Abstract
Mucin type O-glycosylation is a widespread modification of eukaryotic proteins, but its functional requirements remain incompletely understood. It is initiated by the attachment of N-acetylgalactosamine (GalNAc) to Ser or Thr residues, and then elongated by additional sugars. We have examined requirements for mucin-type glycosylation in Drosophila by characterizing the expression and phenotypes of core 1 galactosyltransferases (core 1 GalTs), which elongate O-GalNAc by adding galactose in a β1,3 linkage. Drosophila encode several putative core 1 GalTs, each expressed in distinct patterns. CG9520 (C1GalTA) is expressed in the amnioserosa and central nervous system. A null mutation in C1GalTA is lethal, and mutant animals exhibit a striking morphogenetic defect in which the ventral nerve cord is greatly elongated and the brain hemispheres are misshapen. Lectin staining and blotting experiments confirmed that C1GalTA contributes to the synthesis of Gal-β1,3-GalNAc in vivo. Our results identify a role for mucin-type O-glycosylation during neural development in Drosophila.
Keywords: Drosophila, galactosyltransferase, mucin, nervous system
INTRODUCTION
O-linked glycosylation, in which glycans are attached to the hydroxyl groups of Ser or Thr residues, is a structurally and functionally diverse class of protein modifications (Haltiwanger and Lowe, 2004). One common class of O-linked glycosylation in eukaryotes encompasses glycans attached to extracellular protein domains via an O-linked N-acetylgalactosamine (O-GalNAc) (Hanisch, 2001; Hang and Bertozzi, 2005). This is commonly referred to as mucin-type glycosylation, because of its association with heavily glycosylated mucin proteins. In this context, mucin-type glycosylation is thought to form a protective barrier and extracellular lubricant. However, mucin-type glycosylation is also found on a wide variety of other proteins, and implicated in diverse functions, from lymphocyte homing to sperm-egg binding (Hanisch, 2001; Hang and Bertozzi, 2005). Additionally, aberrant mucin-type glycosylation is also often associated with tumor metastasis (Brooks et al., 2008). In this work, we describe a requirement for mucin-type glycosylation during the development of the Drosophila nervous system.
In mammals, O-GalNAc (Tn antigen) is most often modified by a β1,3 linked Gal to generate the core 1 disaccharide (T antigen), which is then further modified by the addition of other sugars. Several alternate core structures, in which different sugars are attached to O-GalNAc, have also been described, and the core O-linked glycans can then be elongated in diverse ways to generate a wide variety of mucin-type glycans (Hanisch, 2001; Hang and Bertozzi, 2005). The Drosophila genome does not appear to encode homologues of many of the enzymes required for the generation of the complex and diverse mucin-type glycans found in vertebrates (Adams et al., 2000), and mass spectrometry of O-glycans in Drosophila embryos identified mostly disaccharide (T antigen) (North et al., 2006; Aoki et al., 2008). Nonetheless, some monosaccharide (Tn antigen) was detected, and recent analysis of Drosophila O-glycans has also resulted in the detection of smaller amounts of a number of more complex O-GalNAc glycans, including a set of glucouronylated core 1 O-glycans (Aoki et al., 2008; Breloy et al., 2008).
While glycoconjugate analysis suggests that synthesis of mucin-type glycans in Drosophila might be simpler than in vertebrates, in one respect Drosophila exhibit greater complexity than mammals. There exist a large family of enzymes that can catalyze the attachment of GalNAc to protein in mammals (ppGalNAcTs) (Ten Hagen et al., 2003a), but only a single core 1 Galactosyltransferase (core 1 GalT) has been identified (Ju et al., 2002a). Conversely, bioinformatics analysis suggests that Drosophila possess large families both of ppGalNAcTs (Ten Hagen et al., 2003b), and of core 1 GalTs (Correia et al., 2003). Biochemical studies have confirmed that several of these ppGalNAcTs and at least four of the core 1 GalTs possess the expected enzymatic activity (Ten Hagen et al., 2003b; Muller et al., 2005).
Although potential redundancy remains a challenge, genetic studies are beginning to inform our understanding of the biological requirements for mucin-type glycosylation. In mice, four different ppGalNAcTs have been mutated by gene targeting, but without apparent phenotypic effect, presumably due to redundancy among family members (Ten Hagen et al., 2003a). The existence of only a single core 1 GalT in mice is more favorable for genetic analysis, and a gene-targeted mutation in this gene is embryonic lethal with defective angiogenesis (Xia et al., 2004). However, it also remains possible that for some functions core 1 O-linked glycans are functionally redundant with other O-linked or N-linked glycans. Although Drosophila also contain several ppGalNAcTs, expressed in overlapping patterns (Ten Hagen et al., 2003b; Tian and Ten Hagen, 2006b), mutation of at least one ppGalNAcT, l(2)35a, is lethal (Schwientek et al., 2002; Ten Hagen and Tran, 2002), and mutant animals have tracheal defects (Tian and Ten Hagen, 2006a).
In this work, we present a functional analysis of requirements for two of the core 1 GalTs in Drosophila. We report that the members of this gene family are expressed in diverse expression patterns, suggesting that genetic redundancy among them is limited. We created and characterized mutations in the core 1 GalT encoded by GC9520 (C1GalTA), and identified a striking neural phenotypes in mutant animals, in which the ventral nerve cord fails to condense and the brain hemispheres are misshapen. This phenotype implicates mucin-type glycans in morphogenetic processes required for normal neural development.
RESULTS and DISCUSSION
Core 1 GalTs in Drosophila
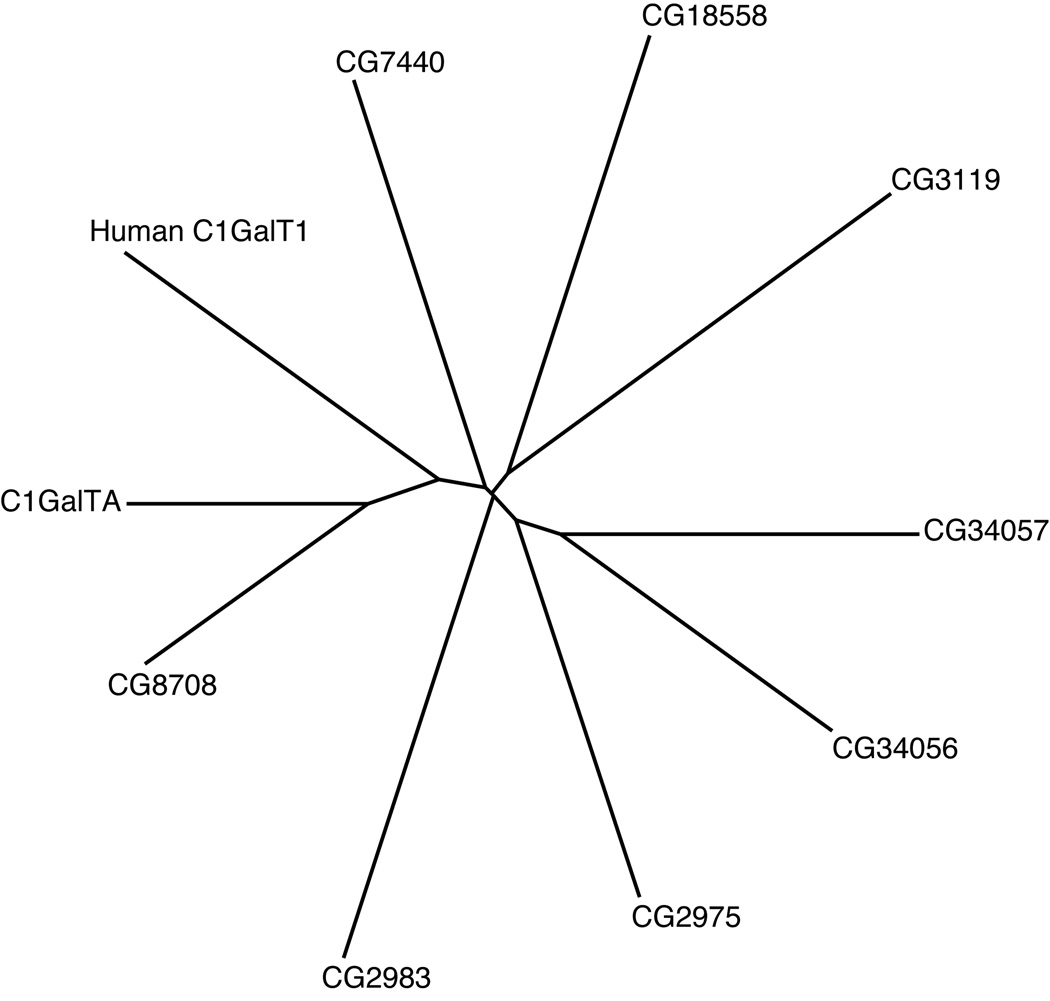
When human core 1 GalT (C1GalT1) was first cloned, it was suggested that Drosophila contain two close homologues, encoded by CG8708 and CG9520 (Ju et al., 2002a). However, based on bioinformatic analysis, we suggested that Drosophila are likely to encode at least eight distinct core 1 GalT domains, which at that time were annotated as seven distinct genes (CG9520, CG8708, CG2975, CG3119, CG7440, CG13904, CG18558), one of which (CG13904) encoded two tandemly repeated core 1 GalT domains (Correia et al., 2003). Another gene, CG2983, shares a high degree of overall sequence similarity with the other putative core 1 GalTs, but appears to lack critical amino acids within the most highly conserved sequence motifs (Correia et al., 2003). In a subsequent refinement of the Drosophila genome annotation, CG13904 has been split into two adjacent genes, CG34056 and CG34057 (Drysdale and Crosby, 2005). We also note that four of the putative core 1 GalTs (CG3119, CG2975, CG18558, and CG2983) are located in close proximity to each other on the second chromosome (Table 1), suggesting that they arose from relatively recent duplication events. By BLASTP (Correia et al., 2003) or ClustalW analysis, CG9520 is both the closest Drosophila homologue of human core 1 GalT1, and the closest homologue of most of the putative Drosophila core 1 GalTs, and thus appears to represent the ancestral core 1 GalT in Drosophila (Fig. 1).
