Abstract
Regulator of G protein signaling 4 (RGS4) regulates intracellular signaling via G proteins and is markedly reduced in the prefrontal cortex (PFC) of patients with schizophrenia. Characterizing the expression of RGS4 within individual neuronal compartments is thus key to understanding its actions on individual G protein–coupled receptors (GPCRs). Here we present an ultrastructural reference map of RGS4 protein in macaque PFC based on immunogold electron microscopic analysis. At the soma, all labeling was asynaptic and affiliated with subsurface cistern microdomains of pyramidal neurons. The nucleus displayed most of immunoreactivity. RGS4 levels were particularly high along proximal apical dendrites and markedly decreased with distance from the soma; clustered label was present at the bifurcation into second-order branches. In distal dendrites and in spines, the protein was found flanking or directly facing the postsynaptic density of symmetric and asymmetric synapses. Axons also expressed RGS4. In fact, the density and distribution of pre- and postsynaptic labeling was correlated with the axon ultrastructure and the type of established synapses. The data indicate that RGS4 is strategically positioned to regulate not only postsynaptic but also presynaptic signaling in response to synaptic and nonsynaptic GPCR activation, having broad yet highly selective influences on multiple aspects of PFC cellular physiology.
Keywords: calcium signaling, dopamine receptor, G protein–coupled receptor, macaque monkey, schizophrenia
Introduction
Many neurotransmitters and neuromodulators bind to G protein–coupled receptors (GPCRs) to trigger intracellular signaling cascades. Modulation of these intracellular pathways offers a prime opportunity for downstream tuning of neurotransmitter actions. The expanding family of regulators of G protein signaling (RGS) negatively regulate alpha subunits of heterotrimeric G proteins by activating a GTPase that drives G proteins into their inactive GDP-bound form (reviewed in Hepler 1999; Willars 2006; Bansal et al. 2007). Such regulatory mechanisms are integral to the normative actions of GPCRs, while dysregulation of GPCR signaling has been associated with mental illness (Arnsten 2007; Catapano and Manji 2007; Di Pietro and Seamans 2007).
RGS4 is perhaps the most widely distributed and highly expressed RGS in the brain (Larminie et al. 2004). It attenuates the intensity and duration of signal transduction via Gαi and Gαq proteins coupled to receptors on plasma membranes (Berman et al. 1996; Watson et al. 1996; Hepler et al. 1997). Interest in RGS4 was spearheaded by cDNA microarray and genomic analyses showing that transcription of RGS4 in prefrontal cortex (PFC) is decreased in a diagnosis-specific manner in patients with schizophrenia, and an association of schizophrenia with polymorphisms of the RGS4 gene (Mirnics et al. 2000, 2001; Chowdari et al. 2002). Several independent cohorts have since marshaled evidence to support RGS4 as a candidate schizophrenia susceptibility gene (Levitt et al. 2006; Talkowski et al. 2006), and have replicated marked loss of RGS4 protein from the PFC of patients with schizophrenia (Erdely et al. 2006). Recent studies in patients and healthy controls also indicate that allelic variations in RGS4 gene are associated with reduced PFC volume (Prasad et al. 2005; Buckholtz et al. 2007) and weaker prefrontal network activity during a working memory task (Buckholtz et al. 2007). In addition, RGS4 mRNA in PFC has been inversely correlated with COMT enzyme activity, another candidate schizophrenia gene (Egan et al. 2001), suggesting a direct link between RGS4, dopamine (DA) turnover, and DA signaling in PFC (Lipska et al. 2006), although the same study found no significant association of RGS4 mRNA with schizophrenia.
RGS4 mRNA in human brain is most highly expressed in the neocortex, with lower levels in hippocampus and basal ganglia, and near undetectable levels in thalamus (Erdely et al. 2004; Larminie et al. 2004). In contrast to global mapping of RGS4 mRNA across brain areas, virtually nothing is known with regard to the precise cellular and subcellular localization of the protein in brain neurons. Knowledge of the cellular compartmentalization of RGS4 (e.g., in proximal dendrites vs. distal dendrites vs. axons or along synaptic vs. nonsynaptic membranes) is needed to inform its functional roles within the PFC circuitry. Such information may clarify how altered RGS4 expression is related to specific paradigms of GPCR dysregulation, as is manifest in mental illness.
This study provides the first subcellular reference map of RGS4 protein in the brain. The data demonstrate that despite wide expression in PFC, RGS4 exhibits strict compartment specificity within individual neurons, and selectively “targets” precise plasma membranes to regulate synaptic and nonsynaptic signaling in prefrontal circuits.
Materials and Methods
Tissue Processing
The 3 adult rhesus macaques in this study were maintained and euthanized in accordance with the guidelines of Yale University Institutional Animal Care and Use Committee. As described in Paspalas and Goldman-Rakic (2004a, 2005), the primates were deeply anesthetized prior to transcardial perfusion of artificial cerebrospinal fluid, followed by 4% paraformaldehyde/0.05–0.1% glutaraldehyde in 100 mM phosphate-buffered saline. Brains were sectioned coronally at 60 μm, cryoprotected, and stored at –80 °C. Sections of the dorsolateral PFC (Walker's area 46) were processed for RGS4 immunocytochemistry. To facilitate penetration of immunoreagents, all sections went through 3 freeze-thaw cycles in liquid nitrogen. Nonspecific reactivity was suppressed with 10% normal donkey serum (NDS) and 2% bovine serum albumin (BSA) in 50 mM Tris-buffered saline (TBS).
Antibodies and Immunoreagents
The immunoaffinity purified RGS4 antibody (IgY) was raised in chicken against amino acids 127–205 of the RGS4 protein C-terminus (Abcam, Cambridge, MA), and recognizes human and rodent RGS4 based on sequence homology. It detects a band migrating at ≈28 kDa in rat total striatal lysates (Schwendt and McGinty 2007), which is identical to the band detected with an antibody raised against the RGS4 full sequence and thoroughly characterized by Krumins et al. (2004). An identical size protein band is detected with RGS4 N-terminus–specific antibodies in human frontal cortex homogenates (Erdely et al. 2006). Human-adsorbed donkey anti-chicken IgG fragments (i.e., bridging antibodies), all normal sera, and IgG-free BSA were purchased from Jackson Immunoresearch (West Grove, PA). The gold-conjugated tertiary antibodies were from Nanoprobes (Yaphank, NY).
RGS4 Peroxidase and Gold Immunocytochemistry
For light microscopy, sections were incubated for 36 h in 1:1000 anti-RGS4 in TBS, plus 0.05% Triton X-100, 2% NDS, and 1% BSA, and for another 3 h in 1:500 biotinylated F(ab′)2. For probing the biotinylated fragments, we used 1:100 avidin–biotin–peroxidase complexes (Vector Laboratories, Burlingame, CA) for 1 h or, alternatively, 1:100 anti-biotin F(ab′)2 conjugated to 1.4 nm gold cluster (nanogold) for 2 h. RGS4 was visualized with diaminobenzidine as a chromogen or with silver enhancement of nanogold after glutaraldehyde fixation (HQ Silver, Nanoprobes; see Paspalas and Goldman-Rakic 2004a, 2005).
