Abstract
Rationale
Previous research indicates that acute nicotine administration enhances the acquisition of contextual fear conditioning and trace cued fear conditioning. Pharmacological inhibition of α4β2 nicotinic acetylcholine receptors (nAChRs), but not α7 nAChRs, blocked the enhancing effect of nicotine on contextual fear conditioning. Similarly, genetic deletion of the β2 nAChR subunit but not the α7 nAChR subunit blocked the enhancing effect of nicotine on contextual fear conditioning.
Objectives
In the present study, nAChR subunit knockout mice were used to compare the involvement of β2 subunit-containing nAChRs and α7 subunit-containing nAChRs in the effects of nicotine on hippocampus-dependent trace cued fear conditioning and contextual fear conditioning.
Methods
β2 nAChR subunit knockout mice, α7 nAChR subunit knockout mice, and their wild-type littermates received either nicotine or saline 5 minutes before training and testing. Mice were trained using five conditioned stimulus (CS; 30 s, 85 dB white noise)—trace (30 s)—unconditioned stimulus (US; 2 s footshock) pairings. Freezing to the context and freezing to the CS were assessed 24 h later.
Results
Both contextual and trace cued fear conditioning were enhanced by nicotine administration in wild-type littermates and in α7 nAChR subunit knockout mice. In contrast, neither contextual fear conditioning nor trace cued fear conditioning was enhanced by nicotine administration in β2 nAChR subunit knockout mice.
Conclusions
These results suggest that β2 subunit-containing nAChRs but not α7 nAChR subunit-containing nAChRs are critically involved in the enhancing effect of nicotine on contextual and trace cued fear conditioning.
Keywords: Beta 2, Alpha 7, Nicotinic receptors, Nicotine, Learning, Hippocampus, Fear conditioning, Knockout mice
The effects of nicotine on learning and memory have been examined using a variety of tasks (for reviews, see Levin 2002; Levin and Simon 1998; Rezvani and Levin 2001; Tinsley et al. 2004) including delay and trace fear conditioning (for review, see Gould 2006). In delay fear conditioning, animals are trained using paired coterminating presentations of a conditioned stimulus (CS) with an unconditioned stimulus (US). Training results in the formation of two associations: an association between the training context and the US (contextual fear conditioning), which depends upon the hippocampus, and an association between the CS and the US (delay-cued fear conditioning; Logue et al. 1997b; Phillips and Ledoux 1992), which does not depend critically upon the hippocampus. In trace fear conditioning, animals are trained using presentations of a CS and a US that are separated by a time period during which no stimuli are presented (called a trace period). As in delay fear conditioning, a training context–CS association and a CS–US association is formed as a result of training. However, the addition of a trace period between the CS and the US is believed to engage working memory and renders the association between the CS and the US hippocampus-dependent (McEchron et al. 1998, 2000; Quinn et al. 2002, 2005).
Previous research indicates that acute nicotine administration enhances hippocampus-dependent fear conditioning (i.e., contextual and trace cued fear conditioning) but not hippocampus-independent fear conditioning (i.e., delay cued fear conditioning; Davis and Gould 2006; Davis et al. 2005, 2006; Gould 2003; Gould et al. 2004; Gould and Higgins 2003; Gould and Lommock 2003; Gould and Wehner 1999). Furthermore, research indicating that administration of mecamylamine, a broad-spectrum nicotinic acetylcholine receptor (nAChR) antagonist, blocks the enhancing effect of nicotine on contextual fear conditioning suggests that the enhancing effect of nicotine is mediated via nAChRs (Gould and Higgins 2003; Gould and Wehner 1999). However, these studies do not identify which nAChR subtypes are involved in the effects of nicotine on fear conditioning.
nAChRs are a family of ligand-gated, ionotropic receptors that mediate fast synaptic transmission throughout the central nervous system. nAChRs have a pentameric structure and are comprised of either α (α7–α10) subunits or a combination of α (α2 –α6) and β (β2–β4) subunits (Decker et al. 1995; Hogg et al. 2003; Jones et al. 1999; McGehee 1999). Two nAChR subtypes that, combined, encompass approximately 90% of all nAChRs are α4β2 nAChRs and α7 nAChRs (Marks and Collins 1982; Whiteaker et al. 1998). These nAChR subtypes are critically involved in some hippocampus-dependent tasks (Barros et al. 2004; Curzon et al. 1996; Felix and Levin 1997; Levin et al. 2002) and have a modulatory role in other hippocampus-dependent tasks (Davis and Gould 2006; Wehner et al. 2004). For example, Barros et al. (2004) demonstrated that intrahippocampal administration of dihydro-beta-erythrodine (DHBE), an antagonist that binds α4β2 nAChRs with high affinity, impaired passive avoidance learning. Similarly, Levin et al. (2002) demonstrated that spatial working memory performance in the radial-arm maze was impaired by hippocampal infusions of DHBE and by hippocampal infusions of the α7 nAChR antagonist, methyllycaconitine (MLA). Barros et al. (2004) and Levin et al. (2002) did not examine if administration of either antagonist blocked the enhancing effect of nicotine on working memory performance in the radial-arm maze. However, Bancroft and Levin (2000) and Bettany and Levin (2001) demonstrated that chronic nicotine administration reversed DHBE but not MLA-induced working memory performance deficits. These data suggest that α7 nAChRs are involved in the effect of chronic nicotine administration on working memory performance in the radial-arm maze (for review, see Levin and Simon 1998).
The involvement of α4β2 nAChRs and α7 nAChRs in the enhancing effect of nicotine on contextual fear conditioning, a task that involves different cellular substrates (El Ghundi et al. 1999; Graves et al. 2002; Peters et al. 2003; Roberts et al. 2004; Voikar et al. 2004) and different subregions of the hippocampus (Burwell et al. 2004; Good and Honey 1997) from those that are involved in spatial working memory in the radial-arm maze, has been examined as well. In a recent study, Davis and Gould (2006) examined the effects of administration of DHBE and the effects of MLA on nicotine enhancement of contextual fear conditioning. The results indicated that DHBE administration but not MLA administration blocked the enhancing effect of nicotine on contextual fear conditioning. Consistent with data from previous studies indicating that mecamylamine has no effect on fear conditioning in the absence of nicotine (Gould and Higgins 2003; Gould and Wehner 1999), administration of DHBE or MLA alone did not impair fear conditioning. These results suggest, then, that α4β2 nAChRs are critically involved in the enhancing effect of nicotine on contextual fear conditioning. Furthermore, α4β2 nAChRs and α7 nAChRs are not necessary for the acquisition of delay fear conditioning. Similar results have been reported by Wehner et al. (2004). The researchers examined the role of α7 and β2 subunit-containing nAChRs in the effects of nicotine on delay fear conditioning using nAChR subunit knockout mice and demonstrated that β2 subunit-containing nAChRs but not α7 subunit-containing nAChRs are critically involved in nicotine enhancement of contextual fear conditioning. Deletion of α7 nAChR subunits and β2 nAChR subunits had no effect on delay fear conditioning in the absence of nicotine (see also Caldarone et al. 2000; Paylor et al. 1998).
