Abstract
Direct physical linkage of MAGUKs to the actin cytoskeleton was first established by the interaction of erythrocyte p55 with the FERM domain of protein 4.1R. Subsequently, it was reported that p55 binds to a 51-amino acid peptide, encoded by exon 10, located within the FERM domain of protein 4.1R. In this study, we investigated the nature of the p55-FERM domain binding interface and show that p55 binds to a second 35-amino acid peptide, encoded by an alternatively spliced exon 5, located within the FERM domain of protein 4.1R. Competition and Surface Plasmon Resonance-binding measurements suggest that the peptides encoded by exons 5 and 10 bind to independent sites within the D5 domain of p55. Interestingly, the full length 135 kDa isoform of protein 4.1R containing both exons 5 and 10 was targeted exclusively to the plasma membrane of epithelial cells whereas the same isoform without exon 5 completely lost its membrane localization capacity. Together, these results indicate that p55 binds to two distinct sites within the FERM domain, and the alternatively spliced exon 5 is necessary for the membrane targeting of protein 4.1R in epithelial cells. Since sequences similar to the exon 5-peptide of protein 4.1R and D5 domain of p55 are conserved in many proteins, our findings suggest that a similar mechanism may govern the membrane targeting of other FERM domain containing proteins.
Keywords: Protein 4.1R, Erythrocyte p55, Membrane associated guanylate kinase homologue, FERM domain, Maltose binding protein, Surface Plasmon Resonance
1. Introduction
Membrane-associated guanylate kinase homologues (MAGUKs) are a family of proteins characterized by the presence of one or three PDZ domains, an SH3 domain, and a catalytically inactive guanylate kinase-like (GUK) domain, all of which function as protein-protein interaction modules [1]. It is believed that MAGUKs serve a scaffolding role by linking the transmembrane proteins to the cytoskeleton, thus regulating events such as synapse formation, cell-cell adhesion, and cell polarity [2, 3]. Erythroid p55, encoded by the Mpp1 gene, is one of the two founding members of the MAGUK family of proteins [4, 5]. It forms a ternary complex with the transmembrane protein glycophorin C and the cytoskeletal protein 4.1R, which is located at the spectrin-actin junctions, also called the junctional complex, in erythrocytes [6–9]. Previously, we localized the protein 4.1R-binding site within a positively charged 39-amino acid region located between the SH3 and GUK domains of p55 [7, 8]. This 39-amino acid sequence in p55, termed D5 domain, is also conserved in the Drosophila discs large tumor suppressor (Dlg) where it has been designated as the HOOK domain and plays a critical role in the membrane targeting of Dlg in vivo [10, 11]. A similar functional role of the HOOK domain has been demonstrated in the membrane targeting of mammalian homologue of Dlg tumor suppressor [12]. The primary structure of the D5 domain of p55 is characterized by a cluster of lysine residues (KKKKYKDK) that are fairly conserved among other members of the MAGUK family including CASK and human Dlg [13, 14]. For example, the HOOK domain of CASK binds to the FERM domain of protein 4.1R [13] whereas the alternatively spliced I3 sequence located within the HOOK domain of human Dlg mediates its binding to the FERM domain of protein 4.1R [12, 14]. In addition, the I3 sequence mediates the targeting of human Dlg to the cell-cell contact sites in epithelial cells [12]. Based on these studies, it is now believed that a specific binding element located within the D5/HOOK/I3 sequence plays a critical role in the targeting of MAGUKs to the actin cytoskeleton in both vertebrate and non-vertebrate cells.
Protein 4.1R (Red cell) is the prototypical member of the protein 4.1 family of cytoskeletal proteins including protein 4.1N (Neuron), 4.1B (Brain), and 4.1G (General) [15–18]. Protein 4.1R is a critical component of the erythrocyte membrane skeleton stabilizing the horizontal interactions between spectrin and actin [15]. The physiological significance of protein 4.1R was first revealed when its absence in patients with inherited hemolytic anemia hereditary elliptocytosis caused significant red cell membrane instability and cell shape abnormalities [19, 20]. In addition, the protein 4.1R null red cells show a secondary loss of p55 and glycophorin C in their plasma membrane, further validating the existence of the ternary complex in vivo [6]. The primary genetic defect in patients with hereditary elliptocytosis is located in the promoter region of 4.1R gene that is specific for the erythroid lineage, and does not affect the expression of the non-erythroid isoforms of protein 4.1R transcribed by at least two alternate promoters [20–23].
Considerable evidence now supports the view that the 135 kDa isoform of protein 4.1R plays a functional role in the regulation of cell division and growth, although the precise physiological basis of this function in the proliferation and differentiation pathways remains unknown [24–26]. Like other family members, the protein 4.1R is expressed in a variety of non-erythroid cells and its gene function is regulated by a complex array of alternatively spliced exons in both erythroid and non-erythroid cells [21, 23]. A single gene encoding protein 4.1R is located on human chromosome 1p36 [27], and a combination of constitutive and alternatively spliced exons generates two major polypeptides of protein 4.1R in the mammalian cells [28, 29]. The 135 kDa isoform of protein 4.1R, transcribed from an upstream initiation codon, is found generally in the non-erythroid cells whereas the 80 kDa isoform, transcribed from the downstream initiation codon of the same gene, is found primarily in the mature red blood cells [21, 30, 31].
Using native p55 and protein 4.1R isolated from human red blood cells, our group first showed that p55 binds to a protease-resistant amino terminal 30 kDa fragment of protein 4.1R, which we later named as the FERM domain [32]. We demonstrated that the native FERM domain of protein 4.1R binds to the D5 domain of p55 [7, 8]. In a subsequent study, Nunomura et al. [33] mapped the p55-binding site within the FERM domain of protein 4.1R and showed that p55 binds to a 51-amino acid peptide encoded by the constitutive exon 10 of protein 4.1R. The same study further mapped the p55-binding site within the 31-amino acid sequence located near the C-terminus of the exon 10-encoded 51-amino acid peptide of protein 4.1R [33]. However, with the elucidation of the crystal structure of the FERM domain of protein 4.1R [34], it became apparent that the charge distribution at the p55-protein 4.1R binding interface is inconsistent to support a robust interaction between the D5 domain of p55 and the exon 10-encoded peptide of the FERM domain of protein 4.1R. These observations also implied that the p55-protein 4.1R interaction would not be amenable to regulation by alternative splicing mechanisms since both the 51-amino acid peptide of protein 4.1R and D5 domain of p55 are encoded by constitutive exons in their respective genes [30, 35]. The binding interface between p55 and protein 4.1R is therefore in contrast to the known interaction between human Dlg and protein 4.1R regulated by alternative splicing of the exon encoding the I3 peptide of human Dlg tumor suppressor [12, 14].
