Abstract
Introduction
One possible mechanism of higher cardiovascular mortality associated with the metabolic syndrome (MetS) may be through abnormal modulation in autonomic tone.
Methods and Results
We examined the association between the MetS and autonomic tone as measured by heart rate variability (HRV) among 288 twins from the Twins Heart Study. Of the 288 participants, 151 (52%) had the MetS. The MetS was associated with decreased HRV across all frequency ranges, and each additional MetS risk factor was associated with lower HRV. After adjustment for several potential confounders, very-low frequency (p<0.001), low frequency (p<0.001), and total power (p=.02) spectra of HRV remained significantly lower in twins with a progressively higher number of MetS components (18-50% decrease comparing twins with 5 risk factors to those with no risk factors). Among 87 twin pairs who were discordant for the number of MetS components, a one-unit increment in MetS components was associated with an 8% smaller very-low frequency (p=0.03) and a 15% smaller low frequency spectrum (p=0.002) comparing each twin with his brother.
Conclusion
MetS was associated with lower HRV in a well-characterized sample of middle-aged male twins. This association persisted even after controlling for genetic and shared environmental factors accounted for by comparison within twin pairs. Abnormalities of autonomic tone, as evidenced by lower HRV, may be partly responsible for the higher rate of atrial fibrillation, coronary heart disease, cardiac death, and overall mortality seen in patients with the MetS.
Keywords: metabolic syndrome, heart rate variability, autonomic nervous system, twins
Introduction
The metabolic syndrome (MetS) is associated with the development of diabetes mellitus and increases the likelihood of cardiovascular disease (CVD) events, including atrial fibrillation, acute coronary syndrome, cardiac death, and overall mortality. 1-6 The risk of future CVD events is more strongly correlated with the MetS than its individual components,2, 4 although this issue is still debated. One possible mechanism underlying the link between MetS and CVD events is through abnormal modulation in autonomic tone, which has been associated both with MetS and CVD risk. 7-9 Specifically, increased sympathetic activity10 and decreased parasympathetic activity11 have been associated with a higher risk of sudden death. Both the sympathetic and parasympathetic nervous system have been implicated in mediating atrial fibrillation.12 Experimental evidence also suggests an interaction of the autonomic nervous system with the development of vascular atheroma and occlusion.13
Heart rate variability (HRV) is a complex measure of heart rate modulation that incorporates both sympathetic and parasympathetic effects as well as their interaction.14, 15 When determined from 24-hour electrocardiographic recordings, depressed HRV predicts adverse cardiovascular events.16-18 In particular, reduced HRV is associated with an increased risk of atrial fibrillation, myocardial infarction, congestive heart failure, coronary heart disease death, and total mortality.16-19
While several pilot studies suggest an association between the MetS and HRV, these studies are limited by modest sample size and short-term HRV recordings.7, 9, 20 Short-term HRV recordings (i.e., 2-5 minutes rather than 24-hour) do not quantify the very-low and ultra-low frequency components of HRV, which are the most powerful predictors of adverse CVD events.14, 16 In addition, prior studies have not adjusted for important confounders such as depression and physical activity, nor have these studies standardized the schedule and activity of participants during ambulatory ECG recording21, 22
Studies using discordant twin pairs provide unique insight because similarities in genetic background and early environment between twin siblings reduce variation resulting from unmeasured potential confounders. In addition, a study utilizing twins may provide insight into the genetic or other familial component of any association. The purpose of this study, therefore, was to further examine the association between the MetS and autonomic tone, as measured by HRV, using a well-controlled study of twins. In addition, a study of MetS and autonomic function utilizing twins provides insight into the genetic and other familial component of any association.
Methods
Participants
The Twins Heart Study (THS) is an investigation of psychological, behavioral and biological risk factors for subclinical cardiovascular disease using twins. Participants were selected from the Vietnam Era Twin (VET) Registry, a cohort of 7369 middle-aged male-male twin pairs both of whom served in the United States military during the Vietnam War.23, 24 Characteristics of the VET Registry have been previously reported.25 The THS included 360 twins from the VET Registry all born between 1946 and 1956 (>90% of the twins in the VET registry fall into this range). The methods of construction of this sample were also described before 26-28. Briefly, the twins were free of a selfreported previous diagnosis of cardiovascular disease based on survey data collected in 199029, including a previous diagnosis of myocardial infarction, coronary heart disease, angina, congestive heart failure or stroke, or previous coronary angioplasty or coronary bypass surgery. From this group, random samples of twins in two strata were selected: one stratum included twins discordant for a lifetime history of major depression; and in a second stratum, neither twin had a history of depression. All twins were examined, in pairs, at the Emory University General Clinical Research Center between March 2002 and March 2006. The GCRC admission lasted approximately 27 hours and the entire data collection for the study occurred during this time. The two twins maintained an identical schedule while in the GCRC under the supervision of study personnel. Activity was limited to ambulation within the Emory facilities. All assessment, including ambulatory ECG monitoring, began and ended at the same time. This protocol was approved by the Institutional Review Board at Emory University, and all participants provided written informed consent.
