Abstract
We used event-related functional magnetic resonance imaging to investigate the neuroanatomical substrates of phonetic encoding and the generation of articulatory codes from phonological representations. Our focus was on the role of the left inferior frontal gyrus (LIFG) and in particular whether the LIFG plays a role in sublexical phonological processing such as syllabification or whether it is directly involved in phonetic encoding and the generation of articulatory codes. To answer this question, we contrasted the brain activation patterns elicited by pseudowords with high– or low–sublexical frequency components, which we expected would reveal areas related to the generation of articulatory codes but not areas related to phonological encoding. We found significant activation of a premotor network consisting of the dorsal precentral gyrus, the inferior frontal gyrus bilaterally, and the supplementary motor area for low– versus high–sublexical frequency pseudowords. Based on our hypothesis, we concluded that these areas and in particular the LIFG are involved in phonetic and not phonological encoding. We further discuss our findings with respect to the mechanisms of phonetic encoding and provide evidence in support of a functional segregation of the posterior part of Broca's area, the pars opercularis.
Keywords: articulation, fMRI, left inferior frontal gyrus, pars opercularis, phonological processing
Introduction
Even though Broca's area has been associated with speech and articulation since the 19th century, the exact role that it plays in the process is still a matter of debate. Characteristically, in recent models on the neuroanatomy of language, Broca's area has been associated with quite different processes. In one viewpoint, Indefrey and Levelt (2004) hypothesized that Broca's area was engaged at the level of phonological processing and was particularly associated with the process of syllabification. In contrast, in a model proposed by Hickok and Poeppel (2004), Broca's area was assigned to phonetic encoding and implementing the mechanism of retrieving or generating the articulatory codes. In the present study, we try to address this issue and examine whether the left inferior frontal gyrus (LIFG) is involved in the phonological or the phonetic level of language processing. We used event-related functional magnetic resonance imaging (fMRI) and manipulated the phonological properties of pseudowords in a way that separates the processes of phonological and phonetic encoding. This manipulation allowed us to identify the key areas involved in the 2 levels of encoding and to disambiguate the function of Broca's area with respect to these 2 levels.
The processes that lead to the generation of an articulatory-motor plan are a matter of debate amongst researchers (Goldrick and Rapp 2007). However, it is commonly accepted that syllabic, metrical, and featural information is specified in a phonological representation prior to the generation of the motor plan (Levelt 1999). In extended reviews of studies on word production by Indefrey and Levelt (2000, 2004), it was suggested that in the final stages prior to phonetic encoding and the generation of the articulatory representation, the phonological code of a given word is spelled out into its different phonemic segments, incrementally clustered into syllables, and assigned a metrical structure. As syllables are created, they are then rapidly turned into sequences of motor gestures, also known as gestural scores (Browman and Goldstein 1988).
In this account of word production, it is assumed that there is a different mechanism for dealing with high- and low-frequency syllables. Based on the notion that speakers tend to reuse only a small number of syllables and on evidence that pseudowords with high-frequency syllables are faster to produce than their low-frequency counterparts (Cholin et al. 2006), it was proposed that the articulatory scores for frequent syllables are precompiled and stored in a repository called the “mental syllabary” (Levelt and Wheeldon 1994). In contrast, the articulatory representations for less-frequent syllables are compiled online (Levelt et al. 1999).
Neuroanatomically, the processes of generating lexical phonological representations have been associated with 2 regions: the middle and posterior superior temporal gyrus (STG), also known as Wernicke's area (Fiez et al. 1999; Indefrey and Levelt 2000; Hickok and Poeppel 2004), and Broca's area, specifically the pars opercularis, roughly corresponding to Brodmann area (BA) 44 (Poldrack et al. 1999; Burton et al. 2000; Indefrey and Levelt 2000). The latter region in particular has been shown to facilitate sublexical processes that require explicit segmentation, such as tasks where subjects perform phonological decisions like phoneme monitoring, phoneme discrimination, or phoneme sequencing (Zatorre et al. 1992, 1996; Demonet et al. 1996; Poldrack et al. 1999; Burton et al. 2000). In the proposed model by Indefrey and Levelt (2004), the LIFG is part of a network related to syllabification, whereas the premotor cortex (BA6) is responsible for compiling and storing the motor codes for the individual syllables, that is, it is the location of the mental syllabary (Levelt and Wheeldon 2004).
In recent review papers, Hickok and Poeppel (2004, 2007) proposed a different model for understanding linguistic processing and the role of the LIFG. Inspired by the theory of the “mirror neuron system” and the idea of sensory–motor integration (di Pellegrino et al. 1992; Rizzolatti and Arbib 1998; Rizzolatti and Craighero 2004), they hypothesized that there is a common interface between speech perception and production. This interface also facilitates phonemic-to-articulatory code translation and supports a “motor theory of speech perception” (Liberman and Mattingly 1985). Broca's area is part of the sensory–motor integration interface, and in this sense, it is directly involved in the generation or retrieval of the articulatory codes. Following a computational model of speech production, the proposed role of the posterior Broca's area (along with the ventral premotor cortex) is to hold a “speech sound map,” that is, representations of phonemes or frequent syllables and their associated motor programs (Guenther et al. 2006).
The concept of the speech sound map is similar to that of the mental syllabary presented by Indefrey and Levelt (2004). Where the 2 theories differ is the role of the posterior part of Broca's area. According to Hickok and Poeppel (2000, 2004, 2007), Broca's area is involved in phonetic encoding and the generation of the articulatory scores because it serves as a store for articulatory representations. On the other hand, according to Indefrey and Levelt, the role of Broca's area is to support syllabification and postlexical phonological processing, that is, processes that are a step before the retrieval or compilation of the articulatory codes.
In this study, we investigated the role of Broca's area in generating an articulatory-motor plan. We specifically wanted to address whether the posterior part of Broca's area (pars opercularis) is involved in phonological processes, such as syllabification, or in directly retrieving or compiling the articulatory gestures. To do this, we used event-related fMRI to monitor the changes in blood oxygenation while subjects performed a delayed pseudoword repetition task. The presented stimuli differed in length (4 vs. 2 syllables) and sublexical frequency of segments and syllables (low vs. high sublexical frequency). We anticipated that we would be able to identify 1) the regions involved in phonetic encoding and 2) disambiguate the role of the pars opercularis in single-word production. Specifically, if Broca's area is involved in syllabification and phonological processing prior to the encoding of the articulatory scores, it would only show a strong effect of length, but not sublexical frequency. On the other hand, if Broca's area is the site of the mental syllabary, we expected to see significant effects of both length and frequency manipulations.
Materials and Methods
Subjects
Fifteen healthy, monolingual native speakers of American English were chosen to participate in the study (8 males and 7 females) with mean age of 26 years (range = 20–35). Two subjects (1 male and 1 female) were excluded from analysis because of excessive head motion. All the volunteers reported that they were right handed, with normal hearing and with no history of previous neurological or psychiatric disease. Volunteers were paid for their participation in the 2-h scanning session, in compliance with the institutional guidelines. Prior to testing, volunteers provided written informed consent as approved by the National Institute on Deafness and Other Communication Disorders–National Institute of Neurological Disorders and Stroke Institutional Review Board (protocol NIH 92-DC-0178).