In this study, we re-evaluated the nature of the p55 binding site(s) within the FERM domain of protein 4.1R using a combination of recombinant fusion proteins expressed in bacteria and insect cells. Our results demonstrate that p55 binds to a second site within the FERM domain of protein 4.1R, and this 35-amino acid binding site is encoded by an alternatively spliced exon 5 of the protein 4.1R gene. In addition, the presence of the exon 5-encoded peptide is essential for the intracellular targeting of full length 135 kDa isoform of protein 4.1R to the plasma membrane of epithelial cells. These findings provide a framework for the selection of appropriate constructs for the ongoing crystallization studies of the p55-FERM domain complex, and may have general implications for the subcellular targeting of other FERM domain containing proteins.
2. Materials and methods
2.1. Recombinant FERM domain, exon 5-peptide, and exon 10-peptide of protein 4.1R
FERM domain constructs of human protein 4.1R were engineered. One construct contained exon 5 (FERM) whereas the second construct had exon 5 deleted (FERMΔE5). Both constructs, starting from the second ATG (nucleotide 801) in exon 4 to phenylalanine in exon 12 (nucleotide 1694), were PCR-amplified with the primer set (5′-CCGGAATTCATGCACTGCAAGGTTTCT-3′ and 5′-AAACTGCAGTTAAAATTTGGATCCTAGCGC-3′) using cDNA from the corresponding protein 4.1R isoforms as templates. Two additional constructs of the FERM domain were made where either exon 10 or both exons 5 and 10 were deleted. The inserts were cloned into pMal-c2X and pMal-p2X vectors (NE BioLabs) that produce N-terminal Maltose Binding Protein (MBP)-fusion proteins when expressed in E. coli DH-5α cells. The pMal-c2X and pMal-p2X vectors are engineered to facilitate recombinant protein expression in the bacterial cytoplasm and periplasm, respectively (NE BioLabs).
The exon 5 and exon 10 encoded sequences of protein 4.1R were amplified from the FERM domain constructs of protein 4.1R with each specific set of primers. Specific primers (5′-ATCGGATCCGTGGAGAAACATGCTAAGGGA-3′ and 5′-CTCGAATTCCTTAGAGGTTGCGTTATCCCA-3′) for exon 5 amplified a 129 bp fragment whereas specific primers for exon 10 (5′-ATCGGATCCGACTTGGAAGGAGTAGATATC-3′ and 5′-CTCGAATTCCTCTCCAGGCCGAATCTTGAT-3′) amplified a 171 bp fragment. Appropriate restriction sites were introduced at the beginning of each primer to facilitate subsequent cloning of the amplified PCR bands. The corresponding inserts were cloned in pMal-c2X and pET32a (Novagen) vectors for the expression of MBP and His-Thioredoxin (Trx)-fusion proteins, respectively. Nucleotide sequences of all clones were verified by DNA sequencing. For the expression of recombinant proteins in E. coli, a single bacterial colony containing the desired plasmid was grown overnight at 37°C in 5.0 ml of Luria Broth (LB) supplemented with 100 µg/ml Ampicillin. The bacterial culture was diluted in 300 ml of the same media, and grown to mid-log phase (optical density of 0.6) before induction with 0.2 mM isopropyl-1-thio-β-D-galactopyranoside (IPTG). The culture was further incubated for 12 h at 25°C. Cells were harvested by centrifugation, the cell pellet was resuspended in 20 ml of lysis buffer (phosphate-buffered saline with 0.1% Triton X-100), and the cell suspension was sonicated. The cell free extract was prepared by centrifugation, and the MBP-fusion proteins and His-Trx-fusion proteins were affinity purified using amylose-coupled resin (NE BioLabs) and Ni-NTA resin (QIAGEN), respectively. It is noteworthy that the Trx-exon 10 encoded peptide was largely insoluble when expressed in bacteria. Some residual recombinant protein could be recovered on beads, but the lack of soluble protein precluded its use in the competition assays.
2.2. Full length protein 4.1R
Full length 135 kDa protein 4.1R cDNA containing exon 5 was made as described previously [27]. This construct was made in the pcDNA3.1/His C vector (Invitrogen), which contains the Xpress epitope at the N-terminus. Full length protein 4.1R construct without exon 5 was made by deleting the exon 5 sequence from the above clone, using the Quick Change site-directed mutagenesis kit (Stratagene). DNA sequencing and Western blot of lysate from transfected cells confirmed the expression of a protein with the correct predicted size.
2.3. Recombinant p55
Recombinant full length human erythrocyte p55 cDNA was expressed in E. coli and Sf9 insect cells and used for protein 4.1-binding experiments. The GST-p55 was expressed and purified as described previously [8]. Human p55 cDNA was cloned in the pQE9 (QIAGEN) vector for expression as a His-tagged p55 fusion protein in E. coli, which was purified using Ni-NTA resin as indicated above. For the expression of p55 in insect cells, the p55 cDNA was cloned in pFastBac HTb vector (Invitrogen) for the expression of His-tagged p55 fusion protein. Recombinant baculovirus was obtained according to the protocol of Bac-to-Bac expression system (Invitrogen). His-tagged p55 was purified from cell free supernatant using the Ni-NTA agarose column. The recombinant p55 protein expressed in bacteria, and the His-p55 fusion expressed in Sf9 cells, behaved identically for binding to the FERM domain of human protein 4.1R.
2.4. In vitro protein-protein interaction assays
Recombinant MBP-fusion proteins of FERM, FERMΔE5, exon 5, and exon 10 of protein 4.1R bound to the maltose-coupled resin were incubated with purified recombinant His-p55 in the binding buffer (PBS supplemented with 0.1% Triton-X 100) at 4°C for 2 hours. The beads were washed four times with the binding buffer, and bound proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and analyzed by Western blotting using an anti-p55 monoclonal antibody. For competition assays, an increasing amount of Trx-exon 5 recombinant protein was used in the binding reaction as a competitor. BSA at 1% concentration was included in the binding buffer (PBS, 0.1% Triton-X 100) to reduce the non-specific background.
