Abstract
Objective and Methods
The neurobiology of late-life anxious depression (LLAD) is poorly characterized despite evidence that this is a common and severe subtype of late-life depression. To identify the neuroanatomical substrate of late-life anxious depression, we examined event-related fMRI data collected in 8 subjects with late-life depression, half of whom had high levels of comorbid anxiety. Subjects were trained on the Preparing to Overcome Prepotency (POP) task, which is an executive control task that reliably activates the lateral prefrontal cortex - anterior cingulate cortex cognitive control circuit.
Results
Time series analysis showed that, when compared with elderly depressed subjects, elderly subjects with anxious depression performing the POP task produced a significantly greater and more sustained signal in three regions: BA 24 (dorsal anterior cingulate), BA31 (posterior cingulate) and BA6 (prefrontal cortex). While elderly subjects with pure depression presented a bimodal activation curve in the dorsal anterior cingulate and the posterior cingulate, elderly subjects with anxious depression presented a sustained unimodal activation pattern.
Conclusions
Our preliminary results suggest specific activation patterns unique to anxious depression that may suggest greater and more sustained efforts of the ACC to carry out cognitive control tasks. Further research is needed to clarify the neuroanatomical basis of late-life anxious depression.
Keywords: Late-life anxious depression, fMRI, cognitive control
INTRODUCTION
Comorbid anxiety is common in depressive disorders, both in middle (Flint, 1994, Kessler et al., 1994, Fava et al., 2004) and later life (Ben-Arie et al., 1987, Beekman et al., 2000, Mulsant et al., 1996, Lenze et al., 2000). Comorbid anxiety is clinically relevant as evidenced by its impact on both acute (Fawcett, 1997, Flint and Rifat, 1997a, Andreescu et al., 2007, Steffens and McQuoid, 2005, Dew et al., 1997, Lenze et al., 2003, Alexopoulos et al., 2005) and maintenance treatment of late-life depression (LLD) (Andreescu et al., 2007, Flint and Rifat, 1997b).
The functional neuroanatomy of LLD includes fronto-striatal disconnectivity associated with an increased burden of white matter hyperintensities (Smith et al., 2007, Aizenstein et al., 2005, Alexopoulos, 2002). Although there is a large body of literature examining the functional neuroanatomy of anxiety (including amygdala, insula and prefrontal hyperactivation as well as altered coupling of the amygdala-prefrontal network) (Cannistraro and Rauch, 2003, Etkin and Wager, 2007, LeDoux, 2000, Deckersbach et al., 2006, Stein et al., 2007, Mobbs et al., 2007, Hariri et al., 2003), the functional neuroanatomy of late-life anxiety is not well defined, which is striking because more than half of the cases of LLD are accompanied by substantial anxiety (Beekman et al., 2000). Given the malignant role of comorbid anxiety in short- and long-term treatment response of LLD, anxious depression appears to be not just a more severe form of depression but possibly a distinctive dimension with a unique neurobiological profile. As the biology of LLD involves vascular and neurodegenerative changes of aging, late life anxiety represents probably more than a geriatric version of middle-age anxiety, and some of its neuroanatomical features are likely conditioned by the vascular and degenerative changes experienced by the aging brain. The identification of such a profile would be instrumental in distinguishing biological predictors or moderators of treatment response in LLD and could allow further design of more efficacious pharmacological strategies for LLD and late-life anxious depression (LLAD).
In an attempt to describe features of the neurobiological profile of LLAD, we performed a post-hoc analysis on an event-related fMRI dataset collected in eight subjects with LLD, half of whom had comorbid anxiety. Based on previous literature reporting increased anxiety-induced hyperfrontality (Mobbs et al., 2007, Rauch et al., 1997), we hypothesized that subjects with both anxiety and depression would have a greater activation of the medial prefrontal cortex (in particular in the anterior cingulate) when compared with depressed subjects with low anxiety.
METHOD
Data for this study were provided by the second and third Maintenance Therapies in Late-life Depression studies (MTLD-II and MTLD-III) conducted at the University of Pittsburgh Advanced Center for Intervention and Services for Late-Life Mood Disorders between 1999 and 2004 (MTLD-II) and between 2004 and 2006 (MTLD-III). Details of the MTLD studies protocols are described elsewhere(Reynolds et al., 2006). In brief, participants were age 65 or older, with a Structured Clinical Interview for DSM-IV (SCID) current diagnosis (First M, 1995) of non-psychotic, non-bipolar major depressive disorder (single-episode or recurrent), a 17-item Hamilton Depression Rating Scale (HDRS) of 15 or higher (Hamilton, 1960) and a Mini Mental State Examination (MMSE) score of 24 or higher (Folstein et al., 1975). Cognitive function was assessed with the Dementia Rating Scale (Mattis, 2004) and the Executive Interview (Royall et al., 1992). Subjects with a lifetime history of psychosis or mania, or with a history of substance dependence in the 6 months prior to the screening were excluded. Symptoms of anxiety were measured using the self-report anxiety scale from the Brief Symptom Inventory [BSI, (Derogatis LR, 1983)]. The BSI is a validated self-report scale developed from the Symptom Checklist Inventory SCL-90-R with strong test-retest and internal consistency reliabilities (Derogatis LR, 1983). For this study we used a categorical BSI anxiety measure and we dichotomized those with higher versus lower anxiety by using a median split (median value for the sample = 0.58). We used a median split because BSI scores tends to be skewed (requiring transformation) and also because this approach demonstrated predictive validity in previous analyses using the BSI (Andreescu et al., 2007). Prior psychotropic medications were discontinued at enrollment. Subjects were treated with nortriptyline (MTLDI) and paroxetine (MTLDII), and lorazepam was allowed on an as needed basis.