Stimulus Materials
Four sets of 36 pseudowords were created (a total of 144 items) varying in length and sublexical frequency: 4-syllable low frequency, 4-syllable high frequency, 2-syllable low frequency, and 2-syllable high frequency. The 4 sets of stimuli consisted of alternating consonant–vowel (CV) biphones plus a final consonant, that is, CVCVC and CVCVCVCVC for 2- and 4-syllable pseudowords, respectively. The 4-syllable pseudowords contained 2 stresses (a primary and a secondary stress). However, the position of the stressed syllables within the pseudowords varied to allow greater flexibility in the creation of the data set and avoiding the creation of ungrammatical syllables. Examples of the stimuli are presented in Table 1 (audio files of the examples are provided online as Supplementary Material). As a measure of length, we chose number of syllables and phonemes, with 2 syllables as the minimum length. Two-syllable pseudowords were preferred over monosyllabic ones to allow better control of phonological neighborhood density, which decreases as the word length increases (Pisoni et al. 1985). As a measure of sublexical frequency, we chose the phonotactic probability (PP) of phonemes and biphones. Phonotactic probability refers to the frequency with which legal phonological segments and sequences of segments (i.e., biphones) occur in a given language (Jusczyk et al. 1994). As observed in the syllable frequency effect, low PP pseudowords have slower response time than high PP ones, reflecting the load in the phonetic encoding process (Vitevitch et al. 1997, 1999; Vitevitch and Luce 1998).
Table 1.
Stimulus features
| Condition | Bigram PP | Phoneme PP |
4 Syllables, high PP, for example 
|
0.0251 (±0.0093) | 0.4888 (±0.0681) |
4 Syllables, low PP, for example 
|
0.0013 (±0.0012) | 0.1251 (±0.025) |
2 Syllables, high PP, for example 
|
0.0181 (±0.007) | 0.2965 (±0.0427) |
2 Syllables, low PP, for example 
|
0.0004 (±0.0004) | 0.061(±0.0194) |
Note: table with examples of the stimuli used in each category (phonetic transcription) and their features. For each category, we include the mean (±SD) PP measures for both biphones and phonemes. Audio samples of the stimuli examples are provided online as Supplementary Material.
All the syllables, with the exception of 2, that were used to construct the pseudowords were chosen from a corpus of previous linguistic studies on the effects of PP (Vitevitch et al. 1997; Frisch et al. 2000) such that they were rare, but not illegal (in the case of low-frequency items), and that they satisfied our criteria for frequency. The 2 additional syllables that we included were /θow/ and  . Both of these syllables had a biphone probability greater than zero and were included to increase the variability of the generated data set. The PP for each biphone and phoneme was calculated (Vitevitch and Luce 2004), and pseudowords were created such that each pseudoword consisted entirely of high- or low-probability segments (depending on its category).
. Both of these syllables had a biphone probability greater than zero and were included to increase the variability of the generated data set. The PP for each biphone and phoneme was calculated (Vitevitch and Luce 2004), and pseudowords were created such that each pseudoword consisted entirely of high- or low-probability segments (depending on its category).
To reduce the amount of similarity between the stimuli, no 2 syllables occurred in the same pseudoword more than once and no pseudoword appeared as a contiguous part within another pseudoword. All items were further checked for immediate phonological neighbors using a “one phoneme change” rule, that is, no stimulus could be turned into a word by 1) changing one phoneme into another, 2) deleting one phoneme, or 3) adding one phoneme. Even though phonological neighborhood density and PP are correlated, we expected that by controlling for immediate neighbors, the differences in neighborhood density between items with different PP would not be emphasized. Effects related to PP would then be related to phonetic encoding and not phonological word retrieval, which would arise by manipulating phonological neighborhood density (Okada and Hickok 2006). As a result, low– and high–sublexical frequency items differed systematically only with respect to the positional frequency of their phonemes and syllables. Finally, to avoid morphological confounds, any sequences that ended with a high-probability final rime, for example, /-æs/ and /-æd/, which could be interpreted as inflectional suffixes, were also omitted from the data set.
To record the stimuli, we recruited a female, monolingual American English volunteer. Prior to the recording, the volunteer was trained to pronounce the data set correctly and rehearsed the items a number of times to familiarize herself with the data set. The stimuli were read from a laptop screen and spoken in isolation as naturally and as clearly as possible. All stimuli were recorded in a single session in a nonechoic, sound-attenuated booth. They were digitally recorded using a Shure SM58 vocal microphone at 44.1-kHz sampling rate and were saved at 16-bit resolution. Two or three recordings were made for every stimulus, which were then edited into individual files and screened for accuracy and fluency. The most accurate recording of each item was chosen for the stimulus list. The chosen stimuli were then transcribed, and their segment and biphone PP was recalculated to take into account the cases where there were some differences in the pronunciation. In the resulting lists, the differences between the average segment and the biphone probabilities over both 4- and 2-syllable pseudowords were statistically significant (phonemes: F1,286 = 920.2, P < 0.001; biphones: F1,286 = 763.9, P < 0.001). Higher frequency pseudowords had higher PP scores than lower frequency pseudowords (see Table 1 for more details on the category PP).
Experimental Design and Procedure
Thirty-six items per condition were presented over the course of 2 experimental fMRI runs. Each item was presented to the subject auditorily using an fMRI compatible (pneumatic) system for auditory delivery (Avotec SS-3100, Silent Scan system). After a delay of 6 s, a probe (1 of 2 versions of a bell sound) was heard instructing the subject to repeat the presented pseudoword either overtly or covertly (depending on the type of probe). During the delay period, the subjects were given specific instructions to rehearse the presented stimulus covertly. They did not know prior to the presentation of the relevant probe whether they would be asked to respond overtly or covertly, and so we expected that they would fully retrieve the articulatory scores for the presented pseudoword. Each trial lasted 8 s (Fig. 1A).
Figure 1.
During the experiment, subjects were asked to listen to pseudowords and to repeat them either overtly or covertly after a 6-s delay. The structure of each trial is shown in (A). The stimulus is presented auditorily at 0 s and then subjects wait for the response probe. During the delay period, they are instructed to covertly rehearse the stimulus and are not aware of the type of response (overt or covert) before they hear the probe. The type of stimulus that will be presented in each trial is determined pseudorandomly by a combination of 3 m-sequences. In (B), we present an example of 3 binary sequences that resemble those used in the experiment. Each sequence is associated with an experimental factor. In the example provided, the top sequence controls the length of the stimulus (1 for 4 syllables and 0 for 2 syllables), the middle sequence controls sublexical frequency (1 for high and 0 for low), and the bottom sequence controls response type (1 for overt and 0 for covert). For example, the combination 0 1 0 would retrieve a 2-syllable, high-frequency pseudoword and the covert response probe.
