Abstract
Neisseria gonorrhoeae is a common sexually transmitted pathogen that significantly impacts female fertility, neonatal health, and transmission of HIV worldwide. N. gonorrhoeae usually causes localized inflammation of the urethra and cervix by inducing production of IL-1β and other inflammatory cytokines. Several NLR (Nucleotide binding domain, Leucine Rich Repeat) proteins are implicated in the formation of pro-IL-1β-processing complexes called inflammasomes in response to pathogens. We demonstrate that NLRP3 (cryopyrin,NALP3) is the primary NLR required for IL-1β/IL-18 secretion in response to N. gonorrhoeae in monocytes. We also show that N. gonorrhoeae infection promotes NLRP3-dependent monocytic cell death via pyronecrosis, a recently described pathway with morphological features of necrosis, including release of the strong inflammatory mediator HMBG1. Additionally, N. gonorrhoeae activates the cysteine protease Cathepsin-B as measured by the breakdown of a Cathepsin B substrate. Inhibition of Cathepsin B shows that this protease is an apical controlling step in the downstream activities of NLRP3 including IL-1β production, pyronecrosis, and HMGB1 release. Non-pathogenic Neisseria strains (N. cinerea and N. flavescens) do not activate NLRP3 as robustly as N. gonorrhoeae. Conditioned media from N. gonorrhoeae contains factors capable of initiating the NLRP3 mediated signaling events. Isolated N. gonorrhoeae lipooligosaccharide, a known virulence factor from this bacterium that is elaborated from the bacterium in the form of outer membrane blebs, activates both NLRP3-induced IL-1β secretion and pyronecrosis. Our findings indicate that activation of NLRP3-mediated inflammatory response pathways is an important venue associated with host response and pathogenesis of N. gonorrhoeae.
INTRODUCTION
Neisseria gonorrhoeae (gonococcus) is one of the most common sexually transmitted bacterial pathogens. Worldwide, N. gonorrhoeae accounts for an estimated 60 million cases of urethritis and cervicitis each year (1). During gonococcal infection, there is a local inflammatory response to mucosal invasion by the organism. In women, N. gonorrhoeae infections also lead to major complications including pelvic inflammatory disease, infertility (via inflammatory scarring of the fallopian tubes), and neonatal disease. The host innate immune responses to N. gonorrhoeae are critical in dictating the local inflammatory response to gonococcus, which, in turn, mediate many of the complications of infection by this organism.
Despite the initial innate immune response, most patients develop little adaptive immunity to N. gonorrhoeae and re-exposure frequently results in recurrent infection (2). The mechanisms leading to this poor adaptive immune response are unknown. N. gonorrhoeae is known to engage immunosuppressive signaling pathways in B and T lymphocytes (3, 4). However, there have been no reports of such immunosuppressive signaling in antigen presenting cells, which act as the bridge between the innate and adaptive immune system.
Several cytokines have been implicated in mediating inflammation associated with gonococcal infection. Experimental infection with N. gonorrhoeae in human subjects has been shown to result in measurable increases in systemic and urethral proinflammatory cytokine levels, including IL-1β (5, 6). In women with naturally acquired gonococcal cervicitis, the levels of systemic inflammatory cytokines are not significantly elevated except in the presence of co-infection with other sexually transmitted infections (7). The source of these cytokines is likely to include local epithelial cells, resident phagocytes, and recruited immune cells. Various immortalized epithelial cell lines have been shown to increase expression of IL-1β and other cytokines after exposure to N. gonorrhoeae (8, 9). Peripheral blood mononuclear cells (primarily lymphocytes) exposed to N. gonorrhoeae produce a number of T-cell-associated cytokines, including IL-2, 4, 8, 10, and 12 (10).
Macrophages and other phagocytes are critical cells in the innate immune response to pathogens that are sensed in the environment and phagocytized. In addition to living freely in the extracellular space, N. gonorrhoeae has the capacity to penetrate the cytoplasm of these phagocytes, posing additional difficulties to its detection and elimination by the innate immune system.
