Abstract
OBJECTIVES
The mucosa of patients with columnar-lined esophagus recognized on endoscopy usually shows epithelium with and without goblet cells. Columnar epithelium with goblet cells (“Barrett’s esophagus”) is generally believed to represent a premalignant lesion and has been shown to contain DNA abnormalities. However, the biological properties of non-goblet columnar epithelium remain unknown. The purpose of this study was to determine the DNA content properties of non-goblet epithelium in patients with metaplastic columnar epithelium of the esophagus.
METHODS
Mucosal biopsies of the esophagus from 68 patients with columnar metaplasia of the esophagus (22 without goblet cells and 46 with goblet cells) and 19 patients with normal gastric mucosa (controls) were histologically evaluated for the density of goblet cells. The latter group was divided into low-density, high-density, and very high—density goblet cell subgroups. Tissue sections of non-goblet epithelium and goblet cell epithelium (where present) were evaluated by image cytometry, and high-fi delity DNA histograms were created to indicate the G0/G1 peak DNA index (DI), DNA content heterogeneity index (HI), and the percentage of cells with DNA exceeding 5N (5N-EC). G0/G1 peaks with DI >1.1 were considered aneuploid.
RESULTS
Normal gastric controls showed a mean peak DI of 1.02±0.03 and an HI of 11.6±0.7. None of the controls revealed aneuploidy or 5N-EC. Patients with metaplastic columnar epithelium with goblet cells showed a DI of 1.15±0.12, HI of 18.2±2.1, mild aneuploidy in 54% of the cases, and 5N-EC in 15% of the cases, all of which were signifi cantly higher than in controls. Patients with metaplastic columnar epithelium without goblet cells showed DNA content results statistically similar to those of patients with metaplastic columnar epithelium with goblet cells, and also revealed signifi cantly higher values compared with those of controls. Furthermore, there were no signifi cant differences in any of the key DNA content abnormalities between non-goblet and goblet cell—containing epithelium in patients with metaplastic columnar epithelium with goblet cells, or between these two types of epithelium according to the density of goblet cells.
CONCLUSIONS
DNA content abnormalities occur with equal frequency and extent in metaplastic columnar epithelium of the esophagus without goblet cells compared with metaplastic columnar epithelium with goblet cells. These findings suggest that metaplastic non-goblet columnar epithelium of the esophagus may have neoplastic potential.
INTRODUCTION
Columnar metaplasia of the esophagus is a complication of gastroesophageal reflux disease and represents the main precursor of esophageal adenocarcinoma (1). The incidence of esophageal adenocarcinoma has been increasing at an alarming rate in the past several decades (2). The initial diagnosis of columnar-lined esophagus is typically established at endoscopy, but the final “definitive”diagnosis is confirmed by histologic examination of biopsy tissue. At the present time, the American College of Gastroenterology (ACG) defines Barrett’s esophagus (BE) as a condition that shows endoscopic evidence of columnar metaplasia of the esophagus and in which goblet cells are identified in biopsy tissue (3,4). However, inclusion of goblet cells as a necessary criterion for BE is controversial (5).
The mucosa of columnar-lined esophagus is composed of several types of metaplastic epithelium (6). The glandular compartment is composed of mucous glands or oxyntic glands, or a combination of both. The surface and pit epithelia are typically composed of mucinous columnar cells, either with or without goblet cells. It is generally presumed that only columnar epithelium with goblet cells is at risk for malignancy, and, as a result, goblet cells are required for the definition of BE and are an indication for endoscopic surveillance in affected patients (3). However, little is known about the biological properties and neoplastic potential of non-goblet columnar epithelium in patients with columnar metaplasia of the esophagus (3). Clinical and epidemiological studies are often used to support the fact that columnar mucosa with goblet cells is the main precursor lesion for adenocarcinoma (3). For instance, the finding of columnar mucosa with goblet cells in patients who progress to adenocarcinoma, and the finding of columnar mucosa with goblet cells in association with adenocarcinoma in resection specimens, are features that support the view that goblet cells are a premalignant lesion (3,7). However, recent studies suggest that columnar mucosa without goblet cells is phenotypically intestinalized, and shows chromosomal abnormalities (8,9). In fact, two recent studies suggest that patients with esophageal columnar mucosa without goblet cells have a similar risk of neoplastic progression to patients with columnar mucosa with goblet cells (10,11).
It is well known that chromosomal and genetic instability is a major factor in the pathogenesis of esophageal adenocarcinoma (12-15). Chromosomal abnormalities include both gains and losses of either parts, or entire, chromosomes, both of which result in a change in the normal diploid number of chromosomes in each affected cell. Chromosomal abnormalities may occur early in the progression of neoplasia and have been shown to increase with disease progression (16). In addition, chromosomal abnormalities and aneuploidy have been shown to be important markers of neoplastic progression in BE-associated adenocarcinoma (12,15).
Cellular chromosomal DNA content abnormalities may be measured by DNA histograms that depict the DNA content of cells in different phases of the cell cycle. A normal DNA histogram of cells in interphase reveals that the majority of cells are in the G0/G1 phase. The DNA content from these cells forms a diploid peak, and the cells contain DNA equal to two copies of chromosomes (2N), and show little variability in their DNA content. Under normal circumstances, very few cells are identified in the S and G2 phases of the cell cycle. Cells in the G2 phase contain DNA equal to four copies of the normal chromosomes. Chromosomal changes in neoplasia include G0/G1 peaks that deviate from the normal diploid location and, as such, are defined as aneuploid (17). As large chromosomal losses are often lethal to cells, viable cells typically show aneuploidy with gains of DNA (14). Neoplastic cells also show increased cellular DNA heterogeneity in addition to aneuploidy (16,18).
