Abstract
Behavior arises from a constant competition between potential actions. For example, movements performed unimanually require selecting one hand rather than the other. Corticospinal (CS) excitability of the nonselected hand is typically decreased prior to movement initiation, suggesting that response selection may involve mechanisms that inhibit nonselected candidate movements. To examine this hypothesis, participants performed a reaction time task, responding with the left, right, or both indexes. Transcranial magnetic stimulation was applied over the right primary motor cortex (M1) to induce motor-evoked potentials (MEPs) in a left hand muscle at various stages during response preparation. To vary the time of response selection, an imperative signal was preceded by a preparatory cue that was either informative or uninformative. Left MEPs decreased following the cue. Surprisingly, this decrease was greater when an informative cue indicated that the response might require the left hand than when it indicated a right hand response. In the uninformative condition, we did not observe additional attenuation of left MEP after an imperative indicating a right hand response. These results argue against the “deselection” hypothesis. Rather, CS suppression seems to arise from “impulse control” mechanisms that ensure that responses associated with potentially selected actions are not initiated prematurely.
Keywords: action selection, inhibition, mirror movement, transcranial magnetic stimulation, unimanual movement
Introduction
Motor behavior can be viewed as resulting from a constant competition between potential actions (Cisek 2007). Choices may involve deciding which restaurant to frequent on a Friday evening or the best route to take to avoid holiday traffic. Competitive processes are also at play in many simple actions. To pick up an object such as a cup of coffee, we can use either the left or right hand. The fluid and flexible manner with which we make such decisions indicates the operation of a selection process that takes into account multiple factors such as the relative position of the hand with respect to the object, handedness, contextual rules, and previous experience (Romo and Salinas 2003). Decision models generally assume the parallel activation of multiple options, with a choice occurring when the activity associated with a particular action reaches a given threshold (Ivry and Spencer 2004; Cisek 2007). Consistent with this view, a large number of studies have shown that the motor system initially specifies the metrics of several potential actions with a final outcome that results in a single coherent movement (Deiber et al. 1996; Schluter et al. 1998; Thoenissen et al. 2002; Bastian et al. 2003; Cisek and Kalaska 2005; Medendorp et al. 2005).
Transcranial magnetic stimulation (TMS) has been used to probe the dynamics of corticospinal (CS) excitability during movement preparation and execution. When one makes unimanual movements, a transient decrease is observed in CS excitability of muscles in the nonselected hand (Duque et al. 2005; Leocani et al. 2000; Liepert et al. 2001; Sohn et al. 2003; Weiss et al. 2003). It has generally been assumed that this CS suppression reflects the operation of inhibitory mechanisms that target cortical representations of the nonselected hand, possibly through transcallosal interactions to facilitate hand (de)selection (Ferbert et al. 1992; Mochizuki et al. 2004; Koch et al. 2006). This “inhibition-for-deselection” is thought to be critical to sharpen hand selection in a competitive setting. Moreover, when both hands are potential responders, inhibitory mechanisms might be essential to negate the likelihood of mirror movements that could result from bilateral planning (Serrien et al. 1999; Swinnen 2002; Duque et al. 2005; Davare et al. 2007; Koch et al. 2006).
Another, nonexclusive, possibility is that CS suppression in the nonselected hand reflects more general inhibitory processes which help prevent actions from being emitted prematurely. Such inhibition would be useful while selection operations are incomplete as well as when the task requires that the selected action be withheld until the onset of an imperative signal (Boulinguez et al. 2008; Jaffard et al. 2008). Consistent with this “impulse control” hypothesis, a series of studies have reported inhibitory changes in CS excitability of movement agonists during the warning period of a RT task (Hasbroucq et al. 1997, 1999; Touge et al. 1998; McMillan et al. 2004; Davranche et al. 2007; Prabhu et al. 2007; van Elswijk et al. 2007).
We set out to evaluate the “deselection” and “impulse control” hypotheses by examining the dynamics of CS excitability as participants prepared to make unimanual or bimanual movements. TMS was applied over the right primary motor cortex (M1) to measure CS excitability in a left hand muscle at various points during response preparation and initiation. Participants were required to make a speeded response, using the left, right, or both index fingers following the presentation of an imperative signal. This signal was preceded by a preparatory cue. In some trials, this cue was informative regarding the required response for the forthcoming movement (Rosenbaum 1980); in other trials, the preparatory cue was uninformative and the required response was only indicated by the imperative signal. In the uninformative task, competitive processes related to hand selection should be maximal just after the imperative signal. In contrast, in the informative task, the participant can prepare the required response in advance of the imperative signal. Hence, competitive demands on hand selection should be reduced at the time of the imperative signal.
To assess how selection demands modulate CS excitability in the left hand, a TMS pulse was applied following either the preparatory cue or the imperative signal. Based on the “inhibition-for-deselection” hypothesis, we made 2 predictions: First, following the preparatory cue, CS excitability of the left hand should be lower when the cue specifies a right hand response than when it specifies a left hand response or when it is uninformative. Second, in the uninformative condition there should be a decrease in the CS excitability of the left hand when the imperative signal specifies a right hand response. The “impulse control” hypothesis leads to 2, very different predictions. First, following the preparatory cue, CS excitability of the left hand should decrease when the cue specifies a left hand response or is uninformative; this inhibition may even be greater to that observed on trials in which the preparatory cue specifies a right hand response. Second, there should be no further decrease in CS excitability of the left hand following an imperative signaling a right hand response.
CS excitability is not solely related to M1 activity. It is also influenced by changes confined to the spinal circuitry. Such changes may arise from extra-pyramidal pathways or direct projections to spinal mechanisms from premotor and parietal cortex (Dum and Strick 1991; Galea and Darian-Smith 1994). In order to determine the extent to which changes in CS excitability were related to changes in M1 excitability, we conducted a second experiment with paired-pulse TMS, using an interpulse interval of 3 ms. This paired-pulse procedure has been used to assess changes in M1 inhibitory activity during motor preparation or prevention (Ridding et al. 1995; Reynolds and Ashby 1999; Coxon et al. 2006; van Elswijk et al. 2007). In brief, a suprathreshold “test” stimulus (TS) is preceded by a subthreshold “conditioning” stimulus delivered through the same coil (Kujirai et al. 1993). The conditioning stimulus is thought to excite Gamma-aminobutyric acid intracortical inhibitory interneurons that suppress activity of CS cells 2–5 ms later (Ziemann et al. 1996; Ilic et al. 2002). As a consequence, motor-evoked potentials (MEPs) evoked by the TS are inhibited in a way that depends on the activity of these short latency inhibitory interneurons in M1. The paired-pulse technique allowed us to assess the dynamics of short intracortical inhibition (sICI) in M1 while participants performed our selection task.
Methods
Participants
Twenty-four right-handed students were tested (Experiment 1: n = 14: 6 women and 8 men; 26 ± 2.0 years old; Experiment 2: n = 10: 6 women and 4 men; 26 ± 2.0 years old). Participants were financially compensated and were naive to the purpose of the study. Handedness was determined via a condensed version of the Edinburgh Handedness inventory (Oldfield 1971). All participants gave written informed consent. The protocol was approved by the Committee for the Protection of Human Subjects at UC, Berkeley, and by the Ethics Committee of the Université catholique de Louvain, Belgium.