Metabolic syndrome
We used the 2005 American Heart Association (AHA), National Heart Lung and Blood Institute (NHLBI) definition of the MetS,30 which is based on any 3 of the following 5 criteria: waist circumference of ≥40 inches, triglycerides ≥150 mg/dL or drug treatment for elevated triglycerides, HDL-C <40 mg/dL or drug treatment for reduced HDL-C, systolic blood pressure (BP) ≥130 mmHg or diastolic BP ≥85 mmHg or drug treatment for hypertension, and fasting glucose ≥100 mg/dL or drug treatment for elevated glucose.30
Heart rate variability
For the measurement of HRV, participants wore an ambulatory ECG (Holter) monitor (GE Marquette SEER digital system) for 24 hours. Twin pairs were studied at the same time and their recording times, schedule, and activity level during Holter monitoring were matched. The methodology of HRV acquisition and analysis has been previously described.16, 31-33 All recordings were manually processed and edited to ensure accurate identification of QRS complexes. For frequency domain analysis, the heart rate spectrum was computed using a fast Fourier transformation with a Parzen window. As described previously,16 the various bands of power spectrum were defined as follows: ultra-low frequency (ULF) < 0.0033 Hz, very-low frequency (VLF) 0.0033 to <0.04 Hz, low frequency (LF) 0.04 to <0.15 Hz, high frequency (HF) 0.15 to <0.40 Hz, and total power (TP) <0.40 Hz. Recordings with >20% interpolation or <18 recorded hours of Holter data were excluded from the analysis.
Other measurements
A thorough medical history and a physical exam were obtained from all participants. Abdominal and hip circumference was measured. Systolic and diastolic blood pressure was measured using a standard sphygmomanometer on the right arm with the participant in the seated position after 10 minutes of rest. Blood pressure was taken as an average of two measurements 5 minutes apart. Venous blood samples were drawn for the measurements of glucose and lipid profile after an overnight fast. Total triglycerides were determined by enzymatic methods (Beckman Coulter Diagnostics, Fullerton, CA). Direct high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol were measured with homogeneous assays (Equal Diagnostics, Exton, PA). Glucose levels were measured on the Beckman CX7 chemistry autoanalyzer. Cigarette smoking was classified into current versus not current smoker. Physical activity was determined by means of a modified version of the Baecke Questionnaire of Habitual Physical Activity.34 For this analysis, we used the global physical activity score, higher scores indicating more physically active. To measure depressive symptoms, we administered the Beck Depression Inventory-II (BDI-II), a standardized scale of depressive symptoms with higher scores indicating more severe depression.35 For this analysis, we used a cut point of 14 to indicate the presence of at least mild depression.35
Statistical analysis
Baseline characteristics were compared between study participants with and without the MetS. P values were corrected for the correlation between co-twins using generalized estimating equations (GEE) for categorical variables and mixed effects models for continuous variables. All HRV measurements were log-transformed to normalize their distribution for analysis.
Two approaches were used to examine the association between MetS (and its individual components) and HRV: a) considering twins as separate individuals, in which all twins were eligible for inclusion regardless of whether their brother was available for analysis; and b) within twin pairs approach (co-twin control analysis). The within-pair effects are inherently controlled for demographic, shared familial (including genetics) and environmental influences; in addition, daily activities and other environmental factors during the ambulatory ECG recording are controlled since co-twins were examined at the same time and under the same conditions. If an association is present both when analyzing twins as individuals and within twin pairs, this suggests that there are not familial confounding factors.
In the analysis of all twins as individuals, we used mixed effects regression models and accounted for the twin pairs using a random effect term for each pair. Analyses were repeated after adjusting for age, current smoking, depression, physical activity, medications (beta-blocker, renin-angiotensin blocker, statin, aspirin, anti-depressant), and creatinine clearance. To see if there was a gradient pattern to the association between MetS and HRV, we compared the mean HRV in each frequency band for each additional component of the MetS (from 0 components to all 5 components). To ease interpretation, and in order to display the actual magnitude of the difference, we expressed the results as percent difference of the nontransformed values by using the following formula: [1-(expβ)] × 100 (%). Spearman correlation coefficients were also calculated between the individual components of the MetS and the spectral components of HRV as continuous variables. Finally, we compared mean HRV between individuals with and without the MetS, again adjusting for potential confounding factors.