The results of Davis and Gould (2006) and Wehner et al. (2004) provide evidence that β2 subunit-containing nAChRs (especially α4β2 nAChRs) are necessary for the enhancing effect of nicotine on hippocampus-dependent contextual fear conditioning. However, given data indicating that trace cued fear conditioning may involve neural substrates that are not critically involved in contextual fear conditioning (Knight et al. 2004; Weitemier and Ryabinin 2004), it is unclear if the activation of β2 subunit-containing nAChRs is critically involved in the enhancement of trace cued fear conditioning by nicotine. Furthermore, although it is evident that α7 nAChRs are not critically involved in the enhancing effect of nicotine on the acquisition of contextual fear conditioning, it is unclear if α7 nAChRs are necessary for nicotine enhancement of trace cued fear conditioning. Thus, in the present study, the role of β2 subunit-containing nAChRs and the role of α7 subunit-containing nAChRs in trace cued fear conditioning and in the ability of nicotine to enhance trace cued fear conditioning was examined using α7 nAChR subunit knockout mice and β2 nAChR subunit knockout mice.
Experimental procedures
Subjects
Heterozygous α7 nAChR subunit knockout mice and heterozygous β2 nAChR subunit knockout mice (original breeding pairs provided by Dr. Arthur Beaudet, Baylor College of Medicine) were bred to obtain male and female α7 nAChR subunit knockout mice (α7 KO; ages 8–12 weeks), β2 nAChR subunit knockout mice (β2 KO; ages 8–12 weeks), and wild-type littermates (α7 WT and β2 WT, respectively; 8–12 weeks of age). Mice were generated in 129/SvEv ES cells (see Orr-Urtreger et al. 1997; Xu et al. 1999 for detailed descriptions of the generation of these mouse lines and genotyping reactions) and backcrossed to C57BL/6 mice for seven generations. Mice were maintained on a 12/12 h light dark cycle (lights on 7:00 a.m.) and housed in groups of two to five with ad libitum access to food and water. All behavioral procedures occurred between the hours of 8:00 a.m. and 5:00 p.m.
Apparatus
Training and testing for freezing to the context occurred in four chambers (17.8×19.1×38.1 cm) housed in sound attenuating boxes (MED, St. Albans, VT, USA). The chamber walls were constructed from Plexiglas in the front, back, and top and stainless steel on the sides. The floor of each chamber, which was constructed of 13 metal rods, was connected to a shock scrambler and generator. Ventilation fans for air exchange and background noise (69 dB) were mounted on the right wall of each sound attenuating box, and speakers for administering the white noise CS were located on the right wall of each chamber. Chambers were interfaced with an IBM-PC computer running MED-PC software to control stimulus administration.
Testing for freezing to the CS occurred in four identical altered chambers (20.3×22.9×17.8 cm) that were housed in sound attenuating boxes and located in a different room from the training chambers. The chamber walls were constructed from Plexiglas on all sides, and the chamber floors were covered in white plastic. Speakers for delivering the CS were mounted on the left wall of each chamber. A vanilla extract olfactory cue was added to further alter these chambers from the training chambers.
Behavioral procedures
Mice were trained in trace fear conditioning using five CS (30 s, 85 dB, white noise)—trace (30 s)—US (2 s, footshock) pairings separated by 90–120 s [randomized intertrial intervals (ITI)]. Previous research (Wehner et al. 2004) has demonstrated that the acquisition of contextual fear conditioning is enhanced by nicotine administration in both α7 WT and α7 KO mice when mice are trained with low footshock intensity but not when they are trained with higher footshock intensity. Thus, mice were trained using either a 0.57-mA footshock US, which has been used previously to examine the effects of nicotine on contextual and trace cued fear conditioning (for review, see Gould 2006), or a 0.29-mA footshock US. The training session started with a 120-s baseline period during which freezing (no movement except respiration) was assessed and after which the first CS–trace–US presentation occurred. Immediate freezing was scored during the ITI between the fourth and the fifth CS–trace–US presentations. The training session ended with a 30-s period during which freezing behavior was not recorded.
Testing for freezing to the context occurred 24 h after training. Mice were placed in the training chambers, and freezing was scored for 5 min. One hour later, testing for freezing to the CS occurred in altered context chambers. During the first 180 s, freezing in the absence of the CS was assessed. During the final 180 s, freezing to the CS was assessed. Freezing was assessed by experimenters who have shown interrater reliability of >90%.
Drugs and administration
Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO, USA) was dissolved in saline. Each mouse received either saline or nicotine (0.09, 0.18, and 0.27 mg/kg; reported as free base) via intraperitoneal injection 5 min before both training and testing. The 0.09 mg/kg dose of nicotine was chosen because it enhances contextual fear conditioning (for review, see Gould 2006) and produces plasma nicotine levels comparable to smokers (Benowitz et al. 1989; Henningfield and Keenan 1993).
Statistical analyses
All data were initially analyzed using either 2 (sex: male, female) × 2 (genotype: wildtype, knockout) × 2 (treatment: 0.09 mg/kg nicotine, saline) ANOVAs or 2 (sex: male, female) × 2 (genotype: wild type, knockout) × 3 (treatment saline, 0.18 mg/kg nicotine, 0.27 mg/kg nicotine) ANOVAs. Because there were no significant interactions with sex, data from males and females were collapsed, and 2×2 or 2×3 ANOVAs were performed (as indicated in the “Results” section). Post hoc analyses were performed using Tukey HSD tests when variances were equal and Games–Howell tests when group variances were unequal.