Table 1. Expression of the Core1 GalT family in Drosophila.
Tissues in which prominent expression of the listed gene was detected are noted, blank cells indicate that the expression was not examined.
| Gene | Cytological Location | Embryo | Larva | Adult Female | Adult Male |
|---|---|---|---|---|---|
| C1GalTA | 29F5 | Amnion serosa, CNS | Gut, nervous system, imaginal discs | Nurse cells | Ejaculatory duct |
| CG8708 | 44B5-7 | Salivary gland | Salivary gland | Not detected | Ejaculatory duct, accessory gland |
| CG7440 | 18A3 | Proventriculus, midgut | Gastric caeca, Proventriculus, nervous system, imaginal discs | Nurse cells | testis |
| CG13904/ CG34056 | 61D4 | Trachea | Imaginal discs and brain | Follicle cells | accessory gland |
| CG34057 | 61D4 | Not detected | |||
| CG2975 | 23B4 | Not detected | Proventriculus | Nurse cells | |
| CG18558 | 23B4 | Not detected | Nurse cells | testis | |
| CG3119 | 23B3 | Not detected | brain | ||
| CG2983 | 23B5 | Not detected | Imaginal discs | Not detected | accessory gland |
Figure 1. Phylogenetic relationship of Core 1 GalTs.
A proportional, radial, phylogenetic tree was constructed to depict the relative sequence divergence among members of the Core 1 GalT family in Drosophila and human. The tree depicts graphically the results of ClustalW analysis.
The enzymatic activity of four putative Drosophila core 1 GalTs, encoded by CG9520, CG8708, CG34056, and CG2975 has been reported (Muller et al., 2005). All four of them exhibit some glycosyltransferase activity on model substrates, transferring Gal onto the 3 position of GalNAc, implying that they can act as core 1 GalTs. CG9520 in particular exhibited high activity on Tn and glycopeptide substrates, although it also exhibited weaker activity on glycolipids. We have independently confirmed the ability of CG9520 to transfer Gal onto a simple GalNAc acceptor, pNp-GalNAc (not shown). We also attempted to detect core 1 GalT activity for CG8708, CG7440 and CG18558 in this same assay, but were unsuccessful. However, we note that Muller et al (2005) reported that the Gal transferase activity of CG8708, CG34056, and CG2975 was very low, approximately a hundred fold less than that of CG9520, and only a few fold above background. The low or undetectable activity on simple model substrates for many members of this gene family might suggest that they do not function as core 1 GalTs, and instead participate in the synthesis of alternative glycoprotein or glycolipid structures. It is also possible that full core 1 GalT activity for most family members can only be achieved on specific, as yet unidentified, substrates. Alternatively, the activity of some family members might require specific co-factors or chaperones (Ju and Cummings, 2002). Conventionally, Drosophila genes are named once genetic or biochemical functions for them have been determined, and on this basis we will refer to CG9520 as Core 1 Galactosyltransferase A (C1GalTA), but because of uncertainty over their principal biochemical functions, continue to refer to other family members by their CG numbers.
Drosophila Core 1 GalT family members exhibit diverse, tissue-specific expression
To gain insight into the potential biological functions of known and putative core 1 GalTs, we examined their expression patterns throughout Drosophila development by in situ hybridization. These expression patterns are summarized in Fig 2–Fig 4 and Table 1. These studies revealed a striking diversity in expression patterns, as many of these genes are uniquely expressed in distinct epithelial tissues. The observation of distinct expression patterns suggests that they have distinct biological functions.
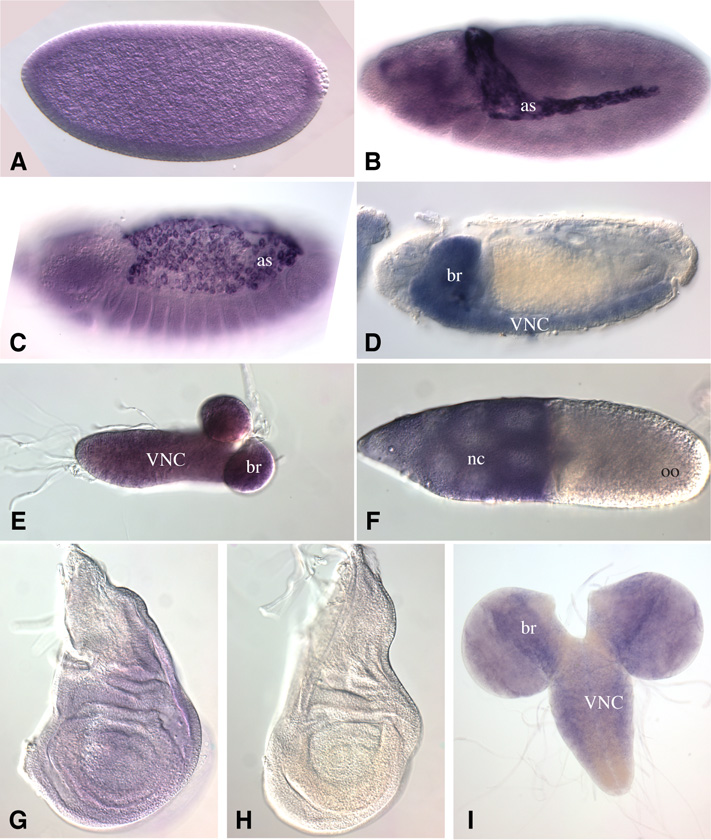
Figure 2. Expression of C1GalTA.
In situ hybridization to Drosophila tissues to detect expression of C1GalTA mRNA. A) Stage 6 embryo. Uniform expression above background is observed in early embryos, which likely includes maternally deposited mRNA. B) Stage 12 embryo. Strong expression is detected in the amnioserosa (as), and weaker expression elsewhere. C) Stage 14 embryo. Strong expression is detected in the amnioserosa. D) Stage 16 embryo. Expression is detected in the VNC and brain (br). E) CNS from first instar larva, expression is detected through out. F) Stage 10 follicle, with strong expression detected in the nurse cells (nc), but not in the oocyte (oo). G) Third instar wing imaginal disc, faint expression is detected. H) Third instar wing imaginal disc hybridized with a sense strand probe as a control, no staining is detected. I) Third instar CNS, C1GaTA is expressed in the brain and VNC.