We then performed within-pair analyses, which examined differences in HRV between co-twins in each pair who were discordant for the MetS component of interest. Initially we constructed twin pair subgroups who were discordant for the MetS and for each individual MetS component and compared HRV values between the discordant twins. Next, to examine the cumulative impact of increasing MetS risk factors on HRV, we defined discordance as a different number of MetS components within a twin pair. We fitted mixed effects models adapted for twin research36 in which the within-pair parameter expressed the difference between twins who were discordant for number of MetS components, and was analyzed as an ordinal variable describing the departure of each twin from the pair average. These analyses were repeated after stratification according to zygosity. A twin study design is a useful tool in uncovering genetic influences not only on specific traits, but also on specific associations. If the association is due to genetic factors, then it should be found within dizygotic (DZ) twin pairs, who share on average only 50% of their genes, but not within monozygotic (MZ) twin pairs, who share 100% of their genes. All of the analyses were performed using Statistical Analysis Software (version 9.13, SAS Institute, Inc, Cary, NC).
Results
Analyses of all twins as individuals
From the 360 twins in this study, 288 (80%) had Holter recordings adequate for HRV analyses (18 hours or more of recording with at least 80% noninterpolated intervals). Of these 288 participants, 151 (52%) had the MetS. This sample included 115 twin pairs and 58 unpaired twins (resulting from exclusion of the co-twin). Twins with the MetS had higher levels of depressive symptoms, were less physically active, and were more likely to be taking beta-blocker, aspirin, renin-angiotensin blocker, statin, and antidepressant medications (Table 1).
Table 1.
Distribution of demographic, behavioral and other characteristics in the entire sample and in 87 twin pairs discordant for the metabolic syndrome.
| All Twins (N=288) | MetS-Discordant Pairs (41 Twin Pairs) | |||||
|---|---|---|---|---|---|---|
| MetS (N=151) |
No MetS (N=137) |
p-value* | MetS (N=41) |
No MetS (N=41) |
p-value* | |
| Age | 54.5±3.0 | 54.2±2.7 | 0.27 | 54 | 54 | |
| Current smoker | 29.6% | 28.9% | 0.92 | 19.5% | 22.0% | 0.90 |
| Number of alcoholic beverages in typical week | 3.80± 5.78 | 5.21±9.31 | 0.13 | 3.58± 6.59 | 5.39± 8.87 | 0.30 |
| Number of caffeinated beverages in typical day | 4.52± 4.10 | 4.37± 5.48 | 0.79 | 4.22± 4.34 | 4.41± 3.07 | 0.81 |
| Beck Depression Inventory ≥ 14 | 14.6% | 7.3% | 0.049 | 14.6% | 7.3% | 0.29 |
| Physical activity | 6.9±1.6 | 7.8±1.9 | <0.001 | 6.9±1.7 | 7.6±2.1 | 0.10 |
| History of coronary heart disease | 12.6% | 3.7% | 0.009 | 4.9% | 2.4% | 0.55 |
| History of diabetes mellitus | 16.6% | 1.5% | <0.001 | 24.4% | 2.4% | 0.007 |
| Medications: | ||||||
| Beta blocker | 11.3% | 2.2% | 0.002 | 9.8% | 2.2% | 0.17 |
| Aspirin | 31.8% | 13.9% | <0.001 | 26.8% | 14.6% | 0.17 |
| Renin-angiotensin blocker (ACE-I or ARB) | 25.8% | 5.8% | <0.001 | 22.0% | 9.8% | 0.13 |
| Statin | 34.4% | 14.6% | <0.001 | 29.3% | 22.0% | 0.45 |
| Antidepressant | 19.2% | 8.8% | 0.01 | 22.0% | 12.2% | 0.24 |
| Creatinine clearance | 0.97± 0.15 | 0.94± 0.13 | 0.13 | 0.92± 0.12 | 0.94± 0.11 | 0.45 |
| Hemoglobin | 14.8 ± 1.1 | 14.9 ± 1.1 | 0.74 | 14.9 ± 1.0 | 14.8 ± 1.4 | 0.63 |
| MetS risk factors: | ||||||
| Triglycerides ≥150 mg/dL | 78.2% | 29.2% | <0.001 | 70.7% | 39.0% | 0.004 |
| HDL ≤40 mg/dL | 87.4% | 35.8% | <0.001 | 82.9% | 58.5% | 0.014 |
| SBP ≥130 or DBP≥85 mm Hg | 80.8% | 39.4% | <0.001 | 75.6% | 41.5% | 0.004 |
| Glucose ≥100 mg/dL | 66.2% | 19.7% | <0.001 | 73.2% | 17.1% | <0.001 |
| Waist ≥40 cm | 56.3% | 7.3% | <0.001 | 46.3% | 12.2% | <0.001 |
p value are corrected for pair cluster
MetS: Metabolic syndrome.