There are now at least four known families of signaling effectors involved in the innate recognition of pathogens: Toll-like receptors (TLRs), NLRs, RIG-I-like helicases (RLHs), and the C-lectin receptors (CLRs) (11–13). While the RLHs are thought to play roles primarily in innate recognition of viral pathogens, the TLRs, CLRs, and NLRs have all been implicated in recognizing or responding to various bacterially derived compounds. Recognition of extracellular gonococcal lipooligosaccharide (LOS) can be mediated by TLR4 and the C-lectin receptor, DC-SIGN (14, 15). Additionally, innate recognition of several N. gonorrhoeae-derived proteins, including Porin and Lip, induces lymphocyte activation that is dependent on TLR2 (16, 17). To date, the role of intracellular NLR proteins in the recognition of N. gonorrhoeae remains uncharacterized.
The NLR gene product NLRP3 (also known as cryopyrin, NALP3, Pypaf1 and CLR1.1), is required for IL-1β induction by the innate immune system in response to a large number of bacterial pathogens and proinflammatory substances (18–23). NLRP3 can also be activated by mutations in its nucleotide-binding domain (24). In humans, these mutations are associated with the periodic fever syndrome CAPS (cryopyrin-associated periodic syndrome) (25). Upon activation, NLRP3 assembles with caspase-1 (the protease responsible for cleaving pro-IL-1β to its mature form), ASC1, Cardinal/TUCAN, and potentially other proteins to produce one of several IL-1β-processing complexes known as “inflammasomes” (26). The activation of the NLRP3 inflammasome has recently been shown to occur both in the setting of exposure to Pathogen Associated Molecular Patterns (PAMPS) and other molecular signs of danger, known as Danger Associated Molecular Patterns (DAMPS). In addition to production of IL-1β, activation of NLRP3 by mutation or pathogens also initiates a pro-inflammatory, necrotic cell death program in monocyte-derived cell lines (27, 28).
We now demonstrate that N. gonorrhoeae potently activates NLRP3-dependent signaling pathways to elicit IL-1β secretion. Our studies suggest that the NLRP3/inflammasome signaling pathway is critical to the secretion of mature IL-1β, which has been observed in humans infected with gonococci. Additionally, we have demonstrated that gonococcus causes the activation of the cysteine protease, Cathepsin B. Inhibition of this protease reduced NLRP3-mediated pyronecrosis and IL-1β secretion in monocyte-derived cells. Isolated gonococcal lipooligosaccharide (LOS), which is shed through membrane blebbing by this organism, also elicited both of these NLRP3-mediated signaling responses. Lipooligosaccharide induced activation of this signaling system likely represents a major component of the inflammatory signaling involved in the pathogenesis of infections caused by N. gonorrhoeae.
MATERIALS AND METHODS
Electrophoresis, immunoblot analysis, and caspase activity analysis
SDS-PAGE electrophoresis was carried out using the NUPAGE system (Invitrogen) according to the manufacturers protocols. Immunoblot analysis for HMGB1, caspase-3, PARP, and actin was performed as described by Willingham et al. (28). Antibodies to HMGB1 were from Immuno Diagnostic Oy; caspase-3 from Cell Signaling; PARP, actin, and HRP conjugated secondary antibodies were all from Santa Cruz Biotechnology. Immunoblot analysis for caspase-1 p10 was carried out using a modified immunoprecipitation/immunoblot protocol described in Williams et al. (29). Cellular lysates for analysis of caspase-1 p10 were prepared by the addition of proteinase inhibitors (complete™, Roche) and NP-40 (final concentration 0.1%) to the treated cell cultures followed by centrifugation at 13,000 ×g for 10 min. Caspase-1 and actin were then simultaneously immunoprecipitated as described using antibodies to caspase-1 p10 (sc-515) and actin (sc-7210) from Santa Cruz Biotechnology. The immunoprecipitated proteins were analyzed by immunoblot with antibodies directed to caspase-1 (IMG-5028, Imgenex) and actin (sc-8432 HRP Santa Cruz Biotechnology).