For immunoelectron microscopy, sections were placed in 1:2000 anti-RGS4 for 48 h, followed by 1:800 biotinylated F(ab′)2, both diluted in TBS with 2% NDS, 0.1% acetylated BSA-C (Aurion, Wageningen, The Netherlands), and 0.01% Tween 20 (gold buffer). Next we applied 1:200 nanogold-anti-biotin F(ab′)2 in gold buffer and used silver autometallography to enhance the nanogold (Paspalas and Goldman-Rakic 2005). As there are multiple gold probes per primary antibody, the 3-layer immunoprocedure is expected to produce multiparticle aggregates, called particle clusters thereafter.
Omission of the anti-RGS4 or substitution with normal chicken serum abolished all reactivity. When bridging biotinylated antibodies were excluded, both nanogold and peroxidase signals were eliminated. Similarly, peroxidase labeling was abolished when blocking the biotinylated probes with avidin/biotin (Vector Laboratories). To control for self-nucleation of the metallographic developer, nanogold was omitted whereas the sections were routinely processed for silver autometallography for 15 min at room temperature. In labeling RGS4, we never exceeded 8 min at 4 °C. All controls were evaluated under the electron microscope. At the light microscopic level, a faint diffuse precipitate remained in control sections treated with diaminobenzidine but was not detectable ultrastructurally (data not shown). Matching sections were processed in parallel for gold immunocytochemistry against antigens with known distribution patterns to test the validity of nuclear labeling for RGS4. These data are summarized in Supplementary Figure 1.
Electron Microscopy and Data Analysis
Sections were processed for electron microscopy as previously described (Paspalas and Goldman-Rakic 2004a, 2005). Layers I–III and IV–VI of PFC were sampled for resectioning and analysis under a JEM1010 (Jeol, Tokyo, Japan) transmission electron microscope at 80 kV. Twenty plastic blocks of each brain were examined using only the 4th to 12th surface-most sections of each block (i.e., 200–600 nm), as signal sharply declined with depth from the tissue surface, possibly due to the larger size of IgY. RGS4 structures were digitally captured at ×25 000 to ×160 000 magnification (Gatan, Pleasanton, CA), and individual panels were adjusted for brightness and contrast using Adobe Photoshop 9.0 image editing software (Adobe Systems Inc., San Jose, CA). Quantitative assessments were performed on series of low magnification micrographs of supragranular PFC, each covering a field of 30 μm2 captured from the 4th and the 12th thin section to avoid recounting a single structure. For profile identification, we adopted the criteria summarized in Peters et al. (1991).
Results
Cellular Expression of RGS4 in PFC
Immunoreactivity to RGS4 was mainly detected in pyramidal neurons in layers II/III (Fig. 1A,B) and V. The nucleus was intensely labeled (see controls in Supplementary Fig. 1) and contrasted with the weakly reactive cytoplasm, especially when visualized with immunogold (e.g., Fig. 1D; compare with the peroxidase reaction filling the entire soma in Fig. 1B). Patches of immunoreactivity on perisomatic membranes (Fig. 1D,E) likely correspond to labeling of the subsurface cisterns (SSC) identified with electron microscopy (see RGS4 in the Soma and Principal Dendrites).
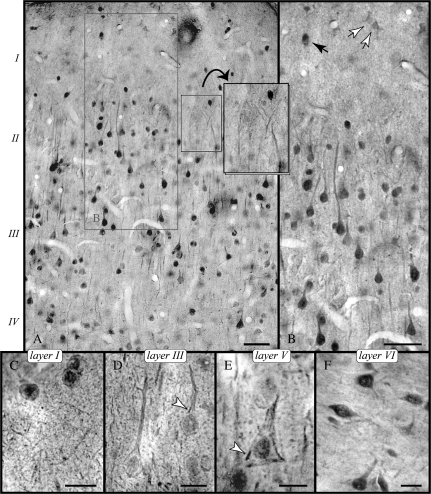
Figure 1.
Cellular expression of RGS4 in PFC. (A) Illustrates immunoperoxidase labeling in layers I through IV; note the highly reactive pyramids. (B) Apical pyramidal dendrites are labeled proximally but lack immunoreactivity as they radiate into layer I containing reactive nonpyramidal neurons (black arrow) and faintly labeled astrocytes (white arrows). Apical tufts of deep layer pyramids can still be observed at the layer I/II border (A, inset). (C–E) RGS4 as demonstrated with immunogold. (C) In layer I neuropil, RGS4 is found in slender processes and puncta. (D and E) show pyramidal neurons with distinctly labeled nuclei in layers III and V; patches of immunoreactivity next to the plasmalemma (arrowheads) likely correspond to labeled SSCs (see Fig. 2B). (F) Immunoreactive nonpyramidal neurons predominate in layer VI. Scale bars: (A, B) 30 μm; (C–F) 10 μm.
Labeled pyramidal neurons featured a prominent apical dendrite and short basal dendrites, though in most cells, basal branches were not reactive (Fig. 1B; see also Fig. 3A,B). RGS4 immunoreactivity of the apical dendrite decreased abruptly after 10–40 μm or about 1–3 soma lengths in the majority of neurons, and hence, we did not visualize the elaborate meshwork of distal apical dendrites invading layer I. The neuropil contained the highly reactive first-order dendrites and high-order branches as well as distinct punctate labeling throughout, including in layer I (Fig. 1C). However, the end-tufts of apical pyramidal dendrites below the pia were not labeled.
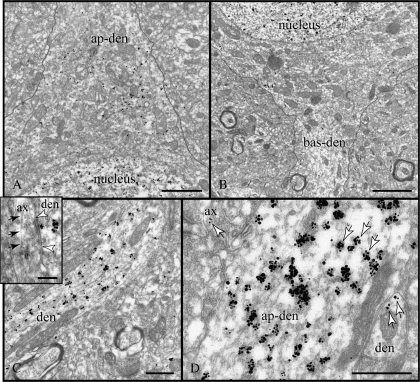
Figure 3.
Expression of RGS4 in principal dendrites. (A, B) The apical and one basal pyramidal dendrite (ap-den, bas-den) are shown emerging from the soma; the plasma membrane has been outlined. Note an increase in RGS4 expression in the tapering ap-den. (C) Particles label the cytoplasm of the apical dendrite and may appear opposite symmetric synapses of varicosities en passant (arrowheads in inset); arrows point to a microtubule running longitudinally inside the stem of the axon. (D) The size of particle clusters (arrows) is indicative of RGS4 levels and is markedly larger in ap-den than in small dendrites and axons in the adjoining neuropil. ax, axon; den, dendrite. Scale bars: (A, B) 1 μm; (C, D) 500 nm; (C, inset) 200 nm.
Immunoreactive nonpyramidal neurons were clearly identified in layer I (Fig. 1C) as well as in layer VI (Fig. 1F). Their dendritic ramifications were not labeled or were only visualized within a short distance. A faint reaction was observed in somata of astrocytes in layer I (Fig. 1B).