Previous studies have shown that columnar mucosa with goblet cells reveal G0/G1 aneuploid peaks, and/or elevated G2 (4N) cells, in about 10% of the cases based on standard flow cytometry (12). However, studies using a more sensitive technique of DNA content analysis, such as high-fidelity DNA histogram analysis by image cytometry on tissue sections, have revealed mild aneuploid G0/G1 peaks in more than 50% of the cases (16). High-fidelity DNA histograms track DNA content of each cell in the histogram and, thus, provide more sensitive information on the status of cells in all phases of the cell cycle, compared with flow cytometry (18).
Information on DNA content abnormalities may also provide information regarding the neoplastic potential of epithelium (12,15). Unfortunately, the DNA content status of esophageal metaplastic columnar epithelium without goblet cells is currently unknown. Therefore, the purpose of this study was to determine whether columnar mucosa without goblet cells, in metaplastic columnar epithelium of the esophagus as defined by endoscopy, has DNA content abnormalities suggestive of chromosomal instability.
METHODS
Study groups
All patients included in this study represent selected cases of columnar-lined esophagus, either with or without goblet cells, found by a retrospective search of the pathology files of the Brigham and Women’s Hospital between 1995 and 2005. All patients had endoscopically confirmed columnar metaplasia of the distal esophagus, and had four-quadrant biopsies obtained of this abnormal mucosa. Overall, 68 patients with columnar metaplasia of the esophagus were studied. Of these 68 patients, 22 revealed columnar metaplasia of the distal esophagus without goblet cells. These patients had short segments of esophageal columnar metaplasia (<3 cm). Of the 46 remaining patients with goblet cells identified in one or more of their biopsies, 17 were considered to have low-density goblet cells based on the fact that they had goblet cells present in less than 50% of the crypts, 15 had high-density goblet cells due to the fact that goblet cells were identified in greater than 50%, but less than 100%, of crypts, and 14 were defined as containing very high—density goblet cells based on the fact that goblet cells were identified in every crypt from all biopsy specimens.
All patients with goblet cells had long segments of columnar metaplasia (>3 cm). As controls, 19 biopsies from normal gastric corpus mucosa (19 patients) were used. Overall, 119 mucosal biopsies from 68 study patients and 19 mucosal biopsies from 19 control patients were analyzed in this study. In addition, for 32 patients with columnar metaplasia with goblet cells, two separate analyses were performed. One was performed on areas of columnar mucosa without goblet cells, and the other was performed on areas of columnar mucosa with goblet cells, from the same patients. A comparison of DNA content abnormalities was made between the different study groups, and controls, and also between the non-goblet- and goblet cell-containing epithelium in patients with columnar metaplasia with goblet cells. The protocol for high-fidelity DNA histograms by image cytometry, and tissue sections, was approved by the Institutional Review Boards of the VA Boston Healthcare System and the Brigham and Women’s Hospital, Boston, MA.
Histologic methods
For all biopsies, two adjacent tissue sections, one 5 μm in thickness and the other 7 μm in thickness, were cut from formalin-fixed tissue blocks. The 5-μm-thick tissue sections were stained with hematoxylin and eosin for histologic analysis. The hematoxylin and eosin-stained sections were evaluated microscopically for the presence or absence, and degree, of goblet cells. None of the patients had dysplasia, acute inflammation, or ulceration. After histologic evaluation, the portions of the biopsies to be evaluated by DNA image cytometry were circled on the slide and submitted to another study participant for image analysis.
Image cytometry and production of high-fidelity DNA histograms
Tissue sections of thickness 7-μm were stained with Feulgen dye for DNA image cytometry. High-fidelity DNA histograms were created by the Automated Cellular Imaging System (ACIS; Clarient, San Juan Capistrano, CA). The technique of image cytometry used to yield high-fidelity DNA histograms has been described in detail elsewhere (16,18,19). Briefly, Feulgen-stained slides were scanned automatically. Only uniformly stained slides were included. A daily quality control was run using a standard calibration kit from the manufacturer. The histological area of interest was identified microscopically and selected for the study. The slides were examined, in a blinded manner, without the knowledge of the study group from which the biopsy specimen was obtained. For each biopsy, at least 50 control stromal cells and 200 target epithelial cells were selected for the analysis. All cell images were digitalized and stored in the ACIS system. The digital images of all nuclei are then converted into a series of pixels that are quantified to obtain an integrated optical density (IOD) value. The IOD represents the DNA content of the cell. The coefficient of variation of IOD of the control stromal cells was less than 6%. The mean IOD of control stromal cells is then assigned a DNA index (DI) value of 1, which serves as an internal diploid standard for DI calculations of the target epithelial cells. A DI value of 1 corresponds to 2N (N represents the number of copies of the chromosomes). DNA histograms are then generated by the ACIS system, which shows plots of different DI values vs. the number of cells with that specific DI value. The histogram represents cells in different phases of the cell cycle. In normal tissue, the overwhelming majority of cells are in the G0/G1 phase. Only few cells are present in the S and G2 phases of the cell cycle.
On the basis of the DI values, the G0/G1 peaks were considered either diploid (DI = 0.9–1.1) or aneuploid (DI>1.1) as previously described. Aneuploidy was further divided into mild (DI=1.1–1.3), moderate (DI=1.3–1.8), and severe (DI=1.8). The heterogeneity index (HI) was defined as the number of clusters of cells with IOD at 0.3 intervals in the histograms, and it was displayed as different columns, or bars, in the histograms. HI values less than 13 were considered normal, 14–20 as moderately elevated, and >20 as severely elevated (16,19,20). Histograms that revealed cells with DI content greater then 5N (5N exceeding cells (5N-EC)) were noted as well. 5N-EC represents cells with DNA content >G2.