To assess caspase inhibition, THP-1 cells were prepared at 1×107 cells/ml and incubated with inhibitors to the following caspases: caspase-1 (Ac-YVAD-CHO, Biomol International), caspase-3 (Ac-DEVD-CHO, Biomol), or pan-caspase (Ac-VAD-CHO, Biomol International) at 20 µM, or DMSO (vehicle) for 4 hours. Cell lysates were prepared by addition of CHAPS detergent (0.5% final concentration) followed by centrifugation at 13,000×g for 10 min. The soluble lysates were added to 1U of recombinant caspase-1 or caspase-3 (Biovision, Mountain View, CA) and after 10 minutes caspase activity in the sample was assessed using Fluorimetric Assay kits for either caspase-1 or caspase-3 (Biovision) according to the manufacturers protocol.
Culture and preparation of N. gonorrhoeae and non-pathogenic Neisseria species
N. gonorrhoeae strain FA1090 was used for all described experiments (30). N. cinerea and N. flavascens were provided to P. F. Sparling by J. Knapp of the Neisseria Reference Lab (31). N. gonorrhoeae can express one or more of thirteen different Opa genes on its surface. Because expression of these proteins shifts with time and may alter cellular adherence and activation of host signaling pathways, a stock of piliated, opaque N. gonorrhoeae was prepared as a mixed population for experimental cell infections. The FA1090 was plated and grown for 20 hours on GCB agar at 37°C in 5%CO2. Approximately 200 optically opaque, piliated colonies were picked using a toothpick and inoculated into GCB media. The media was plated onto GCB agar plates and grown overnight. ~106 colonies were harvested using a sterile swab and inoculated into GC freezing media and frozen at −80° in 50 ul aliquots at a density of ~1×108 cfu/ml. Opa protein expression was determined by whole cell immunoblotting 98 individual colonies and probing with a combination of five specific anti-Opa monoclonal antibodies (32). These studies revealed that the frozen population was greater than 80% Opa expressing and biased toward the optically opaque Opa’s (OpaA, OpaD and OpaI in FA1090). On the day prior to cell infection experiments, a stock aliquot of N. gonorrhoeae was thawed and plated as serial dilutions on GCB agar. After growing overnight, N. gonorrhoeae colonies from a plate containing ~104 colonies were harvested by sterile swab and inoculated into RPMI with 10% FBS. Bacterial density was estimated by measuring the O.D. 600 and confirmed by plating of serial dilutions. N. gonorrhoeae resuspended in RPMI/10% FBS was used to inoculate cultured monocytes, macrophages, or THP-1 cells.
Preparation of N. gonorrhoeae-conditioned media
N. gonorrhoeae was prepared for inoculation into tissue culture as described above. This preparation was placed in a shaking incubator (37° and 5% CO2) for 2 hours. The N. gonorrhoeae-conditioned media was recovered by centrifugation of this preparation at 16,000 ×g for 10 minutes and subsequent filtration of the supernatant through a sterile 0.2 µm filter. This conditioned media was placed in a centrifugal ultrafiltration device (Amicon Ultra-4, Millipore) with a 100kD molecular weight cut-off. The filtrate was collected and the retentate was reconstituted to its original volume with RPMI/10% FBS.
Cell culture and infection with N. gonorrhoeae or treatment with lipopolysaccharide
THP-1 cells and sh-RNA expressing derivatives were cultured in RPMI with 10% FBS supplemented with penicillin and streptomycin (28, 33). Primary human monocytes were isolated from donated human peripheral blood (Red Cross, Durham, NC) using Ficoll-Hypaque centrifugation and adherence, as described by Haskill et al. (34). E. coli K12 lipopolysaccharide was obtained from Invivogen (San Diego, CA). Purification of N. gonorrhoeae lipooligosaccharide from strains PID2 and DOV was carried out using hot phenol extraction as previously described (15, 35). Undifferentiated THP-1 cells were used for all infections with N. gonorrhoeae. THP-1 (and THP-derived) cells and PBMC derived monocytes were washed in antibiotic free media twice, resuspended in antibiotic free media at a density of 0.5 ×106 cells/ml, and inoculated with the indicated dose of gonococcus. The cells were spun at 500 ×g for 10 min. In some experiments the cells were treated after washing and prior to inoculation with N. gonorrhoeae with proteinase inhibitors, including selective Cathepsin B (Ca-074-me, Calbiochem), Cathepsin L (ZFY( OtBu)-COCHO or Cathepsin L Inhibitor V, Calbiochem), caspase-1 (Ac-YVAD-CHO, Biomol International), caspase-3 (Ac-DEVD-CHO, Biomol), or pan-caspase (Ac-VAD-CHO, Biomol International) at indicated concentrations or DMSO (vehicle) for 30 min. prior to addition of bacteria. The cells were re-suspended in the media and transferred to sterile plates. Tissue culture plates were returned to 5% CO2 humidified incubators for indicated times and harvested for analysis of cell death, cytokine secretion, or protein analysis by western blot at indicated times.