RGS4 in the Soma and Principal Dendrites
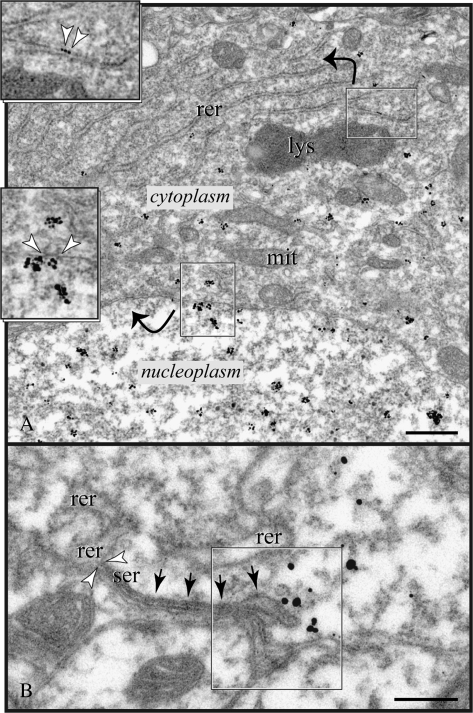
High RGS4 immunoreactivity marked the nucleoplasm; the nuclear envelope was labeled with particle clusters subjacent to nucleopores (Fig. 2A; Supplementary Fig. 2A,B). In comparison, the perikaryon displayed moderate immunoreactivity on smooth endomembranes and vesicles bordering Golgi complexes (data not shown). Rough reticular cisterns were often labeled, but overall RGS4 did not show enrichment in the rough endoplasmic reticulum (Fig. 2A).
Figure 2.
Expression of RGS4 in the soma. (A) Particles label the nucleoplasm and appear subjacent to the nuclear envelope facing nucleopores; arrowheads in bottom inset point to a pore complex with the characteristic cytoplasmic fibril ring (see also Supplementary Fig. 2). Low immunoreactivity in the cytoplasm marks smooth endomembranes and cisterns of the rough endoplasmic reticulum (rer; arrowheads in top inset). (B) RGS4 was not associated with the plasma membrane directly but was found subjacent to portions of the plasmalemma (frame) endowed with SSCs (arrows); the transition of rer to smooth endoplasmic reticulum (ser) is shown between arrowheads. lys, lysosome; mit, mitochondrion. Scale bars: (A) 500 nm; (B) 200 nm.
In general, the somatic plasma membrane was not labeled nor were axosomatic synapses “targeted” in any way by RGS4 particles. Rather, immunoparticles appeared subjacent to SSC-lined membranes and “particularly” next to the vacuolar rim of a cistern (Fig. 2B). It is important to note that SSCs are specialized smooth reticulum endomembranes that hold inositol trisphosphate receptor (IP3R)–gated Ca2+ stores, and may function as junctional and signaling microdomains (see Delmas and Brown 2002; Paspalas and Goldman-Rakic 2004a; and Discussion below).
RGS4 levels increased in the base of the tapering apical dendrite (Fig. 3A; compare to the weak expression in basal dendrite in B), peaked in the stem, and remained high along its proximal path. Label filled the entire cytoplasm (Fig. 3C,D). Although we did find examples of RGS4 localization directly across from symmetric synapses of varicosities en passant (e.g., Fig. 3C, inset), these may have been a reflection of the overall high expression levels of the protein in proximal dendrites rather than a specific association with synaptic membranes.
RGS4 in Dendritic Ramifications
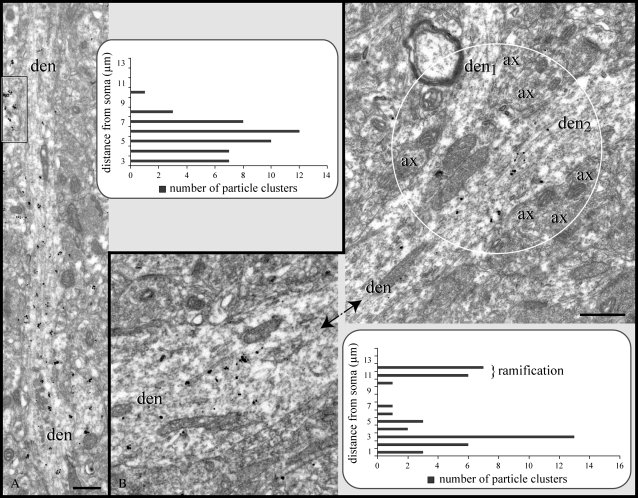
Tagged RGS4 under the electron microscope could be traced for several micrometers in the apical dendrite and occasionally into second-order branches, though immunoreactivity diminished with distance from the soma (Fig. 4A, graph). In section planes capturing apical dendrites along a considerable length, immunoreactivity ended abruptly such that virtually no particles could be found distal to that point. The point where RGS4 could no longer be detected was measured at 7–18 μm from the soma. However, analysis was hindered by the fact that immunoreactive dendrites were often truncated as they left the section plane, suggesting that labeling in certain apical shafts may have extended farther (compare to light microscopic findings in Fig. 1B).
Figure 4.
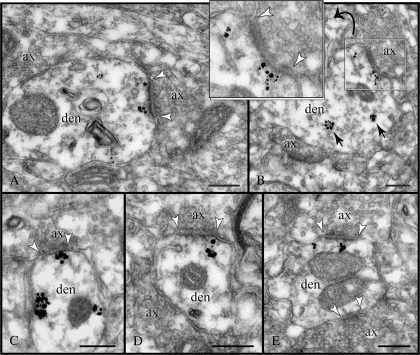
Expression of RGS4 along dendritic ramifications. (A) In most pyramids, RGS4 labeling in first- and second-order apical dendrites drops off precipitously with distance from the soma (see Fig. 1A and corresponding graph), which is not due to penetration artifacts as adjacent profiles are intensely labeled (frame). (B) Even along unlabeled portions of dendrites, RGS4 was detected at points where apical dendrites bifurcate into second-order branches (den1, den2 in circle). Note the numerous axons populating the branch point. Micrographs in (B) capture labeled segments of a single dendrite with an immunonegative segment in between (not illustrated; see graph). ax, axon; den, dendrite. Scale bars: 500 nm.
In apical dendrites where labeling ended before splitting into second-order branches, particle clusters were nonetheless present in the branching point (Fig. 4B). The occurrence of RGS4 in bifurcating dendrites may be of particular significance for signal initiation/transduction (see Discussion), especially as numerous axons populate the branch points (Fig. 4B).
RGS4 in Distal Dendrites and Spines
Distal dendrites (≤0.6 μm in diameter; minor axis) were proportionately the most prominent RGS4 immunoreactive structures in the neuropil (Table 1; Supplementary Fig. 3A–G). They typically contained a few small particle clusters in the cytoplasm, apart from those sections corresponding to synaptic profiles. Indeed, RGS4 in distal dendrites was directly associated with synapses (Fig. 5A–E), which was further confirmed in longitudinally sectioned stems to exclude bias (Supplementary Fig. 3A). Synapse-associated particles were separated by 15–35 nm from the postsynaptic membrane, and rarely were embedded in the postsynaptic density (PSD) per se. Note that the label is distributed across from both central and peripheral portions of the synaptic disk, and involves asymmetric and to a lesser extent symmetric axodendritic synapses, exemplified in Figure 5A,C, respectively. Seventy-six percent of RGS4-labeled axodendritic synapses were type I, asymmetric junctions (see Table 2). However, RGS4 expression in type II, symmetric synapses could be underestimated as we excluded synapses captured at oblique planes, and those that could not be unequivocally categorized in a single section.
Table 1.
