted powdery mildew species (Collins et al., 2003; Bhat et al., 2005; Fig. 1A).
Fig. 1.
Z-projected confocal micrographs depicting focal accumulation of GFP-PEN1 (A) and PEN3–GFP (B). Propidium iodide-stained Blumeria graminis f. sp. hordei appressoria and conidiospores appear red. Scale bars represent 5 μm.
The predicted function of PEN1 and ROR2 and their presence in the plasma membrane, particularly at sites of attempted fungal penetration, strongly suggest a role in targeted trafficking of secretory vesicles to the site of papilla formation. Consistent with a role in papilla deposition, the frequency of papilla formation in pen1-1 mutants in response to Bgh was reduced at early time-points (10, 12, and 14 h), corresponding to a ∼2 h delay in papilla deposition (Assaad et al., 2004). A strong correlation between timing of papilla deposition and penetration success is evident, as resistance of mlo mutants is correlated with the rapid deposition of papillae, whereas enhanced susceptibility to penetration in pen1-1 is correlated with delayed papilla deposition (Skou et al., 1984; Assaad et al., 2004). Thus, Assaad and colleagues speculate that the delay in papilla formation in pen1 mutants is the primary cause of reduced penetration resistance.
Unexpectedly, GFP-PEN1 was found in the apparent interior of the papilla structure in addition to the plasma membrane subtending the papilla (Assaad et al., 2004). Recently, electron micrographs of papillae formed by mlo5 mutant barley in response to Bgh challenge revealed the presence of membrane-like structures and multi-vesicular bodies associated with or appearing within papilla structures (An et al., 2006). The presence of GFP-PEN1 in such membrane structures may explain the observation of GFP-PEN1 within papillae. In addition, the reduced appearance of large (∼1 μm) H2O2-containing vesicles was reported at Bgh penetration sites on barley ror2 mlo5 double mutants compared to mlo5 single mutants (Collins et al., 2003). This finding is in contrast to what would be expected if ROR2 and PEN1 participate only in the fusion of secretory vesicles to the PM. Such a model for ROR2/PEN1 function would predict an increased accumulation of secretory vesicles in the vicinity of the PM rather than a reduction of such vesicles. Collins et al. (2003) hypothesize that ROR2 may also participate in homotypic vesicle fusion to generate the large vesicles frequently observed near fungal penetration sites. Consistent with this hypothesis, GFP-PEN1 was found to localize to endomembrane compartments ∼1 μm in diameter in the vicinity of papillae formed at Bgh penetration sites (Assaad et al., 2004).
Despite the delayed timing, pen1 mutants were able to deposit papillae that were morphologically indistinguishable from those of wild-type plants, suggesting that at least one other syntaxin can substitute for PEN1 in the process of papilla formation, albeit with reduced efficiency (Assaad et al., 2004). The most closely related syntaxin to PEN1 in the Arabidopsis genome is SYP122, a syntaxin for which transcript levels are dramatically up-regulated in response to pathogen challenge and that was found to be rapidly phosphorylated in response to perception of the bacterial flagellin-derived peptide flg22 (Nühse et al., 2003a; Assaad et al., 2004). Interestingly, the pen1 syp122 double mutants developed spontaneous necrotic lesions and showed elevated levels of salicylic acid (SA) and PR1 transcript (Assaad et al., 2004). Triple mutants impaired in the synthesis or perception of SA showed a partial rescue, suggesting additional, and as yet undefined, roles for PEN1 and SYP122 in the modulation of both SA-dependent and -independent cell death (Zhang et al., 2007). Surprisingly, pen1 syp122 mutants were no more susceptible to Bgh penetration than pen1 single mutants (Assaad et al., 2004), suggesting that SYP122 does not contribute significantly to papilla deposition or, alternatively, that further disruption of papilla deposition is not sufficient to allow more frequent Bgh entry.