Experiment 1: Task-Related Changes in CS Excitability
Experimental Procedure
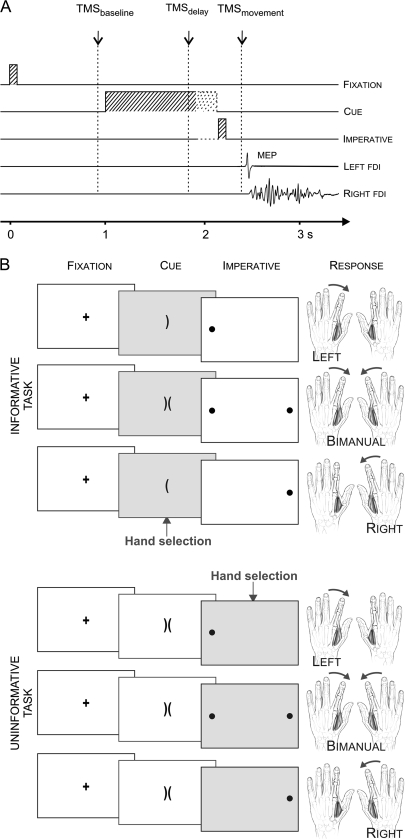
The participants sat in front of a computer screen with both hands resting on a table, palms down and the arms semiflexed. They had to abduct their left or right index finger on unimanual trials, or abduct both index fingers on bimanual trials. The task was described as a virtual “soccer game” in which the required response was indicated by the position of the “ball(s)” on the computer screen. The instructions emphasized that the participant should imagine shooting the ball(s) into the central goal(s) (Fig. 1).
Figure 1.
(A) Time course of individual trials: a fixation marker (100 ms) was followed 900 ms later, by a preparatory cue that lasted for a variable delay (900–1200 ms). Then, the imperative signal appeared (100 ms), indicating that the response should be initiated. A single TMS pulse was applied over the right M1 during 1 of 3 epochs (baseline, delay, movement). (B) The preparatory cue consisted of 1 or 2 brackets, the “soccer goals.” In the informative task (upper examples), the orientation of the goals indicated the forthcoming response. In the uninformative task (lower examples), 2 brackets were always presented. The imperative was always valid in the informative task (congruent with the cue) and indicated the required response in the uninformative task. The imperative consisted of circles, the “soccer balls,” and participants were asked to shoot the ball(s) in the goal(s) by performing the appropriate index finger abduction(s).
Each trial began with the brief presentation (100 ms) of a fixation marker at the center of the screen (see Fig. 1A,B). After a delay of 900 ms, a preparatory cue appeared. In the informative task, the preparatory cue consisted of 1 or 2 brackets (the “goal”), oriented to the left and/or right. In the uninformative task, the cue always consisted of 2 adjacent brackets, one oriented to the left and one to the right. The cue remained visible for a variable interval of 900–1200 ms and was then replaced by an imperative signal (100 ms). For unimanual trials, the imperative signal was a filled circle (the “ball”), positioned on the left or right side of the display; for bimanual trials, 2 circles were presented, one on each side of the display. The participant was instructed to perform the specified abduction movement(s) as quickly as possible following the imperative signal. To prevent the participant from anticipating the imperative signal, we included catch trials in which the circle(s) appeared in the middle of the screen. On these trials, the participant was instructed to not respond.
The preparatory cue was always valid for the informative task; for example, when the bracket pointed to the left, the ball always appeared on the left. The participant was instructed to use this information to prepare the response in advance in order to reduce his or her reaction time (RT). In contrast, in the uninformative task, response selection was not possible until the appearance of the imperative signal.
The participant practiced the 2 tasks in separate blocks for a few minutes to become familiar with the basic procedure. This was followed by 2 no-TMS blocks, one for each task. Each block consisted of 20 left, 20 right, and 20 bimanual trials, as well as a few (4–6) catch trials. The purpose of these blocks was to determine the participant's mean RT for each movement condition (left, right, bimanual) in the absence of TMS. RT for the left and bimanual trials was defined as the time interval between the onset of the imperative signal and a movement-related increase in the electromyography (EMG) activity in the first dorsal interosseous (FDI) of the left hand; for unilateral right trials, RT was measured from the EMG signal of the right FDI.
The main phase of the experiment consisted of 8 blocks with TMS, 4 for each task. In the informative task, there were 66 trials per block, 20 for each response type (left, right, bimanual) and 6 catch trials. In the uninformative task, there were 52 trials per block, 16 for each response type and 4 catch trials. Each block lasted approximately 4 min. The 4 blocks for a given task were run successively with the order of the 2 tasks counterbalanced. A 10-min break was provided prior to the start of the second task.
CS excitability was assessed by measuring MEPs in the left FDI following TMS over the right M1. We focused on a left hand muscle because CS suppression is thought to be stronger in the nondominant hand (Leocani et al. 2000; Duque et al. 2007). Only 1 TMS pulse was applied on each trial, with 3 possible timings (see Fig. 1A). To establish a baseline, stimulation was applied 800 ms after the offset of the fixation marker (100 ms before the onset of the preparatory cue). For the second timing, TMS was applied 800 ms after the cue. Given that the interval between the onset of the cue and the imperative signal was variable, this pulse occurred 100 to 400 ms before the imperative signal. This time point was selected to assess CS excitability changes associated with the delay period. We assumed that the participant had prepared the specified response in the informative condition. In contrast, preparation in the uninformative condition at this time would be limited to general features of stimulus/response anticipation.
For the third timing, TMS occurred after the imperative signal, or what we refer to as the movement period. The timing of this pulse was determined on an individual basis, set to 70 ms before the mean RT as measured on the EMG traces of the no-TMS blocks. This value was selected to provide a probe of CS excitability when response selection was near completion while not contaminated by EMG activity related to the actual movement (Rossini et al. 1988; Chen and Hallett 1999; Reynolds and Ashby 1999; Leocani et al. 2000). Given RT variability, a post hoc analysis was performed to identify the trials to include in the MEP analysis. Only trials in which the TMS pulse occurred between 120 ms and 20 ms prior to EMG onset were included (Duque et al. 2005), a time when CS excitability changes related to movement preparation are the strongest (Chen and Hallett 1999; Leocani et al. 2000).
Transcranial Magnetic Stimulation
TMS was applied using a figure-of-8 magnetic coil (diameter of wings 70 mm) connected to a rapid Magstim 200 magnetic stimulator (Magstim, Whitland, Dyfed, UK). The magnetic coil was placed tangentially on the scalp, over the right M1, with the handle pointing backward and laterally at a 45° angle away from the midline, approximately perpendicular to the central sulcus.
After fitting the participant with a tight EEG cap, we first identified the optimal spot for eliciting MEPs in the left FDI. This hotspot was marked on the EEG cap to provide a reference point for the experimental session. The resting motor threshold (rMT) was defined as the minimal TMS intensity needed to evoke MEPs larger than 50 μV peak-to-peak in the relaxed FDI on 5 out of 10 consecutive trials. Across participants, the rMT corresponded to 67% (SE = 0.72) of maximum stimulator output (MSO). The intensity of TMS for the main experiment was set at 15% above the rMT.
EMG Recording
EMG activity was recorded from surface electrodes placed over the right and left FDI muscles. EMG data were collected for 2000 ms on each trial, starting at least 800 ms before the TMS pulse. The EMG signals were amplified and bandpass filtered online (50–2000 Hz; Delsys, Inc., Boston, MA), and stored on a personal computer for offline analysis. This offline analysis included the measurement of MEP peak-to-peak amplitudes, RTs and mirror activity (MA) during unimanual trials. MA was defined as EMG activity exceeding 50 μV in the nonselected FDI that occurred during the EMG activity in the selected FDI. In order to prevent contamination of our MEP measurements by background EMG activity, the following trials were excluded from the MEP analysis: 1) Trials with any background EMG activity greater than 100 μV in the 200-ms window preceding the TMS artifact (Duque et al. 2005, 2007) and 2) trials with left hand MA (right hand response trials) when TMS was applied during the movement period. We adopted this criterion because left FDI MEPs might also be influenced by MA on such trials. Based on these criteria, a minimum of 16 MEPs were obtained for each TMS timing and movement condition.
Statistical Analysis
To examine the RT data, we used a 3-way repeated-measure ANOVA (ANOVARM) with factors Task (uninformative, informative), Response (left, right, bimanual) and TMS (no TMS, TMSdelay, TMSmovement). Note that trials with TMSbaseline were not included in the RT analysis because we did not have enough of them in each response condition.