Quantitative assessment of RGS4 immunoreactivity in PFC neuropil. Prevalence of RGS4 in cellular compartments
| Principal dendrites | Distal dendrites | Spines | Axons | Glia | N/Da | RGS4 profiles | |
| Non-Glut–like 273/16% | Glut-like 133/7% | ||||||
| 198/11% | 611/35% | 346/20% | 406/23% | 47/3% | 142/8% | 1750/100% | |
A sizable portion of nondetermined (N/D) cellular profiles likely represents dendritic spines.
Figure 5.
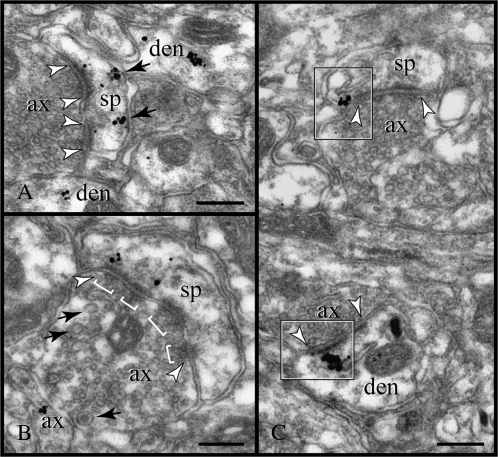
Postsynaptic expression of RGS4 in dendrites. (A–E) The enrichment of RGS4 across from synaptic membranes (between arrowheads) is best appreciated in cross-sectioned dendrites. Particles are sequestered over endomembranes, likely endosomes (arrows in B), but for the most part they cluster next to the plasmalemma with a predilection for synapses. In (C), immunoparticles adorn nonsynaptic membranes as well as the PSD of a symmetric synapse; notice the inconspicuous PSD, the flattened synaptic vesicles, and the narrow synaptic cleft. Compare with the asymmetric synapses shown in panels (D and E). RGS4 is typically found subjacent, at a distance of 15–35 nm, to both medial and lateral portions of the synaptic disk; compare to the axospinous synapses in Figure 6. ax, axon; den, dendrite. Scale bars: 200 nm.
Table 2.
Quantitative assessment of RGS4 immunoreactivity in PFC neuropil. Prevalence of RGS4 in the synaptic compartment
| Synapses type I axodendritic | Synapses type II axodendritic | Synapses type I axospinous | Synapses type II axospinous | RGS4 synapsesa |
| 421/60% | 13219% | 104/15% | 43/6% | 700/100% |
Particle distribution ≤35 nm from the PSD (including perisynaptic).
In favorable section planes, immunoreactive spines arose from labeled, presumably pyramidal dendrites (Supplementary Fig. 3C,D). RGS4 in postsynaptic spines (Table 2) exhibited 2 distinct distribution patterns that were uniquely correlated with inputs from glutamatergic-like (Glut-like) versus non-glutamatergic–like (non-Glut–like) axons (see RGS4 in Axons for definition). Please note that this analysis is not to imply that a given category of spines would receive exclusively symmetric or asymmetric synapses. Rather, it focuses on RGS4 expression on individual axospinous synapses, and attempts to correlate the expression with the axon ultrastructure and synaptology.
In this context, it is imperative also to note that axon varicosities other than glutamatergic may in fact establish asymmetric synapses in PFC (e.g., Aoki et al. 1998), whereas certain symmetric synapses may utilize glutamate as a cotransmitter (Boulland et al. 2008). Therefore, data should be interpreted with the clear understanding that a portion of axons featuring a Glut-like ultrastructure could potentially utilize another neurotransmitter/neuromodulator, whereas axons with non-Glut features might also engage glutamatergic signaling mechanisms.
At asymmetric synapses from Glut-like axons, the particles typically labeled extrasynaptic spine membranes (Fig. 6A), and rarely appeared subjacent to the PSD. However, RGS4 was found at perisynaptic membranes flanking the asymmetric synapse (see below; see also Supplementary Fig. 3B). In those spines receiving symmetric synapses from non-Glut–like axons, RGS4 was found subjacent to the PSD, as in postsynaptic dendrites (Fig. 6B). It should be marked that perisynaptic expression of RGS4 was uniquely associated with spines receiving asymmetric synapses, and not found in spines receiving symmetric synapses or in dendrites receiving either type of synapses (compare the axospinous and axodendritic asymmetric synapses in Fig. 6C).
Figure 6.
Postsynaptic expression of RGS4 in dendritic spines. (A) Immunoparticles face the PSD of an asymmetric synapse (between arrowheads) and appear next to the spine plasmalemma extrasynaptically (arrows). In (B), RGS4 is affiliated with a symmetric synapse (between arrowheads). The synaptic varicosity exhibits the typical ultrastructure of monoaminergic axons, namely multiple, narrow active zones in the synapse (brackets; note the docked vesicles) and pleomorphic vesicles, including large clear and dense-cored vesicles (arrows). (C) Perisynaptic expression of RGS4 was only demonstrated at spine membranes flanking asymmetric synapses (top frame); compare to the axodendritic synapse (bottom frame). ax, axon; den, dendrite; and sp, spine. Scale bars: 200 nm.
RGS4 in Axons
Based on their ultrastructure and synaptology (see Peters et al. 1991 and specific criteria below), axon varicosities in the PFC neuropil were categorized as Glut-like and non-Glut–like (compare ax1 to ax2 in Fig. 7C and in Supplementary Fig. 3C, but see our statement in previous section). Prevalence of RGS4 expression (Table 1) as well as its subcellular distribution clearly differed in Glut-like and non-Glut–like axons.
Figure 7.
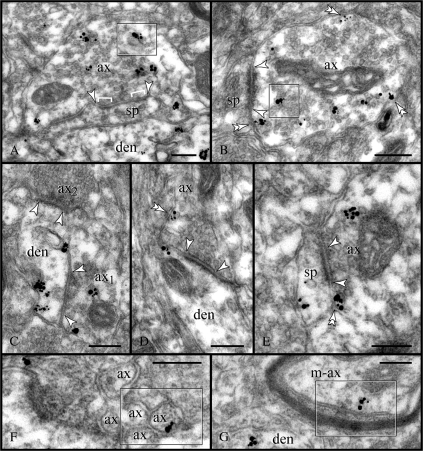
Presynaptic expression of RGS4. (A, B) Labeling was predominantly found in the axoplasm of non-Glut–like varicosities; note the association with endoplasmic vesicles (frames). Relatively few particles labeled the axolemma (double arrowheads), which may indicate a high turnover rate for RGS4 or a corresponding GPCR. Synapses (between arrowheads) were mainly onto immunonegative spines. (C) Convergent synapses (arrowheads) of non-Glut–like and Glut-like axons (ax1 and ax2, respectively) onto a labeled dendrite. Note the atypical particle distribution in the dendrite as immunoreactivity is not associated with PSDs (see Fig. 5). (D, E) Glut-like axons establishing axodendritic and axospinous synapses (arrowheads) displayed low expression of RGS4 and minimal axoplasmic labeling. Rather, RGS4 was associated with extrasynaptic and perisynaptic membranes (double arrowheads). Nonmyelinated (F) and myelinated (G) axon intervaricose segments were also labeled (frames). ax; axon; den, dendrite; m-ax, myelinated axon; and sp, spine. Scale bars: 200 nm.