Most recently, the corresponding Arabidopsis VAMP and SNAP25 homologue proteins that participate in SNARE complex formation with PEN1 were identified. SNAP33 and members of the VAMP72 family of vSNARES were found to immunoprecipitate in complexes with PEN1 (Kwon et al., 2008a). The conditional reduction of VAMP721 and VAMP722 transcript levels using an inducible RNAi construct targeted to the two closely-related VAMPs also resulted in an increased frequency of Bgh penetration, confirming a role for these VAMPs in penetration defence (Kwon et al., 2008a). YFP-SNAP33 was found to accumulate at the sites of attempted Bgh penetration, where it co-localized with CFP-PEN1. GFP-VAMP722, on the other hand, showed a distinct localization to vesicle-like structures near the penetration sites (Kwon et al., 2008a).
PEN2 and PEN3 participate in a pathway distinct from PEN1 to limit powdery mildew penetration
The pen2 mutation occurs in a member of the Arabidopsis family 1 glycosyl hydrolyse group of enzymes (Lipka et al., 2005). These enzymes are thought to participate in the hydrolysis of O- or S-glycosidic bonds of complex carbohydrates or glycosylated metabolites. Mutation of a predicted catalytic residue prevented complementation by the mutant protein, suggesting that enzymatic activity is essential for PEN2 function in limiting powdery mildew penetration (Lipka et al., 2005). A functional PEN2–GFP fusion protein was found to localize to peroxisomes, an organelle that shown to preferentially accumulate at the sites of attempted powdery mildew penetration (Koh et al., 2005; Lipka et al., 2005). The probable enzymatic activity of PEN2 and its localization to peroxisomes suggest that PEN2 may participate in the enzymatic processing of a compound(s) that may contribute to penetration resistance, potentially through antifungal activity (Lipka et al., 2005). Double mutant analysis revealed that pen1 pen2 mutants allow a higher frequency of Bgh penetration than either single mutant, suggesting that PEN1 and PEN2 function in separate pathways contributing to penetration resistance (Lipka et al., 2005). Interestingly, penetration success of another non-adapted powdery mildew, Erysiphe pisi, was enhanced ∼2-fold (55% compared to 25% in WT) in the pen1 mutant and was greatly enhanced to over 80% in the pen2 mutant (Lipka et al., 2005). The penetration success of E. pisi on the pen1 pen2 double mutants was indistinguishable from pen2. Taken together, these data suggest that the contribution of PEN2-mediated defences to penetration resistance against E. pisi is significantly greater than that of PEN1-mediated defences and that defences conferred by PEN1 are insufficient to limit E. pisi entry in the absence of PEN2. In addition, disruption of those defences associated with SA-mediated PCD in pad4 sag101 double mutants, in combination with the disruption of penetration resistance conferred by PEN2, led to the formation of microcolonies and the successful conidiation by Bgh on the pen2 pad4 sag101 triple mutant and to disease development by E. pisi that was indistinguishable from that of the adapted powdery mildew Golovinomyces orontii on wild-type Arabidopsis (Lipka et al., 2005). These results suggest that non-host resistance of Arabidopsis to E. pisi is conferred primarily by defences associated with only two pathways, the pathway involving PEN2 to limit fungal entry, and the SA-mediated pathway involving SAG101 and PAD4 leading to PCD. However, Arabidopsis non-host resistance to Bgh is more complex requiring, in addition, PEN1 and as yet uncharacterized components.
The third characterized pen mutant, pen3, was found to be affected in the Arabidopsis ATP-binding cassette (ABC) transporter PDR8. ABC transporters collectively facilitate the transfer of a vast array of substrates across cellular membranes and participate in numerous cellular processes. A PEN3–GFP fusion protein localized to the plasma membrane in uninoculated leaves and, like PEN1, showed strongly focused accumulation at sites of attempted penetration by Bgh (Stein et al., 2006; Fig. 1B). Unexpectedly, pen3 mutants were more resistant to the adapted Arabidopsis powdery mildew, Golovinomyces cichoracearum, and displayed chlorosis and cell death after inoculation with G. cichoracearum (Stein et al., 2006). Similarly, pen3 mutants showed HR-like cell death upon inoculation with the oomycete Phytophthora infestans and the bacterial pathogen Pseudomonas syringae pv. tomato, suggesting that PEN3 may play a role in interactions with numerous plant pathogens (Kobae et al., 2006). Enhanced resistance to G. cichoracearum was associated with the up-regulation of the SA pathway and mutations disrupting SA biosynthesis or signalling suppressed cell death and the resistance of pen3 mutants to G. cichoracearum (Stein et al., 2006). Interestingly, mutation of PEN2 in pen2 pen3 double mutants partially suppressed the chlorosis and cell death observed in response to inoculation with G. cichoracearum and partially restored susceptibility to the adapted powdery mildew. These results led to the development of a model in which PEN3 exports a potentially fungi-toxic compound that is processed by PEN2 in peroxisomes. It is proposed that the intracellular accumulation of the PEN2 product, and, potentially, other toxic compounds exported by PEN3, leads to the activation of the SA pathway in pen3 mutants, resulting in enhanced resistance to G. cichoracearum (Stein et al., 2006).