To examine the overall dynamics of CS excitability, left FDI MEP amplitudes were analyzed by means of separate 1-way ANOVARM for the informative task (Condition: baseline, delay_left, delay_right, delay_bimanual, mvt_left, mvt_right, mvt_bimanual) and the uninformative task (Condition: baseline, delay, mvt_left, mvt_right, mvt_bimanual). To assess the effect of the cue presentation across tasks, we performed a 1-way ANOVARM with the factor Cue (uninformative, left, right, bimanual) on the TMSdelay MEPs, expressed as a percentage of change with respect to baseline. MEPs at TMSmovement, expressed as a percentage of change with respect to baseline, were also tested by means of a 2-way ANOVARM with factors Task (uninformative, informative) and Response (left, right, bimanual). Finally, to analyze CS excitability changes specifically related to the presentation of the imperative signal in the 2 tasks, we expressed the MEP values during the movement period as a percentage of the MEP values obtained during the delay period ([MEPmovement/MEPdelay] × 100). This dependent variable was evaluated with a 2-way ANOVARM with the factors Task (uninformative, informative) and Response (left, right, bimanual).
In addition, we measured the proportion of trials with MA for each unimanual condition and analyzed these data with a 3-way ANOVARM with Task (uninformative, informative), Response (left, right) and TMS (no TMS, TMS) as factors. Incidence of MA in the TMS conditions was quantified by pooling together TMSdelay and TMSmovement trials.
All post hoc comparisons were conducted using the Fisher's Least Significant Difference (LSD) procedure. All of the data are expressed as mean ± SE.
Experiment 2: Task-Related Changes in M1 Intracortical Inhibition (sICI)
In a second Experiment (n = 10), we investigated whether the CS suppression observed during the delay period in Experiment 1 could be accounted for by changes in M1 excitability. To this end, single- and paired-pulse TMS protocols were applied during the delay period. In the paired-pulse procedure, a suprathreshold TS was preceded by a subthreshold “conditioning” stimulus (interstimulus interval = 3 ms) delivered through the same coil (Kujirai et al. 1993).
Experimental Procedure
In the main session of Experiment 2 (n = 7), participants were required to perform unimanual movements with the left or right index finger (no bimanual trials) following 3 types of preparatory cues; informative left, informative right or uninformative. The imperative signal was always congruent with the preparatory cue or indicated the required response when the preparatory cue had been uninformative. The timing of the different events was the same as in Experiment 1
Each participant performed 4 blocks of 52 trials. Single-pulse TMS was applied during 2 of the blocks and paired-pulse TMS was applied in the other 2 blocks, in a counterbalanced order. Each block included 17 left, 17 right and 18 uninformative preparatory cue trials, selected in a random order. TMS was applied at 2 different times. For baseline measurements, the pulse occurred 800 ms after the offset of the fixation (100 ms before the onset of the preparatory cue). For the delay period, the pulse occurred 800 ms after the cue (100–400 ms before the imperative signal, see Exp. 1). We focused on the delay period rather than the movement period because this epoch showed the strongest CS suppression in Experiment 1. In sum, 24 MEP measurements were acquired at baseline and following each of the 3 preparatory cues (left, right, uninformative) for each TMS protocol (single-pulse, paired-pulse). Three catch trials and 4 trials with no TMS were included in each block to avoid movement or TMS anticipation, respectively.
Transcranial Magnetic Stimulation
A Magstim BiStim (Magstim, Whitland, UK) was used to deliver the TMS pulses. As in Experiment 1, we first identified the hotspot and rMT for the left FDI. On average, the rMT corresponded to 40% (SE = 2.0) of the MSO. Two additional parameters were then determined. First, on single-pulse trials, we identified the TS intensity that yielded an MEP amplitude of about 2 mV in the FDI at rest; on average, the TS intensity was 49% (SE = 2.3) of the MSO, which corresponded to 123% (SE = 2.1) of rMT. Second, on paired-pulse trials (interstimulus interval = 3 ms) we determined the conditioning stimulus intensity required to induce a 50% reduction in the TS MEPs. To do so, the TS intensity was fixed at that established from the single-pulse trials. The conditioning stimulus intensity was initially set at 80% of rMT, a value that should produce a modest degree of sICI (Liepert et al. 1998; Maeda et al. 2002). This intensity was gradually reduced until the amplitude of the TS MEPs reached about 50% of the single-pulse value. On average, the final conditioning stimulus intensity was 27% (SE = 1.9) of the MSO, an intensity that corresponded to 68% (SE = 4.2) of rMT.
sICI was calculated by expressing the amplitude of the conditioned TS MEPs in the paired-pulse trials (MEPC) relative to the amplitude of the nonconditioned TS MEPs (MEPNC): sICI = [MEPC/MEPNC] × 100. As such, the lower the sICI value, the stronger the intracortical inhibition (0% = maximum inhibition, 100% = no inhibition). In the present study, sICI equaled 43% (SE = 4.9) at rest.
A limitation with this procedure is that MEP amplitudes on nonconditioned trials varied across the trial due to changes in CS excitability. Nonetheless, the magnitude of sICI has been shown to be relatively invariant for MEP values between 1 and 4 mV (Kujirai et al. 1993; Ridding et al. 1995; Daskalakis et al. 2002; Rosenkranz and Rothwell 2003; Coxon et al. 2006). To reduce the possibility that task-related MEPNC changes would affect the sICI measurements, we set the baseline MEPNC amplitude at 2 mV. Our goal here was to set a stimulation level that produced MEPs that should fall within the invariant 1- to 4-mV window despite fluctuations in CS excitability.
Furthermore, 6 subjects (3 of whom also participated in the main session of Experiment 2) were tested in a control sICI experiment in which we adjusted the stimulation level such that MEPNC amplitude was matched across conditions. To do so, we first determined the TS intensity required to evoke MEPs of 1 mV in approximately 50% of trials at rest (TS1mVbaseline). This TS intensity (46% [SE = 2.4] of MSO, n = 6) was used to determine sICI at TMSbaseline. We then chose a conditioning stimulus intensity that induced a 50% reduction in the MEPs at rest (23% [SE = 1.3] of MSO). Second, subjects performed the same task as in the main session of Experiment 2 with 2 kinds of preparatory cues (left, right) and we determined the TS intensity that evoked MEPs of 1 mV in at least 50% of trials following a left cue at TMSdelay. We focused on left cue trials because they showed the strongest sICI change in the main session. This TS intensity (TS1mVdelay-left; 50% [SE = 3.0] of MSO) was used to assess sICI at TMSdelay-left. The conditioning stimulus intensity was the same as that used to assess sICI at TMSbaseline.
Once these stimulation levels were set, each participant performed 4 blocks of 60 trials in which TMS was applied during baseline or the delay period on 97% of trials. In the remaining 3% of trials, no TMS was applied to reduce the overall expectancy of a pulse within each block. Single-pulse TMS was applied during 2 of the blocks and paired-pulse TMS was applied in the other 2 blocks, with the order counterbalanced. For one of the blocks of each of the single- and paired-pulse blocks, the stimulation level was set at TS1mVbaseline and for the other, the stimulation level was set at TS1mVdelay-left.
In the analyses of these data, we only used those trials in which the stimulation level was set to the appropriate phase. That is, we analyzed baseline data from the 20 trials when the stimulator intensity was at TS1mVbaseline and analyzed the delay data from the 20 trials following a left cue when the stimulator intensity was at TS1mVdelay-left. This procedure allowed us to assess sICIbaseline and sICIdelay-left with matched ∼1mV MEP amplitudes. All other trials were not included in the analyses reported below.