Non-Glut–like axons featured loose clusters of pleomorphic vesicles, including 80–120 nm clear and dense-cored vesicles. In general, they established symmetric synapses with a 10- to 15-nm-wide cleft and often narrow and multiple active zones. Such axons contained numerous immunoparticles that typically labeled the axoplasm in association with electron-opaque vesicular structures, possibly of the endosomal line (Fig. 7A,B). RGS4 particles were also found at extrasynaptic places along the axolemma but the bulk of immunoreactivity clearly appeared intracellularly. Yet in single sections, most RGS4 non-Glut–like varicosities were asynaptic (see Supplementary Fig. 3C). Serial sectioning revealed spines as their prime postsynaptic target (Fig. 7A,B), which is noteworthy given that dendritic stems would receive the majority of symmetric synapses (presumed γ-aminobutyric acidergic [GABAergic]) in the neocortex (Colonnier 1981). One axodendritic synapse of a labeled non-Glut–like axon is shown in Figure 7C as a part of a synaptic triad involving an immunonegative Glut-like axon.
Glut-like axons featured 30-nm round vesicles docked onto the presynaptic grid and established asymmetric synapses with wide, 20-nm clefts and often a central perforation. They contained many fewer RGS4 particles when compared with the non-Glut–like axons. Immunoparticles, however, were associated with the axolemma at perisynaptic locations and extrasynaptically (Fig. 7D,E); the axoplasm was infrequently labeled. Dendritic spines were prime postsynaptic targets for the RGS4-positive Glut-like axons, with shafts receiving fewer synapses.
Axon intervaricose segments exhibited a sporadic RGS4 immunoreactivity. Thus, it is not clear whether RGS4 in the nonmyelinated axonal profiles (Fig. 7F) may in part represent a functional regulatory component (e.g., for preterminal GPCRs; see Aoki et al. 1998; Muly et al. 2003) or merely indicates protein captured en passant, as could likely be the case in the myelinated profile shown in Figure 7G.
RGS4 at Nonsynaptic Membranes
Immunoparticles aligned with plasma membranes even when synapses were not present, as if the apposed profile directed the accumulation of RGS4. When we screened such appositions in serial sections, synapses were not found nor did RGS4-particles line up with membranes other than those apposing an axon (Fig. 8A,B), suggesting that RGS4 may assemble on the plane of the plasmalemma to potentially regulate focal stimulation of extrasynaptic receptors. In addition, RGS4 was detected in somata and major processes of astrocytes (see also Fig. 1B) but not found in slender astrocytic processes in the neuropil, including those that typically enwrap synapses. Hence, it is unlikely that RGS4 could function as regulator of a GPCR in these processes, such as the glial α2A adrenoceptor (α2A-AR; Aoki et al. 1998; Wang et al. 2007). RGS4 on astrocytic membranes—apparently nonsynaptic—was found across from axons as well as postsynaptic profiles (Fig. 8C).
Figure 8.
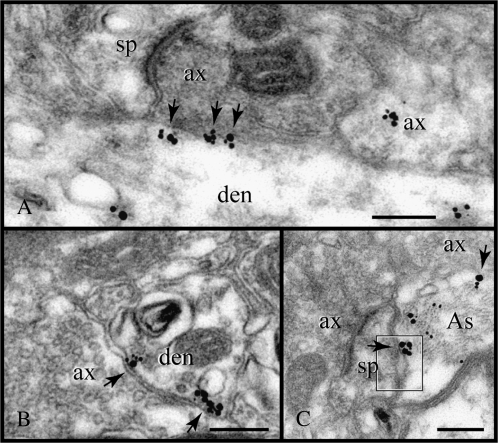
Association of RGS4 with nonsynaptic membranes. (A, B) At axodendritic appositions, the particles (arrows) assemble in a synaptic- or perisynaptic-like pattern although a synapse is not present at this plane or in consecutive sections (data not shown). (C) The association with nonsynaptic membranes is best exemplified in a rare RGS4-reactive astrocyte filled with intermediate filament bundles. Immunoparticles label the glial membrane opposite an axon and a spine (arrows). Note the widening of the intercellular space at the spine apposition (frame). If genuine, it would secure a route for facilitating neurotransmitter diffusion. As, astrocyte; ax, axon; den, dendrite; and sp, spine. Scale bars: 200 nm.
Expression Patterns of RGS4 in PFC
In summary, we have shown that RGS4 exhibited very distinct distribution patterns on the basis of distance from the soma (i.e., proximal vs. distal dendrites), ultrastructure and synaptology (e.g., Glut-like vs. non-Glut–like axons), and presence of specialized plasmalemma (e.g., synaptic and SSC-lined membranes). The data are schematically summarized in Figure 9. As we discuss in the following sections, such patterns may subserve and likely reflect specificity of neuroregulation, for implicit in the proposed actions of RGS4 is its localization at or near plasma membranes where G proteins and GPCRs assemble.
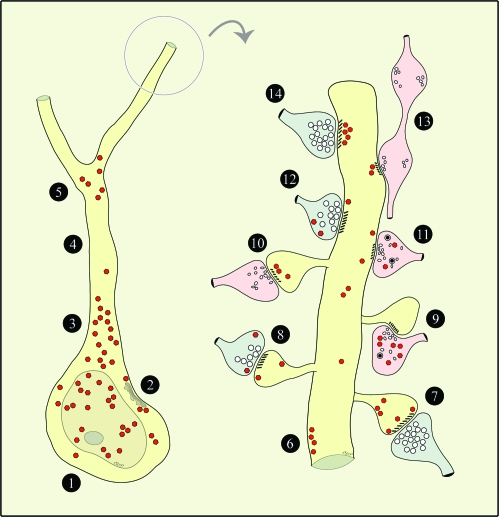
Figure 9.
Schematic depiction of RGS4 expression patterns in the perisomatic region and in a spinous dendrite. For brevity, all axodendritic synapses are shown targeting a pyramidal dendrite, although they could potentially engage nonpyramidal dendrites as well. Axons with Glut-like features are shown in blue; non-Glut–like axons are in pink. Red polygons depict RGS4 labeling. 1) Soma—low and high RGS4 in cytoplasm and nucleoplasm, respectively. 2) Soma—RGS4 subjacent to SSC-lined membranes. 3) Proximal apical dendrite—very high RGS4 in cytoplasm. 4) Distal apical dendrite—paucity of immunoreactivity*. 5) First- to second-order dendrites—RGS4 in bifurcation point. 6) Nonsynaptic membranes/axon appositions—RGS4 subjacent to plasmalemma. 7) Asymmetric axospinous synapse—low RGS4 in spine subjacent to PSD, high RGS4 perisynaptically and extrasynaptically. 8) Asymmetric axospinous synapse—low RGS4 in spine extrasynaptically; low RGS4 in axon perisynaptically and extrasynaptically. 9) Symmetric axospinous synapse—diffuse high RGS4 in axon. 10) Symmetric axospinous synapse—RGS4 in spine subjacent to PSD. 11) Symmetric axodendritic synapse—low RGS4 in dendrite extrasynaptically; diffuse low RGS4 in axon. 12) Asymmetric axodendritic synapse—low RGS4 in dendrite extrasynaptically; low RGS4 in axon extrasynaptically. 13) En passant symmetric axodendritic synapse—RGS4 in dendrite subjacent to PSD. 14) Asymmetric axodendritic synapse—very high RGS4 in dendrite subjacent to PSD. *Not in all cells.
