Abstract
A decade ago, bone morphogenetic protein 1 (BMP1) was shown to provide the activity necessary for proteolytic removal of the C-propeptides of procollagens I–III: precursors of the major fibrillar collagens. Subsequent studies have shown BMP1 to be the prototype of a small group of extracellular metalloproteinases that play manifold roles in regulating formation of the extracellular matrix (ECM). Soon after initial cloning of BMP1, genetic studies showed the related Drosophila proteinase Tolloid (TLD) to be necessary for formation of the dorsal-ventral axis in early embryogenesis. It is now clear that the BMP1/TLD-like proteinases, conserved in species ranging from Drosophila to humans, act in dorsal-ventral patterning via activation of transforming growth factor β (TGFβ)-like proteins BMP2, BMP4 (vertebrates) and decapentaplegic (arthropods). More recently, it has become apparent that the BMP1/TLD-like proteinases are key activators of a broader subset of the TGFβ superfamily of proteins, with implications that these proteinases may be key in orchestrating formation of ECM with growth factor activation and BMP signaling in morphogenetic processes.
Keywords: BMP1, Procollagen, Extracellular matrix, Tolloid, Metalloproteinases, TGFβ
1. Introduction
Collagens I–III, the major fibrous components of vertebrate extracellular matrix (ECM), are synthesized as procollagen precursors with amino- (N-) and carboxyl- (C-) terminal propeptides (Fig. 1). Cleavage of both N- and C-propeptides occurs via highly specific calcium-dependent metalloproteinases with neutral pH optima (Hojima et al., 1985; Hojima et al., 1994; Kessler et al., 1986), and is necessary for releasing the mature triple helical portion of the molecule for proper self-assembly into fibrils (Myllyharju and Kivirikko, 2001; Canty and Kadler, 2005). The smaller N-propetides are processed by proteinases of the ADAMTS (A Disintegrin And Metalloproteinase with ThromboSpondin repeats) family (Greenspan and Wang, 2005); specifically via the structurally similar proteinases ADAMTS2, 3 and 14, which have overlapping but differing distributions in tissues (Colige et al., 1997; Le Goff et al., 2006; Wang et al., 2003). Deficiencies in N-propeptide processing leads to the arthrochalasis and dermatosparaxis forms of Ehlers-Danlos Syndrome (EDS) (Byers, 1995). Interestingly, in these genetic connective tissue disorders, triple helical trimers are still incorporated into collagen fibrils, although the morphology of those fibrils can be aberrant (Byers, 1995). This is consistent with the observation that collagen monomers retaining N-propeptides are efficiently incorporated into elongating fibrils in vitro (Prockop and Hulmes, 1994). In contrast, retention of the bulkier C-propeptides precludes in vitro incorporation of monomers into growing fibrils (Prockop and Hulmes, 1994). The latter observation and the absence of identified genetic disorders in which procollagen C-propeptides remain uncleaved suggest that C-propeptide cleavage is a rate-limiting step necessary to both fibrillogenesis and development itself. Thus, enzymes responsible for removal of the procollagen I–III C-propeptides may be important control points in morphogenesis.
Fig. 1.
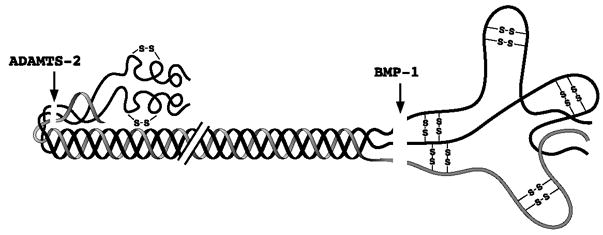
Processing of the N- and C-propeptides of type I procollagen by ADAMTS-2 and BMP-1, respectively. The single, shorter pro-α2 chain and two pro-α1 chains of type I procollagen are shown in grey and black, respectively.
In seminal work, Urist and colleagues demonstrated that protein factor(s) from demineralized bone extracts could induce ectopic bone formation (Urist, 1965; Urist et al., 1973) via a process recapitulating the endochondral bone formation of normal development (Reddi and Huggins, 1972). These factor(s) were designated bone morphogenetic protein (BMP) (Urist et al., 1973), and extensive purification of bone inductive activity from bone extracts led to initial isolation of BMPs 1–7 (Celeste et al., 1990; Wang et al., 1988; Wozney et al., 1988). BMPs 2–7 are members of the TGFβ superfamily of proteins, and over 30 TGFβ-like BMPs have now been identified, primarily via sequence homology, in a broad range of species (Hogan, 1996; Ducy and Karsenty, 2000). BMP1 differs from all other BMPs, in that it is not a TGFβ-like protein. Rather, it contains a metalloproteinase domain and initially was thought to act in bone morphogenesis via activation of the TGFβ-like BMPs with which it co-purified (Wozney, et al., 1988). In 1996 BMP1 was independently shown by two groups to provide the procollagen C-proteinase (pCP) activity responsible for cleaving the C-propeptides from procollagens I–III (Kessler et al., 1996; Li et al., 1996). Subsequently, BMP1 has become the prototype of a small group of proteinases with conserved domain structure, shown to process a variety of precursors into mature functional proteins with roles in ECM formation. It has also become apparent that these proteinases activate a subset of the TGFβ superfamily of proteins and that they play fundamental roles in development, perhaps via the orchestration of ECM formation and growth factor activity. This review details features of this group of proteinases and their functions, with emphasis on recent findings. The reader is directed to earlier reviews for more exhaustive coverage of biosynthetic processing of collagen molecules by various proteinases (Greenspan, 2005) and the developmental aspects of BMP1/TLD-proteinase activities (Ge and Greenspan, 2006).
2. BMP1/TLD family members
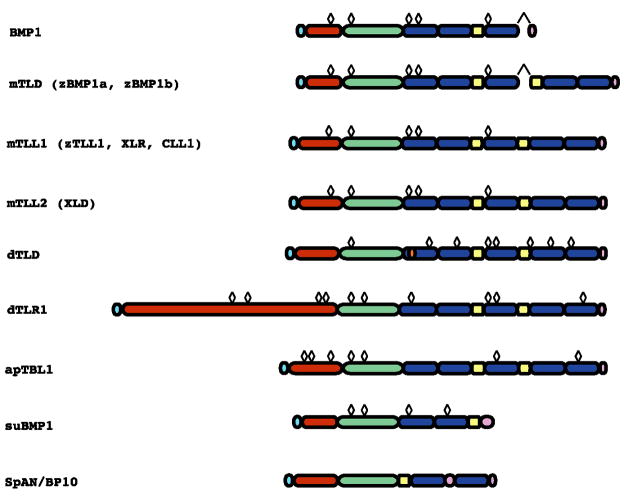
Subsequent to initial cloning of BMP1, the Drosophila protein Tolloid (TLD), the product of one of at least seven zygotically active genes necessary for dorsal-ventral patterning in gastrulation, was found to have a protein domain structure strikingly similar to that of BMP1 (Shimell et al., 1991). This finding demonstrated a previously unsuspected link between morphogenetic patterning and proteases involved in ECM formation. BMP1 has since become the prototype of a small group of metalloproteinases conserved in species ranging from Drosophila to humans. The distinct protein domain structure of BMP1 comprises an amino-terminal prodomain, followed by a conserved protease domain, characteristic of the astacin M12A family of metzincin metalloproteinases (Bond and Beynon, 1995; Stocker et al., 1995), and more carboxyl-terminal CUB (complement-uegf-BMP1) and EGF (epidermal growth factor)-like domains (Fig. 2). Drosophila TLD differs from BMP1 in having additional carboxyl-terminal EGF and CUB motifs. The prodomains are not required for secretion of at least some BMP1/TLD-like proteinases (Marques et al., 1997; Sieron et al., 2000), but are required for maintaining latency (Marques et al., 1997; Sieron et al., 2000; Leighton and Kadler, 2003). The prodomains appear to be proteolytically removed via subtilisin-like proprotein convertases (SPCs), within the trans-Golgi compartments of some cells (Leighton and Kadler, 2003), although some cell types secrete BMP1/TLD-like proteinase as unprocessed forms (Lee et al., 1997; Jasuja et al., 2007). CUB domains are thought to mediate protein-protein interactions (Bork and Beckmann, 1993). EGF-like domains bind Ca2+ in some proteins (Handford et al., 1990), but the Ca2+-dependent proteolytic activites of BMP1/TLD-like proteinases do not appear to rely on Ca2+ binding to EGF motifs (Garrigue-Antar et al., 2004; Ge et al., 2006). Rather, EGF domains bound to Ca2+ may confer a rigid configuration to portions of BMP1/TLD-like proteinase, thereby affecting their structural and functional properties. BMP1 contains a number of sites for Asn-linked glycosylation, some of which are conserved in all family members (Fig. 2). Blocking glycosylation at all sites or at all three CUB domain sites blocks secretion of BMP1, whereas blockage at some of the individual sites, while not blocking secretion, can affect thermal stability and levels of pCP activity (Garrigue-Antar et al., 2002)
Fig. 2.
Domain structures of BMP1/TLD-like proteinases. Light blue, red, green, dark blue, yellow and pink represent signal peptides, prodomains, metalloprotease, CUB and EGF domains and domains unique to each protein, respectively. Chevrons denote alternative splicing that produces BMP1 and mTLD from the same gene. Diamond shapes atop schematics represent potential Asn-linked glycosylation sites conserved in orthologues across all species represented in the figure. In addition, zebrafish BMP1 has two, XLR has one, and XLD has 4 additional prodomain sites; and mTLL2 has one additional site in the fourth CUB domain, not found in corresponding orthologues of other species, and not displayed in the figure.
There are four mammalian BMP1/TLD-like proteinases (Fig. 2), including mammalian TLD (mTLD), which is encoded by alternatively spliced mRNA produced by the Bmp1 gene (Takahara et al., 1994), and mammalian TLD-like 1 and 2 (mTLL1 and mTLL2), which are genetically distinct (Scott et al., 1999; Takahara et al., 1996). The domain structures of all of these are essentially identical to that of Drosophila TLD. Genes that encode alternatively spliced RNAs for a shorter BMP1 form and a longer mTLD-like form appear to be conserved in vertebrates, as such an arrangement has also been reported for Xenopus (Lin et al., 1997) and chick (Reynolds et al., 2000).
Apparently due to partial genome duplication in teleosts, two genes exist for BMP1 in zebrafish (bmp1a and bmp1b) (Muraoka et al., 2006; Jasuja et al., 2006), although only sequences for longer mTLD-like proteinases have been described for these genes, and it has yet to be determined whether they also encode alternatively spliced mRNAs for shorter BMP1 forms. In contrast, cDNA encoding the short form of BMP1 (suBMP), has been described in the sea urchin S. purpuratus (Hwang et al., 1994), with a longer alternatively spliced form yet to be described. Interestingly, suBMP appears to play a role in spiculogenesis (Hwang et al., 1994), or formation of the sea urchin skeleton, thus paralleling at least one of the roles of vertebrate BMP1. Another sea urchin protein with a somewhat different configuration of astacin protease, CUB and EGF domains SpAN/BP10 (Fig. 2), is transiently expressed in the sea urchin blastula (Lepage et al., 1992; Reynolds et al., 1992), and may be involved in modulating BMP signaling (Wardle et al., 1999). Apparent orthologues for mTLL1 have been reported in zebrafish, Xenopus, and chick. In zebrafish this orthologue, TLL1, is the product of a gene originally designated mini fin (mfn) (Connors et al., 1999), but now also known as tll1 (Zebrafish Information Network); whereas in Xenopus and chick, mTLL1 orthologues have been designated Xolloid-related (XLR) (Dale et al., 2002) and Colloid-like 1 (Liaubet et al., 2000), respectively. An apparent mTLL2 orthologue designated Xolloid (XLD) has been reported for Xenopus (Goodman et al., 1998), but an mTLL2 orthologue has yet to be reported for zebrafish.