2.5. Surface Plasmon Resonance measurements
The protein-protein interactions were measured using the BIAcore 1000 system (Pharmacia Biacore AB/GE Healthcare). To analyze interactions between His-p55, MBP-FERM, MBP-FERMΔE5, MBP-exon 5, and MBP-exon 10, the His-p55 fusion protein was immobilized on the CM5 sensor chip. The binding reactions were performed at 25°C. To analyze the strength of various interactions, recombinant proteins (analyte) were passed over the immobilized His-p55 surface at concentrations ranging from 50–400 nM. The experiments were performed at 25°C at 30 µl/min flow rate for the kinetic measurements except the immobilization and regeneration processes, which were carried out at 5.0 µl/min flow rate. The composition of the running buffer was 10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA, and 0.005% P20 (pH 7.4). The composition of immobilization buffer for His-p55 was 10 mM sodium acetate, pH 3.5, and the regeneration buffer was 100 mM NaCl and 10 mM NaOH, pH 11.7. The results shown in Table I represent the outcome of three separate experiments for MBP-FERM, MBP-exon 5, MBP-exon 10, and two experiments for the MBP-FERMΔexon5 construct. Binding data, analyzed by the BIAcore software, were consistent in all cases, and fit well with the 1:1 binding site model.
Table I.
Binding constants for p55 interaction with the FERM domains and individual peptides
| Ligand | Analyte | kass (1/ [M·s]) ×10−4 | kdiss (1/s) × 103 | kD (nM) |
|---|---|---|---|---|
| His-p55 | MBP-FERM | 6.7 ± 0.1 | 4.7 ± 0.1 | 70 ± 3 |
| MBP-FERMΔexon 5 | 2.2 ± 0.04 | 2.9 ± 0.1 | 132 ± 7 | |
| MBP-exon 5 | 16 ± 0.3 | 2.4 ± 0.2 | 15 ± 1.5 | |
| MBP-exon 10 | 6 ± 0.3 | 1.8 ± 0.1 | 30 ± 3 |
2.6. Tissue culture and immunofluorescence analysis
MDCK cells were maintained in the Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. MDCK cells, stably transfected with 135 kDa protein 4.1R either with or without exon 5, were selected by treating the transfected cells with 600 µg/ml Geneticin (Invitrogen) for 2 weeks. DNA transfection was carried out using LipofectAMINE 2000 (Invitrogen). For immunofluorescence analysis, Geneticin-selected cells were plated on the glass cover slips and incubated for 24 hours. Cells were fixed with cold methanol for 10 minutes followed by soaking in cold acetone for 10 seconds, air dried, and processed for staining with the corresponding antibodies. The primary antibody was anti-Xpress monoclonal (Invitrogen) with 1/500 dilution, and the secondary antibody was Texas Red-conjugated goat anti mouse IgG (Molecular Probes) with 1/1000 dilution.
3. Results
3.1. Direct binding of protein 4.1 FERM domain isoforms with p55
To characterize the nature of the p55-binding sites within the FERM domain of human protein 4.1R, we first established in vitro binding assays using purified recombinant proteins. We generated a maltose-binding fusion protein of the FERM domain of protein 4.1R (Fig. 1 and Fig. 2). The MBP-FERM domain protein was immobilized onto the amylose beads and incubated with recombinant His-p55 fusion protein expressed in bacteria. The binding of soluble His-p55 to the beads-bound MBP-FERM domain was erratic particularly when stringent washing conditions were used to remove the unbound material from the beads. While investigating the cause of these variable binding data, we noticed that the FERM domain construct of the human protein 4.1R lacked the alternatively spliced exon 5 but contained the constitutive exon 10, which has been previously shown to encode the p55-binding site [33]. Since this FERM domain construct was missing exon 5, we designated it as MBP-FERMΔExon 5 (Fig. 1B). To examine the possibility that the peptide encoded by exon 5 may facilitate protein 4.1R binding to p55, a second cDNA construct was made that contained both exons 5 and 10 and designated as MBP-FERM domain (Fig. 1B). Except for the presence of exon 5, the two MBP-FERM and MBP-FERMΔExon 5 constructs are identical.
Fig. 1.
Schematic representation of p55 and protein 4.1R domains. (A) Linear schematic diagram of human erythrocyte p55. The D5 domain contains the binding site for the FERM domain of protein 4.1R. (B) Linear domain diagram of human protein 4.1R. The FERM domain construct contains both the alternatively spliced exon 5 and the constitutive exon 10. The mutated FERM (EED→AAA) construct was engineered by deleting the EED sequence to AAA located within exon 5. The LEEDY sequence in exon 5 is known to bind the cytoplasmic domain of band 3 [36]. The FERMΔExon 5 construct was made by deleting exon 5 from the FERM domain construct by in vitro mutagenesis.
Fig. 2.

Expression of FERM domain constructs and p55 binding assay. (A) Coomassie blue stained gel showing recombinant MBP fusion proteins used for the binding assays. MBP (lanes 1), MBP-FERMΔExon 5 (lane 2), and MBP-FERM (lane 3). Fusion proteins shown in lanes 1–3 were expressed in the cytoplasm of E. coli using the pMal-c2X expression vector. Fusion proteins were also expressed in the bacterial periplasm using the pMal-p2X vector (data not shown). Results were identical with both preparations. (B) Coomassie blue stained gel showing recombinant MBP (lane 1), MBP-exon 5 (lane 2), and MBP-exon 10 (lane 3) used for the binding studies. These recombinant proteins were expressed in the bacterial cytoplasm. Western blot based detection of His-p55 recovered by the MBP-fusion protein conjugated amylose-beads is shown on the right. A monoclonal antibody raised against human erythrocyte p55 was used for Western blotting.
The MBP-FERM and MBP-FERMΔExon 5 fusion proteins were expressed and purified under identical conditions from the bacterial cytoplasm (Fig. 2A). Both MBP-FERM domain fusion proteins were soluble when expressed in the bacterial cytoplasm or periplasm (data not shown). We made two additional cDNA constructs of exon 5-peptide and exon 10-peptide expressing individually as MBP fusion proteins (Fig. 2B). The MBP-FERM domain fusion proteins were immobilized to the amylose beads and used to measure the binding of soluble His-p55. All four MBP fusion proteins pulled down His-p55 indicating that both peptides encoded by exons 5 and 10 can bind to p55 under these conditions (Fig. 2). No measurable p55-binding was detected with the MBP protein alone. These results indicate that the erythrocyte p55 can bind to two independent peptides located within the FERM domain of protein 4.1R. It is noteworthy that a previous study has shown direct binding of band 3 to the LEEDY sequence located within the exon 5-encoded peptide of protein 4.1R [36]. To investigate whether both band 3 and p55 can bind to the same binding site on the exon 5-encoded peptide of protein 4.1R, we generated a new FERM domain construct where the LEEDY sequence of exon 5-peptide was changed to LAAAY sequence by in vitro mutagenesis (Fig. 1B). The binding of His-p55 to wild type and mutated FERM domains was similar suggesting that band 3 and p55 do not share the same binding site within the exon 5-peptide (F1 lobe or N-lobe) of protein 4.1R.