Eight subjects with LLD were included in this study. The scans were performed in the first month following each study's baseline evaluation. Informed consent was obtained prior to scanning through procedures approved by the University of Pittsburgh's Institutional Review Board. Each subject was paid $50 for his or her participation.
Procedures
Preparing to Overcome Prepotency (POP) task
After signing informed consent, participants were trained on the Preparing to Overcome Prepotency (POP) task outside the MR scanner until they were familiarized with the task (5-10 minutes). The POP task is an executive control task that reliably activates the dorsolateral prefrontal cortex (dLPFC) - dorsal anterior cingulate cortex (dACC) cognitive control circuit. It is derived from the switching Stroop task described by MacDonald et al. (MacDonald et al., 2000) This task has two components, separated by a delay: an instruction (or cue) phase and a decision (or probe) phase. As in the switching Stroop task, the instruction (cue) phase involves a decision (i.e., conflict monitoring) phase meant to engage the dACC and an executive maintenance component meant to engage the dLPFC. The POP task, which has been validated in several studies (MacDonald et al., 2000, Snitz et al., 2005, Kerns et al., 2004), is particularly well suited for studying anxiety due to the suspected interference of threat monitoring with the conflict-monitoring task phases. Threat monitoring is believed to be a core psychological component of anxiety, and thus we expected it to differentiate the anxious depressed subjects from the purely depressed group.
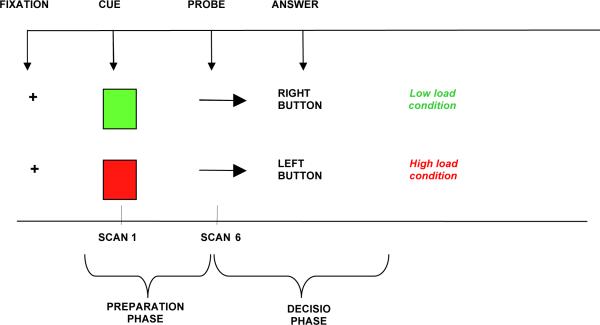
The POP task involves presentation of cues (green/red square) during the preparation phase of the task, indicating whether response to an upcoming probe (arrow on right/left) should be congruent (low-load condition) or incongruent (high-load condition) (see Figure 1). During congruent conditions, the individual has to push a button underneath his left index finger if the arrow is pointing left, and he has to push a button placed underneath his right index finger if the arrow is pointing right. During incongruent tasks, the individual has to invert this order (e.g. push the button underneath his left index if the arrow is pointing right). Red and green cues alternated quasi-randomly during the task to guarantee that 25% of the trials contained a red cue. Indicators of behavioral performance included accuracy rates (number of correct answers/number of total answers), reaction time (RT, in milliseconds) calculated on correct trials only, and percent increase in RT ([average RT in high-load minus average RT in low-load conditions]/average RT in low-load conditions). The percent increase in RT was considered a measure of the load-effect, in that it indicated a proportionally slower response during high load compared to low-load tasks (see Figure 1).
Figure 1.
Individual trial of the POP task. Red or Green square is followed, after a 9.5-second delay, by an arrow pointing to the right or the left.
Scanning Procedures
Data Acquisition
Imaging data were collected with a 1.5T Signa scanner (GE Medical Systems). Highresolution anatomical images - spoiled gradient recalled (SPGRs) were acquired for each subject. Additionally, T1-weighted structural images were acquired with a 3.8 mm thickness (in-plane with the functional images). These had 36 oblique axial slices oriented parallel to a line from the anterior to posterior commissures (the AC-PC line), with an in-plane resolution of 0.9375 mm2 and a field of view of 240 mm2. Functional images were acquired using a one-shot spiral pulse sequence with TE=35ms and TR=2000ms; 26 oblique axial slices were acquired with an in-plane resolution of 64x64 with 3.75 mm2 pixels and a slice thickness of 3.8 mm, and a field of view of 240 mm2. The 4th most inferior slice of the functional images was aligned with the AC-PC line. Images were acquired in four blocks of 24 trials each. There were 10 scans per trial, with each scan lasting 2000 ms. Thus each trial lasted 20 seconds. The cue was presented for 0.5 seconds, followed by 9.5 seconds of a fixation cross in the center of the screen. The probe was then presented for 0.5 seconds. This was then followed by an additional 9.5 seconds of fixation before the next cue.