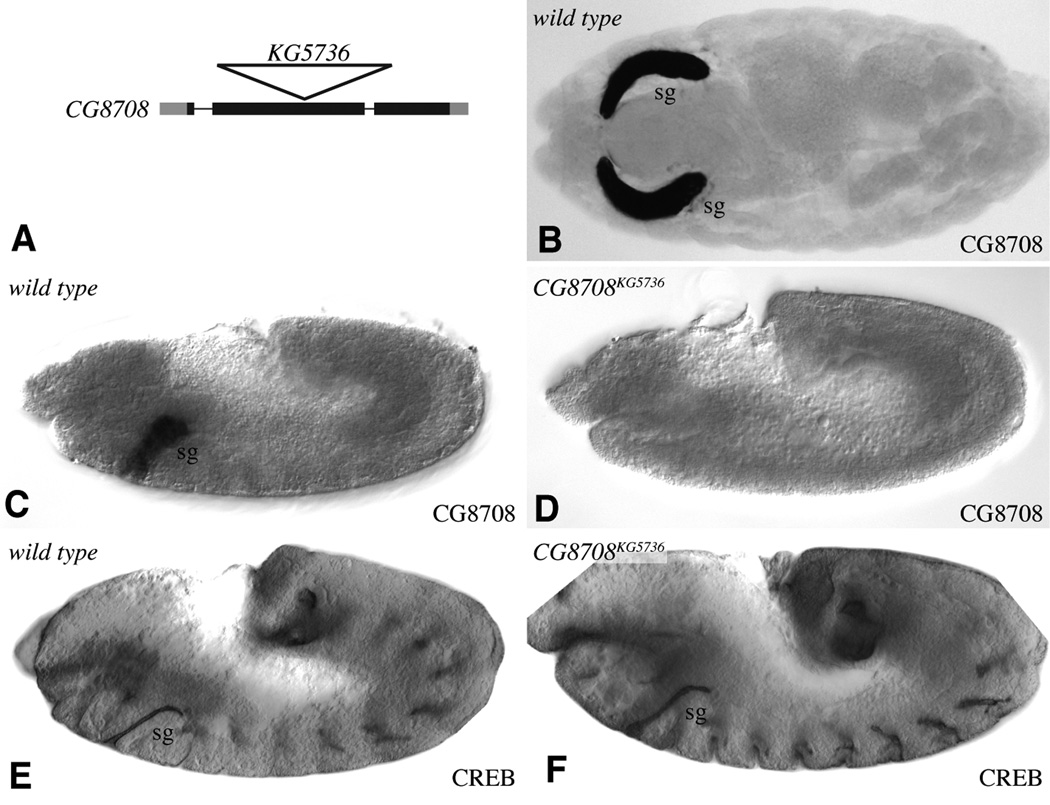
Figure 4. CG8708 mutant does not affect salivary gland morphogenesis.
A) Schematic of CG8708 transcription unit shows introns as thin lines and exons as thick lines, with coding regions in black and non-coding regions in gray. The position of the KG5736 insertion is indicated by the triangle. B) Stage 16 wild-type embryo, with CG8708 expressed specifically in salivary glands (sg). C) Stage 12 wild-type embryo, with CG8708 expressed specifically in the developing salivary glands (sg). D) Stage 12 CG8708KG5736 homozygous embryo; CG8708 expression is not detectable. E) Stage 12 wild-type embryo, anti-CREB staining marks the lumen of the developing salivary glands (sg). D) Stage 12 CG8708KG5736 homozygous embryo; anti-CREB staining is not distinguishable from wild type.
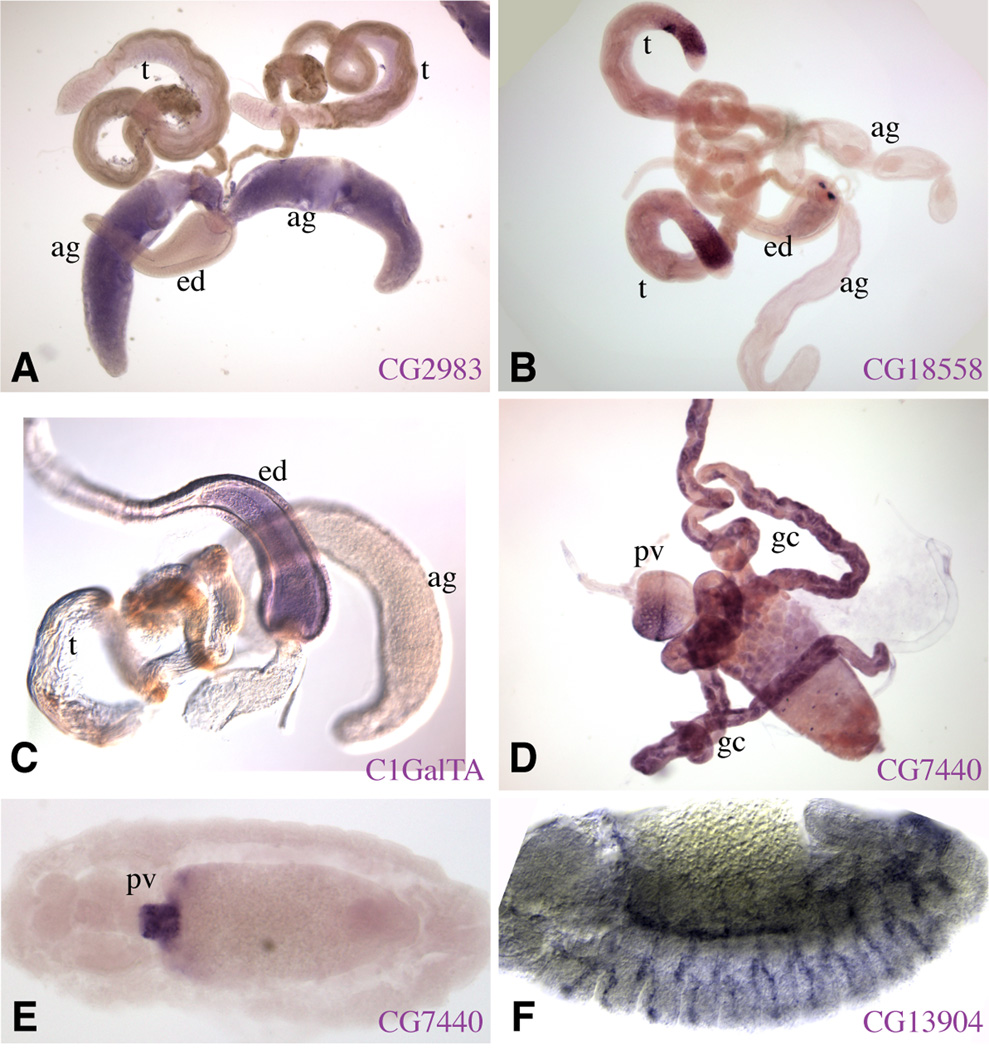
Four of the known and putative core 1 GalTs are expressed during embryonic development. As reported previously (Muller et al., 2005), we find that C1GalTA is expressed by amnioserosa cells (Fig. 2B,C), and CG8708 is expressed by salivary gland cells (Fig. 4B,C). However, in contrast to Muller, we find that CG13904/CG34056 is expressed specifically by tracheal cells rather than salivary gland cells (Fig. 3F), and we also found that during late embryonic development C1GalTA is expressed within the central nervous system (CNS) (Fig. 2D). The tracheal expression of CG13904/CG34056 is intriguing in light of the tracheal defects observed in a ppGalNAcT mutant (Tian and Ten Hagen, 2006a). We also report that CG7440 is expressed by a limited number of cells in the anterior midgut and proventriculus (Fig. 3E). The remaining core 1 GalTs were not detectably expressed during embryogenesis.
Figure 3. Expression of Core 1 GalT family members.
In situ hybridization to Drosophila tissues to detect expression of various members of the Core 1 GalT family. A–C) Male reproductive system, including ejaculatory duct (ed), testis (t), and accessory glands (ag). A) CG2983 is expressed preferentially in the accessory glands. B) CG18558 is expressed preferentially in the distal region of the testis C) C1GalTA is expressed preferentially in the ejaculatory duct. D) portion of the gut from a third instar larva, CG7440 is expressed preferentially in the gastric cecae (gc) and parts of the proventriculus (pv). E) Stage 14 embryo, CG7440 is expressed preferentially in the proventriculus. F) Stage 13 embryo, CG13904 is expressed preferentially in the developing trachea.
We also examined the expression of all family members at later stages of development. During larval development expression of core 1 GalTs was detected in parts of the digestive tract, imaginal discs, and CNS, (Table 1, Fig 2, Fig 3). In the adult, several of the core1 GalTs exhibit distinct regionalized expression patterns in different parts of the male reproductive system (Fig. 3A–C, Table 1), and several are expressed in the nurse cells during oogenesis (Fig. 2F, Table 1).