When the individual components of the MetS were considered as dichotomous variables (as defined by the updated AHA/NHLBI criteria) in the overall sample, most of them showed associations with at least one HRV variable. In adjusted analyses, associations persisted for hypertriglyceridemia (p<0.05 for ULF, VLF, LF, TP, ranging from 16% to 21% lower HRV), hypertension (p<0.05 for VLF, LF, 16% and 20% lower, respectively), hyperglycemia (p<0.05 for LF, 17% lower), and waist circumference (p<0.05 for LF, 18% lower). In contrast, after adjustment for possible known confounders such as physical activity and smoking, HDL did not show significant associations with any of the HRV frequency bands in multivariable analysis.37-40
The MetS was associated with decreased HRV across all frequency ranges when considered as a dichotomous variable (Table 2). After adjustment for possible confounders, the VLF, LF, and TP spectra of HRV remained significantly lower in participants with MetS. In addition, HRV decreased progressively with higher number of MetS components (Figure 1), and participants with all 5 components had HRV values between 18% and 50% lower than those with no risk factors. Exclusion of participants with diabetes or coronary heart disease resulted in no substantial change in these results.
Table 2.
Unadjusted and adjusted mean (and 95% CI) HRV in 288 participants with and without the metabolic syndrome
|
Unadjusted |
Adjusted * |
|||||||
|---|---|---|---|---|---|---|---|---|
| Metabolic syndrome (n=151) |
No metabolic syndrome (n=137) |
% Difference† |
p- value |
Metabolic syndrome |
No metabolic syndrome |
% Difference† |
p-value | |
| LnULF | 9.06 (8.97-9.16) | 9.22 (9.12-9.32) | -14.8 | 0.02 | 9.11 (8.79-9.43) | 9.24 (8.90-9.58) | -12.2 | 0.059 |
| LnVLF | 7.47 (7.37-7.57) | 7.74 (7.64-7.85) | -23.7 | <0.001 | 7.61 (7.29-7.94) | 7.84 (7.49-8.19) | -20.5 | 0.002 |
| LnLF | 6.47 (6.34-6.60) | 6.83 (6.70-6.97) | -30.2 | <0.001 | 6.65 (6.23-7.07) | 6.91 (6.47-7.36) | -22.9 | 0.005 |
| LnHF | 5.26 (5.11-5.41) | 5.50 (5.34-5.66) | -21.3 | 0.02 | 5.43 (4.95-5.91) | 5.59 (5.08-6.10) | -14.8 | 0.14 |
| LnTP | 9.36 (9.27-9.45) | 9.55 (9.45-9.64) | -17.3 | 0.003 | 9.44 (9.15-9.74) | 9.60 (9.28-9.91) | -14.8 | 0.02 |
LnULF= log ultra low frequency, LnVLF = log very low frequency, lnLF = log low frequency, lnHF = log high frequency, lnTP = log total power
Adjusted for age, current smoking, depression, physical activity, medications (beta-blocker, renin-angiotensin blocker, statin, aspirin, antidepressant), and creatinine clearance.
Difference in HRV expressed in original (not log-transformed) units.
Figure 1.