Image Analysis
Images were first pre-processed with movement correction (using a 6 parameter rigid body registration)(Woods et al., 1998). The images were then co-registered to a common space (MNI, colin27) using a fully-deformable registration procedure (Chen, 1999). We previously demonstrated that this procedure overcomes some of the limitations of standard 12-parameter linear affine or smooth non-linear methods (such as SPM), which is particularly relevant for elderly imaging studies (Wu et al., 2006). After registration, the functional images were smoothed with a 4 mm FWHM (full-width half maximum) smoothing kernel. Voxel-wise analysis was then conducted comparing the two groups with high versus low BSI scores. The images were compared using a random-effects (with subject as the random variable) 3-way ANOVA contrasting group (high versus low BSI) by condition (red or green) by scan number (scans 1-10 over the course of each trial). The whole brain 3-way ANOVA was performed using NeuroImaging Software (NIS- http://kraepelin.wpic.pitt.edu/nis). As in previous neuroimaging studies using similar tasks (Carter et al., 2000), we used a voxel-wise threshold of .01 and a contiguity threshold of 8. This threshold is based on work by Forman et. al., which demonstrated that a contiguity of 8 corrects for multiple comparison with voxel-wise p = .01 (Forman et al., 1995) and leads to an image-wise alpha of p < 0.05. This same approach has been used in several other functional imaging studies using a protocol very similar to the current one (Kerns et al., 2004, MacDonald et al., 2000).
RESULTS
Demographic and clinical characteristics of the groups are presented in Table 1.
Table 1.
Demographic and clinical characteristics of the sample
| Variable | High BSI (N=4) | Low BSI (N=4) | T test (two-tail) | DF | P value |
|---|---|---|---|---|---|
| BSI anxiety (Mean/SD) | 1.29(0.56) | 0.2 (0.11) | 2.12 | 3 | 0.05 |
| Current Age - years, mean (SD) | 67.28 (3.4) | 66.5 (4.4) | 0.76 | 3 | 0.25 |
| Age of Depression Onset - years, mean (SD) | 64.1 (4.7) | 59.1 (8.8) | 4.41 | 3 | 0.01 |
| Female N(%) | 2 (50%) | 2 (50%) | |||
| HRSD 17 - mean (SD) | 20.5 (3.4) | 18.5 (7) | 1.88 | 3 | 0.1 |
| DRS - mean (SD) | 140.5 (3.1) | 140 (3.3) | 0.52 | 3 | >0.25 |
| Medication status (use of antihypertensives) - N | 1 | 1 | |||
| EXIT - mean (SD) | 6.7 (2.2) | 6.7 (1.5) | 0 | 3 | |
| Response Accuracy - Congruent Task -mean (SD) | 0.98 (0.01) | 0.98 (0.02) | 0.11 | 3 | 0.91 |
| Response Accuracy - Incongruent Task -mean (SD) | 0.89 (0.11) | 0.95 (0.06) | 0.65 | 3 | 0.55 |
| Reaction Time - Congruent Task -median (msec) | 640 | 989.5 | 1.87 | 3 | 0.15 |
| Reaction Time - Incongruent Task -median (msec) | 775.8 | 988.5 | 0.82 | 3 | 0.47 |
BSIa=Brief Symptom Inventory - anxiety subscale; HRSD= Hamilton Rating Scale for Depression (17 items); DRS= Dementia Rating Scale; EXIT= Executive Interview.
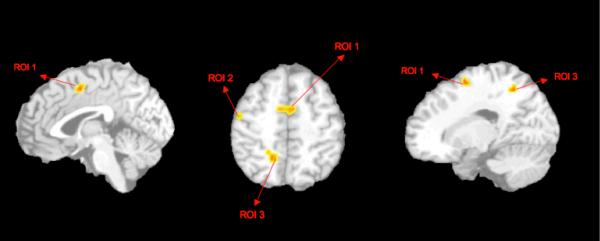
The 3-way ANOVA time series analysis, [thresholded with a Fisher's F-test > 2.755, dF(9, 54), voxel-wise p<0.01, and 8 voxel contiguity threshold] showed a significant effects in the group-by-scan interaction: elderly subjects with higher comorbid anxiety have a significantly greater activation in three regions of the right hemisphere when compared with subjects with pure depression: BA 24 (dorsal ACC - Talairach coordinates x, y, z= 3, 14, 49; Voxel size =16, Max F = 4.39), BA31 (posterior cingulate - Talairach coordinates x, y, z = 23, 52, 46; Voxel size=9, Max F = 3.57) and BA6 (precentral gyrus - supplementary motor area - x, y, z = Talairach coordinates 47, 19, 47; Voxel size = 8, Max F = 3.78). (see Figure 2).