Isolation of mutations in C1GalTA and CG8708
To investigate the biological requirements for mucin-type glycosylation in Drosophila, we sought to identify mutations in core 1 GalTs. We have focused on the two genes that are most closely related by sequence to mammalian C1GalT1, CG8708 and C1GalTA.
In the case of CG8708, a P element insertion within the protein-coding second exon, P[KG05736], was identified by the BDGP gene disruption project (Bellen et al., 2004) (Fig. 4A). This insertion is located between conserved sequence motifs expected to be required for core 1 GalT function. Moreover, in situ hybridization to Drosophila embryos using a probe corresponding to portions of the gene downstream of the insertion site established that downstream sequences are not detectably transcribed in homozygous mutant embryos (Fig. 4D). Thus, this insertion likely corresponds to a null allele. Nonetheless, homozygous mutant animals are viable, fertile, and appear morphologically normal. Examination of salivary gland morphogenesis, using antibodies that stain salivary gland cells, failed to reveal defects in salivary gland development (Fig. 4E,F and data not shown) . CG8708 might be genetically redundant, if, for example, other core 1 GalTs are indeed expressed in salivary gland cells (Muller et al., 2005). Alternatively, CG8708 might have purely physiological rather than developmental roles.
The gene disruption project also identified a P-element insertion within C1GalTA, P[KG02976] (Fig. 5A) (Bellen et al., 2004). Homozygous individuals are viable and appear morphologically normal. This insertion is located within the first intron, and in situ hybridization to mRNA using downstream probes in homozygous animals revealed no detectable effect on C1GalTA expression (not shown), suggesting that it does not impair gene expression. In order to create mutations in C1GalTA, we thus took advantage of the fact that transposase-mediated excision of P elements is often imprecise, resulting in deletion of flanking sequences. From 76 excision events, we isolated 20 homozygous lethal mutant chromosomes. However, genetic complementation tests with chromosomal deficiencies that delete the cytological location of C1GalTA (29F5) revealed that only two of the lethal mutations mapped to this region.
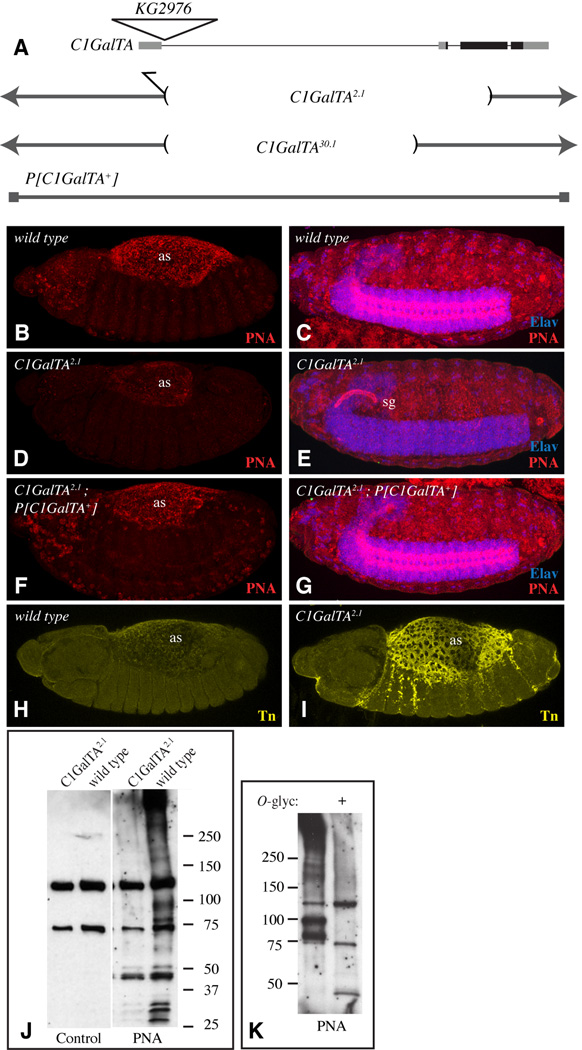
Figure 5. Influence of C1GalTA mutants on PNA and anti-Tn staining.
A) Top line shows a schematic of the C1GalTA transcription unit, with introns as thin lines, exons as thick lines, coding regions in black and non-coding regions in gray. The position of the KG2976 insertion is indicated by the triangle. Lower lines show DNA retained in mutant alleles, and DNA included in the rescue construct. B) Stage 13 wild-type embryo showing PNA staining (red) in the amnioserosa (as). C) Stage 16 wild-type embryo showing PNA staining in the CNS; the CNS is marked by anti-Elav staining (blue). D) Stage 13 C1GalTA2.1 mutant embryo showing reduced PNA staining in the amnioserosa. E) Stage 16 C1GalTA2.1 mutant embryo lacks PNA staining in the CNS, but PNA staining is now detected in the salivary gland (sg). F) Stage 13 C1GalTA2.1 mutant embryo with the P[C1GalTA+] rescue construct, PNA staining is indistinguishable from wild type. G) Stage 16 C1GalTA2.1 mutant embryo with the P[C1GalTA+] rescue construct, PNA staining is indistinguishable from wild type. H) Stage 13 wild-type embryo showing very low anti-Tn staining (yellow) in the amnioserosa. C) Stage 13 mutant embryo showing anti-Tn staining in the amnioserosa. J) PNA lectin blot on lysate from wild-type and C1GalTA2.1 mutant embryos. Two prominent bands detected in both wild-type and mutant lanes are actually background bound by the detection reagent (Avidin-hrp) as revealed by the control blot in which PNA was omitted. K) PNA lectin blot on lysate from wild-type embryos, where indicated (+) lysate was treated with O-glycanase.
These two mutations, which we will refer to as C1GalTA2.1 and C1GalTA30.1, are also lethal in trans to each other, and subsequent molecular and genetic characterization confirmed that they are both alleles of C1GalTA. PCR and DNA sequencing revealed that C1GalTA2.1 is associated with a large deletion of genomic DNA (Fig. 5A), which removes much of the C1GalTA coding region, including motifs predicted to be essential for its catalytic activity. C1GalTA30.1 is associated with a smaller deletion (Fig. 5A). This molecular analysis suggests that C1GalTA2.1 is likely to be a null allele, whereas C1GalTA30.1 might be a hypomorphic allele.
C1GalTA2.1 homozygotes died during embryogenesis, but this was due to an unlinked lethal mutation, as hemizygous mutant animals, created using molecularly defined chromosomal deficiencies (Df(2L)ED647 and Df(2L)Exel7040), can survive until pupal stages. C1GalTA30.1 hemizygotes are also pupal lethal. Confirmation that the lethality is due to mutation of C1GalTA, rather than other genes, was provided by the observation that C1GalTA2.1 or C1GalTA30.1 hemizygotes can be rescued by a transgene (P[C1GalTA+]) that comprises a genomic DNA fragment that only encodes C1GalTA (Fig. 5A). These rescued animals are viable, fertile, and appear morphologically normal.
Glycosylation changes in C1GalTA mutants
The lethality of C1GalTA mutants, together with its normal enzymatic activity, implies that this gene participates in the synthesis of essential mucin-type O-glycans during Drosophila development. To visualize glycosylation changes in mutant animals, we took advantage of the availability of a lectin, peanut agglutinin (PNA), that specifically recognizes T antigen (Gal β1,3 GalNAc) (Wu et al., 1997). In wild-type embryos, PNA preferentially stains the amnioserosa, and at later stages, parts of the CNS (Fig. 5B,C) (Burt and Anderson, 1985; Fredieu and Mahowald, 1994; D’Amico and Jacobs, 1995; Tian and Hagen, 2007). This is reminiscent of the expression of C1GalTA, consistent with the hypothesis that it represents bona fide detection of T antigen in Drosophila. Indeed, when C1GalTA2.1 mutant embryos were stained with PNA, the amnioserosa staining was reduced, and the CNS staining was abolished (Fig. 5D,E). Normal PNA staining was restored in animals with the genomic rescue construct (Fig. 5F,G). These observations implicate C1GalTA as a major T synthase during embryonic development. The residual amnioserosa staining observed in C1GalTA mutants might derive from the maternal contribution of C1GalTA (Fig. 2A,F), or from the activity of other core 1 GalTs. Further support for the conclusion that C1GalTA promotes synthesis of T antigen in vivo was provided by the observation that amnioserosa staining with an antibody that recognizes O-linked GalNAc (anti-Tn) increased in C1GalTA2.1 mutant embryos (Fig. 5H,I). Intriguingly, an obvious PNA staining of the salivary gland was observed C1GalTA2.1 mutant embryos (Fig. 5E), but not in wild-type embryos (Fig. 5C). We suggest that in the absence of C1GalTA, either a CG8708-dependent synthesis of T antigen can now occur, or a pre-existing CG8708-dependent synthesis of T antigen in the salivary gland becomes recognizable by PNA.