Statistical analysis
All values are described as mean±s.d. Statistical comparisons of DNA content results were performed using the paired or unpaired t-test. A P value of <0.05 was considered statistically significant.
RESULTS
DNA content in normal gastric mucosa
The mean DI, percentage of cases with aneuploidy, mean HI, percentage of cases with elevated HI, and the percentage of cases with 5N-EC of the 19 control samples are summarized in Table 1. The mean DI was 1.02 and the mean HI was 11.6. None of the controls showed aneuploidy, elevated HI, or cells with 5N-EC. The upper cutoff values for DI (G0/G1 peak) of 1.1, and HI of 13, fell well within the normal range previously reported for normal gastrointestinal epithelium.
Table 1.
Cellular DNA content in patients with columnar metaplasia with goblet cells
| Patient groups | No. of samples | Mean DI (mean±s.d.) | Aneuploidya (%) | Mean HI (mean±s.d.) | Elevated HIb (%) | 5N-EC (%) |
|---|---|---|---|---|---|---|
| NGM | 19 | 1.02±0.03 | 0 | 11.6±0.7 | 0 | 0 |
| CM-WVHDG | 14 | 1.14±0.15 | 50 | 17.6±1.7 | 14 (100) | 1/14 (7) |
| CM-WHDG | 15 | 1.18±0.12 | 60 | 18.9±2.0 | 15 (100) | 3/15 (20) |
| CM-WLDG | 17 | 1.12±0.10 | 53 | 18.1±2.4 | 17 (100) | 3/17 (17) |
| All CM-WG | 46 | 1.15±0.12 | 54 | 18.2±2.1 | 46 (100) | 7/46 (15) |
5N-EC, 5N exceeding cells; all CM-WG, all case of columnar mucosa with any density of goblet cells; CM-WHDG, columnar mucosa with high-density goblet cells; CM-WLDG, columnar mucosa with low-density goblet cells; CM-WVHDG, columnar mucosa with very high—density goblet cells; NGM, normal gastric mucosa.
Aneuploidy: DI of G0/G1 peak >1.1.
Elevated HI>13.
G0/G1 peak DI and HI values from all the metaplasia groups are significantly higher than normal gastric controls (P values vary between 0.02 and 0.0001). G0/G1 peak DI and HI values among the different goblet cell density groups are not different from each other (P values vary between 0.15 and 0.98).
DNA content of all patients with metaplastic columnar epithelium
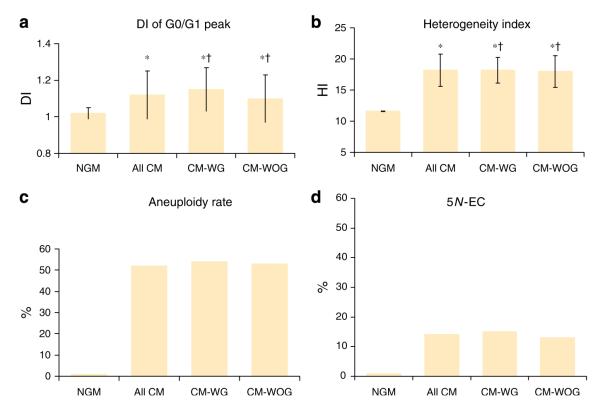
Figure 1 summarizes the DNA content values in controls, patients with columnar mucosa without goblet cells, and patients with columnar mucosa with goblet cells (the latter includes all three goblet cell density subgroups). Overall, in all cases of columnar mucosa, both with and without goblet cells (100 samples), the mean±s.d. value of G0/G1 peaks was 1.12±0.13. The mean HI was 18±2.56. Both of these values were significantly higher than controls (P=0.0053 and 0.001, respectively). In addition, 52% of all patients with columnar metaplasia had an aneuploid peak. Of the patients with aneuploid peaks, the majority (94%) showed mild aneuploidy, and 6% showed moderate aneuploidy. None of the cases from any patient group showed severe aneuploidy. The HI was elevated in 98% of the patients with columnar metaplasia. In total, 13% of the patients showed HI values greater than 20. Furthermore, 14% of the patients with columnar metaplasia showed cells with 5N-EC. Overall, 3% of the histograms showed more than one cell with 5N-EC in the histogram.
Figure 1.
Cellular DNA content in normal gastric mucosa (NGM), all cases of columnar metaplasia (all CM), those with goblet cells (CM-WG), and those with columnar metaplasia without goblet cells (CM-WOG). The bars represent average values±s.d. (a—d) DNA index (DI) values of peaks of the G0/G1 phase, heterogeneity index (HI), aneuploidy rate, and percentage of cells with DI>5N, respectively (a, b). The peak DI and HI values were significantly elevated in all groups of columnar metaplasia compared with normal gastric mucosa (P<0.05), but these values were not different among the columnar metaplasia groups (†P>0.05). (c) An aneuploidy rate of 0 in NGM, and approximately 50% in cases with columnar metaplasia. (d) Normal gastric mucosa had no cells with DI>5N. However, almost 15% of histograms in the columnar metaplasia groups had at least one cell with DI>5N.
There were no significant differences in any of the DNA content parameters between all patients with metaplastic columnar epithelium with goblet cells vs. those without goblet cells (Figure 1). However, both of these groups showed significantly higher DNA content parameters compared with controls. In total, 50% of the cases with goblet cells were aneuploid and 15% contained cells with 5N-EC. Patients with goblet cells showed a mean DI of 1.15±0.12 and a mean HI of 18.2±2.1. The mean DI and mean HI of the patients with metaplastic columnar epithelium without goblet cells was 1.1±0.13 and 17.8±2.9, respectively. Of the patients without goblet cells, 50% showed aneuploidy and 9% contained cells with 5N-EC, which, as mentioned above, was similar to patients with goblet cells.