Figure 2.
Areas of greater activation in elderly anxious depression when compared with elderly non-anxious depression: ROI1= BA24, ROI2 = BA6, ROI 3= BA31
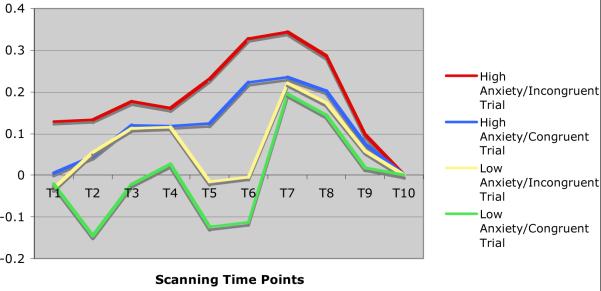
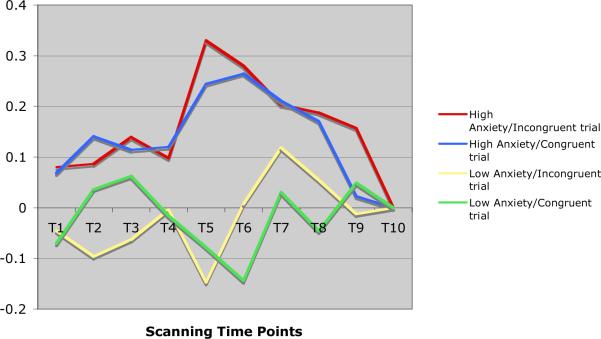
Time series analysis showed that while elderly subjects with pure depression exhibited a bimodal activation curve in the dorsal ACC and the posterior cingulate, elderly subjects with anxious depression presented both a more intense and more sustained pattern of activation, expressed as a unimodal curve (see Figures 3 and 4). Similarly, elderly subjects with pure depression presented with a bimodal activation curve in the prefrontal cortex (supplementary motor area), while elderly subjects with anxious depression presented with a more sustained activation (graphic not shown).
Figure 3.
Pattern of activations of dorsal ACC (BA 24) in elderly depressed and in elderly anxious depressed subjects during the POP task
Figure 4.
Pattern of activations of posterior cingulate (BA 31) in elderly depressed and in elderly anxious depressed subjects during the POP task.
DISCUSSION
To our knowledge, this is the first fMRI study exploring the activation patterns in late-life depression with comorbid anxiety. Consistent with our hypothesis regarding the different functional neuroanatomic profile of anxious depression, we found a specific activation pattern differentiating subjects with anxious depression from subjects with depression without anxiety.
In a group of eight subjects with late-life depression, we report a greater and more sustained activation of the dorsal ACC (BA24), prefrontal cortex - supplementary motor area (BA6) and posterior cingulate (BA31) in subjects with comorbid anxiety, while performing a Stroop-like cognitive probe (POP task).
The dorsal ACC (BA24) is involved in affective awareness and self-referential thought (Gusnard et al., 2001, Pujol et al., 2002) and has been described as a key region in the integration of emotional and cognitive experiences (Phillips et al., 2003). According to the conflict-monitoring hypothesis, the ACC monitors response conflict and thus signals the engagement of control processes that are needed to overcome conflict and perform effectively (Kerns et al., 2004). During the POP task, the ACC is normally activated in a biphasic manner - first wave signaling the identification of a conflict and the second wave subsequently engaging control over the conflict. (Kerns et al., 2004). There is greater conflict for incongruent trials (pushing the button opposite to the direction indicated by the arrow) than for congruent trials. However, monitoring response conflict may be just one of the multiple functions of the ACC, which also monitors internal states for signs of breakdowns in processing and performance that require adjustment in control (Kerns et al., 2004).
Thus, while in “purely” depressed subjects we found a bimodal curve congruent with previously published reports (MacDonald et al., 2000), subjects with anxious depression exhibited a unimodal activation curve. The unimodal curve obtained for subjects with increased anxiety might be interpreted as pathological signaling of conflict even when conflict is absent (see Figure 1 and Figure 3). Dysfunction of the ACC has been implicated in poor antidepressant response in geriatric depression (Alexopoulos et al., 2007). Our findings suggest a further connection between ACC dysfunction and the association of comorbid anxiety with poorer antidepressant treatment response (Andreescu et al., 2007).