Results
The β2 nAChR subunit is involved in the enhancing effect of nicotine on contextual and trace cued fear conditioning
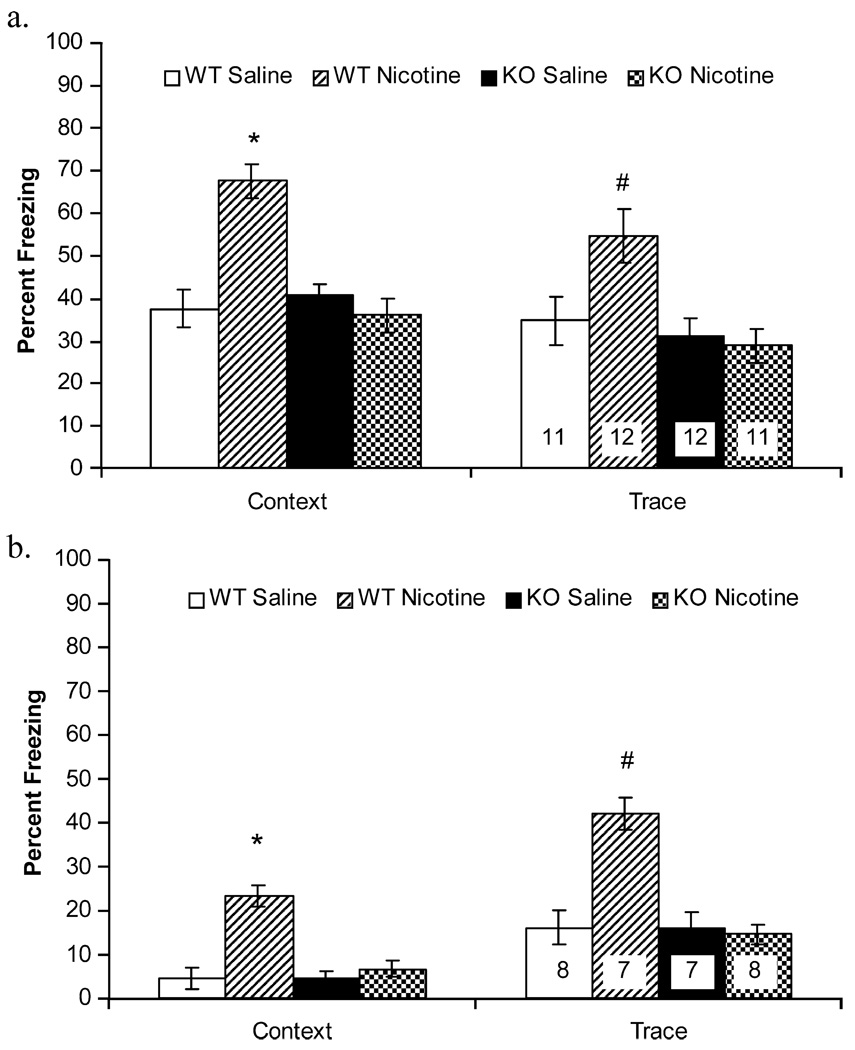
A 2×2 ANOVA revealed a main effect of treatment [F(1, 42)=12.72, p=0.00], a main effect of genotype [F(1, 42)=14.54, p=0.00], and a significant treatment by genotype interaction [F(1, 42)=20.73, p=0.00] for freezing to the context for mice trained using a 0.57-mA footshock US (Fig. 1a; N=11–12 per group). Follow-up Tukey-adjusted comparisons revealed that β2 WT mice treated with nicotine demonstrated higher levels of contextual fear conditioning than saline-treated β2 WT mice [t(42)=5.76, p=0.00], saline-treated β2 KO mice [t(42)=5.30, p=0.00], and nicotine-treated β2 KO mice [t(42)=6.05, p=0.00]. There were no significant differences in contextual fear conditioning among nicotine-treated β2 KO mice, saline-treated β2 KO mice, and saline-treated β2 WT mice (p>0.05 for all comparisons).
Figure 1.
The effects of nicotine (0.09 mg/kg) administration on contextual and trace cued fear conditioning in β2 WT mice and β2 KO mice were examined. a Mice were trained using 5 CS (30 s, 85 dB white noise)—trace (30 s)—US (2 s, 0.570-mA footshock) presentations. Pairwise comparisons revealed that nicotine administration enhances the acquisition of both contextual fear conditioning and trace cued fear conditioning in β2 WT mice but not in β2 KO mice. Error bars represent ±1 SE from the means. Asterisk, number sign significantly different (p<0.05) from all other groups (for contextual and trace fear conditioning respectively). b β2 WT and β2 KO mice were trained using a 0.285-mA footshock US. Nicotine administration enhanced the acquisition of both contextual fear conditioning and trace cued fear conditioning in β2 WT mice but not β2 KO mice. Error bars represent ±1 SE from the means. Asterisk, number sign significantly different (p<0.05) from all other groups (for contextual and trace fear conditioning respectively). The numbers of mice per group are presented in the trace fear conditioning bars; the same mice were tested for contextual and trace cued fear conditioning
A 2×2 ANOVA revealed a significant effect of genotype [F(1, 42)=8.90, p=0.01] and no effect of treatment on trace cued fear conditioning [F(1,42)=2.12, p=0.15]. In addition, there was a significant genotype by treatment interaction [F(1, 42)=6.62, p=0.01]. Follow-up Tukey-adjusted comparisons revealed that nicotine-treated β2 WT mice demonstrated higher levels of trace cued fear conditioning than saline-treated β2 WT mice [t(42)=2.83, p=0.04], saline-treated β2 KO mice [t(42)=3.45, p=0.01], and nicotine-treated β2 KO mice [t(42)=3.69, p=0.00]. There were no differences in trace cued fear conditioning among saline-treated β2 WT mice, saline-treated β2 KO mice, and nicotine-treated β2 KO mice (p>0.05 for all comparisons). In addition, there were no differences among groups in baseline, immediate, and pre-CS freezing (data not shown; p>0.05 for all comparisons) suggesting that differences in contextual and trace cued fear conditioning were not due to differences in locomotor activity or generalized freezing.