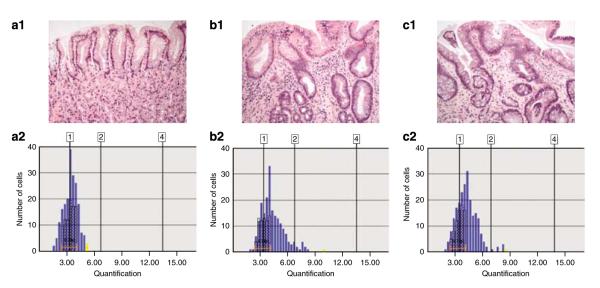
Figure 2 shows representative DNA histograms of three patients, one with columnar mucosa with goblet cells, one with columnar mucosa without goblet cells, and one with normal gastric mucosa. Note that normal gastric mucosa shows a diploid (DI<1.1) G0/G1 peak, an HI of 13 and no cells with DI>5N. The histogram of the patient with columnar mucosa with goblet cells shows an aneuploid G0/G1 peak (DI=1.24), an elevated HI of 20, increased cells in the S phase and one cell with DI>5N. The DNA histogram of the patient with columnar mucosa without goblet cells also shows similar abnormalities, including an aneuploid G0/G1 peak (DI=1.20), an HI of 23, increased S-phase cells and one cell with DI>5N.
Figure 2.
Representative DNA histograms from three separate patients. (a) Normal gastric mucosa, (b) columnar metaplasia without goblet cells, and (c) columnar metaplasia with goblet cells. Note that the DNA histograms of columnar mucosa with or without goblet cells showed similar abnormalities, including aneuploid G0/G1 peaks, elevated heterogeneity index (HI), increased cells in the S phase and occasional cells with DNA index (DI)>5N.
DNA content of patients with columnar metaplasia with goblet cells
The DNA content values for patients with columnar metaplasia with goblet cells are summarized in Table 1. In addition, the values of the normal gastric controls are included for comparison. Overall, the mean DI was 1.15±0.12, and the mean HI was 18.2±2.1. In total, 54% of the patients showed aneuploidy, 100% showed elevated HI, and 15% showed cells with 5N-EC. All of these values were significantly higher than controls. Table 1 also reveals the DNA content parameters of the patients with metaplastic columnar epithelium with goblet cells evaluated according to the density of goblet cells (i.e., very high density, high density, and low density). Overall, there were no significant differences in the mean DI, mean HI, rate of aneuploidy, rate of elevated HI, or 5N-EC fraction between patients with very high—density goblet cells vs. those with high-density goblet cells vs. those with low-density goblet cells. However, each of these groups independently showed significantly higher values compared with controls.
DNA content in metaplastic columnar epithelium without goblet cells, from patients either with or without goblet cells
Table 2 summarizes the DNA content values of columnar epithelium without goblet cells, in patients without goblet cells elsewhere in their esophagus, and also shows the DNA content values for the non-goblet portions of columnar epithelium in patients with either low- or high-density goblet cells elsewhere in the esophagus. Non-goblet columnar epithelium from patients with very high—density goblet cells could not be evaluated as all of the crypts in these cases contained goblet cells. The mean DI and mean HI of the non-goblet epithelium from patients with columnar metaplasia, but without goblet cells, were 1.13±0.16 and 18.4±3.3, respectively. Overall, 64 and 100% of these cases showed aneuploidy and elevated HI, respectively. Only 18% showed an elevated 5N-EC fraction. All of these values were significantly higher than gastric controls. Overall, the DNA content parameters were statistically similar in non-goblet epithelium from patients with low-density goblet cells compared with patients with high-density goblet cells. Furthermore, the non-goblet epithelia from patients with either low- or high-density goblet cells were statistically similar to the non-goblet epithelium from patients with columnar metaplasia, but without goblet cells.
Table 2.
Cellular DNA content in metaplastic columnar epithelium without goblet cells
| Patient groups | No. of samples | Mean DI (mean±s.d.) | Aneuploidya (%) | Mean HI (mean±s.d.) | Elevated HIb (%) | 5N-EC (%) |
|---|---|---|---|---|---|---|
| NGM | 19 | 1.02±0.03 | 0 | 11.6±0.7 | 0 | 0 |
| CM-WOG | 22 | 1.13±0.16 | 64 | 18.4±3.3 | 22 (100) | 4/22 (18) |
| CM-WOG in patients with CM-WLDG |
17 | 1.09±0.10 | 41 | 17.3±1.8 | 17 (100) | 1/17 (6) |
| CM-WOG in patients with CM-WHDG |
15 | 1.09±0.12 | 40 | 17.5±3.2 | 14 (93) | 2/15 (13) |
| All CM-WOG | 54 | 1.10±0.13 | 50 | 17.8±2.9 | 53 (98) | 7/54 (13) |
5N-EC, 5N exceeding cells; all CM-WOG, all case of columnar mucosa without goblet cells; CM-WHDG, columnar mucosa with high-density goblet cells; CM-WLDG, columnar mucosa with low-density goblet cells; CM-WOG, columnar mucosa without goblet cells; NGM, normal gastric mucosa.
Aneuploidy: DI of G0/G1 peak >1.1.
Elevated HI>13.
G0/G1 peak DI and HI values in all of the CM-WOG groups are significantly higher than normal gastric controls (P values vary between 0.02 and 0.0001). G0/G1 peak DI and HI values among the different CM-WOG groups are not different from each other (P values vary between 0.15 and 0.98).