3.2. Protein 4.1R-FERM domain containing both exon 5 and exon 10 binds to p55 with higher affinity
To examine the role of exon 5-encoded peptide on the p55-binding capacity of protein 4.1R in the context of the entire FERM domain, individual interactions were quantified using the BIAcore-based Surface Plasmon Resonance assay. Bacterially expressed His-p55 was immobilized on the CM5 sensor chip and its binding affinity with the MBP-FERM and MBP-FERMΔExon 5 fusion proteins was quantified. MBP at 500 nM concentration was used as a negative control showing negligible interaction with the ligand surface. The kdiss and kD values, which represent the dissociation rate constant and equilibrium constant, respectively, were calculated using a series of analyte concentrations and the BIAevaluation 3.0 software. Analysis using both kinetic and equilibrium methods yielded consistent results. The kdiss and kD values of respective interactions are shown in Table I. The kdiss value between His-p55 and MBP-FERM fusion protein was 4.7 ± 0.1 × 10−3 (1/s), whereas the kdiss value between His-p55 and MBP-FERMΔExon 5 fusion protein was 2.9 ± 0.1 × 10−3 (1/s). The calculated kD values between His-p55 and MBP-FERM, and His-p55 and FERMΔExon 5 fusion proteins were calculated to be 70 ± 3 nM and 132 ± 7 nM, respectively. These measurements indicate that the association rate of MBP-FERM domain to His-p55 is relatively faster than that of MBP-FERMΔExon 5 domain, thus making the overall affinity of His-p55 to MBP-FERM fusion protein approximately two times higher than that of MBP-FERMΔExon 5 domain (Table I). It is noteworthy that the quantitative values, as shown in Table I, were obtained by normalizing the amount of each MBP fusion protein (analyte).
To investigate the individual binding of exon 5 and exon 10 encoded peptides, in vitro binding assays were performed demonstrating that His-p55 can interact with both MBP-exon 5 and MBP-exon 10 peptides but not with the control MBP (Fig. 2). Moreover, the amount of bound MBP fusion peptides was normalized and the ratio of protein to Western blot signal of His-p55 was determined. These results suggested that the binding of His-p55 with MBP-exon 5 peptide was relatively stronger as compared to the binding between His-p55 and MBP-exon 10 peptide (data not shown). To quantify these interactions, His-p55 was immobilized on the CM5 sensor chip and the binding of MBP fusion peptides encoded by exons 5 and 10 was measured using the BIAcore SPR assay. The MBP was used as a negative control. The kdiss values between His-p55 and MBP-exon 5 peptide, and His-p55 and MBP-exon 10 peptide were determined to be 2.4 ± 0.2 × 10−3 (1/s) and 1.8 ± 0.1 × 10−3 (1/s), respectively. The corresponding kD values between His-p55 and MBP-exon 5 peptide, and His-p55 and MBP-exon 10 peptide were calculated to be 15 ± 1.5 nM and 30 ± 3 nM, respectively (Table I). These measurements indicate that both MBP-exon 5 and MBP-exon 10 peptides can bind to His-p55, and the association rate of MBP-exon 5 peptide to His-p55 is relatively faster as compared to the binding between MBP-exon 10 peptide and His-p55, thus making the overall affinity of the MBP-exon 5 peptide to His-p55 approximately two times higher than that of MBP-exon 10 peptide (Table I). At this stage, we do not know the reason why the individual peptides encoded by exons 5 and 10 bind to His-p55 with relatively higher affinity as compared to when they are presented in the context of the entire FERM domain. (Table I). This finding can be rationalized by greater steric accessibility and molar concentration of the binding sites when presented as individual peptides. The FERM domain is a highly compact protease-resistant module, and it is likely that additional regulatory cues may be required to fully expose the individual binding sites in the intact domain. Consistent with this notion, we have previously demonstrated that the length of peptides can influence their binding properties with the PDZ domains even when the extended segments do not encode any binding residues [9].
3.3. Competition studies suggest that both exon 5 and exon-10 peptides bind to independent sites on p55
Next, we examined whether the peptides encoded by exons 5 and 10 can compete for the same binding site on p55. To test this possibility, we made a new Trx-exon 5 construct and expressed it as a soluble fusion protein in the bacterial cytoplasm. The Trx-exon 5 peptide was used as a competitor to inhibit the binding of p55 to the MBP-exon 10 peptide immobilized on the amylose beads (Fig. 3). No inhibition of p55-binding to the immobilized MBP-exon 10 peptide by the Trx-exon 5 peptide was observed suggesting that these peptides bind to independent sites within p55 (Fig. 3A). To further investigate the independence of the two binding sites, we used a GST-p55 fusion protein that was coupled to the glutathione-Sepharose beads and then incubated with the MBP-exon 5 peptide by reciprocal rounds of three incubations in order to saturate all the exon 5-peptide binding sites accessible on immobilized p55. The bead-bound GST-p55 saturated with the MBP-exon 5 peptide was washed and then incubated with the MBP-exon 10 peptide. The binding of MBP fusion proteins to the glutathione beads were analyzed by SDS-PAGE and Western blotting using an anti-MBP antibody. Both fusion peptides of MBP-exon 5 and MBP-exon 10 were pulled down simultaneously with the GST-p55 beads (Fig. 3B, lane 4). Together, these results suggest that the recombinant peptides encoded by exons 5 and 10 bind to two independent binding sites within p55.
Fig. 3.