The data from β2 KO mice and β2 WT mice trained using a 0.29-mA footshock US are presented in Fig. 1b (N=7–8 per group). The 2×2 ANOVAs revealed significant effects of genotype [F(1, 26)=15.52, p=0.00] and treatment on contextual fear conditioning [F(1, 26)=25.65, p=0.00] and significant effects of genotype [F(1, 26)=22.23, p=0.00] and treatment [F(1, 26)=18.20, p=0.00] on trace cued fear conditioning. In addition, the analyses revealed significant interactions between genotype and treatment for contextual [F(1, 26)=25.65, p=0.00] and trace cued fear conditioning [F(1, 26)=17.39, p=0.00]. Follow-up Tukey-adjusted comparisons revealed that β2 WT mice treated with nicotine demonstrated higher levels of contextual fear conditioning [t(26)=7.16, p=0.00; t(26)=6.16, p=0.00; t(26)=6.37, p=0.00 vs saline-treated β2 WT mice, saline-treated β2 KO mice, and nicotine-treated β2 KO mice, respectively] and trace cued fear conditioning [t(26)=5.96, p=0.00; t(26)=6.15, p=0.00; t(26)=6.28, p=0.00 vs saline-treated β2 WT mice, saline-treated β2 KO mice, and nicotine-treated β2 KO mice, respectively] than all other groups. There were no differences in contextual or trace cued fear conditioning among saline-treated β2 WT mice, saline-treated β2 KO mice, and nicotine-treated β2 KO mice (p>0.05 for all comparisons). In addition, no differences existed in baseline, immediate, or pre-CS freezing (data not shown; p>0.05 for all comparisons).
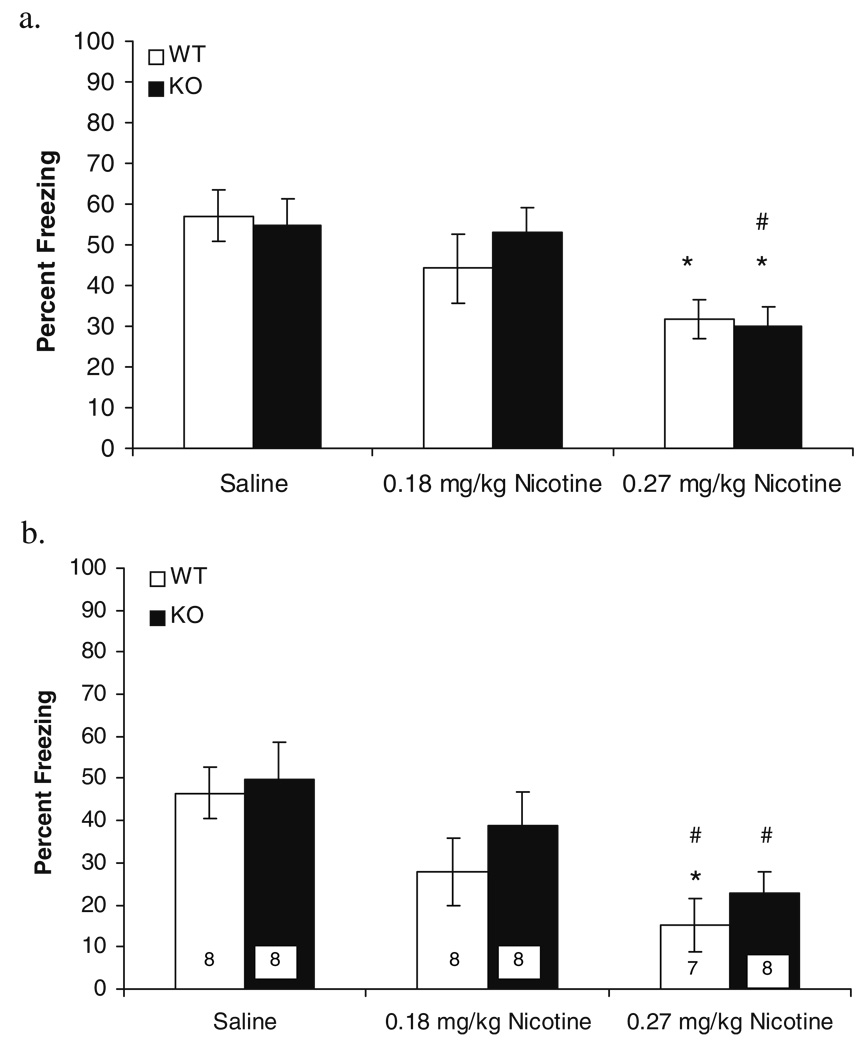
It is possible that genetic deletion of the β2 nAChR subunit altered sensitivity to the effects of nicotine on contextual and trace cued fear conditioning. Such alterations could account for results indicating that the single tested dose of nicotine (0.09 mg/kg) had no effect on contextual or trace cued fear conditioning in β2 KO mice. To assess this possibility, additional experiments were conducted to assess the effects of two higher doses of nicotine (0.18 and 0.27 mg/kg) on these tasks in β2 KO mice and β2 WT mice (N=7–8 per group) trained using the 0.57 mA footshock US. The 2×3 ANOVAs revealed that there was a main effect of treatment on both contextual [F(2, 41)=9.75, p=0.00; Fig. 2a] and trace cued fear conditioning [F(2, 41)=10.23, p=0.00; Fig. 2b]. There were no effects of genotype on contextual [F (1, 31)=0.11, p=0.74] or trace cued fear conditioning [F(1, 41)=2.02, p=0.16] and no significant interactions between genotype and treatment [F(2, 41)=0.58, p=0.56; F (2, 41)=0.18, p=0.84 for contextual and trace cued fear conditioning, respectively]. In addition, there were no significant main effects or interaction for baseline, immediate, and pre-CS freezing (p>0.05 for all comparisons; data not shown). Follow-up Tukey HSD analyses revealed that WT mice treated with 0.27 mg/kg of nicotine froze significantly less to the context than saline-treated WT mice [t(41)=3.01, p=0.05] and significantly less to the CS than saline-treated WT mice [t(41)=3.38, p=0.02] and saline-treated KO mice [t(41)=3.78, p=0.01]. Likewise, KO mice treated with 0.27 mg/kg of nicotine froze significantly less to the context than saline-treated KO [t(41)=3.10, p=0.04] and WT mice [t(41)=3.35, p=0.02] and significantly less to the CS than saline-treated KO mice [t(41)=3.01, p=0.05].