DNA content of non-goblet- vs. goblet-containing epithelium from patients with columnar metaplasia with goblet cells
Table 3 summarizes the DNA content values in the non-goblet- vs. the goblet cell-containing epithelium, only in patients who had metaplastic columnar epithelium with goblet cells elsewhere in the esophagus. Of the patients with metaplastic columnar epithelium with goblet cells, separate comparisons were made for the groups with low- vs. high-density goblet cells. Overall, the mean DI and mean HI of the goblet cell-containing portions of the epithelium was 1.15±0.12 and 18.2±2.1, respectively. Overall, 54 and 100% of the cases showed aneuploidy and elevated HI, respectively, and 15% showed 5N-EC cell populations. These values were statistically similar to the mean DI, mean HI, percentage of aneuploidy, percentage of HI, and 5N-EC fractions in the non-goblet-containing portions of the epithelium from the same patients.
Table 3.
Non-goblet vs. goblet epithelium in patients with columnar metaplasia with goblet cells
| Patient groups | No. of samples | Mean DI (mean±s.d.) | Aneuploidya (%) | Mean HI (mean±s.d.) | Elevated HIb (%) | 5N-EC (%) |
|---|---|---|---|---|---|---|
| NGM | 19 | 1.02±0.03 | 0 | 11.6±0.7 | ||
| CM-WLDG in the esophagus | ||||||
| Non-goblet epithelium | 17 | 1.09±0.12 | 41 | 17.1±1.83 | 100 | 1/17 (6) |
| Goblet cell epithelium | 17 | 1.12±0.10 | 53 | 18.1±2.44 | 100 | 3/17 (18) |
| CM-WHDG in the esophagus | ||||||
| Non-goblet epithelium | 15 | 1.09±0.12 | 40 | 17.5±3.23 | 93 | 2/15 (13) |
| Goblet cell epithelium | 15 | 1.18±0.12 | 60 | 18.9±2.05 | 100 | 3/15 (20) |
| All columnar metaplasia | ||||||
| Non-goblet epithelium | 54 | 1.10±0.13 | 50 | 17.8±2.9 | 98 | 7/54 (13) |
| Goblet cell epithelium | 46 | 1.15±0.12 | 54 | 18.2±2.1 | 100 | 7/46 (15) |
5N-EC, 5N exceeding cells; CM-WHDG, columnar mucosa with high-density goblet cells; CM-WLDG, columnar mucosa with low-density goblet cells; NGM, normal gastric mucosa.
Aneuploidy: DI of G0/G1 peak >1.1.
Elevated HI>13.
G0/G1 peak DI and HI values in all of the corresponding non-goblet and goblet cell epithelium groups were not signifi cantly different (P values vary between 0.07 and 0.84), except the non-goblet and goblet epithelium in patients with high-density goblet cells. In this latter group, the peak DI in goblet epithelium was significantly higher than in the non-goblet epithelium (P=0.035).
Similarly, the DNA content values in the non-goblet epithelium, compared with the goblet cell-containing epithelium, in patients with low-density goblet cells were statistically similar. However, in a comparison of the non-goblet-containing portions of epithelium, compared with the goblet cell-containing portions of epithelium, from patients with high-density goblet cells, a significant difference in the mean DI value was noted. The high-density goblet cell areas of epithelium showed a mean DI of 1.18±0.12 as compared with 1.09±0.12 in the non-goblet-containing portions of the epithelium from the same patients (P=0.035). However, all of the other DNA content parameters were statistically similar between these two types of epithelium from the same patients.
DISCUSSION
Little is known regarding the biological properties of the background non-goblet columnar epithelium in patients with BE, despite the fact that this epithelium comprises the majority of the cell population in patients with this condition. Given that several recent studies suggest that the risk of neoplastic progression in patients with columnar metaplasia of the esophagus is similar in those with, or without, goblet cells, we performed this study to evaluate DNA content in non-goblet epithelium (10,11,21). Our results showed that metaplastic esophageal columnar epithelium with goblet cells shows frequent, albeit early, DNA content abnormalities, including the presence of aneuploid G0/G1 peaks, increased cellular DNA content heterogeneity, and emergence of cells with elevated 5N-EC. We found no difference in the presence, or extent, of DNA content abnormalities in patients with metaplastic columnar epithelium with goblet cells stratified according to the density of goblet cells. Finally, and most importantly, we noted that patients with metaplastic columnar epithelium, without goblet cells (often referred to as cardia-type mucosa) (6,22), also shows frequent DNA content abnormalities similar in type and extent to patients with goblet cells (often referred to as specialized intestinal metaplasia characteristic of BE) (6,23).
The metaplastic columnar epithelium with goblet cells showed a significantly elevated mean DI, and nearly 50% of the cases showed mild aneuploidy. The rate of aneuploid peaks in the cases in our study is higher than that previously reported in studies that evaluated DNA content by flow cytometry (12). It is also higher than previous studies that evaluated DNA content by early production image cytometry systems (15,24). The differences in results are likely due to the high degree of sensitivity of image cytometry in detecting aneuploidy by high-fidelity DNA histograms, and on improvements of hardware and software in the more recently improved image cytometry systems. Nevertheless, the high DNA aneuploidy rate detected in metaplastic columnar epithelium with goblet cells in our study is comparable to the results obtained by comparative genomic hybridization, which has previously shown a high rate of gains of chromosomal fragments, and copy numbers, in patients with BE (25). For instance, Chaves et al. (9) detected chromosomal abnormalities in two-thirds of the patients with BE, the latter defined by the presence of goblet cells in their esophageal mucosal biopsies. In addition, trisomy 7 and 18, and loss of Y, was detected in 40% of the cases. Structural alterations were found in one-third of the cases with recurrent break points at 1Q21, 15Q15, and 15Q22.