Stimulus presentation was in a pseudorandom, fast event-related fashion, whereby the order of occurrence for the conditions was controlled by a combination of 3 binary shifted versions of an m-sequence (one shifted by 9 bins and the other by 18 bins with respect to the first one; see, e.g., Fig. 1B). The use of m-sequences (Buracas and Boynton 2002; Kellman et al. 2003) to control stimulus delivery allowed for a simple and efficient way to increase design efficiency and minimize the chance of significant correlation between the regressors, even in case of post hoc exclusion of incorrect trials. The binary m-sequence used in the study had a length of 63 bins (corresponding to the number of trials per run) and was padded in the beginning with 9 more trials, which were not analyzed for the purposes of this study. The purpose of these onset trials was to allow for the subject to get comfortable with the task and the noisy environment in the scanner.
Prior to the onset of the experiment, all subjects performed a 150-min practice session outside the scanner to allow them to become familiar with the structure of the task and its demands. The material used as the training set (10 items per category) contained pseudowords with features similar to the ones presented during the experimental runs but from an unrelated set (built from different syllables) to avoid habituation and familiarity.
Because of the concern that, during the scanning session, the scanner noise would mask out some of the stimuli, a quality check run was performed prior to the onset of the experimental runs. During this run, a set of pseudowords (not used for the experimental set but recorded in the same session as the experimental set, i.e., with the same amplitude and recording characteristics) was presented to the subject. The volume of the headset was then adjusted based on the subject's feedback to ensure protection from exposure to a noisy environment, comfort, and clear stimulus delivery. Images acquired during this test run were also submitted to a quality check to make sure that they were free from artifacts.
During the scanning session, subject responses were recorded using a dual-channel, noise canceling, fiber optic microphone (Dual-Channel Phone-Or by Optoacoustics Ltd, Or-Yehuda, Israel). This system is specifically designed for use in magnetic resonance imaging (MRI) environments and offers real-time adaptive elimination of the MRI acoustic noise from the signal. This allowed us to record both the subject responses and the timing of their responses. However, due to concerns that the filtering algorithm introduced a small, random delay in the recording of the responses, we did not consider the estimates of the subject response timing reliable. Thus, as a behavioral measurement, we only used subject response accuracy.
fMRI Data Acquisition
Imaging was performed on a 3.0-T MRI system (General Electric, Milwaukee, WI), equipped with Cardiac Resonance Module whole-body gradients. For improved signal-to-noise ratio (SNR) and higher spatial resolution, we used a custom-built 16-channel MRI receive array (Nova Medical, Wilmington, MA; de Zwart et al. 2004) connected to a custom-built 16-channel MRI receiver. For the functional scans, we used single-shot, rate-2, sensitivity-encoded (SENSE), gradient-echo, echo-planar imaging (EPI) (de Zwart et al. 2002). A total of 32 axial slices were acquired interleaved (time echo [TE] = 31 ms, flip angle of 90 degrees, time repetition [TR] = 2 s, and acquisition bandwidth 250 kHz) with an in-plane resolution of 2.3 × 2.3 mm2 (96 × 72 matrix, 22.4 × 16.8 cm2 field of view [FOV]) and slice thickness = 2 mm (gap = 0.3 mm). Four volumes were acquired during each trial. The combination of the dedicated receive array with SENSE EPI allowed a 2- to 4-fold improvement in SNR and a 50% reduction in geometric distortions relative to a conventional setup with a birdcage head coil (de Zwart et al. 2004). The reduced geometrical distortions of SENSE EPI are due to its use of a shortened data acquisition window compared with conventional EPI at the same spatial resolution.
To increase the efficiency of subject motion correction, we acquired isotropic voxels (2.3 mm cube side). However, the resulting smaller-than-usual thickness of the slices put a constraint on the brain volume that could be imaged. We did not have a hypothesis about the involvement of any areas below the superior temporal sulcus (STS), and we therefore acquired images in a slightly oblique position, covering an area from below the STS to the top of the head. By avoiding the lower parts of the cortex (e.g., the inferior temporal areas), we also avoided geometrical distortions and artifacts that are caused by articulatory muscle movement (Birn et al. 2004). To facilitate slice selection, a sagittal 2-dimensional anatomical image was acquired prior to the onset of the functional runs. This image was inspected for specific anatomical landmarks such as the anterior commissure and the STS and was used to make the slice selection. At the end of the scanning session, high-resolution spin-echo T1 anatomical images were acquired at the same location as the functional EPI scans. The scanning parameters for the anatomical image were as follows: TR = 700 ms, TE = 13 ms, 256 × 192 data matrix with a 22.4 × 16.8 cm2 FOV, resulting in 0.86 × 0.86 mm2 in-plane resolution, and 2 mm slice thickness (with 0.3 mm gap).
To minimize head movement during the scanning sessions, we used head padding and a velcro strap, mounted on each side of the head coil and positioned on the subject's forehead at the line just above the eyebrows. The purpose of the strap was to act as a motion reference point for the subject. Head movement, especially in the z (head–foot) direction, would cause a strain on the strap, make the subject aware of the movement and cause him/her to restrict it and return to the original position. Prior to the onset of the scanning session, the subjects were given instructions about how to restrict their head movement and about the function of the velcro strap. Tests were also performed to ensure that the strap was properly placed, and the subjects could feel it when moving during speech.
Image Preprocessing
All analyses and image preprocessing were carried out using the SPM5 software package and associated toolboxes (http://www.fil.ion.ucl.ac.uk/spm/software/spm5). Preprocessing included slice-timing correction and an optimized motion correction routine to ensure good quality registration (Oakes et al. 2005). Images were then registered to the Montreal Neurological Institute (MNI) anatomical template and transformed into MNI stereotactic space to allow for group comparisons. The functional data were then smoothed with an isotropic Gaussian filter kernel of 6 mm (full width at half maximum) to improve SNR.
To quantify the effect of subject movement on the quality of our data, we inspected the data using the ArtRepair toolbox for SPM5 (Mazaika et al. 2007) and examined the realignment parameters provided by the SPM5 motion correction procedure. We were particularly interested in scan-to-scan (incremental) motion during the task, that is, the change in position between the image acquired during the subject response and its immediate preceding image. In previous studies on speech-related motion (Barch et al. 1999), it was shown that speech-related motion is mainly scan-to-scan motion affecting the first scan acquired after the response probe. To assess the effects of speech-related motion on our data, we performed a 3-factor analysis of variance (ANOVA) with within-subject factors response type, stimulus length, and sublexical frequency and dependent variable the 6 motion estimates for incremental (scan-to-scan) movement. The analysis revealed a significant main effect of response type in all directions (F1,12 > 26, P < 0.004 for all directions). In agreement with other studies (Barch et al. 1999; Shuster and Lemieux 2005), the incremental movement was overall quite small and greater for overt response trials (mean ± standard deviation [SD] displacement was 0.039 ± 0.014 mm for translations and 0.034 ± 0.012° for rotations) than covert response ones (mean ± SD was 0.02 ± 0.008 mm for translations and 0.017 ± 0.006° for rotations).