PEN3 has also been found to be rapidly phosphorylated in response to perception of defence elicitors such as flg22 and fungal xylanase, suggesting a potential role for elicitor-induced phosphorylation in PEN3 activation (Nühse et al., 2003b, 2007; Benschop et al., 2007). Most recently, PEN3 (PDR8) was proposed to participate in tolerance to the heavy metals lead (Pb) and cadmium (Cd; Kim et al., 2007). pen3 mutants or RNAi lines grew poorly on medium containing Pb or Cd and accumulated higher levels of Cd, whereas plants overexpressing PEN3 showed enhanced tolerance to Pb and Cd and reduced accumulation of Cd. It is not yet clear whether PEN3′s roles in defence and heavy metal tolerance are somehow related, or simply reflect a broad substrate specificity or promiscuity of transport for PEN3.
The mystery of MLO
MLO is probably the most well-studied player in non-host resistance to powdery mildew fungi. MLO appears to be a negative regulator of defences contributing to powdery mildew resistance, with mutations at the MLO locus of barley conferring broad spectrum resistance to most known isolates of the barley mildew Bgh (Jørgensen, 1992; Piffanelli et al., 2004). The phenomenon of mlo-based resistance to powdery mildew was initially thought to be unique to barley. However, Arabidopsis homologues of barley MLO were recently identified that play a similar negative regulatory role in defence against powdery mildew fungi and, most recently, loss of function at the tomato (Solanum lycopersicum var. cerasiforme) MLO locus SlMLO1 was found to confer resistance to the powdery mildew Oidium neolycopersici (Consonni et al., 2007; Bai et al., 2008).
Arabidopsis encodes 15 genes with high similarity to barley MLO. Mutation of AtMLO2 was found to confer partial resistance to G. orontii that was characterized by reduced penetration success and conidiation (Consonni et al., 2007). The observed partial resistance was found to be due to functional redundancy among Arabidopsis MLO homologues as Atmlo2 Atmlo6 Atmlo12 triple mutants displayed full resistance to G. orontii. Interestingly, mlo2 pen1 double mutants allowed a near wild-type frequency of G. cichoracearum penetration, but no significant increase in conidiation. Like PEN1 and barley ROR2, an MLO–YFP fusion protein was found to accumulate at penetration sites in barley and MLO and AtMLO2 were found physically to interact with ROR2 and PEN1, respectively (Schulze-Lefert, 2004; Bhat et al., 2005; Panstruga, 2005). These results suggest that MLO may negatively regulate defences mediated by PEN1/ROR2 through direct physical interaction with these syntaxins. In addition, Atmlo2 pen2 and Atmlo2 pen3 double mutants also allowed wild-type frequencies of G. cichoracearum penetration, and unlike Atmlo2 pen1 mutants, displayed a significant increase in G. cichoracearum conidiation (Consonni et al., 2007). These results suggest a role for the PEN2/PEN3 pathway in both pre- and post-penetration defences, possibly via continued poisoning of the fungal haustorium through secretion of toxic compounds.