In addition to the above, a second BMP1/Tolloid-like proteinase, tolloid-related 1 (TLR1, also known as tolkin) (Finelli et al., 1995; Nguyen et al., 1994) has been characterized in Drosophila, and differs from other members of the group in having a relatively elongated prodomain (Fig. 2). Sequence comparisons do not show either Drosophila TLD or TLR1 to be more related to one of the vertebrate BMP1/TLD-like proteinases than to another. Thus there is no clear orthologous relationship between either of the Drosophila proteinases and any one of the vertebrate members of this proteinase subgroup. A single BMP1/TLD-like proteinase, Aplysia Tolloid/BMP1-like 1 (apTBL1) has been reported for the mollusk Aplysia (Liu et al., 1997) and has a domain structure identical to that of vertebrate and Drosophila members of this proteinase subgroup. However, apTBL1, which has an implied role in the synaptic plasticity associated with learning, is not a clear-cut orthologue of any one of the vertebrate proteinases, although some homology in the prodomain (Liu et al., 1997), and the placement of potential Asn-linked glycosylation sites in the protease domain (Fig. 2) suggest that it is more closely related to Drosophila TLR1 than to Drosophila TLD.
3. Direct roles of BMP1/TLD-like proteinases in ECM formation
Since the finding that BMP1/TLD-like proteinases provide pCP activity, it has become apparent that these proteinases process various precursors into functional structural proteins and into active enzymes involved in ECM formation in general and collagen fibrillogenesis in particular. A role has also been described for BMP1/TLD-like proteinases in the initiation of mineralization in hard connective tissues.
3.1 Fibrillar collagens
Subsequent to the finding that BMP1 provides pCP activity (Kessler et al., 1996; Li et al., 1996), it was shown that each of the mammalian BMP1/TLD-like proteinases has some level of pCP activity; with BMP1 and mTLL2 showing highest and lowest in vitro pCP levels, respectively (Pappano et al., 2003; Scott et al., 1999). In fact, mTLL2 pCP levels are not observable in vitro, unless procollagen substrates and mTLL2 are co-incubated in the presence of a third protein known to enhance the pCP activity of BMP1/TLD-like proteinases (Pappano et al., 2003; and see below). Nonetheless, small amounts of residual pCP activity in mouse embryos doubly null for the genes which encode BMP1, mTLD and mTLL1, suggest that mTLL2 may be an in vivo pCP (Pappano et al., 2003). BMP1/TLD-like proteinases are secreted molecules, pCP activity has a pH optimum of 8.0 – 8.5 (Hojima et al., 1985; Kessler et al., 1986), and various studies have demonstrated both pCP activity and unprocessed procollagen in the extracellular space in cell and organ cultures. However, it has recently been reported that processing of procollagen C-propeptides of the major fibrillar collagens occurs intracellularly, under presumably acidic conditions, in developing tendon (Canty et al., 2004). Thus, procollagen I-III C-propeptide processing may occur intracellularly or extracellularly, in a cell type-specific/tissue-specific manner. The major fibrillar procollagens appear to form intracellular aggregates prior to secretion (Birk and Trelstad, 1984; Bonfanti et al., 1998; Canty et al., 2004), and these aggregates may constitute the normal in vivo substrates for pCP activity. The latter possibility is supported by the finding that aggregated type I procollagen is processed more rapidly by pCP activity than is monomeric procollagen (Hojima et al., 1994).
In addition to their roles in processing the major fibrillar collagen types I–III, vertebrate BMP1/TLD-like proteinases play roles in biosynthetic processing of the minor fibrillar collagen types V and XI, which combine with collagens types I and II, respectively, and regulate the size and shapes of the resulting heterotypic fibrils (Birk et al., 1990; Li et al., 1995; Mendler et al., 1989; Toriello et al., 1996). The most abundant form of type V collagen monomer is a heterotrimer of two α1(V) chains and one α2(V) chain, although α1(V)3 homotrimers and α1(V) α2(V) α3(V) heterotrimers, with more restricted distributions of expression, are also found (Fessler and Fessler, 1987). Type XI collagen consists of α1(XI)α2(XI)α3(XI) heterotrimers (Morris and Bachinger, 1987). In addition, heterotrimers containing α chains of both type V and type XI collagens have been reported (Kleman et al., 1992; Mayne et al., 1993), suggesting that V and XI may represent a single collagen type with various α chains expressed differentially in a tissue-specific manner. BMP1/TLD-like proteinases cleave the C-propeptides of proα2 (V) chains (Unsold et al., 2002), which are more closely related in protein domain structure to the chains of the major fibrillar procollagens than are the proα1(V), proα1(XI), and proα2(XI) chains. Surprisingly, the BMP1/TLD-like proteinases appear to cleave the latter three chains solely within their relatively large amino-terminal globular sequences, leaving smaller, trimmed amino-terminal globular sequences (Imamura et al., 1998; Pappano et al., 2003; Unsold et al., 2002). These proteinases can also cleave within proα3(V) amino-terminal globular sequences (Gopalakrishnan et al., 2004).
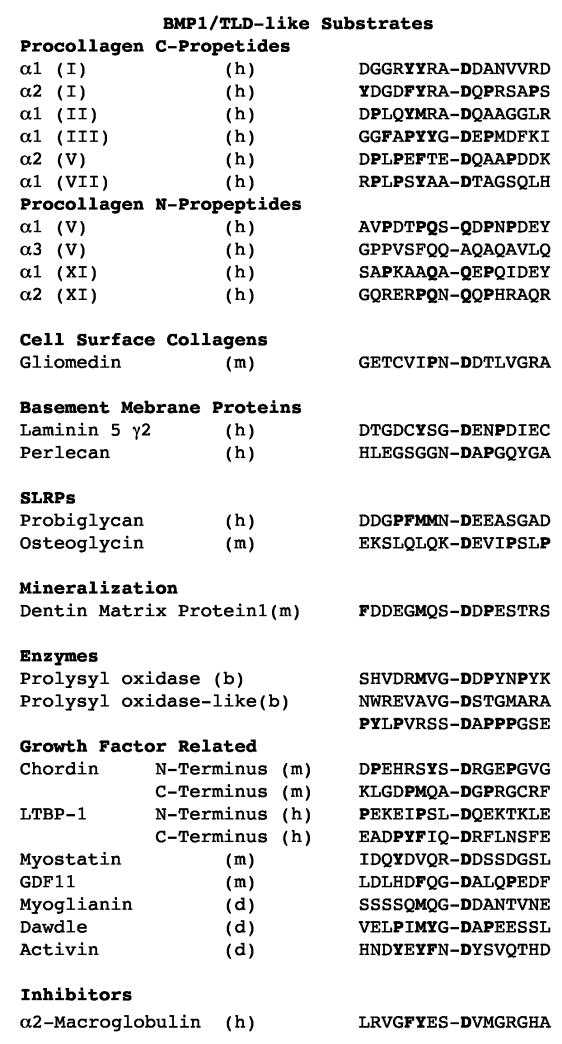
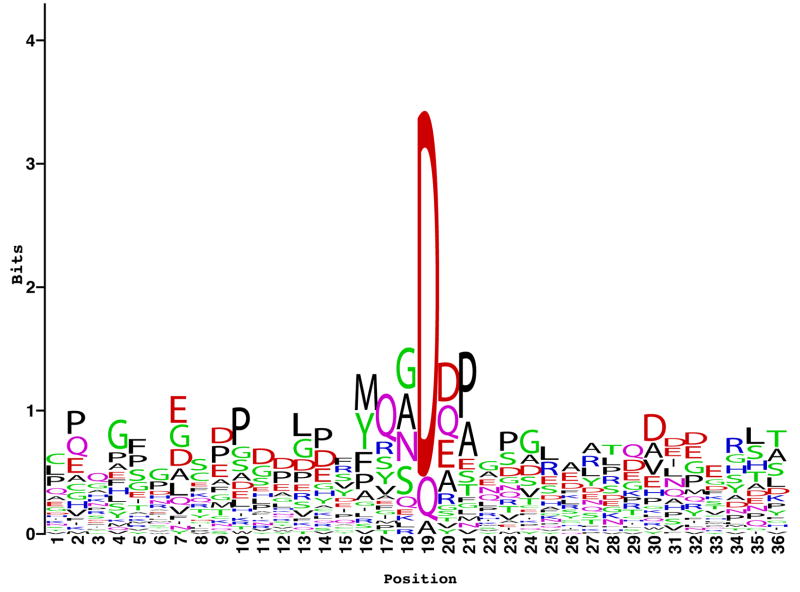
Processing of minor and major fibrillar collagen propeptides by the same proteinases may coordinate deposition of the two types of fibrillar collagens into the same heterotypic fibrils. It is the proteolytically trimmed amino-terminal globular sequences of the minor fibrillar collagen chains that are thought to regulate the shapes and diameters of heterotypic fibrils (Linsenmayer et al., 1993). Thus, proteolytic trimming of the amino-terminal globular sequences of minor fibrillar procollagen chains appears to be another way in which the BMP1/TLD-like proteinases regulate collagen fibrillogenesis. Surprisingly, the cleavage sites at which BMP1/TLD-like proteinases process amino-terminal globular sequences of types V and XI procollagen chains contain glutamine residues at the P1′ positions, rather than the aspartates observed at the P1′ sites of all other known substrates (Ge and Greenspan, 2006; Imamura et al., 1998; Pappano et al., 2003) (Fig. 3). Thus, although there is a clear preference for certain residues at various positions adjoining the cleavage sites of BMP1/TLD-like proteinase substrates (Fig. 4), there is no absolute requirement for a particular residue at any site, and substrate tertiary configuration may play an important role in determining cleavage sites.
Fig. 3.
Cleavage sites of known substrates of BMP1/TLD-like proteinases. Residues in boldface type have previously been reported to be conserved either at the cleavage sites of the N-propeptides of minor fibrillar procollagens, or at the cleavage sites of the majority of other substrates.
Fig. 4.
Amino acid conservation adjoining cleavage sites in known substrates of BMP1/TLD-like proteinases. Amino acid sequence specificity was examined for a 36 amino acid region flanking each cleavage site of the 29 substrates of Fig. 3. The sequence logo of these sites was plotted by the WebLogo software of Crooks et al. (2004). At each position of the logo, in which the cleavage site is between positions 18 and 19, amino acids are arranged in the order of their occurrence frequency from top to bottom, with the highest-frequency amino acid being on top of the stack. The height of the entire stack is proportional to the information measured in bits at that position, which helps in easily identifying the most important positions, whereas the height of each letter is proportional to the occurrence frequency of the corresponding amino acid at that position. For each position, the null hypothesis was tested that the amino acid composition of the site follows a random distribution, which assigns an equal occurrence probability to each amino acid. Nominal p-values from the chi-square goodness-of-fit test of each position showed positions 10 and 16–21 to exhibit strong evidence against the null hypothesis. In addition, although positions 7 and 23 did not pass the Bonferroni adjusted p-value cut-off, which accounts for multiple testing (Westfall et al., 1993), they had notably small p-values (0.0016 and 0.002, respectively).
In addition to processing fibrillar procollagens, the BMP1/TLD-like proteinases affect fibrillogenesis by activating the zymogen of lysyl oxidase (Panchenko et al., 1996; Uzel et al., 2001), an enzyme necessary to forming the covalent crosslinks that provide collagen and elastic fibers with most of their tensile strength. Similarly, BMP1 has been shown to activate the related cross-linking enzyme lysyl oxidase-like (Borel et al., 2001). Another way in which BMP1/TLD-like proteinases affect fibrillar collagen-containing ECM is via cleavage of the non-collagenous Small Integrin-Binding LIgand N-linked Glycoprotein (SIBLING) protein, Dentin Matrix Protein-1 (DMP-1) (Steiglitz et al., 2004), thereby releasing a highly acidic Ca2+ binding domain believed to help initiate mineralization of the type I collagen fibrils of bone and teeth (Butler, 1998, Veis, 1993).