With regard to the lateral prefrontal cortex, although the signal was significantly stronger in elderly anxious depressed subjects than in elderly subjects with pure depression, the activation pattern is less compelling, lacking a defining bimodal or unimodal curve. Moreover, one of the areas identified as significantly hyperactive in this study corresponds to the supplementary motor area. While there is a whole body of literature regarding the role of the prefrontal cortex in depression, including late-life depression, most of these studies have focused on the role of the dLPFC (Aizenstein et al., 2006, Aizenstein et al., 2005, Bae et al., 2006, Taylor et al., 2004, Thomas et al., 2003, Alexopoulos et al., 2002). Hyperactivation of the supplemental motor prefrontal area has been described recently in subjects with generalized anxiety (Zhao, 2006). It might be related to the increased anticipatory preparation for initiating movement in anxious patients as the supplemental motor area is the involved in planning, sequencing and initiating motor activity (like the POP tasks) (Shima and Tanji, 2000).
Both the dorsal regions of the ACC (BA24) and the lateral prefrontal cortex have been described as part of the dorsal system involved in the integration of cognitive processes (Phillips et al., 2003). Moreover, the ACC-dLPFC circuit involved in monitoring response conflict and the execution of cognitive control may be further impaired in the depressed elderly due to altered connectivity (Bae et al., 2006).
The observed difference in activation in the dACC-prefrontal circuit in anxious depression can be related to specific characteristics of information processing in depression and anxiety (Akiskal, 2005, Ingram et al., 1987). Thus, information is processed in parallel by brain systems responsible for identifying emotional aspects of the information and by other systems responsible for identifying the nonemotional aspects of information (Ingram, 1984) and affective processing continues to receive equal weighting in the face of cognitive performance demands (Whalen et al., 1998). Affective interference (Siegle et al., 2002) has been previously related to a particular aspect of anxiety, namely ruminative worry. Rumination might mitigate the ability to shift resources between emotional and cognitive tasks (Whalen et al., 1998). However, the global measure of anxiety used in this study does not allow for differentiation between particular aspects of anxiety. Moreover, it is less clear whether in this group of subjects the sustained hyperactivation of the dACC-prefrontal circuit is related to an “emotional overload” or whether the primary deficit is related to impaired cognitive abilities to monitor and process conflict, which secondarily would provoke performance anxiety. The paradigm used in this study does not allow inferring the direction of the causal effect. However, we can speculate that a dysfunctional hyperactivation of the dACC-prefrontal circuit is one of the specific neural substrates of late-life anxious depression; the unique pattern of activation obtained for the anxious depressed elderly points toward a different dimension gained in the presence of increased anxiety symptoms.
The posterior cingulate appears to serve as sensory association cortex as it is involved in episodic memory, visual-spatial processes and proprioception, having a role in the processing of emotionally salient information (Vogt et al., 1995, Maddock, 1999). Together with the medial frontal cortex, the lateral parietal cortex and the precuneus, the posterior cingulate is considered part of the “default network” that supports passive processes like monitoring the environment as well as one's internal state and emotions (Lustig et al., 2003). Engaging in goal-directed activity, as the POP task, would normally be followed by decreased activity in the default network. Several reports pointed toward increased activity in the posterior cingulate in the context of induced sadness (Mayberg et al., 1999) or major depression (Drevets, 2000, Buchsbaum et al., 1997); altered default network activity has been recently reported in subjects with anxiety disorders (Zhao et al., 2007). The posterior cingulate is strongly connected with the medial temporal cortex and, while it deactivates during goal-directed activities in healthy adults, it becomes hyperactive in older adults in the beginning stages of Alzheimer disease (Sperling et al., 2003, Lustig et al., 2003). Recent data report on the profile of cognitive impairment associated with late-life anxiety, mainly with regard to short-term episodic memory (Mantella et al., 2007) as well as on the role of anxiety symptoms as predictors of MCI conversion to Alzheimer disease (Palmer et al., 2007). Our findings suggest that posterior cingulate's dysfunctional hyperactivation might represent one of the neural substrates for the impaired attention and short-term episodic memory observed in late-life anxious depression. It is worth noting that despite the difference in fMRI activation patterns our two groups did not significantly differ on cognitive performance. This suggests that the observed pattern reflects changes in the functional neuroanatomy and may be a biomarker of early pathology, rather than an epiphenomenon of the behavioral differences.
Our study has several limitations: we had a small number of subjects since the study was intended as a preliminary analysis. The comparison subjects had “pure” depression, though our results would have been more interpretable if we had data from a non-psychiatric healthy control group and/or a comparison group with “pure” anxiety. Nevertheless, given the presence of the “pure” depression control group it is less likely that the results we obtained are related to the effects of late-life depression. Moreover, the severity of depression did not differ in the two groups.
We used a cognitive and not an anxiety-inducing probe and thus we can only make inferences about the impact of increased anxiety on cognitive performance in the context of late-life depression. Also, we used a simple scale - the BSI anxiety - which might not capture entirely the various clinical presentations of anxiety in the elderly.
Moreover, the cluster size threshold was calculated using the Forman et al method (REF), which, although it is a well-established method to, has the risk of increasing the cluster false-positive rate. Thus, our results require further validation on larger samples and with stringent cluster-size threshold (Wager et al., 2007).