Additional significant effects were present for length in the pitch rotation and for both the main effect (F1,12 = 5.9, P < 0.04) and the interaction between length and response type (F1,12 = 19, P < 0.001). Four-syllable pseudowords (mean ± SD pitch displacement was 0.038 ± 0.020°) produced greater movement than 2-syllable pseudowords (mean was 0.034 ± 0.016°) especially during overt responses. Finally, in the y direction, there was a significant main effect of sublexical frequency (F1,12 = 6.3, P < 0.03) and interaction between sublexical frequency and response type (F1,12 = 10.8, P < 0.01). Low-frequency items caused greater movement (mean ± SD 0.021 ± 0.013 mm) than high-frequency items (0.019 ± 0.010 mm), especially during overt response trials. To remove effects related to subject movement, we included the realignment parameters in the design matrix as effects of no interest. Finally, we also inspected the movement parameters for extreme movement. We took into account both incremental movement and absolute movement (the displacement of a scan with respect to the realignment reference scan of the time series, i.e., in our case, the first image in the time series). Our criteria for inclusion in the study were that a subject would not show absolute motion greater than the voxel size and incremental motion greater than 1 mm in translations and 1° in rotations. All subjects met the absolute motion inclusion criteria, but not the incremental motion. Two subjects showed movement greater than our criteria and were consequently excluded from the analysis.
Further examination using the ArtRepair toolbox revealed that in a few cases, incremental movement even as low as 0.5 mm induced global signal changes greater than 1.5% of the mean and “stripe-like” artifacts on the image. To ensure the quality of our data and to completely remove their effect from the analysis, we also added an additional regressor for images that showed changes in the global signal greater than 1.5% of the mean followed by a greater than 0.5 mm incremental movement (Mazaika et al. 2007).
Behavioral Data Analysis
In order to get an estimate of subject performance and ensure that the subjects were performing the task as instructed, we estimated the subject response accuracy. To calculate it, we monitored and phonologically transcribed all subject responses. However, because of the low quality of the recording, resulting from the noise reduction filtering, a precise phonetic transcription of the subject response was not always possible and the nearest phonological transcription was used. Cases where the recording was unintelligible because of noise were not included in the analysis. The resulting transcriptions were compared with the target stimulus phoneme-by-phoneme, and a score was calculated based on the number of correct phonemes (token count). If a phoneme was omitted in the subject response, it was scored as a mismatch, for example, if the target was  and the response was /keb/, the first 2 phonemes were counted as a mismatch and the final phonemes were counted as a match. To determine a match between the target and the response, we used broad phonemic criteria and ignored differences between allophones (Vitevitch and Luce 2005). The scores were then submitted to a 2-way ANOVA with factors length and sublexical frequency.
and the response was /keb/, the first 2 phonemes were counted as a mismatch and the final phonemes were counted as a match. To determine a match between the target and the response, we used broad phonemic criteria and ignored differences between allophones (Vitevitch and Luce 2005). The scores were then submitted to a 2-way ANOVA with factors length and sublexical frequency.
Even though we were not able to extract a very detailed phonetic transcription, our interpretation of the data does not dependent on the subtle phonetic details of the subjects' performance, for example, distinguishing between 2 allophones. The primary reasons for analyzing the behavioral results were to identify incorrect trials, to ensure that the subjects were performing the task as instructed, and that the difference between low– and high–sublexical frequency items was retained in the subject response. For this purpose, we also estimated the PP of the subjects' overt responses in the same way as we did for the stimuli (Vitevitch and Luce 2004). To determine whether there is a significant difference between the 2 conditions, we performed a paired t-test. Finally, we also examined the subject recordings to identify trials that were incorrectly answered (i.e., responses on covert trials or no response on overt trials). These trials were included to a regressor of no interest and excluded from the fMRI data analysis.
fMRI Data Analysis
Statistical analysis of the factorial event-related experiment was performed using SPM5. The hemodynamic response function (HRF) for each trial was modeled using a finite impulse response function (FIR) with 12 bins (duration of 2 s) to capture the temporal components of a delayed response task. Stimulus presentation was modeled as a delta function. A 2-way, random-effects, within-subject ANOVA with factors length (4- vs. 2-syllable pseudowords) and sublexical frequency (low vs. high) was performed. Each of the 4 different resulting types of trials, for example, 4-syllable and low sublexical frequency, was modeled by separate regressors, and the main effects and interactions were evaluated by contrasting within or across (interactions) the levels of each factor. To perform group statistics, the contrast images for each effect and for all subjects were submitted to a 1-way ANOVA (with 12 levels). T-contrasts testing for the predicted shape of the HRF (a canonical, 2 gamma function; Friston et al. 1998) were performed to produce maximum intensity projections and reveal voxels whose differential activity pattern conforms to the shape of the HRF. SPMs were thresholded at P < 0.001 uncorrected at the voxel level and P < 0.05 corrected for familywise error (FWE) at the cluster level (Hayasaka and Nichols 2003). For our study, significant clusters had on average more than 85 voxels.
In order to analyze the contrast estimates for the LIFG, we used the cytoarchitectonic probability map for left hemisphere BA44 (Eickhoff et al. 2005). For each of the main effects of interest (length, frequency, and response type), we identified the voxels within the activated clusters that were part of BA44. We then extracted the average beta weights (over cluster voxels) for each of the 4 conditions of interest in the design (4-syllable low frequency, 4-syllable high frequency, 2-syllable low frequency, and 2-syllable high frequency) and for all subjects. A single value corresponding to the weighted sum of the estimates across the FIR (weighted by the HRF) was then extracted for each of the 4 conditions and subjects and used in multiple 2-sided t-tests testing for effects of frequency, length, or the difference between the 2 conditions within each region. This approach followed the implementation of random-effects analyses in the Marsbar SPM toolbox (Brett et al. 2002). Significance was determined using a threshold of P <0.05. Where appropriate (more than 1 region of interest [ROI]), the P values were adjusted to correct for multiple comparisons (Bonferroni correction).
To ensure that the significant activations observed during the delay period for both the whole-brain and the LIFG analyses were not related to subject motion, we extracted and inspected the parameter estimates for each significantly activated cluster over the window of the FIR (24 s). The time course of movement-related activations is very different from that of blood oxygen level–dependent (BOLD) related activations. Whereas motion-related signal changes appear as large spikes in the signal intensity for the first few images at the time of the subject movement, BOLD-related signal changes follow a curve similar to the HRF (Birn et al. 1999). It should also be noted that significant effects for length and frequency were estimated over both covert and overt responses, and so we expected that the contribution of motion-related artifacts to the significant activations observed would be minimal, if any.
Results
Behavioral Results
To test for effects of length or frequency on subject performance, we measured subject response accuracy. Based on previous results, we expected to find a decrease in response accuracy for low-frequency pseudowords, but we did not expect to find an effect of length. We performed a 2-way ANOVA with within-subject factors: length and sublexical frequency. As expected, we found that there was a significant main effect only for frequency (F1,12 = 14.6, P < 0.003). No other main effects or interactions were significant. Mean (±SD) accuracy rates were 64.5% (±15) for low-frequency pseudowords and 75% (±13) for high. The relatively low accuracy scores were expected, considering the nature of the task (pseudoword repetition) and the noisy environment. All subjects' performance accuracy was within 3 SDs of the group mean (70%, SD = 13).