EMG Recording
EMG activity was recorded from surface electrodes (Neuroline, Medicotest, Oelstykke, Denmark) placed over the right and left FDI muscles. The raw EMG signals were amplified (gain, 1K), bandpass filtered (10–500 Hz; Neurolog; Digitimer, Hertfordshire, UK), and digitized at 2 kHz for offline analysis. The same exclusion criteria were used as in Experiment 1. Based on these criteria, a minimum of 16 MEPs was obtained for each condition.
Statistical Analysis
To examine the dynamics of CS and M1 inhibitory activity in the main session of Experiment 2, the amplitude of left FDI MEPNC and sICI were analyzed using a 1-way ANOVARM with the factor Condition (baseline, delay-left, delay-right, delay-uninformative). Given our strong hypothesis based on results from experiment 1, 1-tailed paired t-tests were used for post hoc comparisons of left FDI MEPNC. The Fisher's LSD procedure was used for post hoc comparisons of the sICI data. All of the data are expressed as mean ± SE. In the control session of Experiment 2, 1-tailed paired t-tests were used to compare sICI at baseline and in the delay-left condition with matched MEP1mV amplitudes.
Results
Experiment 1: Task-Related Changes in CS Excitability
Reaction Time
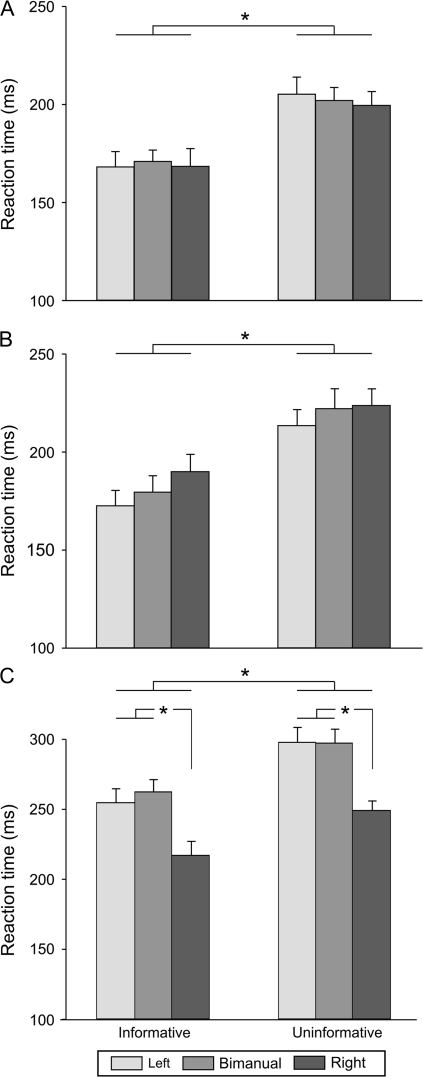
RTs differed for the informative and uninformative Tasks (F = 73.35, p < 0.001, see Fig. 2). As expected, RTs were shorter when the cue specified the forthcoming response. Overall, in blocks with no TMS (Fig. 2A), the mean RT in the informative condition was 169 ms (SE = 7.6), with only a 3 ms increase in mean RT on bimanual trials compared with unimanual trials (171 vs. 168 ms for left and right). The mean RT in the uninformative condition was 202 ms (SE = 7.5), and again, the difference between the 3 movement types was quite small (range 200–205 ms). Shorter RTs in the informative than uninformative blocks were also observed in the main experiment when TMS was applied during the delay period (181 ms [SE = 7.4] vs. 220 ms [SE = 6.3], see Fig. 2B) or the movement period (245 ms [SE = 8.5] vs. 281 ms [SE = 8.1], see Fig. 2C). The finding that RTs were shorter in the informative trials for both the no-TMS and TMS blocks indicates that, as instructed, participants used the informative cues to prepare the appropriate response.
Figure 2.
RTs (ms) when TMS was absent (A), applied during the delay period (B), or during the movement period (C), for the left, bimanual and right hands, in the informative and uninformative tasks. As expected, RTs were faster when the response had been specified by a preparatory cue. Values presented are group means with SE bars (n = 14). *P value < 0.05.
We also found a significant Response × TMS interaction (F = 24.02, P < 0.001). As evident in the means, TMS significantly increased RTs for all response types when applied during the movement period (all P < 0.001 when compared with the no-TMS condition), although this effect was more pronounced for left hand responses (performed unimanually or bimanually). As a consequence, during the movement period, RTs were longer for the left hand responses (left only and bimanual movements) than for the right hand responses (both P < 0.001, see Fig. 2C). This effect is consistent with previous results showing that M1 TMS mainly increases RT in the contralateral hand (Leocani et al. 2000).
CS Excitability
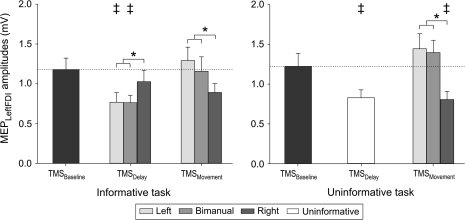
During the “baseline period”, the mean MEP amplitudes were 1.18 mV [SE = 0.14] and 1.23 mV [SE = 0.16] for the informative and uninformative tasks, respectively. These baseline MEP amplitudes were not significantly different from one another (t = 0.62, P = 0.54) but were modulated in distinct ways in the 2 tasks (Fig. 3).
Figure 3.
Group means and SE bars (n = 14) of the amplitude (mV) of MEPs recorded from left FDI following TMS of right M1. MEP amplitudes are shown for the baseline (following fixation), delay (following cue) and movement (following imperative) periods in both the informative (left side) and uninformative (right side) tasks. MEP amplitudes were significantly decreased during the delay period; especially when the forthcoming response would (informative) or might (uninformative) include the left FDI (all except cued right responses). No change was observed between the delay and movement periods in the left FDI on right hand only trials. *P value < 0.05. ‡Significant difference with MEPs at TMSbaseline.
In the informative task, there was a significant effect of Condition (F = 3.45, P = 0.005, Fig. 3, left). During the “delay period,” the MEPs were significantly reduced with respect to baseline when the cue indicated that the response would require a left hand movement (left only or bimanual, both P < 0.03) but not when the cue indicated that the response would require only a right hand movement (P = 0.44). This result is consistent with the “impulse control” hypothesis and at odds with the “inhibition-for-deselection” hypothesis. When TMS was applied during the movement period, MEP amplitudes increased with respect to the delay period when the forthcoming movement required a left hand response (left: P < 0.001, bimanual: P < 0.01). When the imperative signaled a right hand response, the mean value of the left FDI MEP amplitudes remained unchanged with respect to the delay period.
In the uninformative task, the factor Condition was also significant for left FDI MEP amplitudes (F = 7.2, P < 0.001, Fig. 3, right). MEP amplitudes were significantly reduced with respect to baseline during the delay period (P < 0.02), although here, the cue did not specify the forthcoming response. Then, when the imperative stimulus signaled a left hand response, the left FDI MEPs increased during the movement period (left: P < 0.0001, bimanual: P < 0.002) with respect to the delay period. As in the informative condition, no change was observed between the delay and movement periods when the imperative signaled a right hand response. Thus, contrary to the “inhibition-for-deselection” hypothesis, the selection of a right hand response (following the imperative signal in this task) did not lead to additional inhibition of the nonselected left hand MEP (P = 0.76).
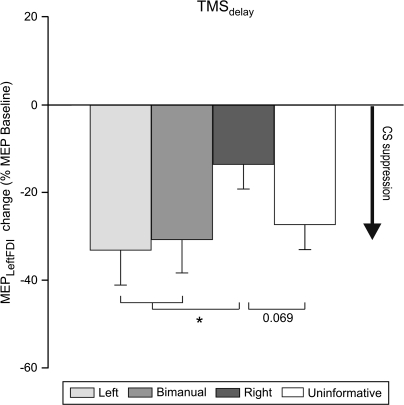
The modulation of the MEPs during the delay period varied across all 4 types of Cues (uninformative, left, right, bimanual, F = 3.05, P = 0.04, Fig. 4). The amplitude of MEPs during the delay period in the uninformative task was attenuated to a similar degree (27% decrease with respect to baseline) as that observed when the cue indicated that the response would require a left hand movement (33% and 31% decrease for left and bimanual movements, respectively). In contrast, the magnitude of this attenuation was much smaller when an informative cue signaled a right hand response (13% decrease only; compared with left and bimanual movements: P < 0.009 and P < 0.02 respectively). The same trend was observed when comparing right hand responses to the uninformative condition (P = 0.069). Thus, even across tasks, CS excitability of the left hand was lower during the delay period when this hand was a potential respondent compared with when it was no longer a viable candidate for selection.