To our knowledge, this is the first study exploring the fMRI activation patterns in late-life depression with comorbid anxiety. Further research is needed to focus on better understanding of the functional neuroanatomy of comorbid anxiety and depression in late-life, including further clarifications of the biological mechanisms underlying the response variability to antidepressant treatment and the greater cognitive decline reported in late-life anxious depression.
Acknowledgments
Supported by NIMH 64678, 072947, 076079, 071944, 070547, the John A. Hartford Foundation Center of Excellence in Geriatric Psychiatry and the University of Pittsburgh Medical Center (UPMC) endowment in Geriatric Psychiatry.
Footnotes
Conflict of Interest: Carmen Andreescu, Meryl Butters, Vijay K. Venkatraman, Megan Nable and Howard Aizenstein have no conflict of interest to report. Eric J. Lenze has received research support from OrthoMcNeill and Forest Pharmaceuticals. Charles F. Reynolds III has received pharmaceutical supplies for his NIH-sponsored research from GlaxoSmithKline, Pfizer Inc., Eli Lilly and Co., Bristol Meyers Squibb, and Forest Pharmaceuticals.
REFERENCES
- AIZENSTEIN HJ, BUTTERS MA, CLARK KA, FIGURSKI JL, ANDREW STENGER V, NEBES RD, REYNOLDS CF, 3RD, CARTER CS. Prefrontal and striatal activation in elderly subjects during concurrent implicit and explicit sequence learning. Neurobiol Aging. 2006;27:741–51. doi: 10.1016/j.neurobiolaging.2005.03.017. [DOI] [PubMed] [Google Scholar]
- AIZENSTEIN HJ, BUTTERS MA, FIGURSKI JL, STENGER VA, REYNOLDS CF, 3RD, CARTER CS. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry. 2005;58:290–6. doi: 10.1016/j.biopsych.2005.04.023. [DOI] [PubMed] [Google Scholar]
- AKISKAL H. Kaplan & Sadock's Comprehensive Textbook of Psychiatry. Vol. 1. 2005. Mood Disorders: Clinical Features; pp. 1611–1652. [Google Scholar]
- ALEXOPOULOS GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–95. [PubMed] [Google Scholar]
- ALEXOPOULOS GS, KATZ IR, BRUCE ML, HEO M, TEN HAVE T, RAUE P, BOGNER HR, SCHULBERG HC, MULSANT BH, REYNOLDS CF., 3RD Remission in depressed geriatric primary care patients: a report from the PROSPECT study. Am J Psychiatry. 2005;162:718–24. doi: 10.1176/appi.ajp.162.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, KIOSSES DN, CHOI SJ, MURPHY CF, LIM KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–32. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, MURPHY CF, GUNNING-DIXON FM, KALAYAM B, KATZ R, KANELLOPOULOS D, ETWAROO GR, KLIMSTRA S, FOXE JJ. Event-related potentials in an emotional go/no-go task and remission of geriatric depression. Neuroreport. 2007;18:217–21. doi: 10.1097/WNR.0b013e328013ceda. [DOI] [PubMed] [Google Scholar]
- ANDREESCU C, LENZE EJ, DEW MA, BEGLEY AE, MULSANT BH, DOMBROVSKI AY, POLLOCK BG, STACK J, MILLER MD, REYNOLDS CF. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. Br J Psychiatry. 2007;190:344–9. doi: 10.1192/bjp.bp.106.027169. [DOI] [PubMed] [Google Scholar]
- BAE JN, MACFALL JR, KRISHNAN KR, PAYNE ME, STEFFENS DC, TAYLOR WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60:1356–63. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- BEEKMAN AT, DE BEURS E, VAN BALKOM AJ, DEEG DJ, VAN DYCK R, VAN TILBURG W. Anxiety and depression in later life: Cooccurrence and communality of risk factors. Am J Psychiatry. 2000;157:89–95. doi: 10.1176/ajp.157.1.89. [DOI] [PubMed] [Google Scholar]
- BEN-ARIE O, SWARTZ L, DICKMAN BJ. Depression in the elderly living in the community. Its presentation and features. Br J Psychiatry. 1987;150:169–74. doi: 10.1192/bjp.150.2.169. [DOI] [PubMed] [Google Scholar]
- BUCHSBAUM MS, WU J, SIEGEL BV, HACKETT E, TRENARY M, ABEL L, REYNOLDS C. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry. 1997;41:15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- CANNISTRARO PA, RAUCH SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37:8–25. [PubMed] [Google Scholar]
- CARTER CS, MACDONALD AM, BOTVINICK M, ROSS LL, STENGER VA, NOLL D, COHEN JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–8. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN M. 3-D deformable registration using a statistical atlas with applications in medicine. Carnegie Mellon University; Pittsburgh: 1999. [Google Scholar]
- DECKERSBACH T, DOUGHERTY DD, RAUCH SL. Functional imaging of mood and anxiety disorders. J Neuroimaging. 2006;16:1–10. doi: 10.1177/1051228405001474. [DOI] [PubMed] [Google Scholar]