3.2 Non-fibrillar collagens
Anchoring fibrils, consisting of type VII collagen, are important structural components that mediate attachment of epidermis to underlying stroma (Sakai et al., 1986). Synthesized and secreted by epidermal keratinocytes, type VII procollagen monomers self-assemble into anti-parallel dimers, which then associate laterally to form anchoring fibrils (Keene et al., 1987; Morris et al., 1986). Initial dimer formation and stabilization through disulfide bonding seems to require retention of the type VII procollagen C-propeptide, also known as the NC2 domain (Chen et al., 2001; Lunstrum et al., 1987). However, proteolytic removal of NC2 seems necessary to anchoring fibril formation, as a mutation that eliminates the cleavage site results in the severe blistering disease dystrophic epidermolysis bullosa (Bruckner-Tuderman et al., 1995). This mutation removes 29 amino acids containing a potential cleavage site with features found in known substrates of BMP1/TLD-like proteinases (Figs. 3 and 4). In vitro cleavage assays have confirmed that BMP1, mTLL1 and mTLL2 are capable of cleaving the NC2 domain (Rattenholl et al., 2002), however type VII collagen appears to be fully processed in the skin of mouse embryos null for the Bmp1 gene, which encodes both BMP1 and mTLD (Rattenholl et al., 2002). Thus, mTLL1 and mTLL2 may fully compensate for loss of Bmp1 in these mice, for removal of the procollagen VII NC2 domain. Direct testing of this possibility is not feasible at this time, as mice lacking the Tll1 gene, which encodes mTLL1, die at ~13.5 days post conception (Clark et al., 1999), at which stage the dermal-epidermal basement membrane zone remains poorly developed; and Tll2−/− mice have not yet been reported. Thus, detailed analysis of in vivo roles for BMP1/TLD-like proteinases in processing type VII procollagen requires development of mice conditionally null for mTLL1and null for mTLL2.
Gliomedin, a recently identified transmembrane collagen containing an olfactomedin domain, is essential to formation of the nodes of Ranvier (Eshed et al., 2005). Expressed on the surface of Schwann cells, gliomedin serves as a ligand for the neuronal Immunoglobulin Cell Adhesion Molecules (IgCAMs) Nr-CAM and neurofascin (Eshed, et al., 2005). Interaction of gliomedin with these IgCAMs induces Na+ channel clustering at nodes that enables saltatory conduction in peripheral myelinated nerve fibers. Although gliomedin is a transmembrane glycoprotein, it is also shed from the cell surface by furin-like proprotein convertases at a juxtamembrane site (Maertens et al., 2007). Thus, in various respects, gliomedin resembles other integral transmembrane proteins containing collagen domains, which are also shed from the cell surface. These include ectodysplasin A and collagens XIII, XVII and XXV (Franzke et al., 2005). It has recently been demonstrated that gliomedin ectodomains shed from the cell surface are further processed by BMP1/TLD-like proteinases, which cleave between the collagen-like and olfactomedin domains (Maertens et al., 2007). Although the physiological relevance of the latter cleavage is not yet clear, such cleavage appears to favor oligomerization of freed olfactomedin domains, which may in turn contribute to maintenance of the nodes of Ranvier (Maertens et al., 2007).
3.3 Non-collagenous matrix proteins
Laminin-5, a trimer comprising α3, β3, and γ2 chains, is a key component of anchoring filaments, which help keratinocytes adhere to the basement membrane (Rousselle, et al., 1991), is. Laminin-5 is secreted as a precursor that undergoes processing of its α3 and γ2 chains (Marinkovich et al., 1992; Rousselle et al., 1991). Matrix metalloprotease 2 (MMP2) is capable of cleaving rat laminin-5 γ2 chains in vitro (Giannelli et al., 1997), although this cleavage site is not conserved in human laminin-5. It has also been reported that Membrane type 1 matrix metalloprotease (MT1-MMP) may be the primary enzyme responsible for cleaving the rat γ2 chain (Koshikawa et al., 2000). However, BMP1/TLD-like proteinases are the only enzymes thus far demonstrated to cleave both rat and human γ2 chains at the sites employed by keratinocytes (Amano et al., 2000). Moreover, analysis of laminin-5 in the skin of Bmp1−/− mice has revealed a deficiency in γ2 chain processing (Veitch et al., 2003). MMP2, MT1-MMP, plasmin, and BMP1/TLD-like proteinases are all capable of processing the α3 chain to a size consistent with that observed in keratinocyte cultures (Amano et al., 2000; Goldfinger et al., 1998; Veitch et al., 2003). However, a small molecule inhibitor specific to BMP1/TLD-like proteinases is able to inhibit processing of both the γ2 and α3 chains in keratinocyte cultures, although inhibition of α3 cleavage required higher inhibitor concentrations and was incomplete (Veitch et al., 2003). Thus, BMP1/TLD-like proteinases may be involved in processing both γ2 and α3 chains. Such processing is of importance, as processed and unprocessed forms of laminin 5 appear to have different properties in regard to cell adhesion and motility, and the invasiveness of squamous cell carcinomas (Giannelli et al., 1997; Tsubota et al., 2005).
Perlecan is a large proteoglycan that is a major structural component of basement membranes (Murdoch et al., 1994). The perlecan carboxyl-terminal domain, domain V, is responsible for most of the molecule’s ability to bind cells and, when cleaved, corresponds to a fragment designated endorepellin, with angiostatic activity (Mongiat et al., 2003). Endorepellin comprises EGF-like repeats and laminin-like globular (LG) motifs. The most C-terminal LG3 motif appears to harbor most of the angiostatic activity (Bix et al., 2004) and is proteolytically removed from endorepellin and from full-length perlecan by BMP1/TLD-like proteinases (Gonzalez et al., 2005). The BMP1/TLD-like proteinase cleavage site for LG3 is identical to the site at which perlecan is cleaved in vivo to produce LG3 fragments found in the urine of patients with end-stage renal disease (Oda et al., 1996) and in the amniotic fluid of pregnant women with ruptured fetal membranes (Vuadens et al., 2003), suggesting that cleavage by these proteinases is physiologically relevant. Recently, the LG3 domain was also shown to promote apoptosis-resistance in fibroblasts via interactions with α2β1 integrins (Laplante et al., 2006). As accumulation of apoptosis resistant fibroblasts may be a hallmark of fibrosis (Arora and McCulloch, 1999; Desmouliere et al., 1995), proteolytic processing of perlecan by BMP1/TLD-like proteinases may have functional implications in both angiogenesis and fibrosis.
Small leucine-rich proteoglycans (SLRPs) are a small family of non-aggregating proteoglycans currently containing 11 members in mammals (Iozzo, 1998; Svensson et al., 2001). SLRPs can be subdivided into four classes. Members of class I (decorin and biglycan) and IV (epiphican, osteoglycin and opticin) SLRPs are synthesized as precursors that are processed in vivo to produce the mature proteins (Funderburgh et al., 1997; Johnson et al., 1997; Roughley et al., 1996). Biglycan has been shown to be processed by BMP1/TLD-like proteinases in vitro and in vivo (Scott et al., 2000). Since biglycan is thought to play a positive role in bone formation (Bianco et al., 1990) and to be involved in regulating TGFβ signaling (Chen et al., 2002), its processing may represent novel mechanisms by which BMP1/TLD-like proteinases affect osteogenesis and TGFβ signaling (also see below), respectively. It should be cautioned however that it is not yet known how processing affects biglycan function. Enzymes responsible for cleavage of decorin have yet to be determined.
Class IV SLRPs osteoglycin and epiphycan N-propeptides are also proteolytically cleaved in vivo (Funderburgh et al., 1997; Johnson et al., 1997). All four BMP1/TLD-like proteinases are capable of cleaving pro-osteoglycin in vitro (Ge et al., 2004), and Bmp1/Tll1 doubly homozygous null mice produce pro-osteoglycin exclusively in the unprocessed form (Ge et al., 2004). Osteoglycin is believed to function as a regulator of collagen fibril diameter (Ge et al., 2004; Tasheva et al., 2002), and in vitro fibrillogenesis assays revealed that mature osteoglycin is more efficient in regulating type I collagen fibrillogenesis than is the pro-form (Ge et al., 2004). This suggests another way in which BMP1/TLD-like proteinases may affect collagen fibrillogenesis. Epiphycan is also processed at a site similar to those observed in substrates of BMP1/TLD-like proteinases (Johnson et al., 1997). However, further studies are required to ascertain whether BMP1/TLD-like proteinases are responsible for epiphycan processing.
4. BMP1/TLD-like proteinase activation of a subset of TGFβ-like proteins
One of the earliest functions ascribed to BMP1/TLD-like proteinases was the potentiation of signaling by TGFβ-like BMPs in dorsal-ventral patterning. It has more recently become apparent that these proteinases are involved in activating a growing list of members of the TGFβ superfamily.
4.1 BMPs 2 and 4
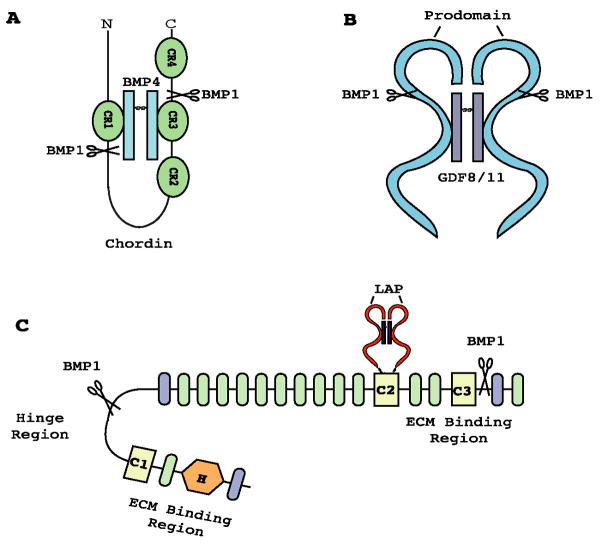
TGFβ-like BMPs play a broad range of roles in processes that include dorsal/ventral patterning, organogenesis, neural development (Hogan, 1996; Furuta et al., 1997; De Robertis and Kuroda, 2004), and osteogenesis (Urist et al., 1973; Wozney et al., 1988). In particular, signaling gradients of BMP2 and BMP4 in vertebrates, and of the Drosophila orthologue decapentaplegic (DPP), are the major determinants for forming the dorsal-ventral axis in gastrulation (Hogan, 1996). It is now known that Drosophila TLD activates DPP by cleaving the extracellular antagonist Short Gastrulation (SOG) (Marques et al., 1997). Similarly, the vertebrate SOG orthologue Chordin is cleaved in vitro, at two sites (Fig. 5A), and counteracted in overexpression assays in Xenopus and zebrafish embryos, by various BMP1/TLD-like proteinases (Blader et al., 1997; Dale et al., 2002; Piccolo et al., 1996; Scott et al., 1999; Wardle et al., 1999). In mammals, BMP1 and mTLL1, but not mTLD or mTLL2, are capable of cleaving Chordin in vitro (Scott et al., 1999); and products of the Bmp1 and Tll1 genes are responsible for cleaving Chordin in vivo (Pappano et al., 2003). It has been postulated that the two most carboxyl-terminal mTLD CUB domains are held by EGF-like motifs in a conformation that blocks Chordinase activity, as removal of one or both EGF motifs provides the truncated mTLD with readily detectable Chordinase activity (Garrigue-Antar et al., 2004).
Fig. 5.
Activation of TGFβ-like growth factors by BMP1/TLD-like growth factors. Scissors represent cleavage by BMP1/TLD-like proteinases within Chordin (A), the prodomains of GDF8 and 11 (B), and LTBP1 (C). Chordin comprises four cysteine-rich (CR) domains connected by linker regions, and binds dimeric BMP4 or BMP2 (A). Cleaved GDF8/11 prodomains (blue) form non-covalent latent complexes with their cognate mature regions (disulfide-linked dimers, shown in purple) (B). LTBP1 has three 8-cysteine domains (C1-3). The LAP prodomain (red) of TGFβ is disulfide bound to C2, and the mature TGFβ dimer (black) is noncovalently bound to LAP. Other LTBP1 domains are Ca2+-binding (green) and non-binding (purple) EGF-like motifs, and a “hybrid” domain (H).