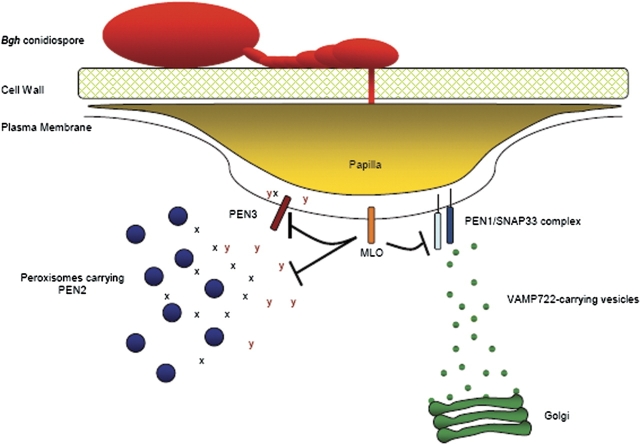
These results also implicate MLO in the modulation of both PEN1 and PEN2/PEN3 pathways leading to penetration resistance. A model depicting the involvement of the PEN proteins and MLO in resistance to powdery mildew is presented in Fig. 2. The three characterized pen mutants appear to represent proteins involved in carrying out the resistance response, rather than signalling components; whereas, MLO may play a regulatory role in modulating defence activation. In this model, the PEN3 transporter exports the product of the PEN2 enzymatic activity (X) and additional, as yet undefined, defence compound(s) (Y). The PEN1/SNAP33 complex mediates fusion of VAMP721/VAMP722-carrying Golgi-derived vesicles at the plasma membrane. These vesicles are presumed to contain materials for the construction of the nascent papilla. Finally, MLO exerts a negative effect on both the PEN1 and PEN2/3 pathways through an unknown mechanism(s).
Fig. 2.
Model depicting the roles of the PEN proteins and MLO in resistance to penetration by powdery mildew fungi. X and Y represent putative PEN3 transport substrates.
Role of cytoskeletal components in penetration resistance
Numerous processes associated with penetration resistance are predicted to involve cytoskeletal components such as actin filaments and microtubules. These include delivery of secretory vesicles to sites of papilla deposition, and cytoplasmic streaming and migration of organelles such as peroxisomes, Golgi, and the nucleus to the site of interaction with the fungus. Consistent with a predicted role for cytoskeletal components in these processes, pharmacological and genetic interference with actin filaments disrupts penetration resistance, leading to increased penetration frequency by various powdery mildew species in several experimental systems (Kobayashi et al., 1997a, b; Yun et al., 2003).
In addition, an intact actin cytoskeleton is also required for full mlo-mediated resistance to powdery mildew (Miklis et al., 2007). Actin filaments have frequently been found to show significant polarization toward sites of attempted fungal penetration and the extent of such actin polarization has been correlated with success or failure of penetration resistance (Opalski et al., 2005; Shimada et al., 2006). Interestingly, genetic disruption of actin filaments in barley through overexpression of the actin depolymerizing factor HvADF3 did not alter the focal accumulation of MLO-YFP or YFP-ROR2 at penetration sites, despite completely disrupting peroxisome motility (Bhat et al., 2005). Similarly, in Arabidopsis, GFP–PEN1 accumulation at penetration sites is not affected by treatment with the actin disrupting agent cytochalasin E (Underwood and Somerville, unpublished results). These results suggest that PEN1/ROR2 and MLO are recruited to penetration sites through actin-independent mechanisms. By contrast, focal accumulation of PEN3–GFP at sites of attempted Bgh penetration is disrupted by cytochalasin E treatment (W Underwood and SC Somerville, unpublished results), suggesting at least two potentially distinct mechanisms for the recruitment of defence-related proteins to the sites of interaction with invading fungi.
While experimental evidence strongly supports a pivotal role for the actin cytoskeleton in penetration resistance, the role of microtubules is not as clear. Disruption of microtubules in some systems was found to confer a modest increase in fungal penetration efficiency, whereas in other systems, similar disruption of microtubules was found to have no effect (Kobayashi et al., 1997a; Takemoto et al., 2003). In addition, the disruption of microtubules did not affect mlo-mediated resistance (Miklis et al., 2007). In general, microtubules appear to have little or no role in defences leading to penetration resistance.