Figure 4.
Change in MEP amplitudes recorded from the left FDI during the delay period, expressed as a function of baseline MEP amplitudes. Histograms are for each of the 3 types of informative cues (left, bimanual, right) and for the uninformative cue. Inhibition of left FDI is greater when a cue indicates that a left hand movement should be prepared than when the cue indicates that a right hand movement should be prepared. *P value < 0.05.
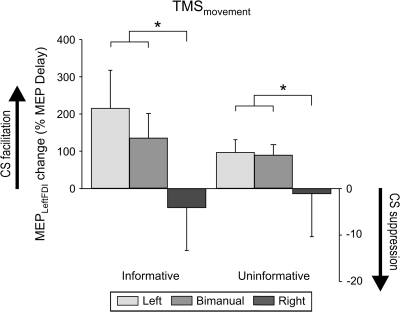
During the movement period, MEP amplitudes varied as a function of the Response (F = 7.16, P < 0.004, see Fig. 3), regardless of the Task. As expected, left MEPs were smaller after an imperative signaling a right hand response than after imperative signals that indicated left hand (P < 0.002) or bimanual (P < 0.007) movements.
Finally, we examined changes in MEP amplitudes following the imperative signal with respect to the MEPs during the delay period ([MEPmovement/MEPdelay] × 100; see Fig. 5). Again, a main effect was only found for Response (F = 5.44, P < 0.01); MEP changes were significantly larger for left hand responses, either produced on unimanual or bimanual trials, than right hand responses (both P < 0.03). Surprisingly, there was no difference between the 2 Tasks for right hand responses (F = 1.2, P = 0.3). Thus, we not only failed to observe a strengthening in left hand inhibition when the right hand response was specified by the imperative signal, but there was no difference in left hand MEPs for the 2 Tasks during the movement period preceding a right hand response. This null effect was obtained despite the fact that hand selection could only be initiated with the presentation of the imperative signal in the uninformative task.
Figure 5.
Change in MEP amplitudes recorded from the left FDI during the movement period, expressed as a function of MEP amplitudes during the delay period. Note that there was no accentuation of left FDI suppression when the imperative signal indicated that the response would only involve the right hand. *P value < 0.05.
Mirror Activity
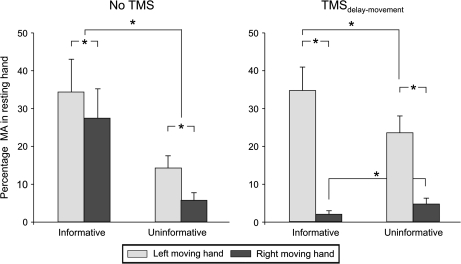
We assessed MA, defined as an increase in EMG activity in the nonresponding hand. Because EMG electrodes were placed on both left and right FDI, we were able to measure MA in both hands. Overall, MA was observed in the nonselected hand on 18% of the trials in which a unimanual response was made (see Fig. 6). The incidence of MA varied with Task (F = 12.48, P < 0.004), and Response (F = 15.13, P < 0.001). Moreover, the 3-way interaction Task × Response × TMS was significant (F = 15.06, P < 0.002, see Fig. 6). In blocks with no TMS, the incidence of MA was significantly higher following an informative than an uninformative cue for both left (P < 0.001) and right hand responses (P < 0.001). The incidence of MA was significantly larger in the right hand (left hand responses) compared with the left hand (right hand responses) in the informative (P < 0.006) and uninformative (P < 0.001) tasks.
Figure 6.
Percentage of trials with MA in the nonselected index finger during unimanual responses (left and right in light and dark gray, respectively) in the informative and uninformative tasks, in the absence (left side) or presence of TMS over the right M1 (right side). In the absence of TMS, incidence of MA was always higher in the informative task compared with the uninformative task, consistent with the smaller inhibition in the nonselected hand in the former condition. *P value < 0.05.
The incidence of MA was modulated by the presence of right M1 TMS pulses. TMS decreased the incidence of MA in the contralateral left hand during right hand responses for the informative condition (P < 0.0001, with respect to the no-TMS condition). In contrast, right M1 TMS increased the incidence of MA in the ipsilateral right hand during left hand responses, but here the effect was limited to the uninformative task (P < 0.001, with respect to the no-TMS condition). Overall, the incidence of MA was much larger in the right hand (left hand response) than in the left hand (right hand responses) on the TMS trials in both informative (P < 0.0001) and uninformative conditions (P < 0.0001).
Experiment 2: Task-Related Changes in M1 Intracortical Inhibition
A new set of participants was recruited to investigate whether CS excitability changes observed during the delay period in Experiment 1 were related to changes in M1 intracortical inhibition (sICI). MEPs on single- and paired-pulse trials were compared with assess sICI.
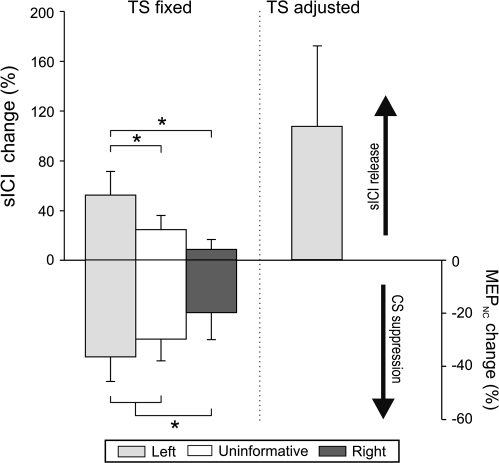
In single-pulse trials of the main session of Experiment 2 (n = 7), the baseline left FDI MEPNC amplitude averaged 2.0 mV (SE = 0.41). Following the cue, a reduction in CS excitability was observed (F = 5.18; P = 0.009, see Fig. 7, lower half). MEPNC amplitudes were significantly lower than baseline following the left and uninformative cues (36% [1-tailed t = 2.38, P < 0.03] and 30% [t = 2.58, P < 0.02] decrease, respectively) whereas a right hand cue only induced a marginal decrease in amplitude (20% decrease [t = 1.83, P = 0.06]). Hence, in the absence of a conditioning stimulus, the MEPs during the delay period were smaller when the left index finger might be involved in the forthcoming movement compared with when the cue indicated a forthcoming right hand response (left: t = 2.28, P = 0.03; uninformative: t = 3.19, P = 0.009), consistent with the results of Experiment 1.
Figure 7.
Left FDI nonconditioned MEP amplitudes (MEPNC, lower half) and sICI ([MEPC/MEPNC] × 100, upper half) during the delay period in Experiment 2. Results in the main session in which the TS intensity was set to produce a 2 mV MEP at rest are illustrated on the left side (fixed TS). The right side shows the results from the control session in which TS intensity was adjusted to evoke MEPs of 1 mV at baseline and during the delay period (adjusted TS). All values are expressed as a function of change with respect to baseline. Note the inverse profiles for the MEP and sICI results. *P value < 0.05; MEPC = conditioned MEP.