In addition to a high prevalence rate of aneuploid G0/G1 peaks, we also demonstrated that almost all cases of metaplastic columnar epithelium with goblet cells show elevated cellular DNA heterogeneity by analysis of high-fidelity DNA histograms. This finding is similar to previous reports (16,19). In fact, increased cellular DNA heterogeneity may result from an increased number of cells in the S/G2 phases of the cell cycle due to increased cell proliferation, or it may be due to the appearance of newly formed neoplastic clones (17). Furthermore, the presence of cells with 5N-EC cannot be explained on the basis of increased cell proliferation (26).
We showed that 15% of the cases of metaplastic columnar epithelium with goblet cells contained DI>5N (5N-EC). The presence of cells with DI>5N in interphase nuclei is always considered abnormal. These cells may represent aneuploid cells in the S or G2 phases of the cell cycle, severely aneuploid cells in the G0/G1 phase, diploid cells in a second S phase joining endoreplication, or a product of diploid cells with unbalanced anaphase, including a G2 phase of the larger segment and a second S phase of the smaller segment. Thus, cells with DI>5N most likely represent abnormal cells with neoplastic potential (17). Previous studies in breast cancer have shown that cells with DNA content outside of the G0/G1 peak, also known as the scatter index, may be a marker of increased chromosomal instability and poor clinical outcome (27). In fact, previous molecular studies in BE have shown aneuploidy and chromosomal instability characterized by abnormalities in P53, P16, and P21 cell cycle genes and proteins (28-30).
Our study, for the first time, demonstrated that metaplastic columnar epithelium without goblet cells is associated with frequent DNA content abnormalities. For instance, almost 50% of the cases of metaplastic columnar epithelium without goblet cells showed a mild aneuploid G0/G1 peak, and the majority of these cases also showed increased cellular DNA content heterogeneity. In addition, 13% showed cells with DI>5N (5N-EC). These abnormalities were present in patients with metaplastic columnar epithelium without goblet cells and were also present in non-goblet epithelium from patients with metaplastic columnar epithelium with goblet cells. In fact, the DNA content abnormalities in the non-goblet epithelium were similar to that observed in goblet-rich epithelium in patients with metaplastic columnar epithelium with goblet cells. Romagnoli et al. (31) also showed molecular alterations, such as loss of heterozygosity and allelic imbalances, in patients with metaplastic columnar epithelium without goblet cells. Furthermore, Chaves et al. (9) reported a similar degree of chromosomal instability in metaplastic columnar epithelium without goblet cells compared with metaplastic columnar epithelium with goblet cells.
Another interesting finding of our study is that aneuploidy is an early event in columnar metaplasia of the esophagus as it is present in both non-goblet and goblet cell epithelium with equal frequency. A progressive rate of deterioration in aneuploidy has been reported with progression to dysplasia and adenocarcinoma in BE (12,16). Thus, the high prevalence rate of early aneuploidy in columnar metaplasia of the esophagus may serve as a model to further investigate the genesis of aneuploidy in this condition.
The relationship between non-goblet- and goblet cell-containing epithelium in patients with columnar metaplasia of the esophagus is poorly understood. It has been suggested that non-goblet columnar metaplasia may be an initial response to gastroesophageal reflux disease (21,32). In addition, studies have shown that the non-goblet columnar epithelium in patients with BE shows phenotypic features of intestinal differentiation, such as expression of CDX-2, Villin, MUC-2, and DAS-1, all of which represent either protein or transcription factors specific to intestinal epithelium (8,33). These data suggest that non-goblet and goblet cell epithelium may have a common cell lineage, and that metaplastic epithelium without, and with, goblet cells may simply represent different ends of the spectrum of intestinal differentiation.
Our findings, including those of others, reveal that metaplastic columnar epithelium without goblet cells shows intestinal differentiation and DNA content and molecular abnormalities, which raise the possibility that this non-goblet epithelium has neoplastic potential similar to goblet-rich metaplastic columnar epithelium. These findings, therefore, have considerable clinical implications. One is with regard to the current controversy regarding the definition of BE (34). For instance, a panel of experts of the ACG have indicated a requirement for histological demonstration of goblet cells in the esophagus (“specialized intestinal metaplasia”) to establish a diagnosis of BE, and limited endoscopic surveillance to patients only with the latter finding (3). In contrast, the British Society of Gastroenterology extends the diagnosis of BE to all patients with columnar metaplasia of the esophagus, including those without goblet cells (5). Our studies, as well as those of several others, support the view that columnar mucosa without goblet cells or“cardia-type”mucosa, is metaplastic and may have neoplastic potential, and, thus, should be included in the definition of BE. The definition of BE has more than just academic/semantic interest, as it has major implications with regard to the economic impact of endoscopic surveillance, health-care insurance and costs, and medical litigation (35). Interestingly, anecdotal information suggests that goblet cells are decreased, or are completely absent, with neoplastic progression in BE. Furthermore, rarely, adenocarcinomas, particularly those of the gastroesophageal junction, may develop in mucosa without any evidence of goblet cell metaplasia. These conclusions are further supported by reports of development of dysplasia or adenocarcinoma in patients with metaplastic columnar epithelium of the esophagus regardless of the presence or absence of goblet cells (10,11,21). It is possible that neoplastic lesions that develop in metaplastic columnar epithelium without goblet cells may progress through a different biological or molecular pathway than cancers that develop in metaplastic epithelium with goblet cells (31). Further studies are needed to test this hypothesis, and to determine the risk of neoplasia in patients with and without goblet cell metaplasia.