Although TLD-null Drosophila have severe dorsal-ventral patterning defects (Childs and O’Connor, 1994; Finelli et al., 1994), mice null for the Bmp1 gene show what appears to be relatively mild dorsal-ventral patterning defects, reflected in failure to close the ventral body wall (Suzuki et al., 1996). The latter may also reflect deficient ECM production, as do abnormal collagen fibrillogenesis and defective membranous bone formation in these mice. Tll1 knockout mice are embryonic lethal at 13.5 days post conception, with defects confined almost entirely to the heart (Clark et al., 1999). The cardiac defects in these mice do not appear to arise from defective collagen fibrillogenesis, which is not affected in an obvious way (Clark et al., 1999). Rather, Tll1-null cardiac abnormalities may arise due to failure to process Chordin in this tissue, resulting in aberrations in the BMP signaling normally involved in septation and looping of the vertebrate heart (Clark et al., 1999). Absence of severe dorsal-ventral patterning defects in Bmp1 and Tll1 null mice suggests functional redundancy. Such redundancy is confirmed by the observation that mouse embryos doubly null for both Bmp1 and Tll1 have less pCP activity than do embryos singly null for either gene; thereby showing products of both genes to be in vivo pCPs (Pappano et al., 2003). However, doubly null embryos die at the same point in development as do Tll1-null embryos, without obvious dorsal-ventral patterning defects (Clark et al., 1999), thus suggesting residual Chordinase activity from an unknown protease(s). mTLL2 may provide such activity in vivo, since it has Chordinase activity in vitro in the presence of the modulator protein twisted gastrulation (TSG) (Scott et al., 2001; also see below). In contrast to mammals, knockdown of the expression of the bmp1a and tll1 genes in zebrafish is sufficient to severely dorsalize embryos (Jasuja et al., 2006; Muraoka et al., 2006), indicating less redundancy for Chordin cleavage in teleosts than in mammals. In addition to their roles in patterning, BMPs 2 and 4 are potently osteoinductive (Wozney et al., 1988). Thus, activation of BMPs 2 and 4 is another way in which BMP1/TLD-like proteinase affect skeletogenesis, in addition to their roles in maturing proteins necessary to normal bone formation (e.g. collagen type I, lysyl oxidase, biglycan, and DMP1).
4.2 Growth and differentiation factors 8 and 11 (GDFs 8 and 11)
All TGFβ-like proteins are synthesized as proproteins, containing an amino-terminal prodomain that must be cleaved by SPCs for full activity of the mature protein (Ducy and Karsenty, 2000; Hogan, 1996). Although such cleavage is sufficient for activating most TGFβ-like proteins (Hogan, 1996), prototypical family members TGFβ 1–3 remain non-covalently bound to their cleaved prodomains in latent complexes (Massague, 1998). More recently it has been demonstrated that GDF8 and 11, which share ~90% sequence identity in their mature regions (Gamer et al., 1999; Ge et al., 2005; Nakashima et al., 1999), are also non-covalently bound to their respective prodomains in latent complexes (Ge et al., 2005; Hill et al., 2002; Lee and McPherron, 2001; Wolfman et al., 2003) (Fig. 5B). Secreted by myocytes, GDF8, also known as Myostatin, is an important negative regulator of skeletal muscle growth (Hill et al., 2002; Lee and McPherron, 2001; McPherron et al., 1997), whereas GDF11, secreted by mature neurons, serves a comparable function in inhibiting neurogenesis (Wu et al., 2003). GDF11 also appears to play roles in anterior-posterior patterning of the axial skeleton (McPherron et al., 1999). BMP1/TLD-like proteinases have been demonstrated to cleave within GDF8 and 11 prodomain sequences, thus liberating the mature proteins and enabling downstream signal transduction (Ge et al., 2005; Wolfman et al., 2003). Interestingly, recombinant GDF8 prodomain with a single amino acid substitution that renders it resistant to cleavage by BMP1/TLD-like proteinases is capable of inducing increased muscle mass when injected into normal adult mice, whereas wild type GDF8 prodomain has no effect (Wolfman et al., 2003). This supports an in vivo role for activation of GDF8 by BMP1/TLD-like proteinases, and suggests mutated GDF8 and 11 prodomain sequences as possible therapeutic tools for reversing the pathologies of muscular dystrophies and degenerative diseases and injuries of neural tissues, respectively.
4.3 Drosophila TLD activates TGFβ-like factor Daw and affects axon guidance
Two recent studies found mutations in the gene for the Drosophila BMP1/TLD-like proteinase TLR, also known as Tolkin, to cause strong defects in fasciculation and guidance of motor axons (Meyer and Aberle, 2006). Since TGFβ-like molecules have been implicated in axon guidance in C. elegans and mouse, and because BMP1/TLD-like proteinases had previously been demonstrated to proteolytically activate GDF8 and 11 (see above), Serpe and O’Connor (Serpe and O’Connor, 2006) sought to determine whether the role of TLR in motor neuron axon guidance might involve activation of TGFβ-like proteins. TLR was found to process the prodomains of three of the four Drosophila non-BMP TGFβ-like proteins: Activin (Act), Dawdle (Daw), and Myostatin homolog Myoglianin (Myo), each of which contains at least one site resembling cleavage sites in known substrates of BMP1/TLD-like proteinases (Fig. 3). Both TLR and Drosophila TLD were capable of cleaving Act, Daw and Myo prodomains in vitro, to produce fragments consistent in size with cleavage having occurred at the predicted sites. Furthermore, alanine substitution for residues at the P3, P2, P1 and P1′ positions at the predicted site of each prodomain inhibited processing by TLR and TLD. Surprisingly, only cleavage of the Daw prodomain was conclusively shown to result in increased signaling, as determined via Smad2 phosphorylation levels in a cell culture-based signaling assay (Serpe and O’Connor, 2006). The distribution of Daw expression in muscle and central nervous system is consistent with a role in fasciculation and axon guidance of motor neurons; whereas the finding that trans-heterozygous combinations of mutations in the tlr and daw genes had enhanced axon guidance defects compared with single heterozygous flies, was consistent with a role for TLR activation of Daw in axon guidance/fasciculation (Serpe and O’Connor, 2006). However, fasciculation defects are substantially milder and more transient in daw than in tlr mutants, suggesting additional roles in axon guidance and fasciculation for TLR, beyond activation of Daw. An additional interesting finding of the Serpe and O’Connor study (Serpe and O’Connor, 2006) was that endogenous TLR normally circulates in the hemolymph. It remains to be determined whether any vertebrate BMP1/TLD-like proteinases are found in the general circulation, although such a finding would have ramifications for our understanding of how these proteinases contribute to morphogenetic processes.
4.4 BMP1/TLD-like proteinases activate TGFβ1 via cleavage of LTBP
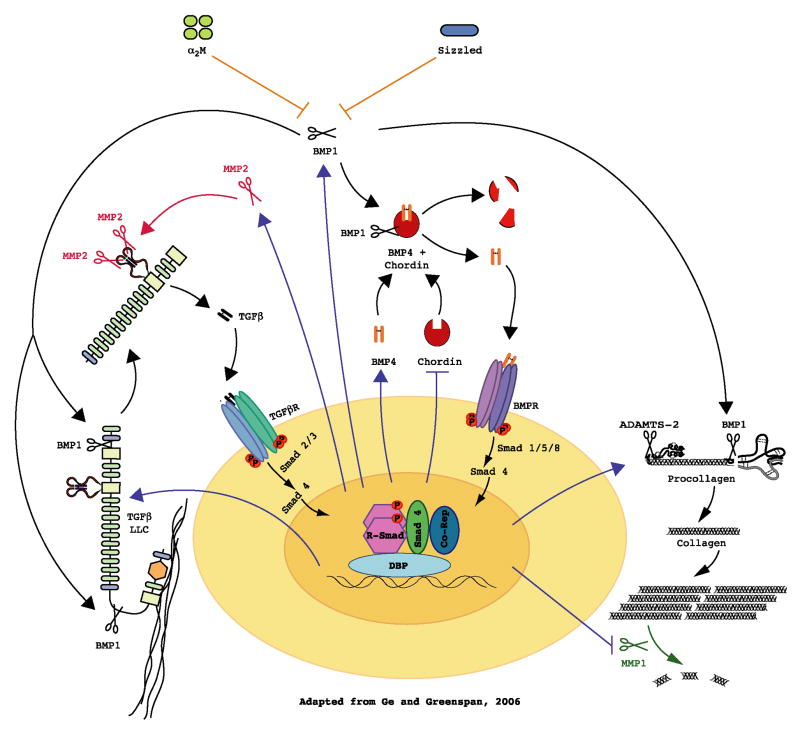
TGFβs 1–3 play fundamental roles in regulating cell differentiation, apoptosis, immune responses, tissue repair and ECM formation (Massague, 1990; Border and Ruoslahti, 1992; Derynck et al., 2001; Letterio and Roberts, 1998), and are key players in the etiology of fibrosis (Border and Ruoslahti, 1992). TGFβ signaling induces a net increase in ECM formation in development, tissue repair, and fibrosis via inhibiting expression of ECM-degrading proteases; and by increasing expression of structural components of the ECM, lysyl oxidase, and the proteinases that process ECM components and lysyl oxidase to their mature and functional forms (Lee et al., 1997; Massague, 1990; Wang et al., 2003). The latter include the BMP1/TLD-like proteinases (Lee et al., 1997). Thus, if BMP1/TLD-like proteinases were involved in activating TGFβ signaling, it would complete a positive feed-back loop in ECM formation. Binding of TGFβ1–3 prodomains to their cognate mature proteins in non-covalent latent complexes, subsequent to cleavage by SPCs, has garnered these prodomains the designation Latency Associated Peptides (LAPs) (Annes et al., 2003). The complex formed by TGFβ and LAP is known as the small latent complex (SLC) (Annes et al., 2003). However, most TGFβ is secreted as a Large Latent Complex (LLC), comprising SLC disulfide bonded, via LAP, to the genetically distinct protein Latent TGFβ-binding Protein (LTBP) (Annes et al., 2003) (Fig. 5C). LLC is in turn bound to the ECM via LTBP, perhaps by covalent binding of LTBP to ECM components (Annes, et al., 2003). TGFβ can be activated via cleavage by MMPs (D’Angelo et al., 2001; Maeda et al., 2001; Yu and Stamenkovic, 2000) or via interactions with thrombospondin (Crawford, et al., 1998) or integrins (Mu et al., 2002; Munger et al., 1999; Yang et al., 2007). However, a role has also recently been described for BMP1/TLD-like proteinases in activating TGFβ LLCs (Ge and Greenspan, 2006). Specifically, although BMP1/TLD-like proteinases do not cleave LAP, they were found to cleave LTBP1 (one of four mammalian LTBPs) at two sites (Figs. 3 and 5C) in vitro (Ge and Greenspan, 2006). In addition, Bmp1/Tll1 doubly homozygous null mouse embryo fibroblasts were found to produce only unprocessed LTBP1, in contrast to wild type (Ge and Greenspan, 2006). Although LTBP1 cleavage by BMP1/TLD-like proteinases does not directly activate TGFβ1, it does appear to liberate LLCs from the ECM, thereby rendering LAP susceptible to cleavage by MMP2, and possibly by MMPs 9 and 14, with consequent TGFβ1 activation (Ge and Greenspan, 2006).