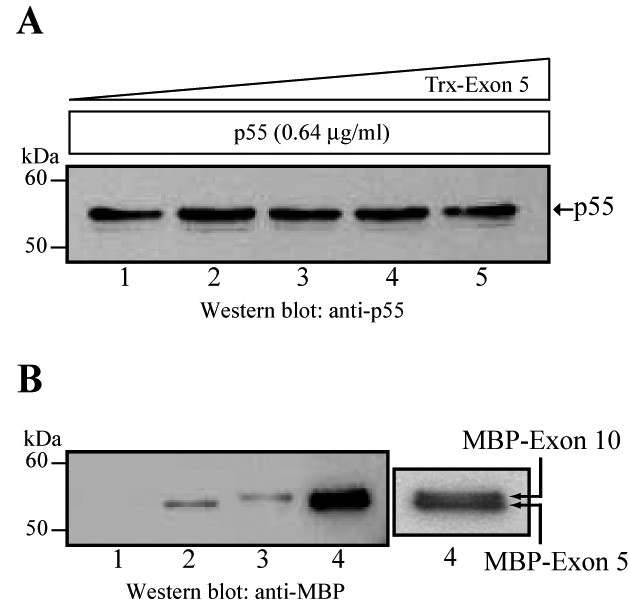
Independent binding of exon 5 and exon 10 encoded peptides of FERM domain to p55. (A) Lack of competition of p55 binding to MBP-exon 10 and Trx-exon 5 fusion proteins. The recombinant His-p55 (0.64 µg/ml or 11.6 nM) was pre-incubated for two hours at 4°C with increasing amounts of Trx-exon 5 peptide (0.2, 0.9, 1.9, and 4.9 µg/ml/196 nM) before the addition of MBP-exon 10 coupled to the amylose beads (~0.5 µg per assay). The mixture was incubated for one hour at 4°C. The beads were washed, harvested, and bound proteins resolved by SDS-PAGE using 10% polyacrylamide gels. The binding of His-p55 to exon 10 was detected by Western blotting using a monoclonal antibody against p55. (B) As an alternate strategy, the MBP-exon 5 fusion protein was incubated with the GST-p55 fusion protein coupled to the glutathione Sepharose beads. The p55 binding sites were saturated by three consecutive incubations of MBP-exon 5 fusion protein. After washing, the glutathione beads were incubated with the MBP-exon 10 fusion protein for two hours at 4°C. Proteins bound to the beads were harvested, washed four times, and analyzed by SDS-PAGE and Western blotting. The blots were developed using a commercial polyclonal antibody against MBP. Note that the closely-spaced doublet of MBP-exon 5 and MBP-exon 10 peptides is more visible at the low exposure of the material shown in lane 4. Lane 1 is the negative control GST-coupled beads incubated with both MBP-Exon 5 and Exon-10, which showed no detectable binding. Lane 2 is the MBP-Exon-5 bound to GST-p55 and lane 3 is the MBP-Exon-10 bound to GST-p55.
The existence of independent binding sites for peptides encoded by exons 5 and -10 on p55 was further investigated using the BIAcore assay. Two experimental approaches were used to quantify these interactions. The experiments were performed at 25°C at 5 µl/min flow rate. Freshly prepared His-p55 was immobilized on the CM5 sensor chip before each assay. First, the MBP-exon 5 fusion peptide was injected on the His-p55 surface, and the surface was washed with the running buffer at 5 µl/min to stabilize the baseline. This step was repeated several times to saturate all the accessible MBP-exon 5 peptide binding sites on His-p55. After the stabilization of the exon 5-peptide bound His-p55 surface, the MBP-exon 10 peptide was injected to determine whether the MBP-exon 10 peptide can bind to the His-p55 surface that is already saturated with the MBP-exon 5 peptide. The SPR measurements show that the MBP-exon 10 peptide can bind to the His-p55 surface that is already saturated with the MBP-exon 5 peptide (Fig. 4D), indicating that the exon 5 and -10 peptides likely bind to non-overlapping sites on p55 and there is no apparent steric hindrance phenomena. In addition, a reverse experiment was performed where the His-p55 surface was first saturated with the MBP-exon 10 peptide, and then the binding of MBP-exon 5 peptide was measured. Again, the MBP-exon 5 peptide bound to the His-p55 surface further confirming the independence of the two binding sites on p55 (data not shown). We have previously shown that the FERM domain isolated from native protein 4.1R binds to 39-amino acid sequence flanked by the SH3 and Guanylate kinase domains of p55 [8]. This 39-amino acid sequence of p55 contains an unusually large number of lysine residues [8]. We believe that a simple mutagenesis or truncation approach may not be feasible to assess the independence of exon 5 and exon 10 peptide binding sites on p55. This is partly because the limited truncation of the 39 amino acid D5 domain of p55 leads to highly unstable D5 domain. Therefore, we believe that the co-crystallization of the p55-FERM domain complex provides the most rigorous means of precisely mapping the binding interface between p55 and protein 4.1R in future studies.
Fig. 4.
BIAcore quantification of p55 interaction with peptides encoded by exons 5 and 10. The Surface Plasmon Resonance (SPR) technique was used to quantify the interaction of p55 with the FERM domain of protein 4.1R with and without exon 5-peptide (A–C). The same technique was used to demonstrate the non-competition of exon 5 and exon 10 peptide-binding sites to His-p55 (D). Sensograms were obtained from SPR analysis of the interaction between His-p55 and the FERM domain constructs of protein 4.1R. Recombinant His-p55 protein was immobilized on the CM5 sensor chip and the MBP-FERM domain fusion proteins were injected as an analyte. The wild type His-p55 protein was expressed either in the bacterial cytoplasm or in Sf9 cells with no effect on its binding properties. (A) The binding properties of MBP-FERM and MBP-FERMΔExon 5 fusion proteins to the immobilized His-p55 were measured at 100 nM concentration of each analyte protein. Sensogram of 100 nM MBP (analyte) binding to His-p55 on the chip was used as a negative control. The sensorgrams were generated using a 30 µl/min analyte flow rate (BIAcore 1000 instrument) and included a 3-minute association and a 5-minute dissociation section. (B) Sensograms of the MBP-FERM domain fusion protein (analyte) injected at various concentrations ranging from 50–400 nM. MBP alone at 500 nM was used as a negative control. The flow rate was 30 µl/min over the immobilized His-p55 surface yielding a kD value of 70 nM. (C) Sensograms of the MBP-FERMΔExon 5 domain fusion protein (analyte) injected at various concentrations ranging from 50–400 nM. The MBP at 500 nM was used as a negative control. The flow rate was 30 µl/min over the immobilized His-p55 surface yielding a kD value of 132 nM. (D) The MBP-exon 5 fusion protein (1 µM) was injected over the His-p55 surface, followed by the washing of the chip surface with the running buffer for 25 min at 5 µl/min flow rate. This step was repeated three times, followed by the injection of 1.0 µM MBP-exon 10 for 25 minutes and washing of the chip surface with the running buffer for 25 minutes. The sensograms show that the exon 10-peptide can bind to the p55 surface that is already saturated with the exon 5-peptide.
3.4. Exon 5-peptide is essential for the membrane targeting of protein 4.1R in epithelial cells
Our results indicate that the exon 5-peptide provides a second binding site on the FERM domain of protein 4.1R for p55, and this binding site is of higher affinity than the exon 10-peptide. To assess the physiological significance of this observation in non-erythroid cells, we generated two mammalian expression constructs of full length protein 4.1R (135 kDa) with and without exon 5. Protein 4.1 family proteins, such as protein 4.1R [37], 4.1N [38], as well as their Drosophila homologue, Coracle [39] are important components of the lateral plasma membrane of polarized epithelial cells. Consistent with these observations, the full length protein 4.1R (135 kDa), which contained both exons 5 and 10, localized efficiently to the lateral membrane when expressed in the MDCK epithelial cells (Fig. 5, upper panel). In contrast, the same protein 4.1R construct from where the alternatively spliced exon 5 was deleted by in vitro mutagenesis completely lost its lateral membrane localization and exhibited a punctuate distribution throughout the cytoplasm (Fig. 5, lower panel). In summary, these results demonstrate that the protein-protein interaction(s) mediated by the exon 5-encoded peptide plays a critical role for the lateral membrane targeting of protein 4.1R in the mammalian epithelial cells.