Another important clinical implication of our findings relates to the fact that we detected DNA content abnormalities in non-goblet epithelium even in patients with very short segments of esophageal columnar metaplasia, some of whom had a marginally displaced (irregular) “Z” line. This is a relatively common finding in patients with gastroesophageal reflux disease and, thus, suggests that DNA abnormalities occur quite early in the pathogenesis of esophageal columnar metaplasia. Clearly, the importance of this finding, and the risk of neoplastic progression, in this subgroup of patients needs to be evaluated in further, preferably prospective, studies.
In summary, we have shown that DNA content abnormalities occur with equal frequency and extent in metaplastic columnar epithelium without goblet cells compared with metaplastic columnar epithelium with goblet cells. In addition, the DNA alterations do not change according to the density of goblet cells in patients with goblet cells. These findings suggest that non-goblet esophageal columnar epithelium may have neoplastic potential. Further studies should be performed to determine the natural history and risk of malignancy in patients with columnar metaplasia of the esophagus, either with, or without, goblet cells.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Barrett’s esophagus is defined by the presence of metaplastic columnar epithelium, with goblet cells, in the distal esophagus.
It is generally presumed that only columnar epithelium with goblet cells is at risk for malignancy.
Chromosomal and genetic instability is a major factor in the pathogenesis of Barrett’s-associated adenocarcinoma.
Little is known regarding the biological properties of non-goblet columnar epithelium in patients with Barrett’s esophagus.
WHAT IS NEW HERE
By using image analysis and high-fidelity histograms, we have shown that DNA content abnormalities occur with equal frequency and extent in metaplastic columnar epithelium without goblet cells, compared with columnar epithelium with goblet cells.
DNA alterations do not change according to the density of goblet cells in patients with Barrett’s esophagus.
On the basis of these findings, esophageal non-goblet columnar epithelium may have neoplastic potential and warrants further study.
ACKNOWLEDGMENTS
We thank Ali Shahsafaei for his help in processing the biopsy samples, Arun Chaudhury, MD, for his help in the preparation of this manuscript, and Xiaowei Dou, PhD, for his help in statistical analysis. This article was presented, in part, at the annual USCAP meeting in San Diego, CA (2008).
Financial support. This study was supported, in part, by an NIH Grant NIDDK 62867 and 1R01CA125711-01A2 and Merit Review Award from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs.
Footnotes
CONFLICT OF INTEREST Guarantor of the article: Robert D. Odze, MD, FRCPC and Raj K. Goyal, MD.
Potential competing interests: None.
REFERENCES
- 1.Hornick JL, Odze RD. Neoplastic precursor lesions in Barrett’s esophagus. Gastroenterol Clin North Am. 2007;36:775–96. doi: 10.1016/j.gtc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Yousef F, Cardwell C, Cantwell MM, et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:237–49. doi: 10.1093/aje/kwn121. [DOI] [PubMed] [Google Scholar]
- 3.Wang KK, Sampliner RE. Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago workshop. Gastro enterology. 2004;127:310–30. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Playford RJ. New British Society of Gastroenterology (BSG) guidelines for the diagnosis and management of Barrett’s oesophagus. Gut. 2006;55:442. doi: 10.1136/gut.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paull A, Trier JS, Dalton MD, et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med. 1976;295:476–80. doi: 10.1056/NEJM197608262950904. [DOI] [PubMed] [Google Scholar]
- 7.Murray L, Watson P, Johnston B, et al. Risk of adenocarcinoma in Barrett’s oesophagus: population based study. BMJ. 2003;327:534–5. doi: 10.1136/bmj.327.7414.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerkhof M, Bax DA, Moons LM, et al. For The Cybar Study Group Does CDX2 expression predict Barrett’s metaplasia in oesophageal columnar epithelium without goblet cells? Aliment Pharmacol Ther. 2006;24:1613–21. doi: 10.1111/j.1365-2036.2006.03163.x. [DOI] [PubMed] [Google Scholar]
- 9.Chaves P, Crespo M, Ribeiro C, et al. Chromosomal analysis of Barrett’s cells: demonstration of instability and detection of the metaplastic lineage involved. Mod Pathol. 2007;20:788–96. doi: 10.1038/modpathol.3800787. [DOI] [PubMed] [Google Scholar]
- 10.Kelty CJ, Gough MD, Van Wyk Q, et al. Barrett’s oesophagus: intestinal metaplasia is not essential for cancer risk. Scand J Gastroenterol. 2007;42:1271–4. doi: 10.1080/00365520701420735. [DOI] [PubMed] [Google Scholar]
- 11.Gatenby PA, Ramus JR, Caygill CP, et al. Relevance of the detection of intestinal metaplasia in non-dysplastic columnar-lined oesophagus. Scand J Gastroenterol. 2008;43:524–30. doi: 10.1080/00365520701879831. [DOI] [PubMed] [Google Scholar]
- 12.Reid BJ, Blount PL, Rabinovitch PS. Biomarkers in Barrett’s esophagus. Gastrointest Endosc Clin N Am. 2003;13:369–97. doi: 10.1016/s1052-5157(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopalan H, Jallepalli PV, Rago C, et al. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. doi: 10.1038/nature02313. [DOI] [PubMed] [Google Scholar]