Discussion
Spatial Specificity of RGS4 as a Means of Securing Specificity of Neuroregulation
In somata, RGS4 was enriched near SSCs, the smooth reticulum that holds IP3R-gated Ca2+ stores (Delmas and Brown 2002; Berridge 2005). So far, SSC-lined membranes have been identified as DA D5 receptor (D5R)–specific microdomains, linking nonsynaptic receptors to internal Ca2+ stores in PFC pyramids (Paspalas and Goldman-Rakic 2004a). D5Rs couple to Gs to elevate cAMP but have also been implicated in triggering Ca2+ signaling cascades via the Gq–phosphoinositide signal transduction system (Pacheco and Jope 1997; Jin et al. 2001). In fact, D1/D5Rs stimulate Ca2+ release from IP3-sensitive stores in perikarya of dissociated cortical neurons (Lezcano and Bergson 2002). Thus, a selective placement of RGS4 in microdomains would potentially allow for regulation of signal transduction triggered by nonsynaptic D5Rs, namely phospholipase C activation, IP3 formation, and IP3R-mediated Ca2+ release from the SSC.
Beyond the soma, RGS4 expression in the pyramidal apical dendrite was markedly higher than in any other cellular compartment, including basal dendrites. Intense labeling of the apical dendrite cannot represent newly synthesized protein en passant, for RGS4 levels rose to a peak proximally, and declined sharply as distance from the soma increased. Thus, it likely represents another substrate for neuroregulation.
Interestingly, the apical dendritic field proximal to the pyramidal soma expresses the highest levels of serotonin 2A receptor (5HT2AR) in PFC, supporting the notion that it is “the ‘hot’ spot for 5HT2AR-mediated physiological actions relevant to normal and ‘psychotic’ functional states of the cerebral cortex” (Jakab and Goldman-Rakic 1998; reviewed in Aghajanian and Marek 2000). The 5HT2ARs couple to Gq to mobilize the phosphoinositide system, and several lines of evidence now implicate altered 5HT2AR signaling in mental illness, including attention deficit hyperactivity disorder, affective and anxiety disorders, and schizophrenia and Alzheimer's disease (Norton and Owen 2005; Tamminga 2006). For example, newer atypical antipsychotics target the 5HT2AR, suggesting that these actions might be essential for antipsychotic effects in PFC (Di Pietro and Seamans 2007). In addition, proximal apical dendrites express D5Rs that are found in SSC-microdomains, like those in the soma, but not D1Rs or D2Rs (Paspalas and Goldman-Rakic 2004a). Future work will determine whether the 5HT2AR or other GPCRs (e.g., nonsynaptic α2A-ARs form patches subjacent to somatic membranes; Aoki et al. 1998) might utilize microdomains as scaffolds for internal Ca2+ signals or even for spatially segregated cAMP signaling events (Rich et al. 2001) in the perisomatic region.
Proximal apical dendrites have further been recognized as the sites of regenerative Ca2+ release from internal stores. Activation of Gq-coupled glutamate receptors (group I mGluRs) in proximal apical dendrites of hippocampal pyramids elicits IP3R-mediated Ca2+ waves that propagate and invade the soma. Basal dendrites and the apical dendrite beyond a sensitive segment of about 2–3 soma lengths are much less responsive (Nakamura et al. 1999). This pattern has been broadly replicated in the neocortex as an integral element of pyramidal cell physiology (reviewed in Ross et al. 2005). Recently, Yeckel's group presented evidence of mGluR-mediated, IP3-sensitive Ca2+ waves in the proximal apical dendrite of PFC pyramidal cells and proposed that internal Ca2+ release may contribute to shaping the firing pattern in PFC and thereby regulate working memory processes (Hagenston et al. 2008). In light of the physiological evidence, our data of RGS4 peak expression in proximal apical dendrites provide a structural framework for RGS4 modulation of mGluR-mediated phosphoinositide signaling in the pyramidal perisomatic region, which also expresses the highest levels of IP3Rs and Ca2+ pumps (Sharp et al. 1993). Moreover, we have found ramifications of apical dendrites to express RGS4 (this work) and IP3R (Paspalas and Goldman-Rakic, unpublished data), which could indicate a regional specialization for signal transduction, as branching apical dendrites are the preferred initiation sites of those synaptically activated Ca2+ waves in hippocampal and neocortical pyramidal neurons (Nakamura et al. 1992; Larkum et al. 2003).
Synaptic Specificity of RGS4 as a Means of Securing Specificity of Neuroregulation
In distal processes, RGS4 was associated with synaptic specializations as well as with nonsynaptic membranes, suggesting regulation of both synaptic and nonsynaptic GPCR signaling components.
RGS4 was found facing the PSD of asymmetric and, to a lesser extent, symmetric axodendritic synapses. The previously described pattern of D2R expression overlaps with that of RGS4; D2Rs are found in dendrites extrasynaptically and within the PSD of symmetric synapses (Paspalas and Goldman-Rakic 2004b). Therefore, it is possible that RGS4 is within range to regulate both synaptic and extrasynaptic Gi-coupled D2Rs in dendrites. Other GPCRs, for example, Gi-coupled α2A-ARs and Gq/Gi-coupled acetylcholine muscarinic receptors 1 and 2 (M1R and M2R), also are located in PSDs of symmetric axodendritic synapses, although the majority are found at asymmetric axospinous synapses (Mrzljak et al. 1993; Aoki et al. 1998). Nonetheless, there are very few reports in PFC describing GPCRs embedded in PSDs. For example, Muly et al. (2003) provided a comprehensive analysis of group I mGluRs in PFC but did not call attention to a synaptic component of either mGluR1α or mGluR5. Thus, the question remains as to why RGS4 is predominantly expressed facing the PSD of asymmetric axodendritic synapses. One possibility is that all previous analyses have missed one or several known receptor subtypes embedded in PSDs, a masking effect that is discussed in Baude et al. (1995). It could also be that RGS4 is placed to regulate an as yet unknown GPCR or other signaling pathway within the asymmetric synapse.
The perisynaptic distribution of RGS4 on spine membranes flanking asymmetric synapses suggests a particularly close partnership with DA receptors of the D1 subtype, given that this pattern is a distinguishing feature of the D1R (Smiley et al. 1994; Paspalas and Goldman-Rakic 2005). Thus, RGS4 would be strategically placed to regulate DA signaling in the distal dendritic field (via D1Rs) as well as proximally (via perisomatic D5Rs). Distal RGS4 could also engage D2Rs, which have a prominent dendritic and a limited spine component (Paspalas and Goldman-Rakic 2004b). Further support for a distal regulation of DA signaling is provided by the presence of RGS4 in spines receiving symmetric monoaminergic-like synapses (e.g., Fig. 6B), for the majority of identifiable DA synapses in PFC target dendritic spines (Smiley and Goldman-Rakic 1993). Very importantly, the location of RGS4 on extrasynaptic and perisynaptic spine membranes might suggest a key role in regulating noradrenergic signaling mechanisms. Spine membranes express extrasynaptic and perisynaptic α2A-ARs and have recently been identified as a substrate for interaction with hyperpolarization-activated cyclic nucleotide-gated channels to strengthen the functional connectivity of PFC networks (Wang et al. 2007). Introducing an RGS4 regulatory component in those spines would shape further the dynamics of network connectivity in PFC.