TGFβ1 activation by BMP1/TLD-like proteinases, MMP2, and potentially MMP9, all of which are potently up-regulated by TGFβ1 (Brown et al., 1990; Lee et al., 1997; Marti et al., 1994; Overall et al., 1991; Sehgal and Thompson, 1999) suggests a fast-forward loop of potential importance to tissue remodeling (Fig. 6). Since mammals appear to possess various molecular mechanisms for latent TGFβ activation, the mechanism involving activation by BMP1/TLD-like proteinases may be limited to some subset of cell types and/or physiological/pathological circumstances. One candidate set of circumstances is development, as LTBP1 is clearly cleaved by BMP1/TLD-like proteinases in mouse embryo fibroblast cultures (Ge and Greenspan, 2006). Another set of circumstances may involve neoplasia, given the key roles of MMP2, other MMPs and TGFβ in the tissue remodeling associated with growth, angiogenesis and invasiveness of tumors (Derynck et al., 2001; Sternlicht and Werb, 2001; Yu and Stamenkovic, 2000), and the identification of BMP1 as among the most up-regulated genes in activated endothelia associated with tumor angiogenesis (St Croix et al., 2000).
Fig. 6.
An overview of roles of BMP1/TLD-like proteinases in morphogenetic events. BMP1/TLD-like proteinases process ECM precursors (e.g. procollagen) to mature functional ECM components. They also activate TGFβ by processing LTBP1, thereby releasing truncated LLC from the ECM, leading to consequent activation via cleavage of LAP cleavage by non-BMP1/TLD-like metalloproteinases (e.g. MMP 2). Activated TGFβ binds cell surface receptors, which activate R-Smads 2 and 3, which combine with Smad4 for translocation to the nucleus and up-regulation (arrows) of BMP1, ECM precursors (e.g. procollagen), MMP 2, and TGFβ itself. TGFβ also down-regulates some MMPs that degrade ECM (e.g. MMP1). The BMP1/TLD-like proteinases thus appear to be involved in a positive feedback/feed-forward loop for tissue remodeling. BMP1-like proteinases also activate BMPs 2/4 by cleaving the antagonist Chordin, thus inducing activation of R-Smads 1, 5, and 8. These may compete with R-Smads 2 and 3 for limiting amounts of Smad4. Chordin might also compete with ECM precursors and LTBP1 for BMP1/TLD-like proteinases. Smad4 and BMP1-like proteinases may thereby represent two levels at which cross-talk between TGFβ and BMP signaling pathways orchestrates tissue remodeling with patterning.
5. Modulators of BMP1/TLD-like Proteinase Activity
It is apparent that the BMP1/TLD-like proteinases play manifold roles in morphogenetic processes. Clearly, modulators of the activities of these proteinases would therefore be important regulators of the same morphogenetic processes. Interestingly, separate positive regulators have now been reported for the pCP and Chordinase activities of these proteinases. More recently, endogenous inhibitors have been described as well.
5.1 Procollagen C-Proteinase Enhancers
While not a proteinase itself, the glycoprotein Procollagen C-Proteinase Enhancer 1 (PCOLCE 1) was found to increase the pCP activity of BMP1/TLD-like proteinases ~10-fold (Kessler and Adar, 1989). More recently, a second PCOLCE (PCOLCE 2) was identified, with an identical protein domain structure and similar levels of pCP-enhancing activity (Xu et al., 2000; Steiglitz et al., 2002). Both PCOLCEs contain two amino-terminal CUB domains followed by a carboxyl-terminal Netrin-like (NTR) domain (Steiglitz et al., 2002; Takahara et al., 1994). Full pCP enhancing activity appears to reside in the CUB domains (Takahara et al., 1994), through which PCOLCEs are thought to bind directly to procollagen C-propetides and -telopeptide sequences, thereby inducing conformational changes that render procollagens more readily cleaved by BMP1/TLD-like proteinases (Kessler and Adar, 1989; Moali et al., 2005; Takahara et al., 1994). The enhancing properties of the PCOLCEs appear to be restricted to pCP activity, as these proteins show no enhancement of cleavage of other known substrates of BMP1/TLD-like proteinases (Petropoulou et al., 2005). Recently, it was shown that PCOLCE1 is bound via the most carboxyl-terminal EGF and/or CUB domains of mTLL1 (Ge et al., 2006) and that full-length, but not carboxyl-terminal truncated forms of BMP1 and mTLL1 are fully-enhanced by PCOLCE1 (Ge et al., 2006; Petropoulou et al., 2005). Thus, PCOLCEs may act via forming a ternary complex between the most carboxyl-terminal domains of BMP1/TLD-like proteinases and their procollagen substrates. The PCOLCE1 NTR domain possesses inhibitory activity against MMPs, and perhaps other proteinases (Mott et al., 2000), and thus may play some role as an inhibitor of collagen catabolism. PCOLCE1-null mice have profoundly abnormal collagen fibrils in both bone and soft tissues, suggesting that PCOLCE1 may play additional in vivo roles beyond enhancement of pCP acitivity (Steiglitz et al., 2006).
5.2 Twisted gastrulation (TSG)
Drosophila TSG is the product of a gene necessary to dorsal-ventral patterning (Mason et al., 1994). TSG orthologues have now been identified in various species, and in which they bind BMP4/DPP and Chordin/SOG in a ternary complex, and appear capable of either promoting or antagonizing BMP signaling in different in vivo contexts (Oelgeschlager et al., 2000; Chang et al., 2001; Ross et al., 2001; Scott et al., 2001). Although TSG does not appear to bind BMP1/TLD-like proteinases, it does enhance the Chordinase activity of BMP1 and mTLL1 (Scott et al., 2001). In fact mTLD, which does not have detectable in vitro Chordinase activity in the absence of TSG (Scott et al., 1999), has such activity when TSG is present (Scott et al., 2001). Perhaps TSG induces a conformational change in Chordin that makes it more readily cleavable by BMP1/TLD-like proteinases. It has also been reported that TSG may increase cleavage of Chordin at an alternative site not used in the absence of TSG (Scott et al., 2001), although this may not be the case in all systems (Xie and Fisher, 2005).
5.3 Inhibitors of BMP1/TLD-like Proteinases
α2-Macroglobulin (α2M) is a homotetrameric member of the α-macroglobulin family (Sottrup-Jensen, 1989). Synthesized primarily in the liver, α2M circulates at high levels (2–4 mg/ml) in blood and is also produced by astrocytes, monocytes/macrophages, lung fibroblasts and some tumor cells (Baker et al., 2002; Borth, 1992). An irreversible suicide inhibitor, each α2M subunit has a “bait region” containing cleavage sites for a wide variety of proteases (Borth, 1992; Feinman, 1994). Cleavage within these bait regions causes a conformational change, such that proteases are entrapped in the α2M interior, thereby inhibiting protease activity via steric hindrance (Baker et al., 2002; Borth, 1992). The conformational change also exposes previously buried reactive thioester groups, which can covalently bind proteases (Baker et al., 2002; Borth, 1992). In addition, the conformational change transforms α2M to an “activated” form capable of binding various cytokines, and of binding to the cell surface receptor, low-density lipoprotein receptor-related protein (LRP) (Borth, 1992). The result of LRP binding is rapid clearance of α2M-protease complexes from the extracellular space and degradation, although activated α2M may also be able to bind cytokines in a reversible manner, such that it serves as a carrier for targeting such proteins to appropriate cell types (Borth, 1992). α2M has been shown to inhibit a wide variety of proteases, including MMP and ADAM family metalloproteinases, but it does not inhibit the astacin-like metalloproteinases α- and β-meprin (Kruse et al., 2004). Thus, α2M was not believed to inhibit any astacin family members (Baker et al., 2002; Kruse et al., 2004), including the BMP1/TLD-like proteinases. However, the α2M bait region contains sequences that resemble previously characterized BMP1/TLD-like proteinase cleavage sites (Fig. 3); and it was recently demonstrated that this site is indeed cleaved by BMP1/TLD-like proteinases, with consequent inhibition of these proteinases by plasma α2M (Fig. 6), with a Ki of 25 nM (Zhang et al., 2006). Interestingly, replacing the bait region with the cleavage site and flanking sequences of probiglycan, which is very efficiently cleaved by BMP1/TLD-like proteinases, produced a recombinant version of α2M that is 16-fold more effective at BMP1/TLD-like proteinase inhibition than is native plasma α2M (and 24-fold more effective than wild type recombinant α2M prepared via the same system as the mutant α2M) (Zhang et al., 2006). Future modified versions of α2M may be further optimized for cleavage by BMP1-like proteinases by substituting in preferred residues (Fig. 4) at positions flanking bait cleavage sites.
Sizzled, a secreted frizzled-related protein (sFRP) originally described as a Wnt signaling antagonist, has surprisingly been identified as the product of the ogon/mercedes locus, that acts as an inhibitor of BMP signaling in zebrafish dorsal-ventral patterning (Martyn and Schulte-Merker, 2003; Yabe et al., 2003). It has recently been shown that Xenopus and zebrafish Sizzled can act by binding BMP1/TLD-like proteinases and inhibiting their chordinase activity (Lee et al., 2006) (Fig. 6). Since Sizzled was effective at inhibiting cleavage of a small fluorogenic peptide by BMP1 and XLR, it has been inferred that it acts exclusively by binding the active sites of these proteinases (Lee et al., 2006). It has also been reported that the related protein mouse sFRP2 is capable of inhibiting the chordinase activity of XLR proteinases (Lee et al., 2006). However, further studies are needed to determine whether sFRPs are physiologically relevant inhibitors of BMP1/TLD-like proteinases in mammals.
6. Perspectives
Biosynthetic processing by the same small group of proteinases of a wide range of proteins involved in ECM formation in general and collagen fibrillogenesis in particular, doubtless provides an element of coordination to these processes. Similarly, the activation of a subset of TGFβ-like proteins by the BMP1/TLD-like proteinases may serve to coordinate signaling by these factors, and to orchestrate such signaling with ECM formation in morphogenetic events (Fig. 6). In addition to previously known roles of the BMP1/TLD-like proteinases in ECM formation and patterning, new roles are beginning to emerge. Among these are the roles that these proteinases appear to play in formation of neural tissues (Liu et al., 1997; Ge et al., 2005; Meyer and Aberle, 2006; Maertens et al., 2007). To further understand the varied roles of the BMP1/TLD-like proteinases will require a further cataloguing of their substrates, which may require high-throughput approaches, such as mass-spectrometric analyses of protein cleavage products produced by wild type cells, but not by cells null for individual, or combinations of BMP1/TLD-like proteinases.
Of great potential interest is the possibility of inhibiting BMP1/TLD-proteinase actions in ways that will constitute therapeutic interventions in human disease. In this regard, inhibition of GDF8 activation by BMP1/TLD-like proteinases has recently been shown to result in increased muscle mass, and represents a possible approach to treating muscular dystrophies (Wolfman et al., 2003). Importantly, the multiple roles of BMP1/TLD-like proteinases in collagen fibrillogenesis make them attractive targets for anti-fibrotic interventions. Approaches towards therapeutic inhibition of BMP1/TLD-like proteinases may take advantage of the manipulation of recently reported endogenous inhibitors (Lee et al., 2006; Muraoka et al., 2006; Zhang et al., 2006) and/or development of small molecule inhibitors (Veitch et al., 2003).