Fig. 5.

Localization of heterologous protein 4.1R in MDCK epithelial cells. The 135 kDa full-length isoform of protein 4.1R including exons 5 and 10 was expressed in the MDCK cells (upper panel), whereas the same isoform of protein 4.1R containing exon 10 but lacking exon 5 was also expressed in the MDCK cells under identical conditions (lower panel). The expression of heterologous proteins was established by staining with an anti-Xpress antibody to detect the Xpress-tagged isoforms of protein 4.1R. The merged images of protein 4.1R in red and DAPI staining in blue are shown in each panel.
4. Discussion
This study was initiated to elucidate the molecular basis of the binding interface between human erythrocyte p55 and the FERM domain of protein 4.1R. We examined the interaction of p55 with the FERM domain of protein 4.1R using several independent approaches. First, we generated and expressed a GST-FERM domain fusion protein of protein 4.1R and tested its binding with the His-p55 recombinant protein. Our pull-down binding assays indicated that the GST-FERM domain was very “sticky” to the Sepharose beads and therefore could not be used reliably in the conventional bead-binding assays. To overcome this limitation, we cloned and expressed the FERM domain as a maltose binding protein (MBP) fusion in bacteria. The FERM domain was cloned into both cytoplasmic and periplasmic expression vectors to take into account the effect of disulphide bond pairing on the refolding of MBP-FERM domain proteins. The histidine-tagged p55 protein was expressed in bacteria as well as in Sf9 cells using the baculovirus expression system. Binding between His-p55 and MBP-FERM domain was measured by the pull-down and BIAcore assays. Our measurements indicated that there was no difference in the binding capacity of the MBP-FERM domain expressed in either the bacterial cytoplasm or periplasm. Also, the binding capacity of His-p55 expressed in either bacteria or Sf9 cells was also similar (data not shown). Using these reagents, our results indicate that both constructs, MBP-FERM domain and MBP-FERMΔExon 5 domain, can bind to His-p55. Moreover, individual peptides encoded by either exon 5 or exon 10 can also bind to p55. These data suggest that p55 binds to a second site within the FERM domain of protein 4.1R, and the presence of exon 5-encoded peptide is necessary for optimal and stable binding between the FERM domain and p55.
Our identification of exon 5-encoded peptide as the higher affinity binding site for p55 is consistent with the charge distribution within the FERM domain of protein 4.1R [34]. The constitutive exon 10-peptide is located in the C-lobe (also called the F3 lobe) of the FERM domain, whereas the alternatively spliced exon 5-peptide is located within the N-lobe (also called the F1 lobe) of the FERM domain of protein 4.1R [34] (Fig. 6). There are several negatively charged residues present within the F1 lobe that could potentially bind to the cluster of positively charged lysine residues present within the D5 domain of p55. It is also noteworthy that we have previously reported the binding stoichiometry of p55-protein 4.1 interaction by demonstrating that a significantly higher molar concentration of p55 is required to saturate all the binding sites on the FERM domain of protein 4.1 [7]. Finally, our findings imply that the binding interaction between p55 and protein 4.1R can be regulated through alternative splicing of exon 5 within the FERM domain of protein 4.1R (Fig. 6). This model is similar to the hDlg tumor suppressor where its alternatively spliced I3 sequence regulates hDlg binding to the FERM domain of protein 4.1R [12, 14]. Together, the p55-4.1R and hDlg-4.1R interactions exemplify two distinct regulatory controls of the same binding interface between MAGUKs and the membrane cytoskeleton.
Fig. 6.

Schematic presentation of p55 interactions with protein 4.1R and membrane targeting in epithelial cells. (A) The D5 domain of p55 contains two binding sites for the FERM domain of protein 4.1R. The exon 5-peptide is located in the F1 lobe whereas the exon 10-peptide is located in the F3 lobe. (B) The presence of alternatively spliced exon 5-peptide is essential for the membrane targeting of protein 4.1R in the epithelial cells. Members of the p55-subfamily of MAGUKs are likely candidates for the recruitment of protein 4.1R to the plasma membrane. (C) Ribbon diagram and charge distribution surface of the FERM domain of human erythrocyte 4.1R; adapted from reference [34]. The respective locations of the p55-binding sites are indicated by arrows in the FERM domain.
The results reported in this manuscript are consistent with a previous study by Nunomura et al. [33] that suggested p55 binds to the peptide encoded by the constitutive exon 10 of the FERM domain of protein 4.1R. However, our finding that p55 binds to the peptide encoded by the alternatively spliced exon 5 of the FERM domain appear to be inconsistent with the Nunomura et al. [33] where they have reported that p55 does not bind to the GST-FERM domain construct that lacks exon 10 but retains exon 5. At this stage, we do not know the reason for this discrepancy. To investigate this issue further, however, we first attempted to express the MBP-FERM domain construct lacking both exons 5 and 10. The deletion of constitutive exon 10 and alternatively spliced exon 5 was not feasible as no recombinant MBP-FERM domain could be detected in bacteria (data not shown). In addition, we made another MBP-FERM domain construct where we deleted the exon 10 but retained exon 5. Again, the expression of this construct resulted in highly unstable protein with most of the recombinant MBP-FERM domain trapped in the bacterial inclusion bodies. We tested multiple clones for protein expression with no success. Based on these observations, we conclude that the deletion of constitutive exon 10 will lead to the disruption of the highly-folded compact structure of the FERM domain of protein 4.1R. Therefore, we recommend that any recombinant FERM domain expressed without exon 10 must be first tested rigorously for its folding and stability properties prior to its use in protein binding assays.