We also examined glycosylation changes in mutant animals by separating proteins on SDS PAGE gels, transferring them to membranes, and then probing them with sugar binding proteins (lectin blots). PNA blotting identified several bands in wild-type embryos (Fig. 5J,K). These bands correspond to O-glycosylated proteins, as PNA staining on blots was greatly reduced by treatment of embryo lysates with O-glycanase (Fig. 5K), which specifically removes O-GalNAc glycans. Notably, these bands were also greatly reduced in C1GalTA2.1 mutants (Fig. 5J). Similar results were observed with another T-antigen binding lectin, Jacalin (not shown). These observations further support the conclusion that C1GalTA is the principal T synthase during embryonic development.
C1GalTA is required for nervous system morphogenesis
In order to identify developmental processes that require C1GalTA–mediated glycosylation, we examined the external and internal morphology of mutant animals. Most embryonic and larval tissues were not visibly abnormal. However, the larval nervous system displayed a striking defect, in which the ventral nerve cord (VNC) was greatly elongated, and the larval brain hemispheres were distorted (Fig. 6A–K); this phenotype was fully rescued by the wild-type transgene (Fig. 6J). Thus, elongation of mucin type O-glycans is apparently required for normal development of the Drosophila nervous system.
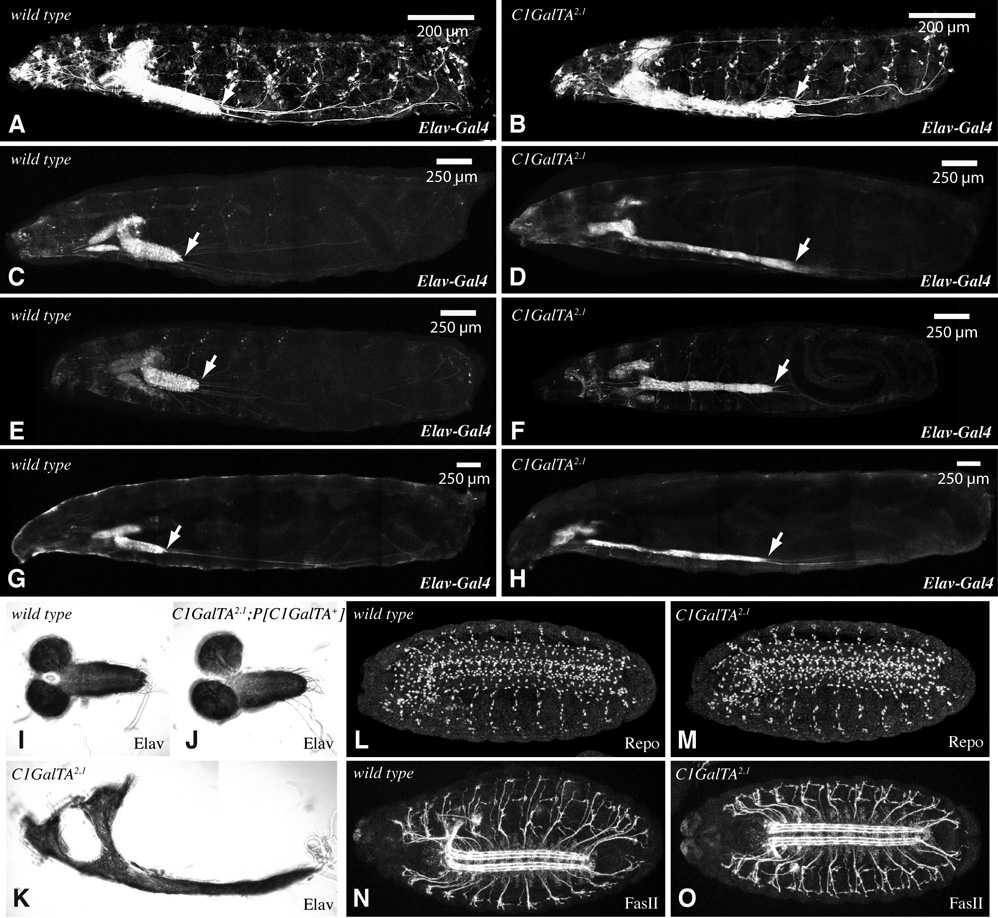
Figure 6. VNC Phenotype of C1GalTA mutants.
A–H) Composite confocal images of larvae with Elav-Gal4 and UAS-GFP transgenes. GFP is expressed in the nervous system and salivary glands, arrow points to the posterior end of the VNC. A–D, G, H are saggital views, E,F is a ventral view. A) wild-type first instar. B) C1GalTA2.1 mutant first instar. C) wild-type second instar. D) C1GalTA2.1 mutant second instar. E) wild-type second instar. F) C1GalTA2.1 mutant second instar. G) wild-type third instar. H) C1GalTA2.1 mutant third instar. I) Dissected late third instar CNS from wild type, stained with anti-Elav. J) Dissected late third instar CNS from C1GalTA2.1 mutant with P[C1GalTA+] rescue construct, stained with anti-Elav. K) Dissected late third instar CNS from C1GalTA2.1 mutant, stained with anti-Elav. L,M) Stage 16 wild-type (L) and C1GalTA2.1 mutant (M) embryos stained with anti-Repo to detect glial cells. N,O) Stage 16 wild-type (N) and C1GalTA2.1 mutant (O) embryos stained with anti-FasII to detect a subset of axon fasicles.
In wild type, the VNC spans the length of the embryo when it first forms, but then towards the end of embryogenesis begins to condense, such that in the larva it spans only a few segments. The elongated length of the larval VNC suggested that this normal condensation process could be defective. To facilitate characterization of VNC condensation, we examined animals in which GFP was expressed in the VNC under nrv2-Gal4 or elav-Gal4 control. In wild type, condensation begins during stage 15, and continues through hatching (Olofsson and Page, 2005). In C1GalTA mutants, VNC condensation occurs, but fails to reach the same extent of condensation as in wild type, as the VNC is obviously elongated in first instar larvae (Fig. 6A,B, Table 2). To probe for defects in neural development during embryogenesis, we stained embryos with antibodies that specifically recognize neurons (anti-Elav), glia (anti-Repo), or axons (anti-Fasciclin II, anti-Neuroglian), but none of these stains revealed obvious differences between wild-type and C1GalTA mutant embryos (Fig. 6L–O and data not shown).
Table 2. Length of the VNC in wild-type and mutant larvae.
Average lengths as measured from confocal micrographs of larvae expressing GFP under elav-Gal4 control (Fig. 5), the variation (+/−) indicates one standard deviation. Ratio indicates VNC length / Larval length.
| Genotype | Instar | Number measured | Larval length (µm) | VNC length (µm) | Ratio (%) |
|---|---|---|---|---|---|
| Wild type | 1st | 20 | 1403 +/− 114 | 351 +/− 36 | 25 |
| C1GalTA2.1 | 1st | 15 | 1231 +/− 125 | 530 +/− 69 | 43 |
| Wild type | 2nd | 24 | 2752 +/− 343 | 350 +/− 46 | 13 |
| C1GalTA2.1 | 2nd | 29 | 2582 +/− 511 | 923 +/− 201 | 36 |
| Wild type | early 3rd | 11 | 4103 +/− 464 | 437 + / − 50 | 11 |
| C1GalTA2.1 | early 3rd | 15 | 4202 +/− 519 | 1554 +/− 253 | 37 |
In wild-type animals the VNC remains short throughout larval development, but in C1GalTA mutants the VNC is even more elongated at third instar than it is at the end of embryogenesis. To examine the progression of the VNC morphology defect, we compared the relative length of the VNC at first instar, second instar, and early third instar. At each stage, the VNC in mutant animals is elongated compared to wild-type animals, and the VNC continues to increase in length throughout larval development (Fig. 6, Table 2). The continued requirement for C1GalTA during larval development is consistent with the observation that it is expressed in the CNS throughout larval development (Fig. 2E, I). The elongated VNC of mutants is noticeably thinner (Fig. 6C–H) and is also more fragile when dissected.