Parallels with other biological systems
Dramatic membrane polarization and focal accumulation of proteins is not unique to plant penetration resistance to powdery mildew fungi. The processes of membrane polarization, focal recruitment of specific proteins, formation of lipid raft-like, sterol rich membrane domains (Bhat et al., 2005), and targeted vesicle-mediated secretion in penetration resistance are strikingly similar to the processes involved in the formation of the immunological synapse in vertebrate immunity (recently reviewed in Kwon et al., 2008b). Similar to the formation of the immune synapse, invasion of human erythrocytes by the malarial parasite Plasmodium falciparum involves the recruitment of lipid raft membrane domains to the site of the junction between parasite and host cell (Murphy et al., 2006). It is thought that the host cell membrane is substantially remodelled ultimately to form the parasitophorous vacuole that accommodates the parasite within the erythrocyte cell (Haldar and Mohandas, 2007). Such a process of recruitment of host components, remodelling of host membranes, and subsequent accommodation of the parasite within a host membrane-derived structure parallels the accommodation of powdery mildew haustoria within plant epidermal cells. Similarly, invasion of intestinal cells by the intracellular parasite Cryptosporidium parvum involves the clustering of lipid raft-like membrane domains to the point of contact between the host cell and the parasite (Nelson et al., 2006). Remodelling of actin filaments within the host cell toward the site of contact with the invading parasite also plays a role in the entry of C. parvum into host cells (Bonnin et al., 1999; Elliott and Clark, 2000; O'Hara and Lin., 2006). However, the mechanisms of recruitment of lipid microdomains and specific proteins to these specialized membrane structures remain enigmatic.
Future perspectives
The development of advanced microscopy techniques including the use of fluorescent tags and probes, together with the isolation of mutants impaired in the ability to resist fungal penetration, opens up a wealth of possibilities for probing the molecular and cell biological aspects of cell wall-based defences, the studies of which have been primarily descriptive until very recently. Along with new possibilities arise fascinating new questions, summarized in Box 1. Understanding the mechanisms underlying the substantial cell polarization and focal accumulation of proteins and lipid raft-like domains to sites of fungal invasion will contribute not only to studies of invasive phytopathogens, but to general cell biology as cell polarization plays a crucial role in many biological processes.
Box 1. Outstanding questions in non-host resistance to powdery mildew
(i) How are PEN1, PEN3, MLO, and other components of penetration resistance recruited to sites of interaction with the invading fungus?
- (ii) How are virulent fungi able to overcome or circumvent cell wall-based defences?
- – Resistance to secreted antimicrobial compounds?
- – Greater efficiency/speed at degrading cell wall polymers?
- – Concealment of pathogen-associated molecular patterns?
- – Manipulation of host defences by effector proteins?
(iii) How do papillae associated with penetration resistance compare to morphologically similar collar-like structures associated with compatible intractions in which haustoria are formed?
(iv) How do papilla structures assembled in response to bacterial invasion relate to analogous (and microscopically indistinguishable) structures assembled at fungal penetration sites? Are some components of papilla assembly and function used for defence against both types of pathogens?
A question of great significance to host–pathogen interactions is what determines the outcome of the interaction, disease development or resistance? Successful powdery mildews are able both to overcome penetration resistance and to avoid the second line of defence associated with HR-like cell death. This may be due to a lack of elicitors that would be perceived by the plant or, alternatively, to active suppression of the cell death response by effector proteins. The identification of R–Avr pairs in the powdery mildew system, such as Bgh AVRa10 and barley MLA10 and the fact that at least some barley R proteins are located within the plant cytoplasm strongly support the notion that powdery mildews translocate effector proteins into plant cells (Ridout et al., 2006). In contrast to the recent cataloguing of effector proteins from several phytopathogenic bacteria, the number and nature of powdery mildew effectors remain enigmatic, as do the mechanisms of their translocation into plant cells. The identification and characterization of effector proteins from powdery mildews will be a significant step forward toward understanding how fungal pathogens manipulate plant cells.
Finally, plants assemble morphologically similar papilla-like structures in response to numerous pathogens including both fungi and bacteria. It is currently not clear whether these structures serve similar defence roles in the context of different classes of pathogens and whether plant cells utilize similar or identical components and mechanisms to deposit these structures.
The study of penetration resistance of plant cells to fungal pathogens represents an interesting interface between plant pathology and cell biology and future insights into the mechanisms underlying cell wall-based defences should contribute significantly to both areas of research.