Acknowledgments
We thank Amanda Branam and Guy Hoffman for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano S, Scott IC, Takahara K, Koch M, Champliaud MF, Gerecke DR, Keene DR, Hudson DL, Nishiyama T, Lee S, Greenspan DS, Burgeson RE. Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 gamma 2 chain. J Biol Chem. 2000;275:22728–22735. doi: 10.1074/jbc.M002345200. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Arora PD, McCulloch CA. The deletion of transforming growth factor-beta-induced myofibroblasts depends on growth conditions and actin organization. Am J Pathol. 1999;155:2087–2099. doi: 10.1016/s0002-9440(10)65527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990;38:1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- Birk DE, Trelstad RL. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J Cell Biol. 1984;99:2024–2033. doi: 10.1083/jcb.99.6.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Fitch JM, Babiarz JP, Doane KJ, Linsenmayer TF. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95 (Pt 4):649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- Bix G, Fu J, Gonzalez EM, Macro L, Barker A, Campbell S, Zutter MM, Santoro SA, Kim JK, Hook M, Reed CC, Iozzo RV. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through alpha2beta1 integrin. J Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader P, Rastegar S, Fischer N, Strahle U. Cleavage of the BMP-4 antagonist chordin by zebrafish tolloid. Science. 1997;278:1937–1940. doi: 10.1126/science.278.5345.1937. [DOI] [PubMed] [Google Scholar]
- Bond JS, Beynon RJ. The astacin family of metalloendopeptidases. Protein Sci. 1995;4:1247–1261. doi: 10.1002/pro.5560040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L, Mironov AA, Jr, Martinez-Menarguez JA, Martella O, Fusella A, Baldassarre M, Buccione R, Geuze HJ, Mironov AA, Luini A. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/s0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel A, Eichenberger D, Farjanel J, Kessler E, Gleyzal C, Hulmes DJS, Sommer P, Font B. Lysyl Oxidase-like Protein from Bovine Aorta. J Biol Chem. 2001;52:48944–48949. doi: 10.1074/jbc.M109499200. [DOI] [PubMed] [Google Scholar]
- Bork P, Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. J Mol Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- Borth W. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. Faseb J. 1992;6:3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- Brown PD, Levy AT, Margulies IM, Liotta LA, Stetler-Stevenson WG. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990;50:6184–6191. [PubMed] [Google Scholar]
- Bruckner-Tuderman L, Nilssen O, Zimmermann DR, Dours-Zimmermann MT, Kalinke DU, Gedde-Dahl T, Jr, Winberg JO. Immunohistochemical and mutation analyses demonstrate that procollagen VII is processed to collagen VII through removal of the NC-2 domain. J Cell Biol. 1995;131:551–559. doi: 10.1083/jcb.131.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler WT. Dentin matrix proteins. Eur J Oral Sci. 1998;106(Suppl 1):204–210. doi: 10.1111/j.1600-0722.1998.tb02177.x. [DOI] [PubMed] [Google Scholar]
- Byers PH. Disorders of collagen biosynthesis and structure. In: Scriver CR, editor. The metabolic and molecular bases of inherited disease. 7. McGraw-Hill; New York: 1995. pp. 4029–4077. [Google Scholar]
- Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol. 2004;165:553–563. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty EG, Kadler K. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, Wozney JM. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci U S A. 1990;87:9843–9847. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Holtzman DA, Chau S, Chickering T, Woolf EA, Holmgren LM, Bodorova J, Gearing DP, Holmes WE, Brivanlou AH. Twisted gastrulation can function as a BMP antagonist. Nature. 2001;410:483–487. doi: 10.1038/35068583. [DOI] [PubMed] [Google Scholar]
- Chen M, Keene DR, Costa FK, Tahk SH, Woodley DT. The carboxyl terminus of type VII collagen mediates antiparallel dimer formation and constitutes a new antigenic epitope for epidermolysis Bullosa acquisita autoantibodies. J Biol Chem. 2001;276:21649–21655. doi: 10.1074/jbc.M100180200. [DOI] [PubMed] [Google Scholar]
- Chen XD, Shi S, Xu T, Robey PG, Young MF. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J Bone Miner Res. 2002;17:331–340. doi: 10.1359/jbmr.2002.17.2.331. [DOI] [PubMed] [Google Scholar]
- Childs SR, O’Connor MB. Two domains of the tolloid protein contribute to its unusual genetic interaction with decapentaplegic. Dev Biol. 1994;162:209–220. doi: 10.1006/dbio.1994.1079. [DOI] [PubMed] [Google Scholar]
- Clark TG, Conway SJ, Scott IC, Labosky PA, Winnier G, Bundy J, Hogan BL, Greenspan DS. The mammalian Tolloid-like 1 gene, Tll1, is necessary for normal septation and positioning of the heart. Development. 1999;126:2631–2642. doi: 10.1242/dev.126.12.2631. [DOI] [PubMed] [Google Scholar]
- Colige A, Li SW, Sieron AL, Nusgens BV, Prockop DJ, Lapiere CM. cDNA cloning and expression of bovine procollagen I N-proteinase: a new member of the superfamily of zinc-metalloproteinases with binding sites for cells and other matrix components. Proc Natl Acad Sci U S A. 1997;94:2374–2379. doi: 10.1073/pnas.94.6.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors SA, Trout J, Ekker M, Mullins MC. The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development. 1999;126:3119–3130. doi: 10.1242/dev.126.14.3119. [DOI] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo M, Billings PC, Pacifici M, Leboy PS, Kirsch T. Authentic matrix vesicles contain active metalloproteases (MMP). a role for matrix vesicle-associated MMP-13 in activation of transforming growth factor-beta. J Biol Chem. 2001;276:11347–11353. doi: 10.1074/jbc.M009725200. [DOI] [PubMed] [Google Scholar]
- Dale L, Evans W, Goodman SA. Xolloid-related: a novel BMP1/Tolloid-related metalloprotease is expressed during early Xenopus development. Mech Dev. 2002;119:177–190. doi: 10.1016/s0925-4773(02)00359-3. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57:2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR, Jr, Peles E. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47:215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Feinman RD. The proteinase-binding reaction of alpha 2M. Ann N Y Acad Sci. 1994;737:245–266. doi: 10.1111/j.1749-6632.1994.tb44316.x. [DOI] [PubMed] [Google Scholar]
- Fessler JH, Fesller LI. Type V collagen. In: Mayne R, Burgeson RE, editors. Structure and function of collagen types. Academic Press; Orlando: 1987. p. 81. [Google Scholar]
- Finelli AL, Bossie CA, Xie T, Padgett RW. Mutational analysis of the Drosophila tolloid gene, a human BMP-1 homolog. Development. 1994;120:861–870. doi: 10.1242/dev.120.4.861. [DOI] [PubMed] [Google Scholar]
- Finelli AL, Xie T, Bossie CA, Blackman RK, Padgett RW. The tolkin gene is a tolloid/BMP-1 homologue that is essential for Drosophila development. Genetics. 1995;141:271–281. doi: 10.1093/genetics/141.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzke CW, Bruckner P, Bruckner-Tuderman L. Collagenous transmembrane proteins: recent insights into biology and pathology. J Biol Chem. 2005;280:4005–4008. doi: 10.1074/jbc.R400034200. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Corpuz LM, Roth MR, Funderburgh ML, Tasheva ES, Conrad GW. Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J Biol Chem. 1997;272:28089–28095. doi: 10.1074/jbc.272.44.28089. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Wolfman NM, Celeste AJ, Hattersley G, Hewick R, Rosen V. A novel BMP expressed in developing mouse limb, spinal cord, and tail bud is a potent mesoderm inducer in Xenopus embryos. Dev Biol. 1999;208:222–232. doi: 10.1006/dbio.1998.9191. [DOI] [PubMed] [Google Scholar]
- Garrigue-Antar L, Hartigan N, Kadler KE. Post-translational modification of bone morphogenetic protein-1 is required for secretion and stability of the protein. J Biol Chem. 2002;277:43327–43334. doi: 10.1074/jbc.M207342200. [DOI] [PubMed] [Google Scholar]
- Garrigue-Antar L, Francois V, Kadler KE. Deletion of epidermal growth factor-like domains converts mammalian tolloid into a chordinase and effective procollagen C-proteinase. J Biol Chem. 2004;279:49835–49841. doi: 10.1074/jbc.M408134200. [DOI] [PubMed] [Google Scholar]
- Ge G, Seo NS, Liang X, Hopkins DR, Hook M, Greenspan DS. Bone morphogenetic protein-1/tolloid-related metalloproteinases process osteoglycin and enhance its ability to regulate collagen fibrillogenesis. J Biol Chem. 2004;279:41626–41633. doi: 10.1074/jbc.M406630200. [DOI] [PubMed] [Google Scholar]
- Ge G, Hopkins DR, Ho WB, Greenspan DS. GDF11 forms a bone morphogenetic protein 1-activated latent complex that can modulate nerve growth factor-induced differentiation of PC12 cells. Mol Cell Biol. 2005;25:5846–5858. doi: 10.1128/MCB.25.14.5846-5858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge G, Greenspan DS. Developmental roles of the BMP1/TLD metalloproteinases. Birth Defects Res C Embryo Today. 2006;78:47–68. doi: 10.1002/bdrc.20060. [DOI] [PubMed] [Google Scholar]
- Ge G, Greenspan DS. BMP1 controls TGFbeta1 activation via cleavage of latent TGFbeta-binding protein. J Cell Biol. 2006;175:111–120. doi: 10.1083/jcb.200606058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge G, Zhang Y, Steiglitz BM, Greenspan DS. Mammalian tolloid-like 1 binds procollagen C-proteinase enhancer protein 1 and differs from bone morphogenetic protein 1 in the functional roles of homologous protein domains. J Biol Chem. 2006;281:10786–10798. doi: 10.1074/jbc.M511111200. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JC. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B, Greenspan DS, Iozzo RV. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J Biol Chem. 2005;280:7080–7087. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- Goodman SA, Albano R, Wardle FC, Matthews G, Tannahill D, Dale L. BMP1-related metalloproteinases promote the development of ventral mesoderm in early Xenopus embryos. Dev Biol. 1998;195:144–157. doi: 10.1006/dbio.1997.8840. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan B, Wang WM, Greenspan DS. Biosynthetic processing of the Pro-alpha1(V)Pro-alpha2(V)Pro-alpha3(V) procollagen heterotrimer. J Biol Chem. 2004;279:30904–30912. doi: 10.1074/jbc.M402252200. [DOI] [PubMed] [Google Scholar]
- Greenspan DS, Wang WM. Overview of ADAMTS proteases and ADAMTS-2 (procollagen III N-proteinase) In: Hooper NM, Lendeckel U, editors. The ADAMs Family of Proteases: Proteases in Biology and Disease. Vol. 4. Springer Verlag; Amsterdam: 2005. pp. 261–282. [Google Scholar]
- Greenspan DS. Biosynthetic Processing of Collagen Molecules. Top Curr Chem. 2005;247:149–183. [Google Scholar]
- Handford PA, Baron M, Mayhew M, Willis A, Beesley T, Brownlee GG, Campbell ID. The first EGF-like domain from human factor IX contains a high-affinity calcium binding site. Embo J. 1990;9:475–480. doi: 10.1002/j.1460-2075.1990.tb08133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem. 2002;277:40735–40741. doi: 10.1074/jbc.M206379200. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bmps: multifunctional regulators of mammalian embryonic development. Harvey Lect. 1996;92:83–98. [PubMed] [Google Scholar]
- Hojima Y, van der Rest M, Prockop DJ. Type I procollagen carboxyl- terminal proteinase from chick embryo tendons. Purification and characterization. J Biol Chem. 1985;260:15996–16003. [PubMed] [Google Scholar]
- Hojima Y, Behta B, Romanic AM, Prockop DJ. Cleavage of type I procollagen by C- and N-proteinases is more rapid if the substrate is aggregated with dextran sulfate or polyethylene glycol. Anal Biochem. 1994;223:173–180. doi: 10.1006/abio.1994.1569. [DOI] [PubMed] [Google Scholar]