C1GalTA and processes implicated in VNC condensation
Condensation of the VNC occurs in many arthropods, but remains a poorly understood process. Some insight into genes and processes involved has come from genetic studies in Drosophila. Mutation of worniu, which encodes a snail family transcription factor, impairs VNC condensation (Ashraf et al., 2004). To investigate whether there was a regulatory link between worniu and the glycosylation effected by C1GalTA , we examined C1GalTA expression and PNA staining in worniu1 mutants, but no differences were observed (not shown). Glia have been implicated in VNC condensation by the observation that VNC condensation is reduced in mutants that affect glial cells, including prospero and repo (Doe et al., 1991; Campbell et al., 1994; Halter et al., 1995). Using anti-Repo staining as a marker, no difference in the number of glial cells was observed in C1GalTA mutant embryos (Fig. 6L,M).
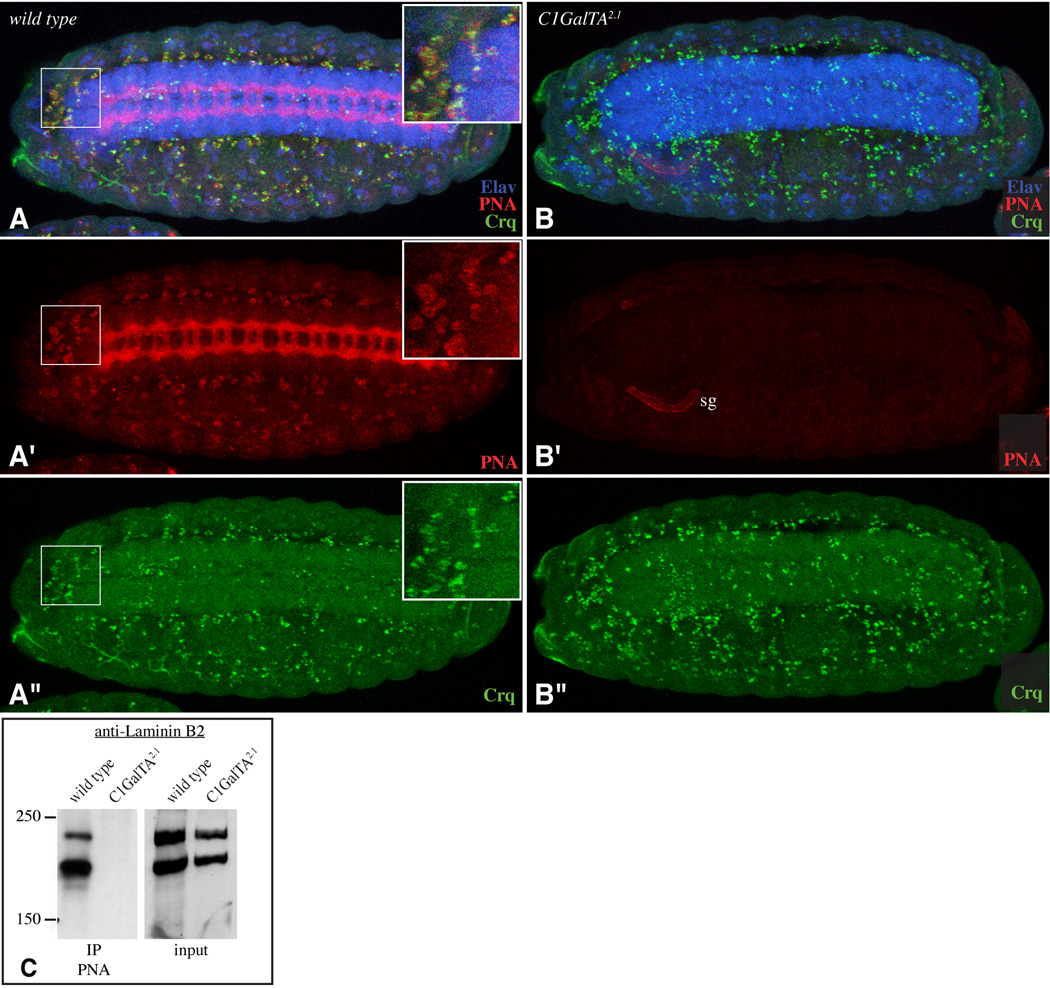
There is also evidence for a critical role of the extracellular matrix and cell-extracellular matrix interactions, as mutations in two different Drosophila integrin subunits have been reported to reduce VNC condensation (Brown, 1994). The secretion of extracellular matrix by hemocytes appears to be particularly important for VNC condensation, as disrupting hemocyte migration impairs both the deposition of extracellular matrix around the VNC and VNC condensation (Olofsson and Page, 2005). Intriguingly, in addition to the PNA reactivity associated with the amnioserosa and VNC, we noticed PNA staining on scattered cells whose number and distribution suggested that they correspond to hemocytes. This suggestion was confirmed by staining with antibodies against the hemocyte-specific protein Croquemort (Crq) (Franc et al., 1996) (Fig. 7A). The PNA reactivity on hemocytes was abolished in C1GalTA mutants (Fig. 7B).
Figure 7. Influence of C1GalTA mutants on PNA staining of hemocytes and Laminin.
A,B show stage 15 embryos stained with PNA (red), anti-Elav (blue), and anti-Crq (green). Panels marked prime show single channels of the embryo above. A) Wild type. PNA staining is detected on hemocytes; inset shows a close-up of the boxed region. B) C1GalTA2.1 mutant; normal PNA staining is lost, although PNA staining is now detected on the salivary gland (sg). C) Western blot using anti-Laminin B2 on lysates from wild-type or C1GalTA2.1 mutant embryos and on material precipitated using PNA-beads. The anti-B2 antibodies cross-react with both Laminin B1 (β, upper band) and Laminin B2 (γ, lower band) (Kumagai et al., 1997).
Laminin is a component of the extracellular matrix and a ligand for integrins. Drosophila Laminin was first identified and purified as a PNA-binding protein (Montell and Goodman, 1988). Drosophila Laminin is a trimer of B1 (β), B2 (γ), and A (α) chains, and binding of Laminin trimers to PNA could be visualized by Western blotting of PNA-bound proteins using antibodies against Laminin B2 (Fig. 7C). By contrast, lysates from C1GalTA mutant embryos lacked detectable association of Laminin with PNA (Fig. 7C). These observations imply that C1GalTA is required for Laminin O-glycosylation. The detection of this requirement for C1GalTA in VNC condensation and Laminin O-glycosylation, together with prior studies implicating extracellular matrix deposition and integrins in VNC condensation, suggest that Laminin could be a critical C1GalTA substrate in Drosophila.
As a number of distinct O-glycosylated proteins are detectable on PNA blots (Fig. 5J,K), further studies will be required to clarify the significance and role of Laminin O-glycosylation. Nonetheless, our observations have identified the product of the C1GalTA gene as a major contributor to T antigen synthesis in the Drosophila embryo, and have clearly established a requirement for elongation of O-GalNAc glycans in the morphogenesis of the Drosophila nervous system.
Experimental Procedures
Drosophila Genetics
Unless otherwise noted, stocks were obtained from the Bloomington Drosophila stock center. For nervous system labeling in larvae we used elav-Gal4[C155] and UAS-mCD8:GFP. Imprecise excisions were generated from a P-element insertion within C1GalTA, P[KG02976] (Bellen et al., 2004) by crossing to a transposase-expressing line (Sp/CyO; ry506 Sb1 P{Δ2–3}99B /TM6B, Ubx). 76 potential excision events were identified by the loss of the w+ marker. From these w− excisions, 20 homozygous lethal mutant chromosomes were isolated. Genetic complementation tests with chromosomal deficiencies (Df(2L)N22-14, Df(2L)ED647, and Df(2L)Exel7040) that delete the cytological location of C1GalTA (29F5), revealed that only two of the lethal mutations, C1GalTA2.1 and C1GalTA30.1, mapped to this region. PNA staining and rescue experiments displayed in the figures were performed using Df(2L)ED647 and Df(2L)Exel7040. Breakpoints for the C1GalTA2.1 and C1GalTA30.1 alleles were obtained by PCR amplification of genomic DNA from homozygous embryos using as primers DELCG9520US-1 5'AGAGAAACAGGCCATTGATAAAT and CG9520DS-1 5'TCAGCCGGCCCAAGGTC. PCR fragments were TA cloned into pGEMTeasy (Promega, Madison, WI) and then sequenced. In C1GalTA2.1, a 6127 bp deletion was detected, which includes the sequences encoding the transmembrane domain and conserved sequence motifs required for core 1 GalT activity. This mutation also retains 1584 bp from the P element insertion. In C1GalTA30.1, 4716 bp of genomic DNA are deleted, which includes most of the first intron of C1GalTA. In CG8708KG05736 (Bellen et al., 2004), the P element insertion is within the protein-coding second exon of CG8708, downstream of conserved motifs i and ii, but upstream of conserved motifs iii and iv (Correia et al., 2003).