- DEROGATIS LR MN. The Brief Symptom Inventory: an introductory report. Psychological Medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- DEW MA, REYNOLDS CF, 3RD, HOUCK PR, HALL M, BUYSSE DJ, FRANK E, KUPFER DJ. Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry. 1997;54:1016–24. doi: 10.1001/archpsyc.1997.01830230050007. [DOI] [PubMed] [Google Scholar]
- DREVETS WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- ETKIN A, WAGER TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVA M, ALPERT JE, CARMIN CN, WISNIEWSKI SR, TRIVEDI MH, BIGGS MM, SHORES-WILSON K, MORGAN D, SCHWARTZ T, BALASUBRAMANI GK, RUSH AJ. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34:1299–308. doi: 10.1017/s0033291704002612. [DOI] [PubMed] [Google Scholar]
- FAWCETT J. The detection and consequences of anxiety in clinical depression. J Clin Psychiatry. 1997;58(Suppl 8):35–40. [PubMed] [Google Scholar]
- FIRST M SR, GIBBON M, WILLIAMS JBW. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P) New York State Psychiatric Institute; New York: 1995. Version 2.0. [Google Scholar]
- FLINT AJ. Epidemiology and comorbidity of anxiety disorders in the elderly. Am J Psychiatry. 1994;151:640–9. doi: 10.1176/ajp.151.5.640. [DOI] [PubMed] [Google Scholar]
- FLINT AJ, RIFAT SL. Anxious depression in elderly patients. Response to antidepressant treatment. Am J Geriatr Psychiatry. 1997a;5:107–15. [PubMed] [Google Scholar]
- FLINT AJ, RIFAT SL. Two-year outcome of elderly patients with anxious depression. Psychiatry Res. 1997b;66:23–31. doi: 10.1016/s0165-1781(96)02964-2. [DOI] [PubMed] [Google Scholar]
- FOLSTEIN MF, FOLSTEIN SE, MCHUGH PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- FORMAN SD, COHEN JD, FITZGERALD M, EDDY WF, MINTUN MA, NOLL DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- GUSNARD DA, AKBUDAK E, SHULMAN GL, RAICHLE ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARIRI AR, MATTAY VS, TESSITORE A, FERA F, WEINBERGER DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- INGRAM R. Toward an information processing analysis of depression. Cogn Ther Res. 1984;8:443–478. [Google Scholar]
- INGRAM RE, KENDALL PC, SMITH TW, DONNELL C, RONAN K. Cognitive specificity in emotional distress. J Pers Soc Psychol. 1987;53:734–42. doi: 10.1037//0022-3514.53.4.734. [DOI] [PubMed] [Google Scholar]
- KERNS JG, COHEN JD, MACDONALD AW, 3RD, CHO RY, STENGER VA, CARTER CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- KESSLER RC, MCGONAGLE KA, ZHAO S, NELSON CB, HUGHES M, ESHLEMAN S, WITTCHEN HU, KENDLER KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- LEDOUX JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LENZE EJ, MULSANT BH, DEW MA, SHEAR MK, HOUCK P, POLLOCK BG, REYNOLDS CF., 3RD Good treatment outcomes in late-life depression with comorbid anxiety. J Affect Disord. 2003;77:247–54. doi: 10.1016/s0165-0327(02)00177-5. [DOI] [PubMed] [Google Scholar]
- LENZE EJ, MULSANT BH, SHEAR MK, SCHULBERG HC, DEW MA, BEGLEY AE, POLLOCK BG, REYNOLDS CF., 3RD Comorbid anxiety disorders in depressed elderly patients. Am J Psychiatry. 2000;157:722–8. doi: 10.1176/appi.ajp.157.5.722. [DOI] [PubMed] [Google Scholar]
- LUSTIG C, SNYDER AZ, BHAKTA M, O'BRIEN KC, MCAVOY M, RAICHLE ME, MORRIS JC, BUCKNER RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–9. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD AW, 3RD, COHEN JD, STENGER VA, CARTER CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MADDOCK RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–6. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- MANTELLA RC, BUTTERS MA, DEW MA, MULSANT BH, BEGLEY AE, TRACEY B, SHEAR MK, REYNOLDS CF, 3RD, LENZE EJ. Cognitive impairment in late-life generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15:673–9. doi: 10.1097/JGP.0b013e31803111f2. [DOI] [PubMed] [Google Scholar]
- MATTIS S. Dementia Rating Scale-2: Professional Manual. Psychological Assessment Resources; Odessa, FL: 2004. [Google Scholar]