To evaluate the biological significance of the exon 5-encoded peptide, we tested the intracellular targeting of full length protein 4.1R (135 kDa) in transfected mammalian epithelial cells. Interestingly, only the protein 4.1R isoform containing exons 5 and 10 was targeted to the plasma membrane whereas protein 4.1R containing constitutive exon 10 but lacking exon 5 failed to associate with the plasma membrane and remained in the cytosol. These results demonstrate that the presence of exon 5-peptide is essential for the intracellular targeting of protein 4.1R to the plasma membrane of epithelial cells. The presence of highly conserved exon 5-peptides in many other proteins including the putative lung tumor suppressor, tyrosine phosphatase, and NF2 tumor suppressor suggest that this sequence could mediate their binding to p55-related MAGUKs in a variety of other tissues. At this stage, we do not know the identity of the protein that binds to exon 5-peptide of protein 4.1R in the kidney epithelial cells. We tested the protein expression level of p55 in the MDCK cells by Western blotting, and found that p55 is not detectable by this technique. Since p55 is very sensitive to proteolysis, it is likely that more sensitive approaches are required to detect p55 in epithelia. Notwithstanding the apparent absence of p55 in the MDCK cells, several p55-like MAGUK proteins are expressed in the MDCK cells including CASK, Dlg2, Dlg3, Pals-1, and Pals-2. Several of them have been shown to localize to the lateral plasma membrane in the MDCK cells, and are known to interact with the FERM domain of protein 4.1R. We speculate that one of these proteins, or a combination of them, is a potential candidate for mediating the exon 5-peptide dependent targeting of protein 4.1R in epithelial cells. In summary, the novel binding interface assembled by the exon 5-peptide of protein 4.1R and the D5 domain of p55 or a related MAGUK suggests a new mechanism for the intracellular targeting of FERM domain containing proteins to the plasma membrane.
Acknowledgements
We are grateful to Dr. Shu-Ching Huang and Dr. Edward J. Benz Jr. of the Dana Faber Cancer Institute, Boston, for providing us the FERM domain construct of human protein 4.1R. This work was supported by the National Institutes Grants HL60755, CA 94414, and the Department of Defense Neurofibromatosis Research Program Career Development Award NF020087 from the Department of Defense.
Abbreviations
- FERM
F for 4.1 protein, E for Ezrin, R for Radixin, M for Moesin
- MBP
maltose binding protein
- 4.1R
human erythrocyte protein 4.1
- MAGUKs
membrane associated guanylate kinase homologues
- GST
glutathione S-transferase
- PCR
polymerase chain reaction
- Dlg
discs large tumor suppressor protein
- p55
human erythrocyte p55
- PBS
phosphate buffered saline
- SPR
Surface Plasmon Resonance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dimitratos SD, Woods DF, Stathakis DG, Bryant PJ. Signaling pathways are focused at specialized regions of the plasma membrane by scaffolding proteins of the MAGUK family. Bioessays. 1999;21:912–921. doi: 10.1002/(SICI)1521-1878(199911)21:11<912::AID-BIES3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery JM, Zamorano PL, Garner CC. MAGUKs in synapse assembly and function: an emerging view. Cell Mol Life Sci. 2004;61:911–929. doi: 10.1007/s00018-003-3364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruff P, Speicher DW, Chishti A. Molecular identification of a major palmitoylated erythrocyte membrane protein containing the src homology 3 motif. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:6595–6599. doi: 10.1073/pnas.88.15.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- 6.Alloisio N, Dalla Venezia N, Rana A, Andrabi K, Texier P, Gilsanz F, Cartron JP, Delaunay J, Chishti AH. Evidence that red blood cell protein p55 may participate in the skeleton-membrane linkage that involves protein 4.1 and glycophorin C. Blood. 1993;82:1323–1327. [PubMed] [Google Scholar]
- 7.Marfatia SM, Lue RA, Branton D, Chishti AH. In vitro binding studies suggest a membrane-associated complex between erythroid p55, protein 4.1, and glycophorin C. Journal of Biological Chemistry. 1994;269:8631–8634. [PubMed] [Google Scholar]
- 8.Marfatia SM, Lue RA, Branton D, Chishti AH. Identification of the protein 4.1 binding interface on glycophorin C and p55, a homologue of the Drosophila discs-large tumor suppressor protein. Journal of Biological Chemistry. 1995;270:715–719. doi: 10.1074/jbc.270.2.715. [DOI] [PubMed] [Google Scholar]
- 9.Marfatia SM, Morais-Cabral JH, Kim AC, Byron O, Chishti AH. The PDZ domain of human erythrocyte p55 mediates its binding to the cytoplasmic carboxyl terminus of glycophorin C. Analysis of the binding interface by in vitro mutagenesis. Journal of Biological Chemistry. 1997;272:24191–24197. doi: 10.1074/jbc.272.39.24191. [DOI] [PubMed] [Google Scholar]
- 10.Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. Journal of Cell Biology. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hough CD, Woods DF, Park S, Bryant PJ. Organizing a functional junctional complex requires specific domains of the Drosophila MAGUK Discs large. Genes & Development. 1997;11:3242–3253. doi: 10.1101/gad.11.23.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanada T, Takeuchi A, Sondarva G, Chishti AH. Protein 4.1-mediated membrane targeting of human discs large in epithelial cells. J Biol Chem. 2003;278:34445–34450. doi: 10.1074/jbc.M305209200. [DOI] [PubMed] [Google Scholar]
- 13.Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells [published erratum appears in J Cell Biol 1998 Aug 24;142(4):following 1156] Journal of Cell Biology. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marfatia SM, Morais Cabral JH, Lin L, Hough C, Bryant PJ, Stolz L, Chishti AH. Modular organization of the PDZ domains in the human discs-large protein suggests a mechanism for coupling PDZ domain-binding proteins to ATP and the membrane cytoskeleton. Journal of Cell Biology. 1996;135:753–766. doi: 10.1083/jcb.135.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conboy JG. Structure, function, and molecular genetics of erythroid membrane skeletal protein 4.1 in normal and abnormal red blood cells. Seminars in Hematology. 1993;30:58–73. [PubMed] [Google Scholar]
- 16.Parra M, Gascard P, Walensky LD, Snyder SH, Mohandas N, Conboy JG. Cloning and characterization of 4.1G (EPB41L2), a new member of the skeletal protein 4.1 (EPB41) gene family. Genomics. 1998;49:298–306. doi: 10.1006/geno.1998.5265. [DOI] [PubMed] [Google Scholar]