In addition, RGS4 was found in Glut-like and particularly in non-Glut–like axon varicosities. Presynaptic active zones were not labeled. Reports of presynaptic GPCRs in monkey PFC include α2A-ARs and M2Rs (Mrzljak et al. 1993; Aoki et al. 1998). These receptors are typically distributed extrasynaptically and often at preterminal segments. D1Rs also have prominent localization in Glut-like axons, appearing perisynaptically and extrasynaptically (Paspalas and Goldman-Rakic 2005), which is very similar to the RGS4 expression in Glut-like axons. Yet RGS4 was mainly found in non-Glut–like axons that rarely formed synapses in single sections. When synapses were identified, those were mostly associated with spines, an unexpected finding as the majority of symmetric synapses in neocortex are GABAergic synapses onto dendritic stems (Colonnier 1981). Among the known transmitter systems, DA afferents are exceptional for selectively targeting and establishing symmetric synapses with pyramidal spines and often for participating in convergent synapses (triads) with a second axon, presumably glutamatergic (Goldman-Rakic et al. 1989). Ongoing studies will establish whether RGS4-reactive varicosities with monoaminergic features are DAergic axons, which would further implicate RGS4 in the regulation of DA release triggered by presynaptic D2 autoreceptors (Missale et al. 1998; Pickel et al. 2002).
RGS4 may also interact with non-GPCR signaling components (Bansal et al. 2007). As such, high-level expression of RGS4 in the nucleus would imply that the protein might have much more complex brain functions than previously thought. Other RGS members also present cytoplasmic/nucleoplasmic localization or are predominantly nuclear in those cell lines examined. It was reported that a molecular determinant for nuclear targeting (or exclusion) is conserved in all RGS proteins, which is independent of their GTPase-activating action (Chatterjee and Fisher 2000). The same study identified sequences that determine nuclear–cytoplasmic export or cytoplasmic retention of RGS proteins, including RGS4. It is very interesting that the cytoplasmic versus nucleoplasmic localization of individual RGS proteins (and RGS4) is subject to signal-induced redistribution (Rimler et al. 2006), as is their recruitment to the plasmalemma from cytoplasmic and nucleoplasmic pools (Roy et al. 2003), which may suggest an as yet obscure adaptive mechanism for G protein–mediated signaling. Yet, it would be premature at this point to draw conclusions with regard to the functional implications of nuclear RGS4 in brain neurons. Burchett (2003) has contributed an excellent review on the nuclear pools of RGS proteins and their potential functions, including their involvement in negatively regulating transcriptional activity, nuclear import processes, and cell cycle control.
In summary, the present results demonstrate prominent RGS4 expression in primate PFC, with subcellular localization patterns consistent with regulation of synaptic as well as volume transmission, internal calcium release, and possibly glia function and nucleocytoplasmic trafficking. Thus, loss of RGS4 in mental illness could compromise many fundamental aspects of PFC physiology.
Supplementary Material
Supplementary figures 1–3 can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institutes of Health (P50MH068789).
Acknowledgments
Conflict of Interest: None declared.
References
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go C-G, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17:i6–i15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther. 2007;116:473–495. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Molnár E, McIlhinney RA, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69:1031–1055. doi: 10.1016/0306-4522(95)00350-r. [DOI] [PubMed] [Google Scholar]
- Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67:1–21. doi: 10.1146/annurev.physiol.67.040103.152647. [DOI] [PubMed] [Google Scholar]
- Boulland J-L, Jenstad M, Boekel AJ, Wouterlood FG, Edwards RH, Storm-Mathisen J, Chaudhry FA. Vesicular glutamate and GABA transporters sort to distinct sets of vesicles in a population of presynaptic terminals. Cereb Cortex. 2009;19:241–248. doi: 10.1093/cercor/bhn077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A, Honea RA, Straub RE, Pezawas L, Egan MF, Vakkalanka R, Kolachana B, Verchinski BA, Sust S, et al. Allelic variation in RGS4 impacts functional and structural connectivity in the human brain. J Neurosci. 2007;27:1584–1593. doi: 10.1523/JNEUROSCI.5112-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchett SA. In through the out door: nuclear localization of the regulators of G protein signaling. J Neurochem. 2003;87:551–559. doi: 10.1046/j.1471-4159.2003.02047.x. [DOI] [PubMed] [Google Scholar]
- Catapano LA, Manji HK. G protein-coupled receptors in major psychiatric disorders. Biochim Biophys Acta. 2007;1768:976–993. doi: 10.1016/j.bbamem.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee TK, Fisher RA. Cytoplasmic, nuclear, and Golgi localization of RGS proteins. Evidence for N-terminal and RGS domain sequences as intracellular targeting motifs. J Biol Chem. 2000;275:24013–24021. doi: 10.1074/jbc.M002082200. [DOI] [PubMed] [Google Scholar]
- Chowdari KV, Mirnics K, Semwal P, Wood J, Lawrence E, Bhatia T, Deshpande SN, Thelma BK, Ferrell RE, Middleton FA, et al. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum Mol Genet. 2002;11:1373–1380. doi: 10.1093/hmg/11.12.1373. [DOI] [PubMed] [Google Scholar]
- Colonnier M. The electron-microscopic analysis of the neuronal organization of the cerebral cortex. In: Schmitt FO, Worden F, Adelman G, Dennis SG, editors. The organization of the cerebral cortex. Cambridge (MA): MIT Press; 1981. pp. 125–152. [Google Scholar]
- Delmas P, Brown DA. Junctional signaling microdomains: bridging the gap between the neuronal cell surface and Ca2+ stores. Neuron. 2002;36:787–790. doi: 10.1016/s0896-6273(02)01097-8. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Seamans JK. Dopamine and serotonin interactions in the prefrontal cortex: insights on antipsychotic drugs and their mechanism of action. Pharmacopsychiatry. 2007;40:S27–S33. doi: 10.1055/s-2007-992133. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Vall08/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdely HA, Lahti RA, Lopez MB, Myers CS, Roberts RC, Tamminga CA, Vogel MW. Regional expression of RGS4 mRNA in human. Eur J Neurosci. 2004;19:3125–3128. doi: 10.1111/j.0953-816X.2004.03364.x. [DOI] [PubMed] [Google Scholar]
- Erdely HA, Tamminga CA, Roberts RC, Vogel MW. Regional alterations in RGS4 protein in schizophrenia. Synapse. 2006;59:472–479. doi: 10.1002/syn.20265. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci USA. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenston AM, Fitzpatrick JS, Yeckel MF. MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cereb Cortex. 2008;18:407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler JR. Emerging roles for RGS proteins in cell signalling. Trends Pharmacol Sci. 1999;20:376–382. doi: 10.1016/s0165-6147(99)01369-3. [DOI] [PubMed] [Google Scholar]