In paired-pulse trials, we found a significant effect of factor Condition on left FDI sICI (F = 12.59, P = 0.0001, Fig. 7, upper half). Compared with baseline (sICI = 47%, SE = 8.2), the attenuation of left FDI MEPs by the conditioning pulse was significantly reduced (higher sICI, or less inhibition) when the left index finger might be involved in the forthcoming movement (left or uninformative cue; Fisher's P < 0.001 and P < 0.009, respectively). When the cue indicated a right hand response, there was no change compared with baseline (P = 0.23). In pairwise comparisons of the 3 cueing conditions, the sICI values were significantly higher following the left cue compared with the right cue (P < 0.0002), again suggesting less intracortical inhibition in left FDI when that muscle will be used in the forthcoming response. However, the sICI release was also significantly stronger after the left cue compared with the uninformative cue (P < 0.01). These results indicate that CS suppression during the delay period, as evidenced in the single-pulse protocols of Experiments 1 and 2, occurs at a time when right hemisphere M1 intracortical inhibition is reduced when preparing a left hand response.
This sICI release at TMSdelay was confirmed in a control session (n = 6) in which the stimulation level was adjusted so as to match the amplitude of MEPNC across the baseline (0.99 mV, SE = 0.22) and delay (1.05 mV, SE = 0.08) epochs. With this procedure, the sICI values were again higher (less inhibition) at TMSdelay-left (sICI = 70% [SE = 7.7]) than at TMSbaseline (sICI = 44% [SE = 8.9]). The increase was significant across participants ([t = 2.42, P = 0.03], see Fig. 7).
Discussion
When performing a unimanual movement, CS signals associated with the nonselected hand are consistently decreased. This suppression in CS activity has generally been assumed to reflect the operation of inhibitory neural processes targeted at the nonselected hand, helping to ensure that this hand is not recruited (Duque et al. 2005; Koch et al. 2006). These mechanisms may, at the cortical level, help facilitate the selection and planning of strictly unimanual actions, and/or, more peripherally, ensure that overt movements are not produced by the nonselected hand (e.g., mirror movements).
However, our results challenge the idea that there are selection processes dedicated to suppressing CS activity of the nonselected hand. Contrary to the predictions of the “inhibition-for-deselection” hypothesis, left FDI MEPs were mainly suppressed following a preparatory cue that indicated that a left hand response would (left hand cue) or might (uninformative cue) be required. In contrast, CS suppression was modest when the cue indicated a right hand response and the magnitude of this suppression was significantly less than in the other conditions. Moreover, we did not observe any additional modulation of left FDI MEPs after an imperative signal indicating a right hand response, regardless of whether the preparatory cue had been informative or uninformative.
In sum, our results suggest a new origin for the suppression frequently observed in CS excitability of the nonselected hand. Rather than reflecting “deselection” processes, this CS suppression seems to be, in large part, related to “impulse control” mechanisms that help prevent premature or inappropriate movement activity during motor preparation in a hand that might be selected for a forthcoming response.
CS Suppression during the Delay Period
Previous investigations of CS excitability changes during a delay period have reported conflicting results showing both facilitatory (Mars et al. 2007; van den Hurk et al. 2007) and inhibitory effects (Hasbroucq et al. 1997, 1999; Touge et al. 1998; McMillan et al. 2004; Davranche et al. 2007; Prabhu et al. 2007; van Elswijk et al. 2007). Our results might seem in conflict with 2 recent studies showing increased CS excitability in a muscle that will be involved in the forthcoming movement and decreased excitability in the homonymous muscle of the nonresponding hand (Mars et al. 2007; van den Hurk et al. 2007). However, a closer look at the experimental design of these studies reveals important differences with the present study including the timing of TMS stimulation, the duration of the delay period, and the expectancy of the imperative signal. It is likely that the methods used in those studies (long delays and variable TMS timing) were optimal to capture facilitatory effects related to motor preparation, whereas our design (short delays and fixed pre-imperative TMS timing) was optimal to investigate inhibitory control mechanisms. Consistent with the present study, CS excitability of response-relevant muscles is constantly observed to decrease at the end of a (short) delay period when an imperative signal is expected (Touge et al. 1998; Hasbroucq et al. 1997, 1999; Prabhu et al. 2007).
These preimperative CS effects arise from multiple preparatory processes occurring in a number of cortical areas (Horwitz et al. 2000; Zang et al. 2003; Beurze et al. 2007), as well as in the cerebellum and basal ganglia (Houk and Wise 1995). Electrophysiological recordings in primates have revealed preparatory neuronal activity in the parietal (Calton et al. 2002), prefrontal (Hoshi 2006), premotor (Hoshi and Tanji 2000; Kurata and Hoshi 2002; Cisek et al. 2003; Cisek and Kalaska 2005; Terao et al. 2007), and primary motor areas (Bastian et al. 2003). This preparatory activity is manifest as an increase in activity specific to the forthcoming movement.
Our results provide important information regarding these pre-imperative CS effects. The decrease in left FDI MEP amplitudes was similar when the cue signaled a left hand response and when it was uninformative. Moreover, CS suppression was greater in these 2 conditions compared with when the cue signaled a forthcoming right hand response. This suggests that, under conditions in which rapid response initiation is emphasized, the motor system not only prepares specified actions (i.e., following informative cues), but also gets ready for a set of potential actions from which the actual response is subsequently selected from (i.e., following uninformative cues) (Cisek and Kalaska 2005; Koch et al. 2006). Part of this preparation process, though, involves the generation of inhibitory signals to ensure that the response is emitted at the appropriate time.
Although less pronounced, we did observe smaller left hand FDI MEPs during the delay period even when the cue indicated that the response would be limited to the other hand. In theory, this suppression could reflect hand selection processes that occur at the time of the informative cue and include the suppression of CS activity associated with the nonselected hand, consistent with the “inhibition-for-deselection” hypothesis (Cisek and Kalaska 2005; Cisek 2007). However, we did not observe any additional MEP suppression of the nonselected hand following the presentation of the imperative signal in the uninformative task (see Fig. 5). If selection processes involve inhibition of nonselected agonists, then an increase of inhibition would be expected at this time too.
A more parsimonious interpretation is that the relatively small CS suppression of the nonselected hand in the informative task may be inherited from inhibitory processes that are activated in anticipation of the cue. In fact, given that the timing of this cue was fixed, the motor system may already be activating representations of potential actions for the 2 hands at the start of the trial (i.e., onset of the fixation), and correspondingly, engage inhibitory mechanisms to ensure that this advance preparation does not induce premature responses at the onset of the cue. Thus, it is plausible that all of the CS suppression during the delay period is due to processes associated with “impulse control” rather than “deselection.” However, we cannot rule out the possibility that CS excitability was decreased during the delay period to a level that made it difficult to detect further suppression following the imperative signal.
M1 Disinhibition during the Delay Period
Using a protocol designed to measure intracortical inhibition in Experiment 2, we found that M1 inhibitory activity was actually lower relative to baseline during the delay period. This attenuated intracortical inhibition in right M1 was most pronounced in the informative left hand condition. This result was obtained in both the main session of Experiment 2 where the stimulation level was fixed for the baseline and delay phases as well as in the control session in which the stimulation level was adjusted to produce matched MEP amplitudes in the baseline and delay phases. Most interestingly, the reduction in local inhibition as measured by sICI was observed at the same time as when the single-pulse protocol indicated pronounced CS suppression. Other studies have similarly showed a parallel decrease in CS excitability during a delay period in which there is an increase in M1 activity (Davranche et al. 2007; Sinclair and Hammond 2008). We propose that the M1 disinhibition arises because of ongoing response preparation processes. However, these processes are accompanied by mechanisms that suppress CS output to help ensure that the response is not initiated prematurely. This CS suppression would prevent overt activity in potentially selected muscles despite the increasingly strong activations in cortical areas.