Molecular Biology
In situ hybridization to mRNA was carried out as described previously (Irvine and Wieschaus, 1994). The templates for C1GalTA, CG8708, CG7440 and CG18558 were cDNAs SD07079 ,GH18356, CK00318, and AT24870 (Rubin et al., 2000), respectively. For CG8708 and C1GalTA, we also created probes corresponding to portions of the gene downstream of the P element insertion sites by PCR using primer pairs including T3 promoter sequence (AATTAACCCTCACTAAAGGG) on one primer and T7 promoter sequence (TAATACGACTCACTATAGGG) on the other (David and Wedlich, 2001). Primer sequences used were: for CG8708 , T7-CTATCCGTACAATCCCGAAACACC, T3-AGCCTGCCAAGTAAACTCAATAAA, and for C1GalTA, T7-CCCCCGCAACGAAGAAAC, T3-TGTCCGAGCAGCAGTCAAGTC. The templates for CG2975, CG13904, CG34056, CG34057, CG3119, and CG2983 were generated by PCR using primer pairs including T3 and T7 promoter sequences. Primer sequences used were: for CG2975, T7- GCTGATGCTAATGTTGCTGAT, T3-GTTCTGTAATTTGGTGTAGTGACG; for CG13904, T7-GTGCTGGGCCTCATCATTGG, T3-CTGCAGGCGCTCGTTCATCT; for CG34056, T7-CCCCCGGCTCGTTTTGTAAG, T3-AGTCGGGGTGGCAGCGTTCTC; for CG34057, T7-TGCGCCAATTGGACACAGC, T3-CCCAAGCGCGACGGAAGAG; for CG3119, T7-ATTATCAGGTTTCTCGGACATTTG, T3-GGCGGAGCTTCGTATATTCTGAGT; for CG2983, T7-GAATACAACGTCGCCATCAAC, T3-ATTTTAGCGCATCTTTTTCAGT.
To confirm the enzymatic activity of C1GalTA, we cloned its C-terminal catalytic domain (amino acids 44–388) into the vector pMT(1B) (Haines and Irvine, 2005; Xu et al., 2007), resulting in the plasmid pMT(1B)- C1GalTA:V5:His. pMT(1B) contains the Drosophila metallothionein promoter (pMT) for inducible expression, the BiP signal sequence for secretion, a V5 epitope tag for detection, and a His-tag for purification. DNA encoding C1GalTA was amplified by PCR from cDNA clone SD07079 using primers YLCG9520-n' 5’-GGGGTACCGGAGCGAAGTGAATTCATG and YLCG9520-c'-tag 5’-GCTCTAGATTGCGTCTTTGTCTCGGCG. Restriction enzyme sites KpnI and XbaI were included in the PCR primers to facilitate cloning. pMT(1B)- C1GalTA:V5:His was transfected into S2 cells, and concentrated media was used for glycosyltransferase assays, as described previously (Ju et al., 2002b; Haines and Irvine, 2005). A similar strategy was used for CG8708, CG7440, and CG18558.
For genomic rescue, a DNA fragment containing the entire C1GalTA region, but none of the neighboring transcription units, was amplified from wild type (Oregon-R) genomic DNA by PCR using primers forward 5’-ATAAGAATGCGGCCGCGTTTTAATGCTTTTGTTGACTTTGTATAAGTG and reverse 5’-CCGCTCGAGCCGCTGGCTAAGACTATGCGATAATTC, and cloned into pCaSpeR4 using NotI and XhoI restriction sites included on the primers, to generate pCaSpeR4- C1GalTA+. This construct was transformed into Drosophila using standard techniques, and insertions on the first P[C1GalTA+ -X] and third chromosomes P[C1GalTA+ −3] were obtained.
Alignment of core 1 GalT sequences by BLASP has been reported previously (Correia et al., 2003). Alignment by ClustalW (Larkin et al., 2007) was performed using the EMBL-EBI server (http://www.ebi.ac.uk/Tools/clustalw2/), and a phylogenetic tree with proportional distances was constructed using the Trex server (http://www.trex.uqam.ca/index.php?action=newick&project=trex) (Makarenkov, 2001).
Immunohistochemistry and blotting
Antibody staining was performed essentially as described previously (Panin et al., 1997), using the primary antibodies: rat-Elav (1:67, 7E8A10, Developmental Studies Hybridoma Bank (DSHB)), mouse anti-Neuroglian (1:125, BP 104, DSHB), mouse anti-CNS axons (1:200, BP 102, DSHB), rat anti-Repo (1:1000, 8D12, DSHB), mouse anti-Fasciclin II (1:200, 1D4, DSHB), rabbit anti-crq antibody (1:2000, N. Franc), mouse anti-Tn (1:10, Biomeda), and rabbit anti-GFP (1:400, Molecular Probes). Staining with biotinylated PNA lectin (Vector) was carried out as described previously (Tian and Hagen, 2007). Biotinylated PNA was used at 0.25ng/ml, and Streptavidin-Cy3 (Jackson ImmunoResearch) was used at 0.5ng/ml.
For lectin blotting 50 micrograms of lysates from both wild type and mutant embryos were run on SDS-PAGE gels and then transferred to membranes. Blots were probed with biotinylated PNA lectin (1:6000, Vector Laboratories) at 5mg/mL concentration followed by avidin and biotinylated HRP complexes using the ABC detection kit (Vector). Blots were then developed using a chemiluminiscence reagent (Perkin Elmer).
O-Glycanase treatment on embryo lysates was performed using GLYKO deglycosylation kit (Prozyme) according to the manufacturer’s instructions. Briefly, 50 µg of embryo lysate was incubated with O-Glycanase in buffer at 37°C for 3 hours. In control samples no enzyme was added. After the treatment samples were run on SDS-PAGE gels, Western blotted and analyzed by PNA lectin staining.
For co-immunoprecipitation experiments, embryo lysates in binding buffer (10 mM HEPES, pH 7.4, 150 mM NaCl) were passed through a PNA-agarose (Vector) column. After washing in high salt buffer (10 mM HEPES, pH 7.4, 500 mM NaCl) to remove unbound proteins, bound proteins were eluted with 0.2M galactose in binding buffer. Eluted proteins were subject to Western blotting using rabbit anti-Laminin B2 antisera (Kumagai et al., 1997)(1:3000).
ACKNOWLEDGEMENTS
We thank T. Okajima, V. Panin, and D. Rogulja for contributions to the initial analysis of core 1 GalT expression, N. Masangkay for assistance with genetic screening, S. Baumgartner, N. Franc, Y. Kitagawa, V. Pirrotta, the Developmental Studies Hybridoma Bank and the Bloomington stock center for plasmids, antibodies and Drosophila stocks, C. Doe for helpful discussion, and C. Rauskolb for comments on the manuscript. This work was supported by a grant from the Mizutani foundation for Glycoscience (060029) and the Howard Hughes Medical Institute.