Figure 2.
The effects of 0.18 and 0.27 mg/kg of nicotine on contextual (a) and trace cued fear conditioning (b) in β2 KO mice and β2 WT mice were examined. Error bars represent ±1 SE from the means. Asterisk significantly less than saline-treated β2 WT mice (p<0.05), number sign significantly less than saline-treated β2 KO mice. The numbers of mice per group are presented in panel b; the same mice were tested for contextual and trace cued fear conditioning
Taken together, the data suggest that β2 subunit-containing nAChRs are critically involved in the enhancing effect of nicotine on contextual and trace cued fear conditioning. Furthermore, it is unlikely that genetic deletion of the β2 nAChR subunit altered sensitivity to the effects of nicotine on contextual and trace cued fear conditioning because higher doses of nicotine failed to enhance freezing to the context and freezing to the CS. Rather, β2 KO and β2 WT mice treated with the highest dose of nicotine froze significantly less to the context and CS than other groups. These data are not surprising as previous studies suggest that high doses of nicotine may produce behavioral and physiological effects that are opposite to those of lower doses of nicotine (for review, see Picciotto 2003).
The α7 nAChR subunit may not be critical for the enhancing effect of nicotine on contextual and trace cued fear conditioning
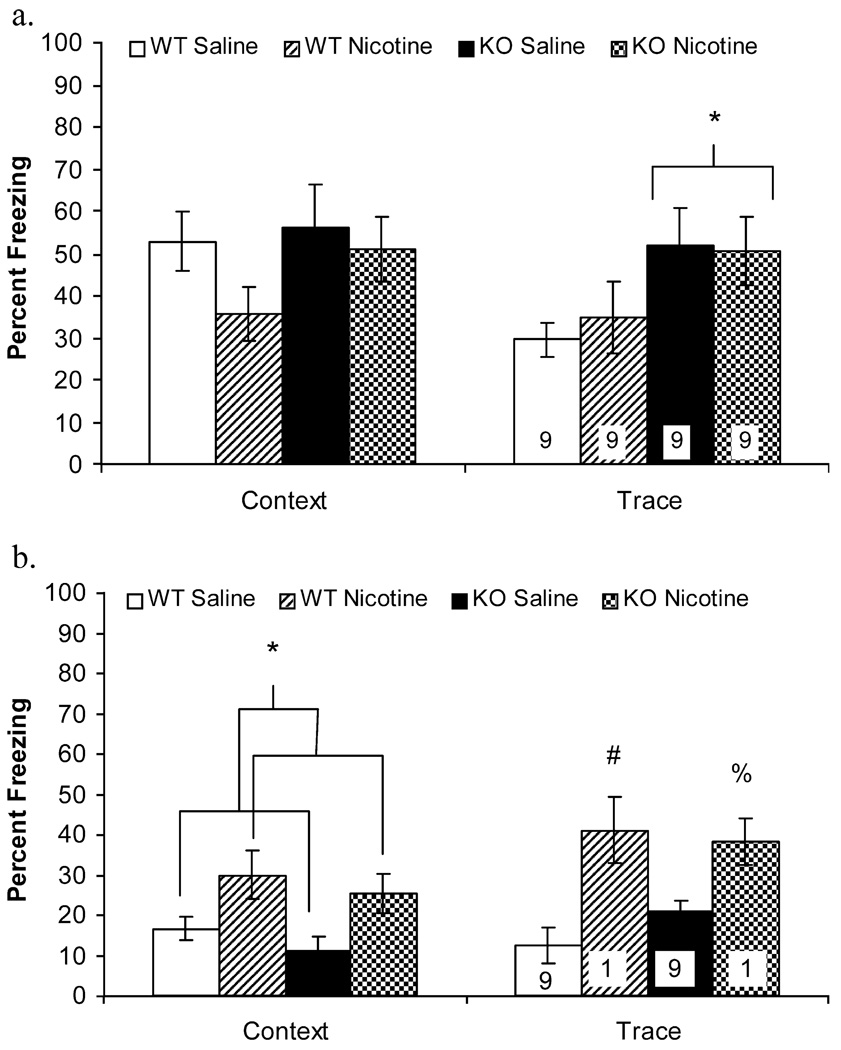
A 2×2 ANOVA revealed no main effect of treatment [F(1, 32)=1.61, p=0.21], or genotype [F(1, 32)=1.04, p=0.32] on freezing to the context, and no significant treatment by genotype interaction [F(1, 32)=0.33, p=0.57] for freezing to the context for mice trained using a 0.57-mA footshock US (Fig. 3a; N=9). Because a visual inspection of the data suggested that a trend for a decrease in contextual fear conditioning in nicotine-treated α7 WT mice existed, Tukey HSD contrasts were performed. Follow-up analyses revealed no significant pairwise differences in contextual fear conditioning (p>0.05 for all comparisons).
Figure 3.
a α7 WT mice and α7 KO mice that received nicotine (0.09 mg/kg) or saline were trained using 5 CS (30 s, 85 dB white noise)—trace (30 s)—US (2 s, 0.570-mA footshock) presentations. There was a significant main effect of genotype on trace cued fear conditioning with α7 KO mice freezing significantly more to the CS than α7 WT mice regardless of treatment. Asterisk significantly different (p<0.05) from levels of trace cued fear conditioning in α7 WT mice. b α7 WT mice and α7 KO mice that received nicotine (0.09 mg/kg) or saline were trained using a 0.285-mA footshock US. Mice treated with nicotine demonstrated higher levels of both contextual fear conditioning and trace cued fear conditioning than mice treated with saline regardless of genotype. Error bars represent ±1 SE from the means. Asterisk significantly different levels of contextual fear conditioning (p<0.05) in saline treated mice; number sign significantly different levels of trace cued fear conditioning (p<0.05) from α7 WT mice that received saline; percent symbol significantly different levels of trace cued fear conditioning (p<0.05) from α7 KO mice that received saline. The numbers of mice per group are presented in the trace fear conditioning bars; the same mice were tested for contextual and trace cued fear conditioning
Analyses of the trace cued fear conditioning data from α7 KO and α7 WT mice trained using the 0.57-mA US indicated that there was no effect of treatment [F(1,32)=0.03, p=0.86] and no interaction between genotype and treatment [F(1, 32)=0.12, p=0.73]. There was a significant main effect of genotype on trace cued fear conditioning [F(1, 32)=7.90, p=0.01] with α7 KO mice freezing significantly more than α7 WT mice. Follow-up Tukey-HSD analyses revealed no significant pairwise differences in freezing to the CS (p>0.05 for all comparisons). The main effect of genotype could suggest that α7 KO mice demonstrated enhanced trace cued fear conditioning after training with a 0.57-mA footshock US compared to α7 WT mice. This effect was not observed for contextual fear conditioning after training with a 0.57-mA footshock US, nor was the deletion of α7 nAChR subunits associated with enhanced trace cued fear conditioning or contextual fear conditioning after training with a 0.29-mA footshock US (results presented below).