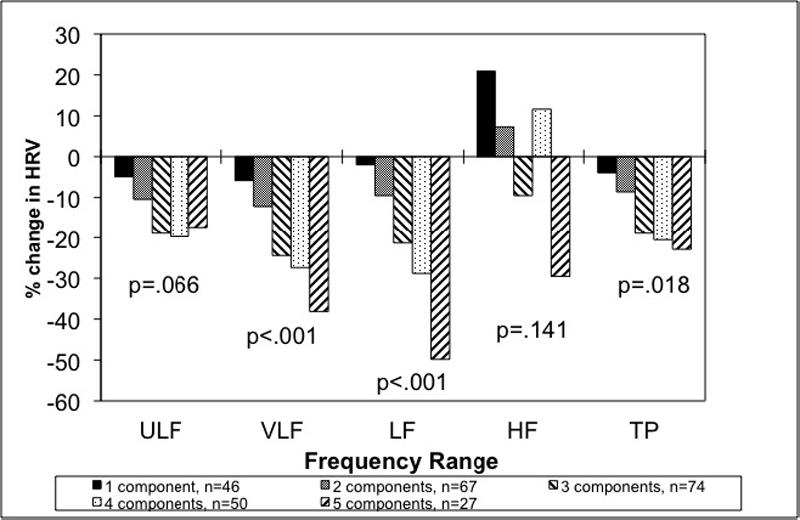
Percent Change in HRV by number of components compared with no components of the metabolic syndrome. P values listed are for the trend of increasing number of metabolic syndrome components. The results are adjusted for age, current smoking, depression, physical activity, medications (beta-blocker, renin-angiotensin blocker, statin, aspirin, antidepressant), and creatinine clearance.
Analyses within twin pairs
Of all twin pairs, 41 were discordant for metabolic syndrome. In general, differences in subject characteristics showed trends similar to the analyses of twins as individuals but were less pronounced within these matched pairs. When analyses were restricted to twin pairs who were discordant for each MetS component, analyses of the individual components of the MetS revealed a similar pattern to analyses of twins as individuals, although differences were somewhat less marked. The limited number of twin pairs who were discordant for each of the MetS components (n=24 to 42, depending on the MetS component), however, minimized the power of this analysis. Only hypertension and waist circumference were significantly associated (p<0.05) with HRV (VLF and LF, 24% to 40% lower for hypertension, respectively, and from 20% to 25% lower HRV for waist circumference) in twin pairs who were discordant for these components. When the analysis was repeated among the 41 twin pairs who were discordant for the MetS, VLF and LF were significantly associated with the MetS in unadjusted analysis and showed a borderline association in adjusted analysis (Table 3).
Table 3.
Unadjusted and adjusted mean (and 95% CI) HRV in 41 twin pairs discordant for the metabolic syndrome.
|
Unadjusted |
Adjusted * |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference for MetS Yes vs. No |
Difference for 1 Risk Factor Increment |
Difference for MetS Yes vs. No |
Difference for 1 Risk Factor Increment |
|||||||||
| Metabolic syndrome (n=41) |
No metabolic syndrome (n=41) |
% Difference† |
p- value |
% Difference† |
p-value | Metabolic syndrome (n=41) |
No metabolic syndrome (n=41) |
% Difference† |
p-value | % Difference† |
p-value | |
| LnULF | 9.15 (8.96-9.33) | 9.13 (8.94-9.32) | 2.0 | 0.87 | 2.1 | 0.60 | 9.01 (8.49-9.53) | 8.98 (8.46-9.51) | 3.0 | 0.79 | 3.0 | 0.46 |
| LnVLF | 7.59 (7.39-7.79) | 7.78 (7.58-7.97) | -17.3 | 0.04 | -10.1 | 0.01 | 7.79 (7.31-8.27) | 7.95 (7.46-8.44) | -14.8 | 0.09 | -8.4 | 0.03 |
| LnLF | 6.64 (6.38-6.91) | 6.90 (6.63-7.17) | -22.9 | 0.03 | -17.5 | 0.0003 | 7.24 (6.58-7.91) | 7.46 (6.78-8.14) | -19.7 | 0.10 | -14.6 | 0.002 |
| LnHF | 5.40 (5.13-5.67) | 5.54 (5.27-5.81) | -13.1 | 0.28 | -8.4 | 0.13 | 5.90 (5.19-6.62) | 6.05 (5.31-6.78) | -13.9 | 0.32 | -5.0 | 0.35 |
| LnTP | 9.46 (9.28-9.63) | 9.49 (9.32-9.76) | -3.0 | 0.63 | -1.3 | 0.72 | 9.44 (8.96-9.91) | 9.45 (8.96-9.94) | -1.0 | 0.86 | -0.2 | 0.95 |
LnULF= log ultra low frequency, LnVLF = log very low frequency, lnLF = log low frequency, lnHF = log high frequency, lnTP = log total power
Adjusted for age, current smoking, depression, physical activity, medications (beta-blocker, renin-angiotensin blocker, statin, aspirin, antidepressant), and creatinine clearance.
Difference in HRV expressed in original (not log-transformed) units.