- Hojima Y, Morgelin MM, Engel J, Boutillon MM, van der Rest M, McKenzie J, Chen GC, Rafi N, Romanic AM, Prockop DJ. Characterization of type I procollagen N-proteinase from fetal bovine tendon and skin. Purification of the 500-kilodalton form of the enzyme from bovine tendon. J Biol Chem. 1994;269:11381–11390. [PubMed] [Google Scholar]
- Hwang SP, Partin JS, Lennarz WJ. Characterization of a homolog of human bone morphogenetic protein 1 in the embryo of the sea urchin, Strongylocentrotus purpuratus. Development. 1994;120:559–568. doi: 10.1242/dev.120.3.559. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Steiglitz BM, Greenspan DS. Bone morphogenetic protein-1 processes the NH2-terminal propeptide, and a furin-like proprotein convertase processes the COOH-terminal propeptide of pro-alpha1(V) collagen. J Biol Chem. 1998;273:27511–27517. doi: 10.1074/jbc.273.42.27511. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Jasuja R, Voss N, Ge G, Hoffman GG, Lyman-Gingerich J, Pelegri F, Greenspan DS. bmp1 and mini fin are functionally redundant in regulating formation of the zebrafish dorsoventral axis. Mech Dev. 2006;123:548–558. doi: 10.1016/j.mod.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Jasuja R, Ge G, Voss NG, Lyman-Gingerich J, Branam AM, Pelegri FJ, Greenspan DS. Bone morphogenetic protein 1 prodomain specifically binds and regulates signaling by bone morphogenetic proteins 2 and 4. J Biol Chem. 2007;12:9053–62. doi: 10.1074/jbc.M610929200. [DOI] [PubMed] [Google Scholar]
- Johnson HJ, Rosenberg L, Choi HU, Garza S, Hook M, Neame PJ. Characterization of epiphycan, a small proteoglycan with a leucine-rich repeat core protein. J Biol Chem. 1997;272:18709–18717. doi: 10.1074/jbc.272.30.18709. [DOI] [PubMed] [Google Scholar]
- Keene DR, Sakai LY, Lunstrum GP, Morris NP, Burgeson RE. Type VII collagen forms an extended network of anchoring fibrils. J Cell Biol. 1987;104:611–621. doi: 10.1083/jcb.104.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler E, Adar R, Goldberg B, Niece R. Partial purification and characterization of a procollagen C-proteinase from the culture medium of mouse fibroblasts. Coll Relat Res. 1986;6:249–266. doi: 10.1016/s0174-173x(86)80010-3. [DOI] [PubMed] [Google Scholar]
- Kessler E, Adar R. Type I procollagen C-proteinase from mouse fibroblasts. Purification and demonstration of a 55-kDa enhancer glycoprotein. Eur J Biochem. 1989;186:115–121. doi: 10.1111/j.1432-1033.1989.tb15184.x. [DOI] [PubMed] [Google Scholar]
- Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science. 1996;271:360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- Kleman JP, Hartmann DJ, Ramirez F, van der Rest M. The human rhabdomyosarcoma cell line A204 lays down a highly insoluble matrix composed mainly of alpha 1 type-XI and alpha 2 type-V collagen chains. Eur J Biochem. 1992;210:329–335. doi: 10.1111/j.1432-1033.1992.tb17425.x. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse MN, Becker C, Lottaz D, Kohler D, Yiallouros I, Krell HW, Sterchi EE, Stocker W. Human meprin alpha and beta homo-oligomers: cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem J. 2004;378:383–389. doi: 10.1042/BJ20031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante P, Raymond MA, Labelle A, Abe J, Iozzo RV, Hebert MJ. Perlecan proteolysis induces an alpha2beta1 integrin- and Src family kinase-dependent anti-apoptotic pathway in fibroblasts in the absence of focal adhesion kinase activation. J Biol Chem. 2006;281:30383–30392. doi: 10.1074/jbc.M606412200. [DOI] [PubMed] [Google Scholar]
- Le Goff C, Somerville RP, Kesteloot F, Powell K, Birk DE, Colige AC, Apte SS. Regulation of procollagen amino-propeptide processing during mouse embryogenesis by specialization of homologous ADAMTS proteases: insights on collagen biosynthesis and dermatosparaxis. Development. 2006;133:1587–1596. doi: 10.1242/dev.02308. [DOI] [PubMed] [Google Scholar]
- Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124:147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Solow-Cordero DE, Kessler E, Takahara K, Greenspan DS. Transforming growth factor-beta regulation of bone morphogenetic protein-1/procollagen C-proteinase and related proteins in fibrogenic cells and keratinocytes. J Biol Chem. 1997;272:19059–19066. doi: 10.1074/jbc.272.30.19059. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton M, Kadler KE. Paired basic/Furin-like proprotein convertase cleavage of Pro-BMP-1 in the trans-Golgi network. J Biol Chem. 2003;278:18478–18484. doi: 10.1074/jbc.M213021200. [DOI] [PubMed] [Google Scholar]
- Lepage T, Ghiglione C, Gache C. Spatial and temporal expression pattern during sea urchin embryogenesis of a gene coding for a protease homologous to the human protein BMP-1 and to the product of the Drosophila dorsal-ventral patterning gene tolloid. Development. 1992;114:147–163. doi: 10.1242/dev.114.1.147. [DOI] [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Li SW, Sieron AL, Fertala A, Hojima Y, Arnold WV, Prockop DJ. The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1. Proc Natl Acad Sci U S A. 1996;93:5127–5130. doi: 10.1073/pnas.93.10.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lacerda DA, Warman ML, Beier DR, Yoshioka H, Ninomiya Y, Oxford JT, Morris NP, Andrikopoulos K, Ramirez F, et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell. 1995;80:423–430. doi: 10.1016/0092-8674(95)90492-1. [DOI] [PubMed] [Google Scholar]
- Liaubet L, Bertrand N, Medevielle F, Pituello F. Identification by differential display of a chicken tolloid-related metalloprotease specifically expressed in the caudal notochord. Mech Dev. 2000;96:101–105. doi: 10.1016/s0925-4773(00)00382-8. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Maeda R, Ong RC, Kim J, Lee LM, Kung H, Maeno M. XBMP-1B (Xtld), a Xenopus homolog of dorso-ventral polarity gene in Drosophila, modifies tissue phenotypes of ventral explants. Dev Growth Differ. 1997;39:43–51. doi: 10.1046/j.1440-169x.1997.00006.x. [DOI] [PubMed] [Google Scholar]
- Linsenmayer TF, Gibney E, Igoe F, Gordon MK, Fitch JM, Fessler LI, Birk DE. Type V collagen: molecular structure and fibrillar organization of the chicken alpha 1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J Cell Biol. 1993;121:1181–1189. doi: 10.1083/jcb.121.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Hattar S, Endo S, MacPhee K, Zhang H, Cleary LJ, Byrne JH, Eskin A. A developmental gene (Tolloid/BMP-1) is regulated in Aplysia neurons by treatments that induce long-term sensitization. J Neurosci. 1997;17:755–764. doi: 10.1523/JNEUROSCI.17-02-00755.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunstrum GP, Kuo HJ, Rosenbaum LM, Keene DR, Glanville RW, Sakai LY, Burgeson RE. Anchoring fibrils contain the carboxyl-terminal globular domain of type VII procollagen, but lack the amino-terminal globular domain. J Biol Chem. 1987;262:13706–13712. [PubMed] [Google Scholar]
- Maeda S, Dean DD, Gay I, Schwartz Z, Boyan BD. Activation of latent transforming growth factor beta1 by stromelysin 1 in extracts of growth plate chondrocyte-derived matrix vesicles. J Bone Miner Res. 2001;16:1281–1290. doi: 10.1359/jbmr.2001.16.7.1281. [DOI] [PubMed] [Google Scholar]
- Maertens B, Hopkins D, Franzke CW, Keene DR, Bruckner-Tuderman L, Greenspan DS, Koch M. Cleavage and oligomerization of gliomedin, a transmembrane collagen required for node of Ranvier formation. J Biol Chem. 2007 doi: 10.1074/jbc.M611339200. In press. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Burgeson RE. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem. 1992;267:17900–17906. [PubMed] [Google Scholar]
- Marques G, Musacchio M, Shimell MJ, Wunnenberg-Stapleton K, Cho KW, O’Connor MB. Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell. 1997;91:417–426. doi: 10.1016/s0092-8674(00)80425-0. [DOI] [PubMed] [Google Scholar]
- Marti HP, Lee L, Kashgarian M, Lovett DH. Transforming growth factor-beta 1 stimulates glomerular mesangial cell synthesis of the 72-kd type IV collagenase. Am J Pathol. 1994;144:82–94. [PMC free article] [PubMed] [Google Scholar]
- Martyn U, Schulte-Merker S. The ventralized ogon mutant phenotype is caused by a mutation in the zebrafish homologue of Sizzled, a secreted Frizzled-related protein. Dev Biol. 2003;260:58–67. doi: 10.1016/s0012-1606(03)00221-5. [DOI] [PubMed] [Google Scholar]
- Mason ED, Konrad KD, Webb CD, Marsh JL. Dorsal midline fate in Drosophila embryos requires twisted gastrulation, a gene encoding a secreted protein related to human connective tissue growth factor. Genes Dev. 1994;8:1489–1501. doi: 10.1101/gad.8.13.1489. [DOI] [PubMed] [Google Scholar]
- Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Mayne R, Brewton RG, Mayne PM, Baker JR. Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J Biol Chem. 1993;268:9381–9386. [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22:260–264. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- Mendler M, Eich-Bender SG, Vaughan L, Winterhalter KH, Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989;108:191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F, Aberle H. At the next stop sign turn right: the metalloprotease Tolloid-related 1 controls defasciculation of motor axons in Drosophila. Development. 2006;133:4035–4044. doi: 10.1242/dev.02580. [DOI] [PubMed] [Google Scholar]
- Moali C, Font B, Ruggiero F, Eichenberger D, Rousselle P, Francois V, Oldberg A, Bruckner-Tuderman L, Hulmes DJ. Substrate-specific modulation of a multisubstrate proteinase. C-terminal processing of fibrillar procollagens is the only BMP-1-dependent activity to be enhanced by PCPE-1. J Biol Chem. 2005;280:24188–24194. doi: 10.1074/jbc.M501486200. [DOI] [PubMed] [Google Scholar]
- Mongiat M, Sweeney SM, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- Morris NP, Keene DR, Glanville RW, Bentz H, Burgeson RE. The tissue form of type VII collagen is an antiparallel dimer. J Biol Chem. 1986;261:5638–5644. [PubMed] [Google Scholar]
- Morris NP, Bachinger HP. Type XI collagen is a heterotrimer with the composition (1 alpha, 2 alpha, 3 alpha) retaining non-triple-helical domains. J Biol Chem. 1987;262:11345–11350. [PubMed] [Google Scholar]
- Mott JD, Thomas CL, Rosenbach MT, Takahara K, Greenspan DS, Banda MJ. Post-translational proteolytic processing of procollagen C-terminal proteinase enhancer releases a metalloproteinase inhibitor. J Biol Chem. 2000;275:1384–1390. doi: 10.1074/jbc.275.2.1384. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Muraoka O, Shimizu T, Yabe T, Nojima H, Bae YK, Hashimoto H, Hibi M. Sizzled controls dorso-ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol. 2006;8:329–338. doi: 10.1038/ncb1379. [DOI] [PubMed] [Google Scholar]
- Murdoch AD, Liu B, Schwarting R, Tuan RS, Iozzo RV. Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissues as detected by a novel monoclonal antibody against domain III and by in situ hybridization. J Histochem Cytochem. 1994;42:239–249. doi: 10.1177/42.2.7507142. [DOI] [PubMed] [Google Scholar]
- Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med. 2001;33:7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Toyono T, Akamine A, Joyner A. Expression of growth/differentiation factor 11, a new member of the BMP/TGFbeta superfamily during mouse embryogenesis. Mech Dev. 1999;80:185–189. doi: 10.1016/s0925-4773(98)00205-6. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Jamal J, Shimell MJ, Arora K, O’Connor MB. Characterization of tolloid-related-1: a BMP-1-like product that is required during larval and pupal stages of Drosophila development. Dev Biol. 1994;166:569–586. doi: 10.1006/dbio.1994.1338. [DOI] [PubMed] [Google Scholar]
- Oda O, Shinzato T, Ohbayashi K, Takai I, Kunimatsu M, Maeda K, Yamanaka N. Purification and characterization of perlecan fragment in urine of end-stage renal failure patients. Clin Chim Acta. 1996;255:119–132. doi: 10.1016/0009-8981(96)06395-4. [DOI] [PubMed] [Google Scholar]