The 2×2 ANOVAs revealed that there were no main effects or interactions in baseline or preCS freezing (p>0.05 for all comparisons; data not shown). There was no main effect of genotype on immediate freezing [F(1,32)=2.96, p=0.10] and no significant interaction between genotype and treatment [F(1,32)=3.45, p=0.07]. However, there was a main effect of treatment on immediate freezing [F(1, 32)=6.47, p=0.02; data not shown]. Follow-up Tukey HSD analyses revealed that saline-treated α7 KO mice demonstrated significantly higher levels of immediate freezing than nicotine-treated α7 KO mice [t(32)=3.11, p=0.02] and nicotine-treated α7 WT mice [t(32)=3.01, p=0.03]. These data could suggest that 0.09 mg/kg of nicotine altered the training experience for α7 KO mice trained with the 0.57-mA footshock US.
Previous research (Wehner et al. 2004) indicates that the enhancing effect of nicotine on contextual fear conditioning is dependent on shock intensity in both α7 KO mice and α7 WT mice. Similar to the results of Wehner et al. (2004), the results of the present study indicate that nicotine did not enhance contextual or trace cued fear conditioning in α7 WT mice or α7 KO mice trained with a 0.57-mA footshock US. Therefore, separate groups of α7 KO mice and α7 WT mice were trained using a lower intensity footshock US (0.29 mA, see Fig. 3b).
The 2×2 ANOVAs revealed no significant effects of genotype on contextual [F(1, 34)=1.36, p=0.25] or trace cued fear conditioning [F(1, 34)=0.28, p=0.60] and no interactions between genotype and treatment [F(1, 34)=0.01, p=0.92; F(1, 34)=1.05, p=0.31 for contextual and trace cued fear conditioning, respectively]. There were, however, significant main effects of treatment on contextual fear conditioning [F(1, 34)=9.89, p=0.00] and trace cued fear conditioning [F(1, 34)=17.10, p=0.00]. Follow-up Games–Howell analyses revealed that α7 WT mice treated with nicotine did not freeze significantly more to the context than α7 WT mice treated with saline [t(34)=2.15, p=0.19], and α7 KO mice treated with nicotine did not freeze significantly more to the context than α7 KO mice treated with saline [t(34)=2.43, p=0.11]. These results indicate that the enhancing effect of nicotine on contextual fear conditioning was only evident when the data were collapsed across genotype. In contrast, follow-up analyses of the trace cued fear conditioning data revealed that α7 WT mice that received nicotine froze significantly more to the CS than α7 WT mice that received saline [t(34)=3.21, p=0.03], and α7 KO mice treated with nicotine froze significantly more to the CS than did their saline-treated counterparts [t(34)=2.91, p=0.05]. Thus, the enhancing effect of nicotine on trace cued fear conditioning was evident in both the α7 WT mice and the α7 KO mice. There were no significant main effects or interactions in baseline, immediate, or preCS freezing (p>0.05 for all comparisons; data not shown).
Discussion
The present research indicates that β2 subunit-containing nAChRs mediate the enhancing effect of nicotine on trace cued fear conditioning; nicotine did not enhance trace cued fear conditioning in β2 KO mice trained with either a 0.29- or 0.57-mA footshock US. The results using two different US intensities suggest that the lack of nicotine enhancement of trace cued fear conditioning in the β2 KO mice was not due to the strength of conditioning. Furthermore, the lack of nicotine enhancement of trace cued fear conditioning in the β2 KO mice was not due to a shift in sensitivity to nicotine in the β2 KO mice; higher doses of nicotine did not enhance conditioning. In fact, the highest dose of nicotine tested disrupted trace conditioning in both WT and β2 KO mice. This result also suggests that the disruptive effect of the highest dose of nicotine is not mediated by β2 subunit-containing nAChRs.
In addition, the present results replicate previous research that used pharmacological and genetic inhibition of nAChRs to demonstrate that β2 subunit-containing nAChRs mediate the enhancement of contextual fear conditioning by nicotine (Davis and Gould 2006; Wehner et al. 2004). The studies by Davis and Gould (2006) and Wehner et al. (2004) trained the mice with two trials. The current study used five trials and tested two different US shock levels but still found no enhancing effects of nicotine on contextual fear conditioning, suggesting that the lack of enhancement seen in the β2 KO mice is independent of the strength of training. Also, as seen for trace fear conditioning, the null effect of nicotine administration on enhancement of contextual conditioning in the β2 KO mice was not due to altered sensitivity to nicotine because doses above the dose previously found to enhance contextual fear conditioning (for review, see Gould 2006) failed to enhance contextual fear conditioning in β2 KO mice. The highest dose of nicotine did, however, disrupt contextual fear conditioning in both WT and β2 KO mice. Together, data from the β2 KO experiments and prior work suggest the β2 subunit-containing nAChRs are involved in the enhancement of both contextual and trace fear conditioning.
α7 nAChRs do not appear to have the same level of involvement in enhancement of contextual and trace fear conditioning as β2 subunit-containing nAChRs. The data from the present research suggest that α7 nAChRs may not be critical for the enhancing effects of nicotine on trace cued fear conditioning. Both nicotine-treated WT mice and nicotine-treated α7 KO mice trained using the 0.29-mA footshock US demonstrated enhanced levels of trace cued fear conditioning compared to saline-treated α7 KO mice and WT mice. It should be noted that it is possible that as of yet undemonstrated developmental compensatory alterations in neural function associated with the α7 KO could have contributed to the results.