CS Excitability Changes during the Movement Period
Following the imperative signal, MEPs became larger in the agonist muscle of the selected hand prior to the movement-related increase in EMG. This observation is consistent with many previous reports (Chen et al. 1998; Chen and Hallett 1999; Leocani et al. 2000). Note that we observed similar MEPs for the selected hand in the informative and uninformative conditions, despite the fact that the RTs were much faster in the former. This effect is likely due to the fact that we chose to stimulate at a fixed point of time relative to EMG onset, approximately 70 ms. Thus, the timing of the movement phase pulse is different in the 2 conditions and the similar amplitude of the MEPs likely reflects the fact that we are probing at similar points of “readiness” by using a criterion based on EMG onset.
Contrary to what we would have expected based on the “inhibition-for-deselection” hypothesis, we did not observe any change in CS excitability of the left hand when the imperative signaled a right hand response. This null result was observed in both the informative and uninformative tasks, further challenging the assumption that inhibitory mechanisms are recruited as part of the selection process per se. If this was so, we would expect larger inhibition in the uninformative than informative condition, because (right) hand selection is only possible at the onset of the imperative signal in the former condition. Consistent with the interpretation of the changes observed during the delay period, persistent inhibition of the nonselected hand during the movement period may primarily be a manifestation of “impulse control” mechanisms.
Our decision to only stimulate right M1 and measure MEPs in the left hand was based on previous evidence that MEP attenuation is typically stronger in the nondominant than dominant hand (Leocani et al. 2000). Motivated by the “deselection” hypothesis, this observation has led to the idea that inhibitory processes are more strongly engaged when the dominant hand is selected than when the nondominant hand is selected. Given that our results favor an “impulse control” mechanism for CS suppression, even in a nonselected hand, the reason for the asymmetry in CS suppression is less clear. That is, why would impulse control signals be stronger to the nondominant hand compared with the dominant hand? At present, we do not have an answer to this question, although various studies suggest a prominent role for the right hemisphere in inhibitory control processes (e.g., Aron et al. 2004). Alternatively, given that MEP amplitudes result from the sum of facilitatory and inhibitory influences, it is also possible that the asymmetry results from more facilitation (associated with movement preparation) of the dominant hand, coupled with the symmetric effects of impulse control mechanisms. These are interesting questions for future study.
Possible Mechanisms of CS Suppression
The exact level at which the suppression of MEPs occurs is unknown. One possibility is that CS suppression arises from an increase in M1 inhibition. This hypothesis is unlikely given that M1 intracortical inhibitory activity was actually lower during the delay period. Alternatively, CS suppression may result from activity in other brain regions. For example, frontal, cingular, and parietal cortices are known to affect the excitability of cortical and/or spinal components of the CS tract (Davidoff 1990; Dum and Strick 1991, 1996; Galea and Darian-Smith 1994; Tokuno and Nambu 2000; Maier et al. 2002; Davare et al. 2008; Schmidlin et al. 2008). Spinal excitability changes have been reported during motor preparation but the nature and time course of these changes suggest that decreased MEP amplitudes cannot be solely accounted for by decreased spinal excitability (Touge et al. 1998; Hasbroucq et al. 1999; Prut and Fetz 1999; Duclos et al. 2008). Therefore, it is likely that CS suppression results from “impulse control” processes that synchronize changes in both the cortical and spinal components of the CS tract.
Which neural region(s) might be responsible for ensuring that movements are not prematurely initiated during response preparation? One candidate for this form of inhibitory control is the prefrontal cortex. Prefrontal cortex plays a critical role in integrating response selection within a context that is defined by internal goals or acquired rules (Wallis et al. 2001; Bunge 2004; Koechlin and Summerfield 2007). In the present study, a primary instruction was to respond as fast as possible, but only after the imperative signal. Although the stimulus-response associations might be represented in premotor cortex (Wise and Murray 2000; Hoshi and Tanji 2006), prefrontal mechanisms could provide the control signals to ensure that the activation of these representations does not lead to the premature initiation of a selected action. Ventrolateral PFC has been linked to the inhibition of planned actions (Aron 2007; Aron et al. 2007; Chambers et al. 2007). Another candidate is the medial aspect of PFC, a region shown to be activated during earlier stages of processing associated with response preparation and selection (Boulinguez et al. 2008; Jaffard et al. 2008).
Mirror Activity
MA was frequently observed in the homologous FDI. Interestingly, MA in the nonselected hand was more prominent in the informative task, when the response could be prepared in advance, than in the uninformative task, when the response was only specified by the imperative signal. At first sight, this result is counterintuitive; one might expect reduced MA when the participant has known well in advance that a given hand will not be involved in the response. However, the present results indicate that suppression in activity of the nonselected hand was small following an informative cue (Fig. 3). It is reasonable to assume that the likelihood of MA would be greater when inhibition is weak. Moreover, these results are consistent with the view that inhibitory processes during movement preparation are primarily related to controlling the activity in a potentially selected hand, rather than suppressing activity in a nonselected hand.
TMS applied over right M1 substantially decreased the occurrence of MA in the (contralateral) left hand during right hand responses but increased the incidence of MA in the (ipsilateral) right hand during left hand responses. These TMS-related effects are puzzling because we cannot distinguish between effects directly resulting from the “virtual lesion” induced by TMS of M1 and changes in excitability associated with the pulse that could influence activity in M1 and interconnected regions.
Conclusion
The present results suggest a new interpretation of the role of inhibitory processes observed during movement preparation. The standard assumption has been that decreased CS excitability of a nonselected hand reflects the operation of inhibitory mechanisms that promote response “deselection.” As an alternative, we propose that the primary role for CS suppression is to ensure that increased preparatory activity in M1 associated with a potential response does not lead to a premature or inappropriate response. By this view, CS suppression in the nonselected hand is seen as an indirect consequence of “impulse control” mechanisms, modulated by the degree of uncertainty and timing in response preparation.
Funding
National Institute of Health (NS040813); the National Science Foundation (BCS 0726685); the Belgian American Educational Foundation; and the Fulbright program.
Acknowledgments
We are grateful to Etienne Olivier for his comments on an earlier version of this manuscript. We would also like to thank Tim Verstynen for his technical support. J.D. is currently a postdoctoral Researcher at the Belgian National Funds for Scientific Research (FRS-FNRS). Conflict of Interest: None declared.