- Oelgeschlager M, Larrain J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall CM, Wrana JL, Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem. 1991;266:14064–14071. [PubMed] [Google Scholar]
- Panchenko MV, Stetler-Stevenson WG, Trubetskoy OV, Gacheru SN, Kagan HM. Metalloproteinase activity secreted by fibrogenic cells in the processing of prolysyl oxidase. Potential role of procollagen C-proteinase. J Biol Chem. 1996;271:7113–7119. doi: 10.1074/jbc.271.12.7113. [DOI] [PubMed] [Google Scholar]
- Pappano WN, Steiglitz BM, Scott IC, Keene DR, Greenspan DS. Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/tolloid-like metalloproteinases. Mol Cell Biol. 2003;23:4428–4438. doi: 10.1128/MCB.23.13.4428-4438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulou V, Garrigue-Antar L, Kadler KE. Identification of the minimal domain structure of bone morphogenetic protein-1 (BMP-1) for chordinase activity: chordinase activity is not enhanced by procollagen C-proteinase enhancer-1 (PCPE-1) J Biol Chem. 2005;280:22616–22623. doi: 10.1074/jbc.M413468200. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Hulmes DJS. Assembly of collagen fibrils de novo from soluble precursors: polymerization and copolymerization of procollagen, pN-collagen, and mutated collagens. In: Yurchenko PD, Birk DE, Mechan RP, editors. Extracellular matrix assembly and structure. Academic Press; New York: 1994. p. 47. [Google Scholar]
- Rattenholl A, Pappano WN, Koch M, Keene DR, Kadler KE, Sasaki T, Timpl R, Burgeson RE, Greenspan DS, Bruckner-Tuderman L. Proteinases of the bone morphogenetic protein-1 family convert procollagen VII to mature anchoring fibril collagen. J Biol Chem. 2002;277:26372–26378. doi: 10.1074/jbc.M203247200. [DOI] [PubMed] [Google Scholar]
- Reddi AH, Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972;69:1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Angerer LM, Palis J, Nasir A, Angerer RC. Early mRNAs, spatially restricted along the animal-vegetal axis of sea urchin embryos, include one encoding a protein related to tolloid and BMP-1. Development. 1992;114:769–786. doi: 10.1242/dev.114.3.769. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Zhang D, Puzas JE, O’Keefe RJ, Rosier RN, Reynolds PR. Cloning of the chick BMP1/Tolloid cDNA and expression in skeletal tissues. Gene. 2000;248:233–243. doi: 10.1016/s0378-1119(00)00114-1. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, Hermanson S, Ekker SC, O’Connor MB, Marsh JL. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410:479–483. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- Roughley PJ, White RJ, Mort JS. Presence of pro-forms of decorin and biglycan in human articular cartilage. Biochem J. 1996;318 (Pt 3):779–784. doi: 10.1042/bj3180779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Morris NP, Burgeson RE. Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol. 1986;103:1577–1586. doi: 10.1083/jcb.103.4.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KW, Greenspan DS. Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- Scott IC, Imamura Y, Pappano WN, Troedel JM, Recklies AD, Roughley PJ, Greenspan DS. Bone morphogenetic protein-1 processes probiglycan. J Biol Chem. 2000;275:30504–30511. doi: 10.1074/jbc.M004846200. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KW, Greenspan DS. Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature. 2001;410:475–478. doi: 10.1038/35068572. [DOI] [PubMed] [Google Scholar]
- Sehgal I, Thompson TC. Novel regulation of type IV collagenase (matrix metalloproteinase-9 and -2) activities by transforming growth factor-beta1 in human prostate cancer cell lines. Mol Biol Cell. 1999;10:407–416. doi: 10.1091/mbc.10.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe M, O’Connor MB. The metalloprotease tolloid-related and its TGF-beta-like substrate Dawdle regulate Drosophila motoneuron axon guidance. Development. 2006;133:4969–4979. doi: 10.1242/dev.02711. [DOI] [PubMed] [Google Scholar]
- Shimell MJ, Ferguson EL, Childs SR, O’Connor MB. The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell. 1991;67:469–481. doi: 10.1016/0092-8674(91)90522-z. [DOI] [PubMed] [Google Scholar]
- Sieron AL, Tretiakova A, Jameson BA, Segall ML, Lund-Katz S, Khan MT, Li S, Stocker W. Structure and function of procollagen C-proteinase (mTolloid) domains determined by protease digestion, circular dichroism, binding to procollagen type I, and computer modeling. Biochemistry. 2000;39:3231–3239. doi: 10.1021/bi992312o. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L. Alpha-macroglobulins: structure, shape, and mechanism of proteinase complex formation. J Biol Chem. 1989;264:11539–11542. [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Steiglitz BM, Keene DR, Greenspan DS. PCOLCE2 encodes a functional procollagen C-proteinase enhancer (PCPE2) that is a collagen-binding protein differing in distribution of expression and post-translational modification from the previously described PCPE1. J Biol Chem. 2002;277:49820–49830. doi: 10.1074/jbc.M209891200. [DOI] [PubMed] [Google Scholar]
- Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem. 2004;279:980–986. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
- Steiglitz BM, Kreider JM, Frankenburg EP, Pappano WN, Hoffman GG, Meganck JA, Liang X, Hook M, Birk DE, Goldstein SA, Greenspan DS. Procollagen C proteinase enhancer 1 genes are important determinants of the mechanical properties and geometry of bone and the ultrastructure of connective tissues. Mol Cell Biol. 2006;26:238–249. doi: 10.1128/MCB.26.1.238-249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker W, Grams F, Baumann U, Reinemer P, Gomis-Ruth FX, McKay DB, Bode W. The metzincins--topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 1995;4:823–840. doi: 10.1002/pro.5560040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Labosky PA, Furuta Y, Hargett L, Dunn R, Fogo AB, Takahara K, Peters DM, Greenspan DS, Hogan BL. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development. 1996;122:3587–3595. doi: 10.1242/dev.122.11.3587. [DOI] [PubMed] [Google Scholar]
- Svensson L, Oldberg A, Heinegard D. Collagen binding proteins. Osteoarthritis Cartilage. 2001;9:S23–S28. doi: 10.1053/joca.2001.0440. [DOI] [PubMed] [Google Scholar]
- Takahara K, Kessler E, Biniaminov L, Brusel M, Eddy RL, Jani-Sait S, Shows TB, Greenspan DS. Type I procollagen COOH-terminal proteinase enhancer protein: identification, primary structure, and chromosomal localization of the cognate human gene (PCOLCE) J Biol Chem. 1994;269:26280–26285. [PubMed] [Google Scholar]
- Takahara K, Lyons GE, Greenspan DS. Bone morphogenetic protein-1 and a mammalian tolloid homologue (mTld) are encoded by alternatively spliced transcripts which are differentially expressed in some tissues. J Biol Chem. 1994;269:32572–32578. [PubMed] [Google Scholar]
- Takahara K, Brevard R, Hoffman GG, Suzuki N, Greenspan DS. Characterization of a novel gene product (mammalian tolloid-like) with high sequence similarity to mammalian tolloid/bone morphogenetic protein-1. Genomics. 1996;34:157–165. doi: 10.1006/geno.1996.0260. [DOI] [PubMed] [Google Scholar]
- Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, Song M, Fox N, Conrad GW. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis. 2002;8:407–415. [PubMed] [Google Scholar]
- Toriello HV, Glover TW, Takahara K, Byers PH, Miller DE, Higgins JV, Greenspan DS. A translocation interrupts the COL5A1 gene in a patient with Ehlers-Danlos syndrome and hypomelanosis of Ito. Nat Genet. 1996;13:361–365. doi: 10.1038/ng0796-361. [DOI] [PubMed] [Google Scholar]
- Tsubota Y, Yasuda C, Kariya Y, Ogawa T, Hirosaki T, Mizushima H, Miyazaki K. Regulation of biological activity and matrix assembly of laminin-5 by COOH-terminal, LG4-5 domain of alpha3 chain. J Biol Chem. 2005;280:14370–14377. doi: 10.1074/jbc.M413051200. [DOI] [PubMed] [Google Scholar]
- Unsold C, Pappano WN, Imamura Y, Steiglitz BM, Greenspan DS. Biosynthetic processing of the pro-alpha 1(V)2pro-alpha 2(V) collagen heterotrimer by bone morphogenetic protein-1 and furin-like proprotein convertases. J Biol Chem. 2002;277:5596–5602. doi: 10.1074/jbc.M110003200. [DOI] [PubMed] [Google Scholar]
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Urist MR, Iwata H, Ceccotti PL, Dorfman RL, Boyd SD, McDowell RM, Chien C. Bone morphogenesis in implants of insoluble bone gelatin. Proc Natl Acad Sci U S A. 1973;70:3511–3515. doi: 10.1073/pnas.70.12.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzel MI, Scott IC, Babakhanlou-Chase H, Palamakumbura AH, Pappano WN, Hong HH, Greenspan DS, Trackman PC. Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J Biol Chem. 2001;276:22537–22543. doi: 10.1074/jbc.M102352200. [DOI] [PubMed] [Google Scholar]
- Veis A. Mineral-matrix interactions in bone and dentin. J Bone Miner Res 8 Suppl. 1993;2:S493–497. doi: 10.1002/jbmr.5650081312. [DOI] [PubMed] [Google Scholar]
- Veitch DP, Nokelainen P, McGowan KA, Nguyen TT, Nguyen NE, Stephenson R, Pappano WN, Keene DR, Spong SM, Greenspan DS, Findell PR, Marinkovich MP. Mammalian tolloid metalloproteinase, and not matrix metalloprotease 2 or membrane type 1 metalloprotease, processes laminin-5 in keratinocytes and skin. J Biol Chem. 2003;278:15661–15668. doi: 10.1074/jbc.M210588200. [DOI] [PubMed] [Google Scholar]
- Vuadens F, Benay C, Crettaz D, Gallot D, Sapin V, Schneider P, Bienvenut WV, Lemery D, Quadroni M, Dastugue B, Tissot JD. Identification of biologic markers of the premature rupture of fetal membranes: proteomic approach. Proteomics. 2003;3:1521–1525. doi: 10.1002/pmic.200300455. [DOI] [PubMed] [Google Scholar]
- Wang EA, Rosen V, Cordes P, Hewick RM, Kriz MJ, Luxenberg DP, Sibley BS, Wozney JM. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci U S A. 1988;85:9484–9488. doi: 10.1073/pnas.85.24.9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WM, Lee S, Steiglitz BM, Scott IC, Lebares CC, Allen ML, Brenner MC, Takahara K, Greenspan DS. Transforming growth factor-beta induces secretion of activated ADAMTS-2. A procollagen III N-proteinase. J Biol Chem. 2003;278:19549–19557. doi: 10.1074/jbc.M300767200. [DOI] [PubMed] [Google Scholar]
- Wardle FC, Angerer LM, Angerer RC, Dale L. Regulation of BMP signaling by the BMP1/TLD-related metalloprotease, SpAN. Dev Biol. 1999;206:63–72. doi: 10.1006/dbio.1998.9127. [DOI] [PubMed] [Google Scholar]
- Westfall PH, Young SS. Resampling-based multiple testing: Examples and Methods for p-value adjustment. Wiley; New York: 1993. [Google Scholar]
- Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci U S A. 2003;100:15842–15846. doi: 10.1073/pnas.2534946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Xie J, Fisher S. Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development. 2005;132:383–391. doi: 10.1242/dev.01577. [DOI] [PubMed] [Google Scholar]
- Xu H, Acott TS, Wirtz MK. Identification and expression of a novel type I procollagen C-proteinase enhancer protein gene from the glaucoma candidate region on 3q21-q24. Genomics. 2000;66:264–273. doi: 10.1006/geno.2000.6229. [DOI] [PubMed] [Google Scholar]
- Yabe T, Shimizu T, Muraoka O, Bae YK, Hirata T, Nojima H, Kawakami A, Hirano T, Hibi M. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development. 2003;130:2705–2716. doi: 10.1242/dev.00506. [DOI] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGF{beta}1 activation in vivo recapitulates the phenotype of TGF{beta}1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ge G, Greenspan DS. Inhibition of bone morphogenetic protein 1 by native and altered forms of alpha2-macroglobulin. J Biol Chem. 2006;281:39096–9104. doi: 10.1074/jbc.M601362200. [DOI] [PubMed] [Google Scholar]