The present results also support prior results indicating that α7 nAChRs may not be critically involved in the nicotine enhancement of contextual fear conditioning (Davis and Gould 2006; Wehner et al. 2004). The α7 antagonist MLA did not significantly attenuate nicotine enhancement of contextual fear conditioning (Davis and Gould 2006) and Wehner et al. (2004) found that nicotine-treated α7 KO mice continue to demonstrated enhanced contextual fear conditioning. In the present study, a main effect of nicotine administration of contextual fear conditioning was seen following training with the 0.29-mA footshock US; nicotine enhanced contextual fear conditioning.
Results with the 0.57-mA footshock US in α7 KO and WT littermates replicate previous findings (Wehner et al. 2004) but are difficult to interpret. Wehner et al. (2004) found that nicotine only enhanced contextual fear conditioning in α7 KO mice or WT littermates at a lower footshock US intensity. Similarly, we found that the enhancing effects of nicotine on trace fear conditioning were evident when α7 KO and α7 WT mice were trained with a 0.29-mA footshock US, but not when they were trained with a 0.57-mA footshock US. It is not clear why the responses of α7 WT to the 0.57- and 0.29-mA footshock stimuli differed from those of the β2 WT mice. However, it must be noted that the WT type mice that are littermates of the α7 KO mice and the WT type mice that are littermates of the β2 KO mice are not genetically homogeneous. It is possible that differences in gene expression and epistasis resulting from initial generation of the separate lines on a mixed 129 SvEv and C57BL/6 background (Orr-Urtreger et al. 1997; Xu et al. 1999) could account for these behavioral differences in the α7 WT mice and the β2 WT mice. Studies indicating that there are behavioral differences among strains of mice support this contention (Logue et al. 1997a,b; Owen et al. 1997).
The present results suggest that α7 nAChRs are not critically involved in the enhancement of trace cued fear conditioning by nicotine but do not preclude involvement of α7 nAChRs in trace fear conditioning. α7 KO mice trained with the 0.57-mA footshock demonstrated significantly higher levels of trace cued fear conditioning than their WT counterparts regardless of treatment with saline or nicotine. This suggests that α7 nAChRs could modulate trace cued fear conditioning. Other studies have suggested that inhibition of α7 nAChRs could enhance some forms of learning and synaptic plasticity (Davis and Gould 2006; Fujii et al. 2000; Yamazaki et al. 2002). Further examination the role α7 nAChRs in learning and memory is warranted, as the cellular effects of activation of these receptors are not well understood. Likewise, additional studies should be carried out to assess how learning-related signaling is altered after nicotine-stimulated β2 subunit-containing nAChR activation.
Differences in localization, density, and function of nAChR subtypes (Flores et al. 1992; Alkondon and Albuquerque 1993; Decker et al. 1995; Graham et al. 2003; Ramirez-Latorre et al. 1998; Seguela et al. 1993; Wada et al. 1988; and for review, see Picciotto et al. 2001) may contribute to or, rather, define the involvement of particular nAChR subtypes in the effects of nicotine on learning. Furthermore, increased neurotransmitter release resulting from presynaptic nAChR activation (for review, see Kaiser and Wonnacott 1998) and/or alterations that result from both presynaptic and postsynaptic nAChR-associated activation of second messenger signaling cascades that support learning and memory, such as extracellular-regulated kinase/mitogen-activated protein kinase, Ca2+ calmodulin-dependent protein kinase II/IV, and protein kinase A (Chang and Berg 2001; Hu et al. 2002; Nakayama et al. 2001; Tang et al. 1998; Valjent et al. 2004; and for reviews, see Hyman and Malenka 2001; Nestler 2002), may be involved in the effects of nicotine on learning. Current research in our laboratory is addressing these issues.
Acknowledgements
The authors would like to thank Dr. Arthur Beaudet for generously providing original breeding pairs for these studies. The authors would like to acknowledge grant support from the National Institute on Drug Abuse (DA017949 TG) and the National Cancer Institute/National Institute on Drug Abuse Transdisciplinary Tobacco Use Research Center Grant (P5084718 PI: Caryn Lerman, Ph.D.). Jennifer Davis was supported by a NIH/NIDA training grant (T32DA07237).
References
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Bancroft A, Levin ED. Ventral hippocampal α4β2 nicotinic receptors and chronic nicotine effects on memory. Neuropharmacology. 2000;39:2770–2778. doi: 10.1016/s0028-3908(00)00099-x. [DOI] [PubMed] [Google Scholar]
- Barros DM, Ramirez MR, Dos Reis EA, Izquierdo I. Participation of hippocampal nicotinic receptors in acquisition, consolidation and retrieval of memory for one trial inhibitory avoidance in rats. Neuroscience. 2004;126:651–656. doi: 10.1016/j.neuroscience.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P. Nicotine dependence and tolerance in man: pharmacokinetic and pharmacodynamic investigations. Prog Brain Res. 1989;79:279–287. doi: 10.1016/s0079-6123(08)62487-5. [DOI] [PubMed] [Google Scholar]
- Bettany JH, Levin ED. Ventral hippocampus α7 nicotinic receptor blockade and chronic nicotine effects on memory performance in the radial-arm maze. Pharmacol Biochem Behav. 2001;70:467–474. doi: 10.1016/s0091-3057(01)00643-8. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Saddoris MP, Bucci DJ, Wiig KA. Corticohippocampal contributions to spatial and contextual learning. J Neurosci. 2004;24:3826–3836. doi: 10.1523/JNEUROSCI.0410-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarone BJ, Duman CH, Picciotto MR. Fear conditioning and latent inhibition in mice lacking the high affinity subclass of nicotinic acetylcholine receptors in the brain. Neuropharmacology. 2000;39:2779–2784. doi: 10.1016/s0028-3908(00)00137-4. [DOI] [PubMed] [Google Scholar]
- Chang KT, Berg DK. Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron. 2001;32:855–865. doi: 10.1016/s0896-6273(01)00516-5. [DOI] [PubMed] [Google Scholar]
- Curzon P, Brioni JD, Decker MW. Effect of intraventricular injections of dihydro-beta-erythroidine (DHβE) on spatial memory in the rat. Brain Res. 1996;714:185–191. doi: 10.1016/0006-8993(95)01536-1. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning. Psychopharmacology (Berl) 2006;184:345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett. 2006;394:202–205. doi: 10.1016/j.neulet.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker MW, Brioni JD, Bannon AW, Arneric SP. Diversity of neuronal nicotinic acetylcholine receptors: lessons from behavior and implications for CNS therapeutics. Life Sci. 1995;56:545–570. doi: 10.1016/0024-3205(94)00488-e. [DOI] [PubMed] [Google Scholar]