- Hepler JR, Berman DM, Gilman AG, Kozasa T. RGS4 and GAIP are GTPase-activating proteins for Gq alpha and block activation of phospholipase C beta by gamma-thio-GTP-Gq alpha. Proc Natl Acad Sci USA. 1997;94:428–432. doi: 10.1073/pnas.94.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L-Q, Wang H-Y, Friedman E. Stimulated D1 dopamine receptors couple to multiple Gα proteins in different brain regions. J Neurochem. 2001;78:981–990. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- Krumins AM, Barker SA, Huang C, Sunahara RK, Yu K, Wilkie TM, Gold SJ, Mumby SM. Differentially regulated expression of endogenous RGS4 and RGS7. J Biol Chem. 2004;279:2593–2599. doi: 10.1074/jbc.M311600200. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Watanabe S, Nakamura T, Lasser-Ross N, Ross WN. Synaptically activated Ca2+ waves in layer 2/3 and layer 5 rat neocortical pyramidal neurons. J Physiol. 2003;549:471–488. doi: 10.1113/jphysiol.2002.037614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larminie C, Murdock P, Walhin J-P, Duckworth M, Blumer KJ, Scheideler MA, Garnier M. Selective expression of regulators of G-protein signaling (RGS) in the human central nervous system. Mol Brain Res. 2004;122:24–34. doi: 10.1016/j.molbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Lezcano N, Bergson C. D1/D5 dopamine receptors stimulate intracellular calcium release in primary cultures of neocortical and hippocampal neurons. J Neurophysiol. 2002;87:2167–2175. doi: 10.1152/jn.00541.2001. [DOI] [PubMed] [Google Scholar]
- Levitt P, Ebert P, Mirnics K, Nimgaonkar VL, Lewis DA. Making the case for a candidate vulnerability gene in schizophrenia: convergent evidence for regulator of G-protein signaling 4 (RGS4) Biol Psychiatry. 2006;60:534–537. doi: 10.1016/j.biopsych.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Mitkus S, Caruso M, Hyde TM, Chen J, Vakkalanka R, Straub RE, Weinberger DR, Kleinman JE. RGS4 mRNA expression in postmortem human cortex is associated with COMT Val158Met genotype and COMT enzyme activity. Hum Mol Genet. 2006;15:2804–2812. doi: 10.1093/hmg/ddl222. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry. 2001;6:293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Levey AJ, Goldman-Rakic PS. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci USA. 1993;90:5194–5198. doi: 10.1073/pnas.90.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muly EC, Maddox M, Smith Y. Distribution of mGluR1α and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol. 2003;467:521–535. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- Norton N, Owen MJ. HTR2A: association and expression studies in neuropsychiatric genetics. Ann Med. 2005;37:121–129. doi: 10.1080/07853890510037347. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Barbara J-G, Nakamura K, Ross WN. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron. 1999;24:727–737. doi: 10.1016/s0896-6273(00)81125-3. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Lasser-Ross N, Nakamura K, Ross WN. Spatial segregation and interaction of calcium signaling mechanisms in rat hippocampal CA1 pyramidal neurons. J Physiol. 1992;543:465–480. doi: 10.1113/jphysiol.2002.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco MA, Jope RS. Comparison of [3H]phosphatidylinositol and [3H]phosphatidylinositol 4,5-biphosphate hydrolysis in postmortem human brain membranes and characterization of stimulation by dopamine D1 receptors. J Neurochem. 1997;69:639–644. doi: 10.1046/j.1471-4159.1997.69020639.x. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. J Neurosci. 2004a;24:5292–5300. doi: 10.1523/JNEUROSCI.0195-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Receptor compartmentalization for defining input-specificity of dopamine volumetric signaling: a study of D1, D2 and D5 receptor subtypes in primate prefrontal cortex. Soc Neurosci Abstr. 2004b;30:277–279. [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Presynaptic D1 dopamine receptors in primate prefrontal cortex: target specific expression in the glutamatergic synapse. J Neurosci. 2005;25:1260–1267. doi: 10.1523/JNEUROSCI.3436-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HdeF. The fine structure of the nervous system: neurons and their supporting cells. New York: Oxford University Press; 1991. p. 494. [Google Scholar]
- Pickel VM, Garzón M, Mengual E. Electron microscopic immunolabeling of transporters and receptors identifies transmitter-specific functional sites envisioned in Cajal's neuron. Prog Brain Res. 2002;136:145–155. doi: 10.1016/s0079-6123(02)36014-x. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Chowdari KV, Nimgaonkar VL, Talkowski ME, Lewis DA, Keshavan MS. Genetic polymorphisms of the RGS4 and dorsolateral prefrontal cortex morphometry among first episode schizophrenia patients. Mol Psychiatry. 2005;10:213–219. doi: 10.1038/sj.mp.4001562. [DOI] [PubMed] [Google Scholar]
- Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci USA. 2001;98:13049–13054. doi: 10.1073/pnas.221381398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimler A, Jockers R, Lupowitz Z, Sampson SR, Zisapel N. Differential effects of melatonin and its downstream effector PKCalpha on subcellular localization of RGS proteins. J Pineal Res. 2006;40:144–152. doi: 10.1111/j.1600-079X.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Ross WN, Nakamura T, Watanabe S, Larkum M, Lasser-Ross N. Synaptically activated Ca2+ release from internal stores in CNS neurons. Cell Mol Neurobiol. 2005;25:283–295. doi: 10.1007/s10571-005-3060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AA, Lemberg KE, Chidiac P. Recruitment of RGS2 and RGS4 to the plasma membrane by G proteins and receptors reflects functional interactions. Mol Pharmacol. 2003;64:587–593. doi: 10.1124/mol.64.3.587. [DOI] [PubMed] [Google Scholar]
- Schwendt M, McGinty JF. Regulator of G-protein signaling 4 interacts with metabotropic glutamate receptor subtype 5 in rat striatum: relevance to amphetamine behavioral sensitization. J Pharmacol Exp Ther. 2007;323:650–657. doi: 10.1124/jpet.107.128561. [DOI] [PubMed] [Google Scholar]
- Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunocytochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Goldman-Rakic PS. Heterogeneous targets of dopamine synapses in monkey prefrontal cortex demonstrated by serial section electron microscopy: a laminar analysis using the silver-enhanced diaminobenzidine sulfide (SEDS) immunolabeling technique. Cereb Cortex. 1993;3:223–238. doi: 10.1093/cercor/3.3.223. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkowski ME, Seltman H, Bassett AS, Brzustowicz LM, Chen X, Chowdari KV, Collier DA, Cordeiro Q, Corvin AP, Deshpande SN, et al. Evaluation of a susceptibility gene for schizophrenia: genotype based meta-analysis of RGS4 polymorphisms from thirteen independent samples. Biol Psychiatry. 2006;60:152–162. doi: 10.1016/j.biopsych.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA. The neurobiology of cognition in schizophrenia. J Clin Psychiatry. 2006;67:e11. [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, et al. α2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Watson N, Linder ME, Druey KM, Kehrl JH, Blumer KJ. RGS family members: GTPase-activating proteins for heterotrimeric G-protein alpha-subunits. Nature. 1996;383:172–175. doi: 10.1038/383172a0. [DOI] [PubMed] [Google Scholar]
- Willars GB. Mammalian RGS proteins: multifunctional regulators of cellular signalling. Semin Cell Dev Biol. 2006;17:363–376. doi: 10.1016/j.semcdb.2006.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.