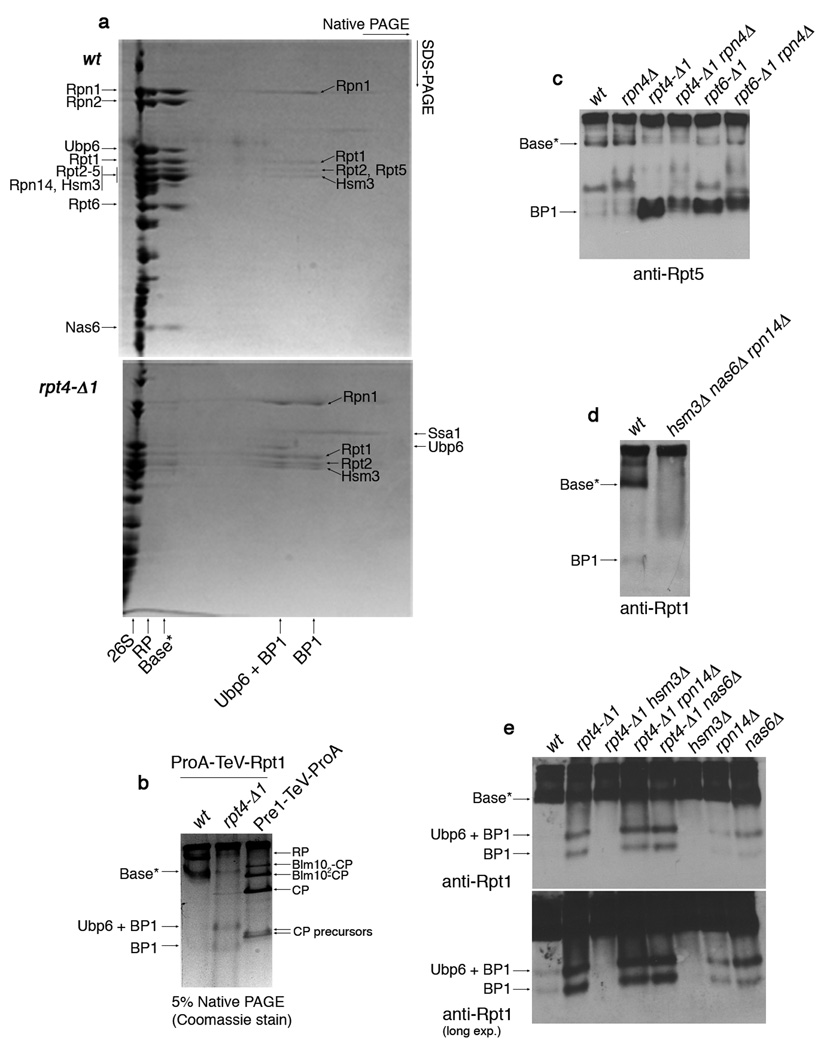
Figure 2. Identification of a base assembly intermediate.
a, 2-D native/SDS-PAGE of affinity-purifications with ProA-TeV-Rpt1 (Protein A tag appended to the N-terminus of Rpt1, a base subunit). Following a first-dimension native PAGE (5%), native gel lanes were individually excised and subjected to second-dimension SDS-PAGE (12.5%). Gels were stained with Coomassie blue. Individual spots of the base*, BP1, and Ubp6 + BP1 complexes were excised for mass spectrometry. Labels on the left indicate spots within base*. The presence of Rpn10 and Rpn13 in base* was not determined. Note that Rpt5 is absent from BP1 purified from rpt4-Δ1 mutants, but present in BP1 in untagged rpt4-Δ1 extracts (panel 4c). This appears to reflect a labile association of Rpt5 with BP1 in rpt4-Δ1 mutants. The presence of Ubp6 in BP1 is explained by its interaction with Rpn125. Note: Base* and BP1 species appear comparable between rpt6-Δ1 and rpt4-Δ1 mutants in their level and composition.
b, 5% Native PAGE following affinity purification via a ProA-TeV-Rpt1 as in (a) or a Pre1 (CP subunit)-TeV-ProA. Native gels were stained with Coomassie blue.
c, d, Immunoblotting following 5% native PAGE of whole cell extracts (100 µg).
e, Immunoblotting following 5% native PAGE of affinity-purified samples (2 µg) from indicated strains carrying ProA-TeV-Rpt1.