- 14.Duesberg P, Li R, Rasnick D. Aneuploidy approaching a perfect score in predicting and preventing cancer: highlights from a conference held in Oakland, CA in January, 2004. Cell Cycle. 2004;3:823–8. [PubMed] [Google Scholar]
- 15.Fang M, Lew E, Klein M, et al. DNA abnormalities as marker of risk for progression of Barrett’s esophagus to adenocarcinoma: image cytometric DNA analysis in formalin-fixed tissues. Am J Gastroenterol. 2004;99:1887–94. doi: 10.1111/j.1572-0241.2004.30886.x. [DOI] [PubMed] [Google Scholar]
- 16.Yu C, Zhang X, Huang Q, et al. High-fidelity DNA histograms in neoplastic progression in Barrett’s esophagus. Lab Invest. 2007;87:466–72. doi: 10.1038/labinvest.3700531. [DOI] [PubMed] [Google Scholar]
- 17.Steinbeck RG. Dysplasia in view of the cell cycle. Eur J Histochem. 2004;48:203–11. [PubMed] [Google Scholar]
- 18.Huang Q, Yu C, Zhang X, et al. Comparison of DNA histograms by standard flow cytometry and image cytometry on sections in Barrett’s adenocarcinoma. BMC Clin Pathol. 2008;8:5. doi: 10.1186/1472-6890-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Huang Q, Goyal RK, et al. DNA ploidy abnormalities in basal and superficial regions of the crypts in barrett’s esophagus and associated neoplastic lesions. Am J Surg Pathol. 2008;32:1327–35. doi: 10.1097/PAS.0b013e31816b6459. [DOI] [PubMed] [Google Scholar]
- 20.Hornick JL, Mino-Kenudson M, Lauwers GY, et al. Buried Barrett’s epithelium following photodynamic therapy shows reduced crypt proliferation and absence of DNA content abnormalities. Am J Gastroenterol. 2008;103:38–47. doi: 10.1111/j.1572-0241.2007.01560.x. [DOI] [PubMed] [Google Scholar]
- 21.Takubo K, Aida J, Namoto Y, et al. Cardiac rather than intestinal-type background in endoscopic resection specimens of minute Barrett adenocarcinoma. Hum Pathol. 2009;40:65–74. doi: 10.1016/j.humpath.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasoma PT, Der R, Ma Y, et al. Histology of the gastro-esophageal junction—an autopsy study. Am J Surg Pathol. 2000;24:402–9. doi: 10.1097/00000478-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Trier JS. Morphology of the epithelium of the distal esophagus in patients with mid esophageal peptic strictures. Gastroenterology. 1970;58:444–61. [PubMed] [Google Scholar]
- 24.Huang Q, Yu C, Klein M, et al. DNA index determination with Automated Cellular Imaging System (ACIS) in Barrett’s esophagus: comparison with CAS 200. BMC Clin Pathol. 2005;5:7. doi: 10.1186/1472-6890-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walch AK, Zitzelsberger HF, Bruch J, et al. Chromosomal imbalances in Barrett’s adenocarcinoma and the metaplasia—dysplasia—carcinoma sequence. Am J Pathol. 2000;156:555–66. doi: 10.1016/S0002-9440(10)64760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenzato M, Abboud P, Masure M, et al. Image cytometry detection of breast cancer cells with >5C DNA content and minor DNA stemlines. Anal Quant Cytol Histol. 2000;22:199–205. [PubMed] [Google Scholar]
- 27.Kronenwett U, Huwendiek S, Ostring C, et al. Improved grading of breast adenocarcinomas based on genomic instability. Cancer Res. 2004;64:904–9. doi: 10.1158/0008-5472.can-03-2451. [DOI] [PubMed] [Google Scholar]
- 28.Reid BJ, Prevo LJ, Galipeau PC, et al. Predictors of progression in Barrett’s esophagus II: baseline 17p (p53) loss of heterozygosity identifies a patient subset at increased risk for neoplastic progression. Am J Gastroenterol. 2001;96:2839–48. doi: 10.1111/j.1572-0241.2001.04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong DJ, Paulson TG, Prevo LJ, et al. P16(INK4a) lesions are common, early abnormalities that undergo clonal expansion in Barrett’s metaplastic epithelium. Cancer Res. 2001;61:8284–9. [PubMed] [Google Scholar]
- 30.Koppert LB, Wijnhoven BP, van Dekken H, et al. The molecular biology of esophageal adenocarcinoma. J Surg Oncol. 2005;92:169–90. doi: 10.1002/jso.20359. review. [DOI] [PubMed] [Google Scholar]
- 31.Romagnoli S, Roncalli M, Graziani D, et al. Molecular alterations of Barrett’s esophagus on microdissected endoscopic biopsies. Lab Invest. 2001;81:241–7. doi: 10.1038/labinvest.3780232. [DOI] [PubMed] [Google Scholar]
- 32.Lord RV, Wickramasinghe K, Johansson JJ, et al. Cardiac mucosa in the remnant esophagus after esophagectomy is an acquired epithelium with Barrett’s-like features. Surgery. 2004;136:633–40. doi: 10.1016/j.surg.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Glickman JN, Wang H, Das KM, et al. Phenotype of Barrett’s esophagus and intestinal metaplasia of the distal esophagus and gastroesophageal junction: an immunohistochemical study of cytokeratins 7 and 20, Das-1 and 45 MI. Am J Surg Pathol. 2001;25:87–94. doi: 10.1097/00000478-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Spechler SJ, Goyal RK. The columnar-lined esophagus, intestinal metaplasia, and Norman Barrett. Gastroenterology. 1996;110:614–21. doi: 10.1053/gast.1996.v110.agast960614. [DOI] [PubMed] [Google Scholar]
- 35.Rubenstein JH, Saini SD, Kuhn L, et al. Influence of malpractice history on the practice of screening and surveillance for Barrett’s esophagus. Am J Gastroenterol. 2008;103:842–9. doi: 10.1111/j.1572-0241.2007.01689.x. [DOI] [PubMed] [Google Scholar]