Finally, to verify that there is a significant difference in sublexical frequency between the responses, we calculated the phoneme and biphone PP of the subjects' overt responses and performed a 2-sided t-test to compare high- versus low-frequency responses. For both biphone and phoneme measurements, the differences were significant (t12 = 14.66, P < 0.001, for biphones and t12 = 15.74, P < 0.001, for phonemes). Mean (±standard error [SE]) PP scores for high-frequency responses were 0.0193 (±0.0009) for biphones and 0.3656 (±0.0145) for phonemes. Low-frequency PP scores were 0.0025 (±0.0006) for biphones and 0.1187 (±0.0091) for phonemes. From the above results, we can conclude that the subjects perceived the differences between low- and high-frequency targets and performed the task according to the instructions.
fMRI Results
Phonological Encoding
To map the areas involved in phonological encoding, we compared the activation levels invoked for processing 4- versus 2-syllable pseudowords (over both low- and high-frequency syllables). A significant main effect of length (4- greater than 2-syllable stimuli) was observed in a large perisylvian network extending bilaterally across the STG, the precentral gyrus (PrCG), and the supplementary motor area (SMA), as well as the LIFG (cf., Fig. 2A for whole-brain results and Fig. 2C for significantly activated voxels within the LIFG). The largest activations were observed in the left hemisphere for a cluster that covered both the PrCG and STG. In particular for the STG, the cluster covered a large portion of the middle and posterior STG including the upper banks of the STS and an area in the junction between the parietal and the temporal lobe also referred to as the Sylvian parietotemporal area (SPT; cf., Table 2 for the coordinates of the significantly activated areas). The left STG (LSTG) has been previously implicated in phonological processing (Indefrey and Levelt 2000, 2004; Graves et al. 2007), whereas the left PrCG is a known premotor area and as such it has been associated with phonetic encoding. A similar effect could also be observed for the LIFG. The activated area was located on pars opercularis and ran along the inferior frontal sulcus (IFS). In accordance to our hypothesis, we expected that both phonological and phonetic encoding processes would show an effect of length. What distinguishes the 2 processes is their sensitivity to sublexical frequency. If a region is involved in phonological processing, we would not expect it to show significant sublexical frequency effects. On the other hand, if it is, we would expect it to show significant effects for both conditions, length and sublexical frequency.
Figure 2.
Surface renderings of significant activations in the whole-brain group analysis for length (A) and sublexical frequency (B). In (A), an extended perisylvian and premotor activation including the LIFG showed significantly higher activation for 4 versus 2 syllables. In (B), premotor areas including the dorsal PrCG and the IFG bilaterally showed significantly higher activation for low- versus high-frequency pseudowords. In (C), we show the main effect of length within left BA44 (significantly activated voxels appear in magenta) using a small volume correction approach (SVC). BA44 (shaded area) was defined using a cytoarchitectonic probability map of the area (Eickhoff et al. 2005). Maps are thresholded voxelwise at P <0.001 uncorrected and clusterwise at P <0.05 FWE corrected. Color grading in (A) and (B) reflects depth, with brighter voxels on the surface. The maximum depth of the projected voxels is 20 mm. L, sagittal view of the left hemisphere.
Table 2.
Brain regions modulated by length and frequency
| Contrast | Region | Coordinates | T | No. of voxels | ||
| x | y | z | ||||
| 4 > 2 Syllables | Left PrCG | −56 | −4 | 44 | 7.87 | 2097 |
| LSTGa | −60 | −12 | 4 | 6.76 | ||
| Left SPT junctiona | −56 | −38 | 20 | 5.82 | ||
| LIFGa | −60 | 4 | 20 | 4.63 | ||
| LSMA | −4 | 10 | 68 | 7.21 | 388 | |
| Right STG | 50 | −22 | 8 | 5.45 | 393 | |
| Right SPT junctiona | 64 | −32 | 10 | 5.24 | ||
| Right PrCG | 50 | −4 | 40 | 5.30 | 176 | |
| Low > high frequency | Left PrCG | −52 | 2 | 40 | 4.77 | 138 |
| LSMA | −4 | 14 | 58 | 4.51 | 122 | |
| LIFG | −54 | 12 | 12 | 4.01 | 119 | |
| Right IFG | 50 | 18 | 4 | 4.23 | 97 | |
Note: regions significantly activated in the group analysis (t144 > 3.1, P < 0.05 FWE corrected for cluster size). Displayed are the contrasts, the coordinates for the voxels of greatest activity within the activated clusters in MNI stereotaxic space, an anatomical description of the region, the T value, and the number of significantly activated voxels.
In the case of very large clusters, multiple peak voxels are reported. They are clustered together with the last entry to include number of voxels.
Phonetic Encoding
Comparing pseudowords with low versus high PP syllables and segments revealed regions that showed an effect for sublexical frequency. Based on our hypothesis, areas that showed a frequency effect reflect the process of phonetic encoding, that is, articulatory code generation (Indefrey and Levelt 2000). Four regions showed significant main effects of frequency: the left hemisphere dorsal PrCG, the left hemisphere SMA (LSMA), and the inferior frontal gyrus (IFG) bilaterally (cf., Table 2 for a detailed list of the activated regions and Fig. 2B for a map of the significantly activated areas). Activity in the LSTG did not reach significance (P < 0.2 cluster size, FWE corrected).
We also tested for the opposite contrast, high- versus low-frequency pseudowords in order to see whether the areas associated with retrieving high-frequency, precompiled syllables from the mental syllabary are different from the ones associated with online generation of articulatory scores. No areas showed higher activation for high- versus low-frequency syllables. There were also no significant interaction effects between length and sublexical frequency.
Left IFG
To further test our hypothesis about the involvement of Broca's area in phonetic processing, we performed an ROI analysis. A region corresponding to left hemisphere BA44 (center of mass x = −53, y = 12, z = 19, size = 1160 voxels) was defined using a cytoarchitectonic probability map of area BA44 (Eickhoff et al. 2005). In a random-effects 2-way ANOVA with factors length (4 vs. 2 syllables) and sublexical frequency (low vs. high), the LIFG showed a main effect for both factors (t12 = 1.97, P < 0.04, and t12 = 2.56, P < 0.02, for length and frequency, respectively).
Because the LIFG showed effects for both length and frequency, we further investigated whether there were any signs of functional segregation within the IFG and in particular the pars opercularis, as had been observed in other studies (Molnar-Szakacs et al. 2005). For the 2 conditions, length and frequency, we observed 2 clusters within the LIFG, which were only partly overlapping (9 voxels out of 82 and 79, respectively, for the 2 clusters; Fig. 3). The distance between their center of mass was 9 mm, that is, a factor of 1.5 greater than the smoothing kernel (6 mm), with the cluster showing a greater effect of length following the anterior banks of the IFS and extending more lateral, posterior, and dorsal to the cluster showing a greater effect of frequency. We will refer to the cluster identified during the length condition as dorsal pars opercularis (dPOp) and the cluster identified for the frequency condition as ventral pars opercularis (vPOp) because of their anatomical differences and in agreement with previous evidence.