When we considered the number of MetS components, there were 87 pairs who were discordant for number of MetS components. Within-pair analyses for differences in HRV between co-twins who differed for number of components confirmed a significantly lower VLF and LF spectra of HRV for each incremental component of the MetS. After adjustment for other covariables as above, a one-unit increment in MetS components was associated with an 8% lower VLF (p=0.03) and a 15% lower LF spectrum (p=0.002) comparing each twin with his brother (Table 3). Results were no different within MZ and DZ twin pairs examined separately. Among MZ twins, who share 100% of their genetic material, for each additional MetS component there was a 10% lower VLF (p=0.03) and a 15% lower LF (p=0.01) HRV comparing each twin with his brother.
We repeated analyses without adjusting for medications, which may have been prescribed for treatment of MetS risk factors (beta-blockers, renin-angiotensin blockers and statins). The results were very similar and are not shown.
Discussion
We found a strong and consistent association between the MetS and lower HRV in a well-characterized sample of middle-aged male twins. There was a gradient to the association such that participants with an increasing number of components of the MetS had progressively lower HRV. In addition, within-pair analyses of co-twins, which accounted for unmeasured sociodemographic, lifestyle and familial factors shared by twins being raised in the same family, as well as environmental influences during ECG monitoring, demonstrated a robust association of the MetS with HRV (specifically VLF and LF). Because we demonstrated that an association between MetS and lower HRV was present in both analyses of twins as individuals as well as within twin pairs, we also can eliminate familial confounding factors. Of all the HRV spectra, VLF and LF showed persistent associations in within-pair analyses, and each incremental MetS component was associated with progressively lower VLF and LF HRV comparing co-twins.
Several prior studies have examined the association between the MetS and HRV.7, 9, 20, 41-43 The presence of the MetS in 2359 patients from the Atherosclerosis Risk in Communities (ARIC) study was associated with reduced LF and HF components of HRV.20 In a study of 2197 participants in the Whitehall II study, each individual component of the MetS and the MetS as a whole were associated with reduced LF and HF power components of HRV.9 In the Cardiovascular Health Study, the presence of >2 components of the MetS was associated with decreased ULF and TP components of HRV.43 Most of these prior studies, however, have been limited by their measurement of short-term (LF and HF) HRV. Importantly, short-term HRV does not measure fluctuation in beat-to-beat intervals with cycles longer than 5 minutes. It is the VLF and ULF spectra that have the greatest prognostic utility for CVD.14, 16 Although VLF can be calculated in short-term recordings (≤ 5 min), it is a dubious measure and should be avoided.31 Other prior studies did not adjust for important potential confounders, such as depression and physical activity, nor was HRV recorded in a controlled setting.43 Our study also has the advantage of twins analyses, which may eliminate other unmeasured confounders.
There are several important implications of our findings. These results support the integral role of the autonomic nervous system in patients with the MetS. Whether the inciting factor leading to the MetS is obesity44 or a primary abnormality of autonomic tone leading to a disturbance in the hypothalamus-pituitary-adrenal axis45 is unclear. Due to the cross-sectional design of our study, this cannot be determined from our results. As demonstrated in the postmyocardial infarction setting and in patients without overt coronary heart disease, abnormal HRV predicts mortality, cardiac death, and arrhythmic death.16, 18, 46 In particular, the ULF and VLF components of HRV are more important predictors than the LF and HF components. The VLF component, which showed a robust association with the MetS in our study, is dependent on the presence of parasympathetic activity47 and is associated with arrhythmic death.16
There are several strengths of our study. We assessed and adjusted for important confounding factors associated with HRV. In addition, because participants in our study were twins, within-pair analyses of co-twins accounted for familial and genetic factors shared by twins. Because co-twins were examined at the same time, environmental influences during ECG monitoring were also controlled. There are, however, several limitations of our study to consider. Despite our controlled design, it is possible that the association between the MetS and HRV may be influenced by other unmeasured confounding variables. In particular, obstructive sleep apnea has been associated with both the MetS and HRV but was unmeasured in our population.48, 49 Also, the participants included in our study were all male middle-aged military veterans, so the results may not generalize to other populations.
In conclusion, in predominantly healthy middle-aged men, the MetS is associated with decreased HRV. These findings suggest that abnormalities of the autonomic tone as evidenced by HRV may be partly responsible for the adverse cardiovascular events such as atrial fibrillation, sudden death, or acute coronary syndrome seen in patients with the MetS.