Acknowledgments
We thank Kian Hématy, Shundai Li, and Ying Gu for critical reading of the manuscript. Funding was provided in part by the Carnegie Institution for Science and by an NIH post-doctoral fellowship to WU, award number 1F32GM083439-01 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
References
- Aist JR. Papillae and related wound plugs of plant cells. Annual Review of Phtyopathology. 1976;14:145–163. [Google Scholar]
- An Q, Hückelhoven R, Kogel K-H, van Bel AJE. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cellular Microbiology. 2006;8:1009–1019. doi: 10.1111/j.1462-5822.2006.00683.x. [DOI] [PubMed] [Google Scholar]
- Assaad FF, Qiu J-L, Youngs H, et al. The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Molecular Biology of the Cell. 2004;15:5118–5129. doi: 10.1091/mbc.E04-02-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Pavan S, Zheng Z, et al. Naturally occurring broad-spectrum powdery mildew resistance in a Central American tomato accession is caused by loss of Mlo function. Molecular Plant–Microbe Interactions. 2008;21:30–39. doi: 10.1094/MPMI-21-1-0030. [DOI] [PubMed] [Google Scholar]
- Benschop JJ, Mohammed S, O'Flaherty MO, Heck AJR, Slijper M, Menke FLH. Quantitative phospho-proteomics of early elicitor signaling in Arabidopsis. Molecular and Cellular Proteomics. 2007;6:1198–1214. doi: 10.1074/mcp.M600429-MCP200. [DOI] [PubMed] [Google Scholar]
- Bhat RA, Miklis M, Schmelzer E, Schulze-Lefert P, Panstruga R. Recruitment and interaction dynamics of plant penetration resistance components in a plasma membrane microdomain. Proceedings of the National Academy of Sciences, USA. 2005;102:3135–3140. doi: 10.1073/pnas.0500012102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Lapillonne A, Petrella T, Lopez J, Chaponnier C, Gabbiani G, Robine S, Dubremetz JF. Immunodetection of the microvillous cytoskeleton molecules villin and ezrin in the parasitophorous vacuole wall of Cryptosporidium parvum (Protozoa: Apicomplexa) European Journal of Cell Biology. 1999;78:794–801. doi: 10.1016/S0171-9335(99)80030-2. [DOI] [PubMed] [Google Scholar]
- Collins NC, Thordal-Christensen H, Lipka V, et al. SNARE-protein-mediated disease resistance at the plant cell wall. Nature. 2003;425:973–977. doi: 10.1038/nature02076. [DOI] [PubMed] [Google Scholar]
- Consonni C, Humphry ME, Hartmann HA, et al. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nature Genetics. 2006;38:716–720. doi: 10.1038/ng1806. [DOI] [PubMed] [Google Scholar]
- Elliott DA, Clark DP. Cryptosporidium parvum induces host cell actin accumulation at the host-parasite interface. Infection and Immunity. 2000;68:2315–2322. doi: 10.1128/iai.68.4.2315-2322.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freialdenhoven A, Peterhänsel C, Kurth J, Kreuzaler R, Shulze-Lefert P. Identification of genes required for the function of non-race-specific mlo resistance to powdery mildew in barley. The Plant Cell. 1996;8:5–14. doi: 10.1105/tpc.8.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar K, Mohandas N. Erythrocyte remodeling by malaria parasites. Current Opinion in Hematology. 2007;14:203–209. doi: 10.1097/MOH.0b013e3280f31b2d. [DOI] [PubMed] [Google Scholar]
- Heath MC. Nonhost resistance and nonspecific plant defences. Current Opinion in Plant Biology. 2000;3:315–319. doi: 10.1016/s1369-5266(00)00087-x. [DOI] [PubMed] [Google Scholar]
- Howard RJ. Breaching the outer barriers: cuticle and cell wall penetration. In: Carroll GC, Tudzynski P, editors. The Mycota. Vol. V. Heidelberg: Springer-Verlag; 1997. pp. 43–60. [Google Scholar]
- Jørgensen JH. Discovery, characterization and exploitation of Mlo powdery mildew resistance in barley. Euphytica. 1992;63:141–152. [Google Scholar]