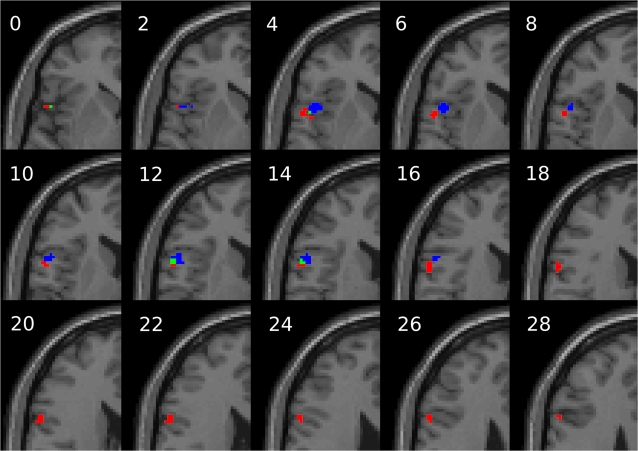
Figure 3.
Significant activations within left hemisphere BA44 as defined by a cytoarchitectonic probability map of the area (Eickhoff et al. 2005). Shown in red are voxels significantly more activated for 4 versus 2 syllables. This cluster extends from z = −2 (slice not shown) to z = 28. The largest effect for length is located dorsally, at [−60 4 20]. Shown in blue are voxels significantly more activated for low versus high sublexical frequency. The largest effect for frequency is located at [−54 12 12]. Finally, shown in green are voxels that are overlapping for both conditions (size of overlap = 9 voxels). Activations are thresholded at P <0.001 uncorrected voxelwise and P <0.05 FWE corrected clusterwise. Z coordinates are in MNI space.
Both the dPOp and the vPOp exhibited effects of frequency and length, though the frequency effect for dPOp was just slightly above significance (dPOp frequency: t12 = 2.5, P < 0.06; vPOp length: t12 = 3.2, P < 0.02 corrected for 2 ROIs). This difference already suggests that there might be a functional segregation within the pars opercularis of the LIFG. To further examine whether there is a functional difference in the activation between the 2 clusters, we examined the region (dPOp vs. vPOp) by experimental condition (length vs. frequency) interaction (Friederici et al. 2006). We performed a 2-sided paired t-test on the region-specific differences between the length and the frequency conditions and found a significant region-by-condition interaction (t12 = 3.1, P < 0.01), indicating that there is a robust difference between the 2 clusters in terms of their response to length and sublexical frequency effects. DPOp shows greater activation for length rather than sublexical frequency (mean ± SE length over frequency difference is 0.093 ± 0.051), whereas in vPOp, there is almost no difference between the levels of activation for the 2 conditions (mean ± SE length over frequency difference is 0.002 ± 0.026).
Discussion
In this study, we were able to delineate the cortical areas involved in the phonemic-to-articulatory translation that is necessary for the generation of articulatory codes. By directly contrasting targets with varying length, we manipulated the load on the system of postlexical articulatory-motor production and were able to identify a number of key regions underlying articulation and the overall process of transforming phonological word forms to articulatory codes. In summary, these regions included bilateral (although strongly left lateralized) mid and posterior superior temporal and frontal regions, the premotor cortex, and the SMA. These results are in agreement with current models on word production that describe a left-lateralized, perisylvian network (Indefrey and Levelt 2000, 2004; Hickok and Poeppel 2004, 2007).
To further identify the roles of the different components of the network and in particular to resolve the conflict on the role of the LIFG, we probed the network by manipulating sublexical frequency. Our hypothesis was that only regions that are directly involved in phonemic-to-articulatory translation would show an effect for frequency manipulation. Targets with components of different sublexical frequency (high vs. low) are processed differently (Guenther et al. 2006). High-frequency clusters are precompiled and their articulatory codes are retrieved, as suggested by the fact that they are processed faster than the ones with less-frequent components (Vitevitch and Luce 1998, 2005). The latter are thought to be compiled online on a segment-to-segment basis (Guenther et al. 2006).
In our experiment, we identified 4 regions that showed an effect related to sublexical frequency (higher activation for low vs. high frequency): the LSMA, the left hemisphere PrCG, and the IFG bilaterally. From previous studies on motor planning and production, it is known that the SMA has a role in motor planning and the preparation of movements. Even though its function is not specifically associated with linguistic processes, it is also part of linguistic motor planning (Riecker et al. 2005). In a recent fMRI study, the pre-SMA was shown to be sensitive to sequence complexity effects both within and beyond the syllable boundaries (Bohland and Guenther 2006). The present findings are in agreement with the current theories on the function of the SMA. The observed frequency effect could simply represent the increased load that is associated with producing new and unfamiliar motor plans (low–sublexical frequency pseudowords) compared with familiar, more rehearsed, and precompiled ones (high–sublexical frequency pseudowords).
The significant activation difference for low– versus high–sublexical frequency pseudowords in the left PrCG is also in agreement with current models on word production (Hickok and Poeppel 2004; Indefrey and Levelt 2004; Guenther et al. 2006). It is worth highlighting that only a small area in the dorsal PrCG was significantly active and that this area has been previously involved in studies examining sensory–motor mapping (Hickok and Poeppel 2004). Hickok and Poeppel propose the existence of a “dorsal stream” in speech processing, which is involved in mapping sound onto articulatory-based representations. The regions that are part of this stream include a posterior inferior frontal area (including Broca's area), a dorsal premotor site, and area SPT (Hickok et al. 2003). The latter region, which lies within the boundaries of the planum temporale, is traditionally associated with acoustic and phonological processing, as well as speech production as the interface for the sound-to-gesture transformation.
In our study, we found that the STG bilaterally shows a greater effect for target length, though the results are strongly left lateralized, and in the left hemisphere, particularly, the effect extends further in the posterior direction to area SPT (Fig. 2A). Bilateral STG activation has been observed during both speech perception and production and reflects the processing of the acoustic and phonological properties of the target stimulus (Hickok and Poeppel 2004). This is in contrast to area SPT, which is thought to be involved in translating between acoustic and motor representations. However, in the current study, both STG and area SPT show a similar behavior and a significant main effect for length only and not for sublexical frequency. Therefore, these findings raise doubts on the role of SPT as an auditory–motor interface and suggest that its role is not that different from the rest of the STG, that is, it could also be involved in phonological processes, such as syllabification and segmentation. This claim would be in agreement with initial claims made by Indefrey and Levelt (2000), whereby a portion of the superior temporal lobe was considered as a possible candidate region for syllabification. Another candidate was the LIFG.
In the current study, we found significant bilateral activation in the IFG. The presence of a sublexical frequency effect in the right IFG was surprising because this region has not been included in any of the neuroanatomical models of speech production previously discussed (Hickok and Poeppel 2000, 2004, 2007; Indefrey and Levelt 2000, 2004). Activation in this region has been previously found during pitch processing and specifically for the integration of accent patterns (Geiser et al. 2008). In the current study, the stress pattern between the 2 categories was controlled, and there were no systematic differences. However, it is possible that the increased processing demands for low–sublexical frequency pseudowords also affected the processing of metrical structure. Further research would be needed to identify the exact nature of the differences.