- MAYBERG HS, LIOTTI M, BRANNAN SK, MCGINNIS S, MAHURIN RK, JERABEK PA, SILVA JA, TEKELL JL, MARTIN CC, LANCASTER JL, FOX PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- MOBBS D, PETROVIC P, MARCHANT JL, HASSABIS D, WEISKOPF N, SEYMOUR B, DOLAN RJ, FRITH CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–83. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULSANT BH, REYNOLDS CF, 3RD, SHEAR MK, SWEET RA, MILLER M. Comorbid anxiety disorders in late-life depression. Anxiety. 1996;2:242–7. doi: 10.1002/(SICI)1522-7154(1996)2:5<242::AID-ANXI6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- PALMER K, BERGER AK, MONASTERO R, WINBLAD B, BACKMAN L, FRATIGLIONI L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68:1596–602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- PHILLIPS ML, DREVETS WC, RAUCH SL, LANE R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- PUJOL J, LOPEZ A, DEUS J, CARDONER N, VALLEJO J, CAPDEVILA A, PAUS T. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage. 2002;15:847–55. doi: 10.1006/nimg.2001.1004. [DOI] [PubMed] [Google Scholar]
- RAUCH SL, SAVAGE CR, ALPERT NM, FISCHMAN AJ, JENIKE MA. The functional neuroanatomy of anxiety: a study of three disorders using positron emission tomography and symptom provocation. Biol Psychiatry. 1997;42:446–52. doi: 10.1016/S0006-3223(97)00145-5. [DOI] [PubMed] [Google Scholar]
- REYNOLDS CF, 3RD, DEW MA, POLLOCK BG, MULSANT BH, FRANK E, MILLER MD, HOUCK PR, MAZUMDAR S, BUTTERS MA, STACK JA, SCHLERNITZAUER MA, WHYTE EM, GILDENGERS A, KARP J, LENZE E, SZANTO K, BENSASI S, KUPFER DJ. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354:1130–8. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- ROYALL DR, MAHURIN RK, GRAY KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40:1221–6. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- SHIMA K, TANJI J. Neuronal activity in the supplementary and presupplementary motor areas for temporal organization of multiple movements. J Neurophysiol. 2000;84:2148–60. doi: 10.1152/jn.2000.84.4.2148. [DOI] [PubMed] [Google Scholar]
- SIEGLE GJ, STEINHAUER SR, THASE ME, STENGER VA, CARTER CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- SMITH GS, GUNNING-DIXON FM, LOTRICH FE, TAYLOR WD, EVANS JD. Translational research in late-life mood disorders: implications for future intervention and prevention research. Neuropsychopharmacology. 2007;32:1857–75. doi: 10.1038/sj.npp.1301333. [DOI] [PubMed] [Google Scholar]
- SNITZ BE, MACDONALD A, 3RD, COHEN JD, CHO RY, BECKER T, CARTER CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162:2322–9. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- SPERLING RA, BATES JF, CHUA EF, COCCHIARELLA AJ, RENTZ DM, ROSEN BR, SCHACTER DL, ALBERT MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEFFENS DC, MCQUOID DR. Impact of symptoms of generalized anxiety disorder on the course of late-life depression. Am J Geriatr Psychiatry. 2005;13:40–7. doi: 10.1176/appi.ajgp.13.1.40. [DOI] [PubMed] [Google Scholar]
- STEIN MB, SIMMONS AN, FEINSTEIN JS, PAULUS MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- TAYLOR WD, MACFALL JR, PAYNE ME, MCQUOID DR, PROVENZALE JM, STEFFENS DC, KRISHNAN KR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–6. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- THOMAS AJ, PERRY R, KALARIA RN, OAKLEY A, MCMEEKIN W, O'BRIEN JT. Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late-life depression. Int J Geriatr Psychiatry. 2003;18:7–13. doi: 10.1002/gps.720. [DOI] [PubMed] [Google Scholar]
- VOGT BA, NIMCHINSKY EA, VOGT LJ, HOF PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- WAGER TD, LINDQUIST M, KAPLAN L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHALEN PJ, BUSH G, MCNALLY RJ, WILHELM S, MCINERNEY SC, JENIKE MA, RAUCH SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–28. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- WOODS RP, GRAFTON ST, WATSON JD, SICOTTE NL, MAZZIOTTA JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–65. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- WU M, ROSANO C, BUTTERS M, WHYTE E, NABLE M, CROOKS R, MELTZER CC, REYNOLDS CF, 3RD, AIZENSTEIN HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–42. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO XH, WANG PJ, LI CB, HU ZH, XI Q, WU WY, TANG XW. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol. 2007;63:373–8. doi: 10.1016/j.ejrad.2007.02.006. [DOI] [PubMed] [Google Scholar]
- ZHAO XH, WANG PJ, LI CB, WANG JH, YANG ZY, HU, ZH, WU, WY Prefrontal and superior temporal hyperactivity as a biological substrate of generalized anxiety disorder. Zhonghua Yi Xue Za Zhi. 2006;86:955–60. [PubMed] [Google Scholar]