REFERENCES
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Aoki K, Porterfield M, Lee SS, Dong B, Nguyen K, McGlamry KH, Tiemeyer M. The diversity of O-linked glycans expressed during Drosophila melanogaster development reflects stage- and tissue-specific requirements for cell signaling. J Biol Chem. 2008 doi: 10.1074/jbc.M804925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf SI, Ganguly A, Roote J, Ip YT. Worniu, a Snail family zinc-finger protein, is required for brain development in Drosophila. Dev Dyn. 2004;231:379–386. doi: 10.1002/dvdy.20130. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breloy I, Schwientek T, Lehr S, Hanisch FG. Glucuronic acid can extend O-linked core 1 glycans, but it contributes only weakly to the negative surface charge of Drosophila melanogaster Schneider-2 cells. FEBS Lett. 2008;582:1593–1598. doi: 10.1016/j.febslet.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Brooks SA, Carter TM, Royle L, Harvey DJ, Fry SA, Kinch C, Dwek RA, Rudd PM. Altered glycosylation of proteins in cancer: what is the potential for new anti-tumour strategies. Anticancer Agents Med Chem. 2008;8:2–21. doi: 10.2174/187152008783330860. [DOI] [PubMed] [Google Scholar]
- Brown NH. Null mutations in the alpha PS2 and beta PS integrin subunit genes have distinct phenotypes. Development. 1994;120:1221–1231. doi: 10.1242/dev.120.5.1221. [DOI] [PubMed] [Google Scholar]
- Burt R, Anderson H. Patterns of peanut agglutinin binding within the developing grasshopper central nervous system. J Embryol Exp Morphol. 1985;90:49–56. [PubMed] [Google Scholar]
- Campbell G, Goring H, Lin T, Spana E, Andersson S, Doe CQ, Tomlinson A. RK2, a glial-specific homeodomain protein required for embryonic nerve cord condensation and viability in Drosophila. Development. 1994;120:2957–2966. doi: 10.1242/dev.120.10.2957. [DOI] [PubMed] [Google Scholar]
- Correia T, Papayannopoulos V, Panin V, Woronoff P, Jiang J, Vogt TF, Irvine KD. Molecular genetic analysis of the glycosyltransferase Fringe in Drosophila. Proc Natl Acad Sci U S A. 2003;100:6404–6409. doi: 10.1073/pnas.1131007100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico P, Jacobs JR. Lectin histochemistry of the Drosophila embryo. Tissue Cell. 1995;27:23–30. doi: 10.1016/s0040-8166(95)80005-0. [DOI] [PubMed] [Google Scholar]
- David R, Wedlich D. PCR-based RNA probes: a quick and sensitive method to improve whole mount embryo in situ hybridizations. Biotechniques. 2001;30:769–772. 774. [PubMed] [Google Scholar]
- Doe CQ, Chu-LaGraff Q, Wright DM, Scott MP. The prospero gene specifies cell fates in the Drosophila central nervous system. Cell. 1991;65:451–464. doi: 10.1016/0092-8674(91)90463-9. [DOI] [PubMed] [Google Scholar]
- Drysdale RA, Crosby MA. FlyBase: genes and gene models. Nucleic Acids Res. 2005;33:D390–D395. doi: 10.1093/nar/gki046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franc NC, Dimarcq JL, Lagueux M, Hoffmann J, Ezekowitz RA. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity. 1996;4:431–443. doi: 10.1016/s1074-7613(00)80410-0. [DOI] [PubMed] [Google Scholar]
- Fredieu JR, Mahowald AP. Glycoconjugate expression during Drosophila embryogenesis. Acta Anat (Basel) 1994;149:89–99. doi: 10.1159/000147562. [DOI] [PubMed] [Google Scholar]
- Haines N, Irvine KD. Functional analysis of Drosophila beta1,4-N-acetlygalactosaminyltransferases. Glycobiology. 2005;15:335–346. doi: 10.1093/glycob/cwi017. [DOI] [PubMed] [Google Scholar]
- Halter DA, Urban J, Rickert C, Ner SS, Ito K, Travers AA, Technau GM. The homeobox gene repo is required for the differentiation and maintenance of glia function in the embryonic nervous system of Drosophila melanogaster. Development. 1995;121:317–332. doi: 10.1242/dev.121.2.317. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS, Lowe JB. Role of Glycosylation in Development. Annual Review of Biochemistry. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- Hang HC, Bertozzi CR. The chemistry and biology of mucin-type O-linked glycosylation. Bioorg Med Chem. 2005;13:5021–5034. doi: 10.1016/j.bmc.2005.04.085. [DOI] [PubMed] [Google Scholar]
- Hanisch FG. O-glycosylation of the mucin type. Biological Chemistry. 2001;382:143–149. doi: 10.1515/BC.2001.022. [DOI] [PubMed] [Google Scholar]
- Ju T, Brewer K, D’Souza A, Cummings RD, Canfield WM. Cloning and expression of human core 1 beta1,3-galactosyltransferase. J Biol Chem. 2002a;277:178–186. doi: 10.1074/jbc.M109060200. [DOI] [PubMed] [Google Scholar]
- Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci U S A. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Cummings RD, Canfield WM. Purification, characterization, and subunit structure of rat core 1 Beta1,3-galactosyltransferase. J Biol Chem. 2002b;277:169–177. doi: 10.1074/jbc.M109056200. [DOI] [PubMed] [Google Scholar]
- Kumagai C, Kadowaki T, Kitagawa Y. Disulfide-bonding between Drosophila laminin beta and gamma chains is essential for alpha chain to form alpha betagamma trimer. FEBS Lett. 1997;412:211–216. doi: 10.1016/s0014-5793(97)00780-1. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Makarenkov V. T-REX: reconstructing and visualizing phylogenetic trees and reticulation networks. Bioinformatics. 2001;17:664–668. doi: 10.1093/bioinformatics/17.7.664. [DOI] [PubMed] [Google Scholar]
- Montell DJ, Goodman CS. Drosophila substrate adhesion molecule: sequence of laminin B1 chain reveals domains of homology with mouse. Cell. 1988;53:463–473. doi: 10.1016/0092-8674(88)90166-3. [DOI] [PubMed] [Google Scholar]
- Muller R, Hulsmeier AJ, Altmann F, Ten Hagen K, Tiemeyer M, Hennet T. Characterization of mucin-type core-1 beta1-3 galactosyltransferase homologous enzymes in Drosophila melanogaster. Febs J. 2005;272:4295–4305. doi: 10.1111/j.1742-4658.2005.04838.x. [DOI] [PubMed] [Google Scholar]
- North SJ, Koles K, Hembd C, Morris HR, Dell A, Panin VM, Haslam SM. Glycomic studies of Drosophila melanogaster embryos. Glycoconj J. 2006;23:345–354. doi: 10.1007/s10719-006-6693-4. [DOI] [PubMed] [Google Scholar]
- Olofsson B, Page DT. Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev Biol. 2005;279:233–243. doi: 10.1016/j.ydbio.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Schwientek T, Bennett EP, Flores C, Thacker J, Hollmann M, Reis CA, Behrens J, Mandel U, Keck B, Schafer MA, et al. Functional conservation of subfamilies of putative UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosaminyltransferases in Drosophila, Caenorhabditis elegans, and mammals. One subfamily composed of l(2)35Aa is essential in Drosophila. Journal of Biological Chemistry. 2002;277:22623–22638. doi: 10.1074/jbc.M202684200. [DOI] [PubMed] [Google Scholar]
- Ten Hagen KG, Fritz TA, Tabak LA. All in the family: the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology. 2003a;13:1R–16R. doi: 10.1093/glycob/cwg007. [DOI] [PubMed] [Google Scholar]
- Ten Hagen KG, Tran DT. A UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase is essential for viability in Drosophila melanogaster. Journal of Biological Chemistry. 2002;277:22616–22622. doi: 10.1074/jbc.M201807200. [DOI] [PubMed] [Google Scholar]
- Ten Hagen KG, Tran DT, Gerken TA, Stein DS, Zhang Z. Functional characterization and expression analysis of members of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family from Drosophila melanogaster. Journal of Biological Chemistry. 2003b;278:35039–35048. doi: 10.1074/jbc.M303836200. [DOI] [PubMed] [Google Scholar]
- Tian E, Hagen KG. O-linked glycan expression during Drosophila development. Glycobiology. 2007;17:820–827. doi: 10.1093/glycob/cwm056. [DOI] [PubMed] [Google Scholar]
- Tian E, Ten Hagen KG. A UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase is required for epithelial tube formation. J Biol Chem. 2006a doi: 10.1074/jbc.M606268200. [DOI] [PubMed] [Google Scholar]
- Tian E, Ten Hagen KG. Expression of the UDP-GalNAc: polypeptide N-acetylgalactosaminyltransferase family is spatially and temporally regulated during Drosophila development. Glycobiology. 2006b;16:83–95. doi: 10.1093/glycob/cwj051. [DOI] [PubMed] [Google Scholar]
- Wu AM, Song SC, Sugii S, Herp A. Differential binding properties of Gal/GalNAc specific lectins available for characterization of glycoreceptors. Indian J Biochem Biophys. 1997;34:61–71. [PubMed] [Google Scholar]
- Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, Lupu F, Cummings RD, McEver RP. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J Cell Biol. 2004;164:451–459. doi: 10.1083/jcb.200311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Haines N, Dlugosz M, Rana NA, Takeuchi H, Haltiwanger RS, Irvine KD. In vitro reconstitution of the modulation of Drosophila Notch-ligand binding by Fringe. J Biol Chem. 2007;282:35153–35162. doi: 10.1074/jbc.M707040200. [DOI] [PubMed] [Google Scholar]