With respect to the LIFG, the pars opercularis showed consistent effects for both length and sublexical frequency (4 vs. 2 syllables and low vs. high frequency, respectively), as well as evidence of functional segregation. The more dorsal part of the area (dPOp) was modulated by differences in stimulus length, whereas the ventral part (vPOp) was modulated by differences in both length and sublexical frequency. The idea that Broca's area is functionally segregated into its 3 anatomical parts (pars opercularis, triangularis, and orbitalis) is well known and well founded (Bokde et al. 2001; Chein et al. 2002; Devlin et al. 2003; Heim et al. 2007). Recently, however, there have also been claims concerning a functional segregation within pars opercularis (Molnar-Szakacs et al. 2005). In a meta-analysis of imaging studies on imitation and action observation, Molnar-Szakacs et al. (2005) identified 2 distinct foci within the pars opercularis, a dorsal and a ventral one, that serve different functions. DPOp shows mirror neuron properties and is significantly active during both action observation and imitation, whereas vPOp shows only motor properties and is only active during imitation.
In agreement with this segregation, we also identified 2 distinct clusters within the pars opercularis with one extending more dorsally than the other. The more dorsal cluster is located closer to the IFS and the premotor cortex and shows greater activation for length manipulation. The vPOp, on the other hand, shows both a main effect of length and sublexical frequency. In the current study, the dPOp is part of a wider area of activation in the left hemisphere PrCG. Therefore, based on its relation to premotor areas, as well as the fact that it is only active for the length condition, we can conclude that the dPOp is involved in phonological encoding and syllabification as proposed by Indefrey and Levelt (2000, 2004). This role is in agreement with other proposed roles such as sequencing discrete units (Gelfand and Bookheimer 2003) or sublexical processing requiring explicit segmentation (Zatorre et al. 1996; Burton et al. 2000; Chein et al. 2002).
The vPOp on the other hand shows a significant effect of both length and frequency, which is in agreement with a role as the cite of the speech sound map or mental syllabary that has been proposed by Guenther et al. (2006). These results are also partially in agreement with the claims made by Molnar-Szakacs and colleagues, who propose that it holds a form of representation of the motor plans that is communicated to the posterior part of the STS (Molnar-Szakacs et al. 2005). In this account, the vPOp is not the location of the speech sound map but only holds a copy of the articulatory codes. The codes themselves are generated elsewhere. The only other possible candidate in our case would be the dorsal premotor cortex, which also showed a significant effect of sublexical frequency. Based on our results, we cannot exclude either possibility.
Research into the functional segregation of the pars opercularis is still in a preliminary phase. The anatomy of the LIFG is highly variable across subjects (Amunts et al. 1999), which makes it difficult to draw any precise conclusions about the exact anatomical borders of the hypothesized segregation of the pars opercularis based on group-averaged results. For the purposes of this study, we have also described the functional segregation of the region using gross anatomical terms such as ventral and dorsal and only in terms of the group tendency. Future research using higher spatial resolution at the single-subject level will be needed to further verify and specify the exact anatomical features of this functional segregation.
Finally, we also note that we did not find any regions showing significant effects for the inverse contrast high– versus low–sublexical frequency. Based on our hypothesis, we would expect that a significant activation for this contrast would reveal the location of the mental syllabary versus the network underlying articulatory code generation. However, based on the computational model proposed by Guenther et al. (2006), the speech sound map (the equivalent of the mental syllabary) does not just contain precompiled frequent syllables but also motor representations for phonemes, common words, phrases, etc. The speech sound map is therefore involved in both processes, though the online compilation of articulatory codes would be computationally more demanding than the retrieval of precompiled gestural scores. This would explain why we do not see increased activity for high- versus low-frequency stimuli because it would be the same network that is underlying both processes.
To conclude, in this fMRI study, we investigated the process of phonological-to-articulatory translation and the role of the LIFG. Based on our findings, we conclude that the LIFG, BA44 in particular, is functionally segregated into 2 subregions following a dorsal–ventral gradient. The dorsal part seems to be involved at the level of phonological encoding as suggested by Indefrey and Levelt (2000, 2004), whereas the ventral part seems to be involved at the level of phonetic encoding and possibly in the translation between phonemic and articulatory representations as proposed by Hickok and Poeppel (2000, 2004, 2007). This finding is in agreement with recent observations on the functional segregation of the pars opercularis and further clarifies the role of the LIFG in language production.
Supplementary Material
Supplementary material can be found at http://www.cercor.oxfordjournals.org/.
Funding
Neuroinformatics Doctoral Training Centre studentship; UK Engineering and Physical Sciences Research Council; Greek Bakalas Bros Foundation to MP; Intramural Research Program of the National Institute on Deafness and Other Communication Disorders of the US National Institutes of Health; Intramural Research Program of the National Institute of Neurological Disorders and Stroke of the US National Institutes of Health to JAdZ and JMJ.
Acknowledgments
We would also like to thank Drs Jason Smith, Jieun Kim, Fatima Husain, David McGonigle, Allen Braun, and Jeff Duyn for their support and helpful comments during the design and execution of the study. This work has made use of the resources provided by the Edinburgh Compute and Data Facility (ECDF) (http://www.ecdf.ed.ac.uk). The ECDF is partially supported by the e-Science Data, Information and Knowledge Transformation (eDIKT) initiative. Conflict of Interest: None declared.