- 17.Walensky LD, Blackshaw S, Liao D, Watkins CC, Weier HU, Parra M, Huganir RL, Conboy JG, Mohandas N, Snyder SH. A novel neuron-enriched homolog of the erythrocyte membrane cytoskeletal protein 4.1. Journal of Neuroscience. 1999;19:6457–6467. doi: 10.1523/JNEUROSCI.19-15-06457.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parra M, Gascard P, Walensky LD, Gimm JA, Blackshaw S, Chan N, Takakuwa Y, Berger T, Lee G, Chasis JA, Snyder SH, Mohandas N, Conboy JG. Molecular and functional characterization of protein 4.1B, a novel member of the protein 4.1 family with high level, focal expression in brain. Journal of Biological Chemistry. 2000;275:3247–3255. doi: 10.1074/jbc.275.5.3247. [DOI] [PubMed] [Google Scholar]
- 19.Shohet SB, Mohandas N, Tchernia G. Homozygous hereditary elliptocytosis: implications for the function of membrane protein band 4.1. Prog Clin Biol Res. 1982;97:45–52. [PubMed] [Google Scholar]
- 20.Conboy JG, Chasis JA, Winardi R, Tchernia G, Kan YW, Mohandas N. An isoform-specific mutation in the protein 4.1 gene results in hereditary elliptocytosis and complete deficiency of protein 4.1 in erythrocytes but not in nonerythroid cells. Journal of Clinical Investigation. 1993;91:77–82. doi: 10.1172/JCI116203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang TK, Qin Z, Leto T, Marchesi VT, Benz EJ., Jr Heterogeneity of mRNA and protein products arising from the protein 4.1 gene in erythroid and nonerythroid tissues. J Cell Biol. 1990;110:617–624. doi: 10.1083/jcb.110.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra MK, Tan JS, Mohandas N, Conboy JG. Intrasplicing coordinates alternative first exons with alternative splicing in the protein 4.1R gene. Embo J. 2008;27:122–131. doi: 10.1038/sj.emboj.7601957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conboy JG, Chan J, Mohandas N, Kan YW. Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells. Proc Natl Acad Sci U S A. 1988;85:9062–9065. doi: 10.1073/pnas.85.23.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang SC, Liu ES, Chan SH, Munagala ID, Cho HT, Jagadeeswaran R, Benz EJ., Jr Mitotic regulation of protein 4.1R involves phosphorylation by cdc2 kinase. Mol Biol Cell. 2005;16:117–127. doi: 10.1091/mbc.E04-05-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang SC, Jagadeeswaran R, Liu ES, Benz EJ., Jr Protein 4.1R, a microtubule-associated protein involved in microtubule aster assembly in mammalian mitotic extract. J Biol Chem. 2004;279:34595–34602. doi: 10.1074/jbc.M404051200. [DOI] [PubMed] [Google Scholar]
- 26.Krauss SW, Lee G, Chasis JA, Mohandas N, Heald R. Two protein 4.1 domains essential for mitotic spindle and aster microtubule dynamics and organization in vitro. J Biol Chem. 2004;279:27591–27598. doi: 10.1074/jbc.M402813200. [DOI] [PubMed] [Google Scholar]
- 27.Huang S, Lichtenauer UD, Pack S, Wang C, Kim AC, Lutchman M, Koch CA, Torres-Cruz J, Huang SC, Benz EJ, Jr, Christiansen H, Dockhorn-Dworniczak B, Poremba C, Vortmeyer AO, Chishti AH, Zhuang Z. Reassignment of the EPB4.1 gene to 1p36 and assessment of its involvement in neuroblastomas. Eur J Clin Invest. 2001;31:907–914. doi: 10.1046/j.1365-2362.2001.00892.x. [DOI] [PubMed] [Google Scholar]
- 28.Huang SC, Baklouti F, Tang TK, Benz EJ., Jr Differential utilization of translation initiation sites in alternatively spliced mRNAs arising from the protein 4.1 gene. Trans Assoc Am Physicians. 1992;105:165–171. [PubMed] [Google Scholar]
- 29.Conboy JG, Chan JY, Chasis JA, Kan YW, Mohandas N. Tissue- and development-specific alternative RNA splicing regulates expression of multiple isoforms of erythroid membrane protein 4.1. Journal of Biological Chemistry. 1991;266:8273–8280. [PubMed] [Google Scholar]
- 30.Baklouti F, Huang SC, Vulliamy TJ, Delaunay J, Benz EJ., Jr Organization of the human protein 4.1 genomic locus: new insights into the tissue-specific alternative splicing of the pre-mRNA. Genomics. 1997;39:289–302. doi: 10.1006/geno.1996.4512. [DOI] [PubMed] [Google Scholar]
- 31.Gascard P, Lee G, Coulombel L, Auffray I, Lum M, Parra M, Conboy JG, Mohandas N, Chasis JA. Characterization of multiple isoforms of protein 4.1R expressed during erythroid terminal differentiation. Blood. 1998;92:4404–4414. [PubMed] [Google Scholar]
- 32.Chishti AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, Liu SC, Low PS, Rouleau GA, Mohandas N, Chasis JA, Conboy JG, Gascard P, Takakuwa Y, Huang SC, Benz EJ, Jr, Bretscher A, Fehon RG, Gusella JF, Ramesh V, Solomon F, Marchesi VT, Tsukita S, Hoover KB, et al. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci. 1998;23:281–282. doi: 10.1016/s0968-0004(98)01237-7. [DOI] [PubMed] [Google Scholar]
- 33.Nunomura W, Takakuwa Y, Parra M, Conboy J, Mohandas N. Regulation of protein 4.1R, p55, and glycophorin C ternary complex in human erythrocyte membrane. Journal of Biological Chemistry. 2000;275:24540–24546. doi: 10.1074/jbc.M002492200. [DOI] [PubMed] [Google Scholar]
- 34.Han BG, Nunomura W, Takakuwa Y, Mohandas N, Jap BK. Protein 4.1R core domain structure and insights into regulation of cytoskeletal organization. Nat Struct Biol. 2000;7:871–875. doi: 10.1038/82819. [DOI] [PubMed] [Google Scholar]
- 35.Kim AC, Metzenberg AB, Sahr KE, Marfatia SM, Chishti AH. Complete genomic organization of the human erythroid p55 gene (MPP1), a membrane-associated guanylate kinase homologue. Genomics. 1996;31:223–229. doi: 10.1006/geno.1996.0035. [DOI] [PubMed] [Google Scholar]
- 36.Jons T, Drenckhahn D. Identification of the binding interface involved in linkage of cytoskeletal protein 4.1 to the erythrocyte anion exchanger. Embo J. 1992;11:2863–2867. doi: 10.1002/j.1460-2075.1992.tb05354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattagajasingh SN, Huang SC, Hartenstein JS, Benz EJ., Jr Characterization of the interaction between protein 4.1R and ZO-2. A possible link between the tight junction and the actin cytoskeleton. J Biol Chem. 2000;275:30573–30585. doi: 10.1074/jbc.M004578200. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Mizutani A, Hisatsune C, Higo T, Bannai H, Nakayama T, Hattori M, Mikoshiba K. Protein 4.1N is required for translocation of inositol 1,4,5-trisphosphate receptor type 1 to the basolateral membrane domain in polarized Madin-Darby canine kidney cells. J Biol Chem. 2003;278:4048–4056. doi: 10.1074/jbc.M209960200. [DOI] [PubMed] [Google Scholar]
- 39.Ward REt, Lamb RS, Fehon RG. A conserved functional domain of Drosophila coracle is required for localization at the septate junction and has membrane-organizing activity. J Cell Biol. 1998;140:1463–1473. doi: 10.1083/jcb.140.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]