Acknowledgements
We gratefully acknowledge the continued cooperation and participation of the members of the Vietnam Era Twin Registry. Without their contribution this research would not have been possible.
This work was supported by NIH (R01 HL68630, R01 AG026255, and K24HL077506); the American Heart Association (0245115N), and Emory University General Clinical Research Center (MO1-RR00039). The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; NIH; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University.
References
- [1].Aguilar D, Fisher MR, O'Connor CM, Dunne MW, Muhlestein JB, Yao L, Gupta S, Benner RJ, Cook TD, Edwards D, Pfeffer MA. Metabolic syndrome, C-reactive protein, and prognosis in patients with established coronary artery disease. Am Heart J. 2006;152:298–304. doi: 10.1016/j.ahj.2005.11.011. [DOI] [PubMed] [Google Scholar]
- [2].Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- [3].Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164:1066–1076. doi: 10.1001/archinte.164.10.1066. [DOI] [PubMed] [Google Scholar]
- [4].Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- [5].Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- [6].Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, Aizawa Y. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117:1255–1260. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GD, Stansfeld SA, Marmot MG. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106:2659–2665. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- [8].Grassi G, Seravalle G. Autonomic imbalance and metabolic syndrome: unravelling interactions, mechanisms and outcomes. J Hypertens. 2006;24:47–49. doi: 10.1097/01.hjh.0000198040.47128.4c. [DOI] [PubMed] [Google Scholar]
- [9].Hemingway H, Shipley M, Brunner E, Britton A, Malik M, Marmot M. Does autonomic function link social position to coronary risk? The Whitehall II study. Circulation. 2005;111:3071–3077. doi: 10.1161/CIRCULATIONAHA.104.497347. [DOI] [PubMed] [Google Scholar]
- [10].Miyazaki T, Zipes DP. Pericardial prostaglandin biosynthesis prevents the increased incidence of reperfusion-induced ventricular fibrillation produced by efferent sympathetic stimulation in dogs. Circulation. 1990;82:1008–1019. doi: 10.1161/01.cir.82.3.1008. [DOI] [PubMed] [Google Scholar]
- [11].Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr., Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- [12].Chen PS, Tan AY. Autonomic nerve activity and atrial fibrillation. Heart Rhythm. 2007;4:S61–64. doi: 10.1016/j.hrthm.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hamaad A, Lip GY, MacFadyen RJ. Heart rate variability estimates of autonomic tone: relationship to mapping pathological and procedural stress responses in coronary disease. Annals of medicine. 2004;36:448–461. doi: 10.1080/07853890410015810. [DOI] [PubMed] [Google Scholar]
- [14].Kleiger RE, Stein PK, Bigger JT., Jr. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51:1725–1733. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- [16].Bigger JT, Jr., Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- [17].Kleiger RE, Miller JP, Bigger JT, Jr., Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- [18].Tsuji H, Larson MG, Venditti FJ, Jr., Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- [19].Vikman S, Makikallio TH, Yli-Mayry S, Nurmi M, Airaksinen KE, Huikuri HV. Heart rate variability and recurrence of atrial fibrillation after electrical cardioversion. Annals of medicine. 2003;35:36–42. doi: 10.1080/07853890310004110. [DOI] [PubMed] [Google Scholar]
- [20].Liao D, Sloan RP, Cascio WE, Folsom AR, Liese AD, Evans GW, Cai J, Sharrett AR. Multiple metabolic syndrome is associated with lower heart rate variability. The Atherosclerosis Risk in Communities Study. Diabetes Care. 1998;21:2116–2122. doi: 10.2337/diacare.21.12.2116. [DOI] [PubMed] [Google Scholar]
- [21].Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O'Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- [22].Pannier B, Thomas F, Eschwege E, Bean K, Benetos A, Leocmach Y, Danchin N, Guize L. Cardiovascular risk markers associated with the metabolic syndrome in a large French population: the “SYMFONIE” study. Diabetes Metab. 2006;32:467–474. doi: 10.1016/s1262-3636(07)70305-1. [DOI] [PubMed] [Google Scholar]