References
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412(2):319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, Cohen JD. Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. Neuroimage. 1999;10(6):642–657. doi: 10.1006/nimg.1999.0500. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Hum Brain Mapp. 1999;7(2):106–114. doi: 10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage. 2004;23(3):1046–1058. doi: 10.1016/j.neuroimage.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32(2):821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Tagamets MA, Friedman RB, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30(2):609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Proceedings of the 8th International Conference on Functional Mapping of the Human Brain in Sendai, Japan. 2002;Vol. 16 Available on CD-ROM in Neuroimage. [Google Scholar]
- Browman CP, Goldstein L. Some notes on syllable structure in articulatory phonology. Phonetica. 1988;45(2–4):140–155. doi: 10.1159/000261823. [DOI] [PubMed] [Google Scholar]
- Buracas GT, Boynton GM. Efficient design of event-related fMRI experiments using M-sequences. Neuroimage. 2002;16:801–813. doi: 10.1006/nimg.2002.1116. [DOI] [PubMed] [Google Scholar]
- Burton MW, Small SL, Blumstein SE. The role of segmentation in phonological processing: an fMRI investigation. J Cogn Neurosci. 2000;12(4):679–690. doi: 10.1162/089892900562309. [DOI] [PubMed] [Google Scholar]
- Chein JM, Fissell K, Jacobs S, Fiez JA. Functional heterogeneity within Broca's area during verbal working memory. Physiol Behav. 2002;77(4–5):635–639. doi: 10.1016/s0031-9384(02)00899-5. [DOI] [PubMed] [Google Scholar]
- Cholin J, Levelt WJ, Schiller NO. Effects of syllable frequency in speech production. Cognition. 2006;99:205–235. doi: 10.1016/j.cognition.2005.01.009. [DOI] [PubMed] [Google Scholar]
- de Zwart JA, Ledden PJ, van Gelderen P, Bodurka J, Chu R, Duyn JH. Signal-to-noise ratio and parallel imaging performance of a 16-channel receive-only brain coil array at 3.0 Tesla. Magn Reson Med. 2004;51(1):22–26. doi: 10.1002/mrm.10678. [DOI] [PubMed] [Google Scholar]
- de Zwart JA, van Gelderen P, Kellman P, Duyn JH. Reduction of gradient acoustic noise in MRI using SENSE-EPI. Neuroimage. 2002;16(4):1151–1155. doi: 10.1006/nimg.2002.1119. [DOI] [PubMed] [Google Scholar]
- Demonet JF, Fiez JA, Paulesu E, Petersen SE, Zatorre RJ. PET studies of phonological processing: a critical reply to Poeppel. Brain Lang. 1996;55(3):352–379. doi: 10.1006/brln.1996.0109. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15(1):71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91(1):176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24(1):205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Fiebach CJ, Schlesewsky M, Bornkessel ID, von Cramon DY. Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb Cortex. 2006;16(12):1709–1717. doi: 10.1093/cercor/bhj106. [DOI] [PubMed] [Google Scholar]
- Frisch SA, Large NR, Pisoni DB. Perception of wordlikeness: effects of segment probability and length on the processing of nonwords. J Mem Lang. 2000;42:481–496. doi: 10.1006/jmla.1999.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7(1):30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Geiser E, Zaehle T, Jancke L, Meyer M. The neural correlate of speech rhythm as evidenced by metrical speech processing. J Cogn Neurosci. 2008;20(3):541–552. doi: 10.1162/jocn.2008.20029. [DOI] [PubMed] [Google Scholar]
- Gelfand JR, Bookheimer SY. Dissociating neural mechanisms of temporal sequencing and processing phonemes. Neuron. 2003;38(5):831–842. doi: 10.1016/s0896-6273(03)00285-x. [DOI] [PubMed] [Google Scholar]
- Goldrick M, Rapp B. Lexical and post-lexical phonological representations in spoken production. Cognition. 2007;102(2):219–260. doi: 10.1016/j.cognition.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gordon JK. A neural signature of phonological access: distinguishing the effects of word frequency from familiarity and length in overt picture naming. J Cogn Neurosci. 2007;19(4):617–631. doi: 10.1162/jocn.2007.19.4.617. [DOI] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 2006;96(3):280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. Neuroimage. 2003;20(4):2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Ischebeck AK, Friederici AD, Stephan KE, Amunts K. Effective connectivity of the left BA 44, BA 45, and inferior temporal gyrus during lexical and phonological decisions identified with DCM [Internet] Hum Brain Mapp. 2007 doi: 10.1002/hbm.20512. Available from: URL http://dx.doi.org/10.1002/hbm.20512. Accessed 6 January 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory-motor interaction revealed by fMRI: speech, music, and working memory in area Spt. J Cogn Neurosci. 2003;15(5):673–682. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Science. 2000;4(4):131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt W. The neural correlates of language production. In: Gazzaniga M, editor. The new cognitive neurosciences. Cambridge (MA): MIT Press; 2000. pp. 845–865. [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jusczyk P, Luce P, Charles-Luce J. Infants' sensitivity to phonotactic patterns in the native language. J Mem Lang. 1994;33:630–645. [Google Scholar]
- Kellman P, van Gelderen P, de Zwart JA, Duyn JH. Method for functional MRI mapping of nonlinear response. Neuroimage. 2003;19(1):190–199. doi: 10.1016/s1053-8119(03)00056-9. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. Models of word production. Trends Cogn Sci. 1999;3(6):223–232. doi: 10.1016/s1364-6613(99)01319-4. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behav Brain Sci. 1999;22(1):1–38. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Wheeldon L. Do speakers have access to a mental syllabary? Cognition. 1994;50(1–3):239–269. doi: 10.1016/0010-0277(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Mattingly IG. The motor theory of speech perception revised. Cognition. 1985;21(1):1–36. doi: 10.1016/0010-0277(85)90021-6. [DOI] [PubMed] [Google Scholar]
- Mazaika P, Whitfield-Gabrieli S, Reiss A. Artifact repair for fMRI data from high motion clinical subjects. Poster presented at: 13th Annual Meeting of the Organization for Human Brain Mapping; Chicago, IL. Hum Brain Mapp Conf. 2007 [Google Scholar]
- Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC. Functional segregation within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cereb Cortex. 2005;15(7):986–994. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- Oakes TR, Johnstone T, Walsh KSO, Greischar LL, Alexander AL, Fox AS, Davidson RJ. Comparison of fMRI motion correction software tools. Neuroimage. 2005;28(3):529–543. doi: 10.1016/j.neuroimage.2005.05.058. [DOI] [PubMed] [Google Scholar]
- Okada K, Hickok G. Identification of lexical-phonological networks in the superior temporal sulcus using functional magnetic resonance imaging. Neuroreport. 2006;17(12):1293–1296. doi: 10.1097/01.wnr.0000233091.82536.b2. [DOI] [PubMed] [Google Scholar]
- Pisoni DB, Nusbaum HC, Luce PA, Slowiaczek LM. Speech perception, word recognition and the structure of the lexicon. Speech Commun. 1985;4(1–3):75–95. doi: 10.1016/0167-6393(85)90037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64(4):700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends Neurosci. 1998;21(5):188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Shuster LI, Lemieux SK. An fMRI investigation of covertly and overtly produced mono- and multisyllabic words. Brain Lang. 2005;93(1):20–31. doi: 10.1016/j.bandl.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Vitevitch M, Luce P. When words compete: levels of processing in perception of spoken words. Psychol Sci. 1998;9(4):325–329. [Google Scholar]
- Vitevitch MS, Luce PA. A web-based interface to calculate phonotactic probability for words and nonwords in English. Behav Res Methods Instrum Comput. 2004;36(3):481–487. doi: 10.3758/bf03195594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitevitch MS, Luce PA. Increases in phonotactic probability facilitate spoken nonword repetition. J Mem Lang. 2005;52(2):193–204. [Google Scholar]
- Vitevitch MS, Luce PA, Charles-Luce J, Kemmerer D. Phonotactics and syllable stress: implications for the processing of spoken nonsense words. Lang Speech. 1997;40(Pt 1):47–62. doi: 10.1177/002383099704000103. [DOI] [PubMed] [Google Scholar]
- Vitevitch MS, Luce PA, Pisoni DB, Auer ET. Phonotactics, neighborhood activation, and lexical access for spoken words. Brain Lang. 1999;68(1–2):306–311. doi: 10.1006/brln.1999.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992;256(5058):846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Meyer E, Gjedde A, Evans AC. PET studies of phonetic processing of speech: review, replication, and reanalysis. Cereb Cortex. 1996;6(1):21–30. doi: 10.1093/cercor/6.1.21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.