Maintainance and care of Nlrp3−/−and Asc−/− mice and generation of bone marrow derived macrophage
The generation of Nlrp3 −/− and Asc −/− mice has been described previously (22, 36). The mice were kindly provided by Millenium Pharmaceuticals and Dr. Vishva Dixit at Genentech, respectively, and were subsequently backcrossed onto the C57BL/6J genetic background for at least nine generations. Age- and sex-matched C57BL/6J mice purchased from The Jackson Laboratory (Bar Harbor, ME) were used as wild-type controls. The mice were maintained according to institutional policies. Bone marrow derived macrophages were isolated as previously described and cultured for 7 days in 30% L929 cell-conditioned media (28). The cells were treated with N. gonorrhoeae as noted above for THP-1 cells and secreted Il-1β was determined after this treatment without the addition of exogenous ATP. All protocols used in this study were approved by the Institutional Animal Care and Use Committees at the University of North Carolina.
Cytokine Analysis
Secreted IL-1β, IL-18, and TNF-α were detected in cell culture supernatants using ELISA kits from R&D Systems (Minneapolis, MN). Multiplex cytokine bead arrays were performed by the UNC Center for Oral and Systemic Diseases – GCRC Bioanalytical Core Lab using the Human Fluorokine MAP Base Kit, Panel A kit (R&D Systems, Minneapolis, MN). For anti-cytokine array analysis, cell free supernatants were harvested from N. gonorrhoeae (MOI=4, 20 hour) infected THP-1 cells. Supernatants were analyzed using RayBio® Human Cytokine Antibody Array G Series 2000 (RayBiotech Inc., Norcross, GA). Axon scanner 4000B with GenePix software was used to collect fluorescence intensities from cytokine-bound antibody spots. These values were normalized to the ratio of positive control values for each sample. Afterward, the total normalized florescence values of replicate spots were averaged and expressed as fold increase over the non-infected sample. Where the raw fluorescence values of replicate spots deviated more than 2 fold from each other, the cytokine was removed from the data set.
Analysis of Cell Death
Trypan Blue exclusion was performed by mixing cultured cells in a 1:1 ratio with Trypan Blue solution (0.4 %, Sigma-Aldrich) that was filtered through a 0.2 micron filter. Twenty microliters of the mixtures were applied to Cellometer slides (2 or three independent slides for each sample) and counted using the automated Cellometer Auto T4 (Nexcelom Bioscience, Lawrence, MA) according to the manufacturers protocols. Cell death was also assessed by measuring release of cytoplasmic LDH into the culture media using the Cytotox-ONE Homogenous Membrane Integrity Assay from Promega. Cell death was examined qualitatively by fluorescent microscopy as follows. THP-1 cells were infected with N. gonorrhoeae at MOI=0.2 as detailed above. After 16 hours, bacteria-induced cell death in THP-1 cells was assessed by incubation of cells with medium containing Hoechst 33342 (10 µM) and propidium iodide (PI) (20 µM) for 10 minutes at 37°C. Results were visualized and imaged under a Zeiss fluorescent inverted microscope with a UV filter, Representative fields are shown from experiments performed in triplicate. In experiments using staurosporine to induce apoptosis, 1mM Staurosporine in DMSO (Staurosporine Ready Made Solution, Sigma-Aldrich) was added to cultures to a final concentration of 1 µM.