- El Ghundi M, Fletcher PJ, Drago J, Sibley DR, O’Dowd BF, George SR. Spatial learning deficit in dopamine D(1) receptor knockout mice. Eur J Pharmacol. 1999;383:95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- Felix R, Levin ED. Nicotinic antagonist administration into the ventral hippocampus and spatial working memory in rats. Neuroscience. 1997;81:1009–1077. doi: 10.1016/s0306-4522(97)00224-8. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha4 and beta2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Fujii S, Ji Z, Sumikawa K. Inactivation of alpha7 ACh receptors and activation of non-alpha7 ACh receptors both contribute to long term potentiation induction in the hippocampal CA1 region. Neurosci Lett. 2000;286:134–138. doi: 10.1016/s0304-3940(00)01076-4. [DOI] [PubMed] [Google Scholar]
- Good M, Honey RC. Dissociable effects of selective lesions of hippocampal subsystems on exploratory behavior, contextual learning, and spatial learning. Behav Neurosci. 1997;111:487–493. doi: 10.1037//0735-7044.111.3.487. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning. Mol Neurobiol. 2006 doi: 10.1385/MN:34:2:93. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. Nicotine produces a within subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integr Physiol Behav Sci. 2003;38:124–132. doi: 10.1007/BF02688830. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci. 2003;117:1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav Brain Res. 2004;155:167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Graham AJ, Ray MA, Perry EK, Jaros E, Perry RH, Volsen SG, Bose S, Evans N, Lindstrom J, Court JA. Differential nicotinic acetylcholine receptor subunit expression in the human hippocampus. J Chem Neuroanat. 2003;25:97–113. doi: 10.1016/s0891-0618(02)00100-x. [DOI] [PubMed] [Google Scholar]
- Graves L, Dalvi A, Lucki I, Blendy JA, Abel T. Behavioral analysis of CREB alphadelta mutation on a B6/129 F1 hybrid background. Hippocampus. 2002;12:18–26. doi: 10.1002/hipo.10003. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Hu M, Liu QL, Chang KT, Berg DK. Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol Cell Neurosci. 2002;21:616–625. doi: 10.1006/mcne.2002.1202. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Wonnacott S. Nicotinic receptor modulation of neurotransmitter release. In: Arneric SP, Brioni JD, editors. Neuronal nicotinic receptors: pharmacology and therapeutic opportunities. New York: Wiley; 1998. pp. 141–159. [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. J Neurosci. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–639. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal α7 and α4β2 nicotinic receptors and working memory. Neuroscience. 2002;109:757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997a;80:1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997b;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Collins AC. Characterization of nicotine binding in mouse brain and comparison with the binding of alpha-bungarotoxin and quinuclidinyl benzilate. Mol Pharmacol. 1982;22:554–564. [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Tseng W, Disterhoft JF. Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus. 2000;10:739–751. doi: 10.1002/1098-1063(2000)10:6<739::AID-HIPO1011>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- McGehee DS. Molecular diversity of neuronal nicotinic acetylcholine receptors. Ann N Y Acad Sci. 1999;868:565–577. doi: 10.1111/j.1749-6632.1999.tb11330.x. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem. 2001;79:489–498. doi: 10.1046/j.1471-4159.2001.00602.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. α7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Neurobiol Learn Mem. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- Peters M, Mizuno K, Ris L, Angelo M, Godaux E, Giese KP. Loss of Ca2+/calmodulin kinase kinase β affects the formation of some, but not all, types of hippocampus-dependent long-term memory. J Neurosci. 2003;23:9752–9760. doi: 10.1523/JNEUROSCI.23-30-09752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, Ledoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Nicotine as a modulator of behavior: beyond the inverted U. Trends Pharmacol Sci. 2003;24:493–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Crabtree G, Turner J, Role L. Molecular compositions and biophysical characteristics of nicotinic receptors. In: Arneric SP, Brioni JD, editors. Neuronal nicotinic receptors: pharmacology and therapeutic opportunities. New York: Wiley; 1998. pp. 43–64. [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Krucker T, Levy CL, Slanina KA, Sutcliffe JG, Hedlund PB. Mice lacking 5HT7 receptors show specific impairments in contextual learning. Eur J Neurosci. 2004;19:1913–1922. doi: 10.1111/j.1460-9568.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K, Wu H, Mahata SK, O’Connor DT. A crucial role for the mitogen-activated protein kinase pathway in nicotinic cholinergic signaling to secretory protein transcription in pheochromocytoma cells. Mol Pharmacol. 1998;54:59–69. doi: 10.1124/mol.54.1.59. [DOI] [PubMed] [Google Scholar]
- Tinsley MR, Quinn JJ, Fanselow MS. The role of muscarinic and nicotinic cholinergic neurotransmission in aversive conditioning: comparing pavlovian fear conditioning and inhibitory avoidance. Learn Mem. 2004;11:35–42. doi: 10.1101/lm.70204. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault J, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Voikar V, Rossi J, Rauvala H, Airaksinen MS. Impaired behavioural flexibility and memory in mice lacking GDNF family receptor α2. Eur J Neurosci. 2004;20:308–312. doi: 10.1111/j.1460-9568.2004.03475.x. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha2, alpha3, alpha4, and beta2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1988;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Weitemier A, Ryabinin AE. Subregion-specific differences in hippocampal activity between delay and trace fear conditioning: an immunohistochemical analysis. Brain Res. 2004;995:55–65. doi: 10.1016/j.brainres.2003.09.054. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Sharples CGV, Wonnacott S. Agonist-induced upregulation of α4β2 nicotinic acetylcholine receptors in M10 cells: pharmacological and spatial definition. Mol Pharmacol. 1998;53:950–962. [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the β2 and the β4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Hamaue N, Sumikawa K. Nicotine compensates for the loss of cholinergic function to enhance long-term potentiation. Brain Res. 2002;946:148–152. doi: 10.1016/s0006-8993(02)02935-9. [DOI] [PubMed] [Google Scholar]