References
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bastian A, Schoner G, Riehle A. Preshaping and continuous evolution of motor cortical representations during movement preparation. Eur J Neurosci. 2003;18:2047–2058. doi: 10.1046/j.1460-9568.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- Beurze SM, de Lange FP, Toni I, Medendorp WP. Integration of target and effector information in the human brain during reach planning. J Neurophysiol. 2007;97:188–199. doi: 10.1152/jn.00456.2006. [DOI] [PubMed] [Google Scholar]
- Boulinguez P, Jaffard M, Granjon L, Benraiss A. Warning signals induce automatic EMG activations and proactive volitional inhibition: evidence from analysis of error distribution in simple RT. J Neurophysiol. 2008;99:1572–1578. doi: 10.1152/jn.01198.2007. [DOI] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci. 2004;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Calton JL, Dickinson AR, Snyder LH. Non-spatial, motor-specific activation in posterior parietal cortex. Nat Neurosci. 2002;5:580–588. doi: 10.1038/nn0602-862. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Gould IC, English T, Garavan H, McNaught E, Kamke M, Mattingley JB. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. J Neurophysiol. 2007;98(6):3638–47. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- Chen R, Hallett M. The time course of changes in motor cortex excitability associated with voluntary movement. Can J Neurol Sci. 1999;26:163–169. doi: 10.1017/s0317167100000196. [DOI] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol. 1998;44:317–325. doi: 10.1002/ana.410440306. [DOI] [PubMed] [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci. 2007;362:1585–1599. doi: 10.1098/rstb.2007.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol. 2003;89:922–942. doi: 10.1152/jn.00607.2002. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol. 2006;95:3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Duque J, Vandermeeren Y, Thonnard JL, Olivier E. Role of the ipsilateral primary motor cortex in controlling the timing of hand muscle recruitment. Cereb Cortex. 2007;17:353–362. doi: 10.1093/cercor/bhj152. [DOI] [PubMed] [Google Scholar]
- Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol. 2008;586:2735–2742. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff RA. The pyramidal tract. Neurology. 1990;40:332–339. doi: 10.1212/wnl.40.2.332. [DOI] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 2007;25:3766–3774. doi: 10.1111/j.1460-9568.2007.05588.x. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Sadato N, Hallett M. Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol. 1996;75:233–247. doi: 10.1152/jn.1996.75.1.233. [DOI] [PubMed] [Google Scholar]
- Duclos Y, Schmied A, Burle B, Burnet H, Rossi-Durand C. Anticipatory changes in human motoneuron discharge patterns during motor preparation. J Physiol. 2008;586:1017–1028. doi: 10.1113/jphysiol.2007.145318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex. 2005;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb Cortex. 1994;4:166–194. doi: 10.1093/cercor/4.2.166. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamai CA. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res. 1997;5:185–192. doi: 10.1016/s0926-6410(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamai CA. The time-course of preparatory spinal and cortico-spinal inhibition: an H-reflex and transcranial magnetic stimulation study in man. Exp Brain Res. 1999;124:33–41. doi: 10.1007/s002210050597. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Deiber MP, Ibanez V, Sadato N, Hallett M. Correlations between reaction time and cerebral blood flow during motor preparation. Neuroimage. 2000;12:434–441. doi: 10.1006/nimg.2000.0632. [DOI] [PubMed] [Google Scholar]
- Hoshi E. Functional specialization within the dorsolateral prefrontal cortex: a review of anatomical and physiological studies of non-human primates. Neurosci Res. 2006;54:73–84. doi: 10.1016/j.neures.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature. 2000;408:466–470. doi: 10.1038/35044075. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol. 2006;95:3596–3616. doi: 10.1152/jn.01126.2005. [DOI] [PubMed] [Google Scholar]
- Houk JC, Wise SP. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb Cortex. 1995;5:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Jaffard M, Longcamp M, Velay JL, Anton JL, Roth M, Nazarian B, Boulinguez P. Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage. 2008;42:1196–1206. doi: 10.1016/j.neuroimage.2008.05.041. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, Rothwell JC. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;26:7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K, Hoshi E. Movement-related neuronal activity reflecting the transformation of coordinates in the ventral premotor cortex of monkeys. J Neurophysiol. 2002;88:3118–3132. doi: 10.1152/jn.00070.2002. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123(Pt 6):1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Liepert J, Classen J, Cohen LG, Hallett M. Task-dependent changes of intracortical inhibition. Exp Brain Res. 1998;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- Liepert J, Dettmers C, Terborg C, Weiller C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol. 2001;112:114–121. doi: 10.1016/s1388-2457(00)00503-4. [DOI] [PubMed] [Google Scholar]
- Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2002;113:376–382. doi: 10.1016/s1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 2002;12:281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- Mars RB, Bestmann S, Rothwell JC, Haggard P. Effects of motor preparation and spatial attention on corticospinal excitability in a delayed-response paradigm. Exp Brain Res. 2007;182:125–129. doi: 10.1007/s00221-007-1055-4. [DOI] [PubMed] [Google Scholar]
- McMillan S, Nougier V, Byblow WD. Human corticospinal excitability during a precued reaction time paradigm. Exp Brain Res. 2004;156:80–87. doi: 10.1007/s00221-003-1772-2. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Crawford JD, Vilis T. Integration of target and effector information in human posterior parietal cortex for the planning of action. J Neurophysiol. 2005;93:954–962. doi: 10.1152/jn.00725.2004. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol. 2004;561:331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Prabhu G, Voss M, Brochier T, Cattaneo L, Haggard P, Lemon R. Excitability of human motor cortex inputs prior to grasp. J Physiol. 2007;581:189–201. doi: 10.1113/jphysiol.2006.123356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut Y, Fetz EE. Primate spinal interneurons show pre-movement instructed delay activity. Nature. 1999;401:590–594. doi: 10.1038/44145. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53:730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487(Pt 2):541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Salinas E. Flutter discrimination: neural codes, perception, memory and decision making. Nat Rev Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA. Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen. 1980;109:444–474. doi: 10.1037//0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol. 2003;551:649–660. doi: 10.1113/jphysiol.2003.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Zarola F, Stalberg E, Caramia M. Pre-movement facilitation of motor-evoked potentials in man during transcranial stimulation of the central motor pathways. Brain Res. 1988;458:20–30. doi: 10.1016/0006-8993(88)90491-x. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121(Pt 5):785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Schmidlin E, Brochier T, Maier MA, Kirkwood PA, Lemon RN. Pronounced reduction of digit motor responses evoked from macaque ventral premotor cortex after reversible inactivation of the primary motor cortex hand area. J Neurosci. 2008;28:5772–5783. doi: 10.1523/JNEUROSCI.0944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien DJ, Bogaerts H, Suy E, Swinnen SP. The identification of coordination constraints across planes of motion. Exp Brain Res. 1999;128:250–255. doi: 10.1007/s002210050845. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Hammond GR. Reduced intracortical inhibition during the foreperiod of a warned reaction time task. Exp Brain Res. 2008;186:385–392. doi: 10.1007/s00221-007-1241-4. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Jung HY, Kaelin-Lang A, Hallett M. Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Exp Brain Res. 2003;148:176–185. doi: 10.1007/s00221-002-1292-5. [DOI] [PubMed] [Google Scholar]
- Swinnen SP. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci. 2002;3:348–359. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- Terao Y, Furubayashi T, Okabe S, Mochizuki H, Arai N, Kobayashi S, Ugawa Y. Modifying the cortical processing for motor preparation by repetitive transcranial magnetic stimulation. J Cogn Neurosci. 2007;19:1556–1573. doi: 10.1162/jocn.2007.19.9.1556. [DOI] [PubMed] [Google Scholar]
- Thoenissen D, Zilles K, Toni I. Differential involvement of parietal and precentral regions in movement preparation and motor intention. J Neurosci. 2002;22:9024–9034. doi: 10.1523/JNEUROSCI.22-20-09024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuno H, Nambu A. Organization of nonprimary motor cortical inputs on pyramidal and nonpyramidal tract neurons of primary motor cortex: an electrophysiological study in the macaque monkey. Cereb Cortex. 2000;10:58–68. doi: 10.1093/cercor/10.1.58. [DOI] [PubMed] [Google Scholar]
- Touge T, Taylor JL, Rothwell JC. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 1998;109:489–495. doi: 10.1016/s0924-980x(98)00050-2. [DOI] [PubMed] [Google Scholar]
- van den Hurk P, Mars RB, van Elswijk G, Hegeman J, Pasman JW, Bloem BR, Toni I. Online maintenance of sensory and motor representations: effects on corticospinal excitability. J Neurophysiol. 2007;97:1642–1648. doi: 10.1152/jn.01005.2006. [DOI] [PubMed] [Google Scholar]
- van Elswijk G, Kleine BU, Overeem S, Stegeman DF. Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci. 2007;19:121–131. doi: 10.1162/jocn.2007.19.1.121. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Weiss AC, Weiller C, Liepert J. Pre-movement motor excitability is reduced ipsilateral to low force pinch grips. J Neural Transm. 2003;110:201–208. doi: 10.1007/s00702-002-0780-x. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA. Arbitrary associations between antecedents and actions. Trends Neurosci. 2000;23:271–276. doi: 10.1016/s0166-2236(00)01570-8. [DOI] [PubMed] [Google Scholar]
- Zang Y, Jia F, Weng X, Li E, Cui S, Wang Y, Hazeltine E, Ivry R. Functional organization of the primary motor cortex characterized by event-related fMRI during movement preparation and execution. Neurosci Lett. 2003;337:69–72. doi: 10.1016/s0304-3940(02)01236-3. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496(Pt 3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]