- [23].Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Genet Med Gemellol (Roma) 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- [24].Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Res. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- [25].Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- [26].Dai J, Miller AH, Bremner JD, Goldberg J, Jones L, Shallenberger L, Buckham R, Murrah NV, Veledar E, Wilson PW, Vaccarino V. Adherence to the mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: a twin study. Circulation. 2008;117:169–175. doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vaccarino V, Lampert R, Bremner JD, Lee FA, Su S, Maisano C, Murrah NV, Jones L, Jawed F, Afzal N, Ashraf A, Goldberg J. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom Med. 2008 doi: 10.1097/PSY.0b013e31817bcc9e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhao J, Cheema FA, Reddy U, Bremner JD, Su S, Goldberg J, Snieder H, Vaccarino V. Heritability of flow-mediated dilation: a twin study. J Thromb Haemost. 2007;5:2386–2392. doi: 10.1111/j.1538-7836.2007.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Scherrer JF, Xian H, Bucholz KK, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of depression symptoms, hypertension, and heart disease in middle-aged men. Psychosom Med. 2003;65:548–557. doi: 10.1097/01.psy.0000077507.29863.cb. [DOI] [PubMed] [Google Scholar]
- [30].Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr., Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- [31].Heart rate variability standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- [32].Rottman JN, Steinman RC, Albrecht P, Bigger JT, Jr., Rolnitzky LM, Fleiss JL. Efficient estimation of the heart period power spectrum suitable for physiologic or pharmacologic studies. Am J Cardiol. 1990;66:1522–1524. doi: 10.1016/0002-9149(90)90551-b. [DOI] [PubMed] [Google Scholar]
- [33].Lampert R, Ickovics JR, Viscoli CJ, Horwitz RI, Lee FA. Effects of propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta-Blocker Heart Attack Trial. Am J Cardiol. 2003;91:137–142. doi: 10.1016/s0002-9149(02)03098-9. [DOI] [PubMed] [Google Scholar]
- [34].Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Jr., Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24:685–693. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- [35].Beck A, Steer R, Brown G. Beck Depression Inventory Manual. 2nd ed. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- [36].Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34:1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- [37].Aadahl M, Kjaer M, Jorgensen T. Associations between overall physical activity level and cardiovascular risk factors in an adult population. European journal of epidemiology. 2007;22:369–378. doi: 10.1007/s10654-006-9100-3. [DOI] [PubMed] [Google Scholar]
- [38].Carnethon MR, Prineas RJ, Temprosa M, Zhang ZM, Uwaifo G, Molitch ME. The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care. 2006;29:914–919. doi: 10.2337/diacare.29.04.06.dc05-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen CC, Li TC, Chang PC, Liu CS, Lin WY, Wu MT, Li CI, Lai MM, Lin CC. Association among cigarette smoking, metabolic syndrome, and its individual components: the metabolic syndrome study in Taiwan. Metabolism. 2008;57:544–548. doi: 10.1016/j.metabol.2007.11.018. [DOI] [PubMed] [Google Scholar]
- [40].Poulsen PL, Ebbehoj E, Hansen KW, Mogensen CE. Effects of smoking on 24-h ambulatory blood pressure and autonomic function in normoalbuminuric insulin-dependent diabetes mellitus patients. Am J Hypertens. 1998;11:1093–1099. doi: 10.1016/s0895-7061(98)00115-0. [DOI] [PubMed] [Google Scholar]
- [41].Aso Y, Wakabayashi S, Nakano T, Yamamoto R, Takebayashi K, Inukai T. High serum high-sensitivity C-reactive protein concentrations are associated with relative cardiac sympathetic overactivity during the early morning period in type 2 diabetic patients with metabolic syndrome. Metabolism. 2006;55:1014–1021. doi: 10.1016/j.metabol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- [42].Kuch B, Hense HW, Sinnreich R, Kark JD, von Eckardstein A, Sapoznikov D, Bolte HD. Determinants of short-period heart rate variability in the general population. Cardiology. 2001;95:131–138. doi: 10.1159/000047359. [DOI] [PubMed] [Google Scholar]
- [43].Stein PK, Barzilay JI, Domitrovich PP, Chaves PM, Gottdiener JS, Heckbert SR, Kronmal RA. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: the Cardiovascular Health Study. Diabet Med. 2007;24:855–863. doi: 10.1111/j.1464-5491.2007.02163.x. [DOI] [PubMed] [Google Scholar]
- [44].Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2006;1083:129–152. doi: 10.1196/annals.1367.010. [DOI] [PubMed] [Google Scholar]
- [45].Buijs RM, Kreier F. The metabolic syndrome: a brain disease? J Neuroendocrinol. 2006;18:715–716. doi: 10.1111/j.1365-2826.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- [46].Sandercock GR, Brodie DA. The role of heart rate variability in prognosis for different modes of death in chronic heart failure. Pacing Clin Electrophysiol. 2006;29:892–904. doi: 10.1111/j.1540-8159.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- [47].Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- [48].Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- [49].Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98:1071–1077. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]