- Kim D-Y, Bovet L, Maeshima M, Martinoia E, Lee Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. The Plant Journal. 2007;50:207–218. doi: 10.1111/j.1365-313X.2007.03044.x. [DOI] [PubMed] [Google Scholar]
- Kobae Y, Sekino T, Yoshioka H, Nakagawa T, Marinoia E, Maeshima M. Loss of AtPDR8, a plasma membrane ABC transporter of Arabidopsis thaliana, causes hypersensitive cell death upon pathogen infection. Plant and Cell Physiology. 2006;47:309–318. doi: 10.1093/pcp/pcj001. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kobayashi I, Funaki Y, Fujimoto S, Takemoto T, Kunoh H. Dynamic reorganization of microfilaments and microtubules is necessary for the expression of non-host resistance in barley coleoptile cells. The Plant Journal. 1997a;11:525–537. [Google Scholar]
- Kobayashi Y, Yamada M, Kobayashi I, Kunoh H. Actin microfilaments are required for the expression of nonhost resistance in higher plants. Plant and Cell Physiology. 1997b;38:725–733. [Google Scholar]
- Koh S, André A, Edwards H, Ehrhardt D, Somerville S. Arabidopsis thaliana subcellular responses to compatible Erysiphe cichoracearum infections. The Plant Journal. 2005;44:516–529. doi: 10.1111/j.1365-313X.2005.02545.x. [DOI] [PubMed] [Google Scholar]
- Kwon C, Neu C, Pajonk S, et al. Co-option of a default secretory pathway for plant immune responses. Nature. 2008a;451:835–840. doi: 10.1038/nature06545. [DOI] [PubMed] [Google Scholar]
- Kwon C, Panstruga R, Schulze-Lefert P. Les liaisons dangereuses: immunological synapse formation in animals and plants. Trends in Immunology. 2008b;29:159–166. doi: 10.1016/j.it.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Lipka V, Dittgen J, Bednarek P, et al. Pre- and postinvasion defences both contribute to nonhost resistance in Arabidopsis. Science. 2005;310:1180–1183. doi: 10.1126/science.1119409. [DOI] [PubMed] [Google Scholar]
- McLusky SR, Bennett MH, Beale MH, Lewis MJ, Gaskin P, Mansfield JW. Cell wall alterations and localized accumulation of feruloyl-3′-methoxytyramine in onion epidermis at sites of attempted penetration by Botryis allii are associated with actin polarization, peroxidase activity and suppression of flavenoid biosynthesis. The Plant Journal. 1999;17:523–534. [Google Scholar]
- Miklis M, Consonni C, Bhat R, Lipka V, Schulze-Lefert P, Panstruga R. Barley MLO modulates actin-dependent and actin-independent antifungal defence pathways at the cell periphery. Plant Physiology. 2007;144:1132–1143. doi: 10.1104/pp.107.098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SC, Hiller NL, Harrison T, Lomasney JW, Mohandas N, Haldar K. Lipid rafts and malaria parasite infection of erythrocytes. Molecular Membrane Biology. 2006;23:81–88. doi: 10.1080/09687860500473440. [DOI] [PubMed] [Google Scholar]
- Nelson JB, O'Hara SP, Small AJ, Tietz PS, Choudhury AK, Pagano RE, Chen X-M, LaRusso NJ. Cryptosporidium parvum infects human cholangiocytes via sphingolipid-enriched membrane microdomains. Cellular Microbiology. 2006;8:1932–1945. doi: 10.1111/j.1462-5822.2006.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Boller T, Peck SC. A plasma membrane syntaxin is phosphorylated in response to the bacterial elicitor flagellin. Journal of Biological Chemistry. 2003a;278:45248–45254. doi: 10.1074/jbc.M307443200. [DOI] [PubMed] [Google Scholar]
- Nühse TS, Bottrill AR, Jones AM, Peck SC. Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. The Plant Journal. 2007;51:931–940. doi: 10.1111/j.1365-313X.2007.03192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Stensballe A, Jensen ON, Peck SC. Large-scale analysis of in vivo phosphorylated membrane proteins by immobilized metal ion affinity chromatography and mass spectrometry. Molecular and Cellular Proteomics. 2003b;2:1234–1243. doi: 10.1074/mcp.T300006-MCP200. [DOI] [PubMed] [Google Scholar]
- O'Hara SP, Lin JJ-C. Accumulation of tropomyosin isoform 5 at the infection sites of host cells during Cryptosporidium invasion. Parasitology Research. 2006;99:45–54. doi: 10.1007/s00436-005-0117-4. [DOI] [PubMed] [Google Scholar]
- Opalski KS, Schultheiss H, Kogel K-H, Hückelhoven R. The receptor-like MLO protein and the RAC/ROP family G-protein RACB modulate actin reorganization in barley attacked by the biotrophic powdery mildew fungus Blumeria graminis f. sp. hordei. The Plant Journal. 2005;41:291–303. doi: 10.1111/j.1365-313X.2004.02292.x. [DOI] [PubMed] [Google Scholar]
- Panstruga R. Serpentine plant MLO proteins as entry portals for powdery mildew fungi. Biochemical Society Transactions. 2005;33:389–392. doi: 10.1042/BST0330389. [DOI] [PubMed] [Google Scholar]
- Piffanelli P, Ramsey L, Waugh R, Benabdelmouna A, D'Hont A, Hollricher K, Jørgensen JH, Schulze-Lefert P, Panstruga R. A barley cultivation-associated polymorphism conveys resistance to powdery mildew. Nature. 2004;430:887–891. doi: 10.1038/nature02781. [DOI] [PubMed] [Google Scholar]
- Pryce-Jones E, Carver T, Gurr SJ. The roles of cellulase enzymes and mechanical force in host penetration by Erysiphe graminis f. sp. hordei. Physiological and Molecular Plant Pathology. 1999;55:175–182. [Google Scholar]
- Ridout CJ, Skamnioti P, Porritt O, Sacristan S, Jones JDG, Brown JKM. Multiple avirulence paralogues in cereal powdery mildew fungi may contribute to parasite fitness and defeat of plant resistance. The Plant Cell. 2006;18:2402–2414. doi: 10.1105/tpc.106.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Assaad FF, Raikhel NV. The Arabidopsis genome. An abundance of N-ethylmaleimide sensitive factor adaptor protein receptors. Plant Physiology. 2000;124:1558–1569. doi: 10.1104/pp.124.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lefert P. Knocking on the heaven's wall: pathogenesis of and resistance to biotrophic fungi at the cell wall. Current Opinion in Plant Biology. 2004;7:377–383. doi: 10.1016/j.pbi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Shimada C, Lipka V, O'Connell RO, Okuno T, Schulze-Lefert P, Takano Y. Nonhost resistance in Arabidopsis–Colletotrichum interactions acts at the cell periphery and requires actin filament function. Molecular Plant–Microbe Interactions. 2006;19:270–279. doi: 10.1094/MPMI-19-0270. [DOI] [PubMed] [Google Scholar]
- Skou JP, Jorgensen J, Lilholt U. Comparative studies on callose formation in powdery mildew compatible and incompatible barley. Phytopathologische Zeitschrift. 1984;109:147–168. [Google Scholar]
- Stein M, Dittgen J, Sánchez-Rodriquez C, Hou B-H, Molina A, Schulze-Lefert P, Lipka V, Somerville S. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. The Plant Cell. 2006;18:731–746. doi: 10.1105/tpc.105.038372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D, Jones DA, Hardham AR. GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. The Plant Journal. 2003;33:775–792. doi: 10.1046/j.1365-313x.2003.01673.x. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H. Fresh insights into processes of non-host resistance. Current Opinion in Plant Biology. 2003;6:351–357. doi: 10.1016/s1369-5266(03)00063-3. [DOI] [PubMed] [Google Scholar]
- Yun BW, Atkinson HA, Gaborit C, Greenland A, Read ND, Pallas JA, Loake GJ. Loss of actin cytoskeleton function and EDS1 activity, in comination, severely compromises non-host resistance in Arabidopsis against wheat powdery mildew. The Plant Journal. 2003;34:768–777. doi: 10.1046/j.1365-313x.2003.01773.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Feechan A, Pederson C, Newman M-A, Qiu J-L, Oleson KL, Thordal-Christensen H. A SNARE-protein has opposing functions in penetration resistance and defence signalling pathways. The Plant Journal. 2007;49:302–312. doi: 10.1111/j.1365-313X.2006.02961.x. [DOI] [PubMed] [Google Scholar]