Electron microscopy
THP-1 cells were infected with N. gonorrhoeae at MOI=2 as described above. The cells were pelleted by centrifugation and washed in Phosphate Buffered Saline then fixed in 2% paraformaldehyde, 2.5% glutaraldehyde in 0.15M sodium phosphate (pH 7.4) 4 hours post infection. Electron microscopy was performed at the UNC Microscopy Services Laboratory.
Analysis of Cathepsin B Activation
THP-1 cells were incubated with or without the cell permeable cathepsin B inhibitor (Ca-074-me, [L-3-trans-(Propylcarbamoyl)oxirane-2-carbonyl]-L-isoleucyl-L-proline Methyl Ester) for 15 min. The cells were then exposed to media with or without N. gonorrhoeae at an MOI of 2 as described above. All cells were then incubated with Magic Red™ Cathepsin B substrate (Immunochemistry Technologies, Bloomington, MN) for 2 hours. The cells were pelleted after centrifugation and washed with phosphate buffered saline and fixed with 0.1% paraformaldehyde. The levels of fluorescent Magic Red™ Cathepsin B substrate present in the cells were quantitated using flow cytometry.
RESULTS
N. gonorrhoeae induces chemotactic and inflammatory cytokine production in monocyte derived cells and primary monocytes
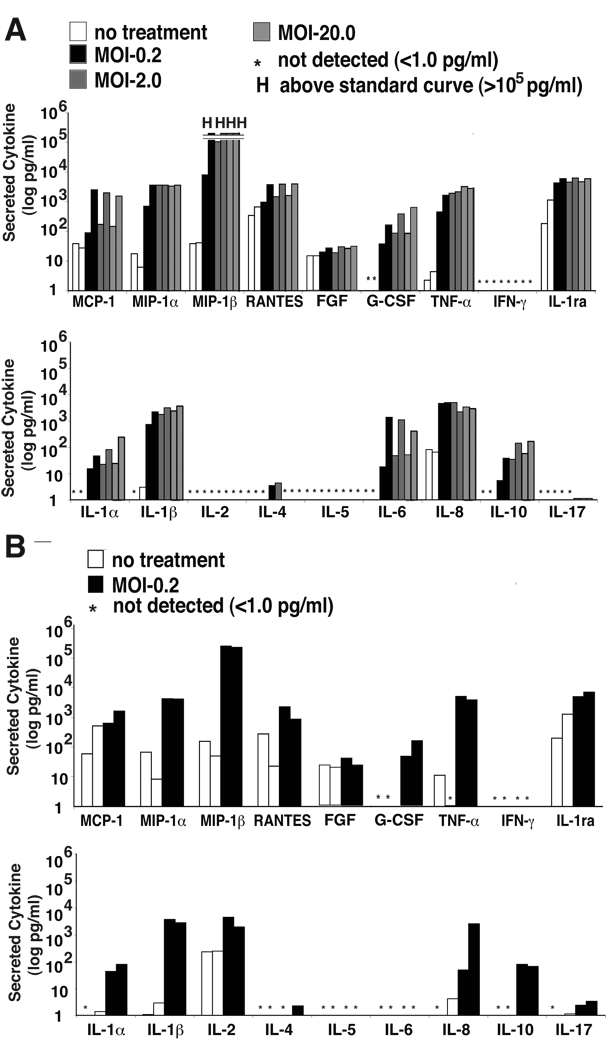
We broadly examined the production of inflammatory mediators from the human monocyte derived cell line, THP-1, in response to N. gonorrhoeae exposure qualitatively using anti-cytokine antibody arrays, which contain antibodies to 175 inflammatory mediators (Table 1, Supplementary Table 1). The most dramatically induced mediators in these cells included the chemotactic cytokines MIP1α, MIP1β, MCP2, and MIP3. Of the other secreted inflammatory mediators, IL-1β, IL-10, and IL-15 were the most highly induced secreted interleukins and the secreted proteinases MMP-9 and MMP-13 were among the most highly induced factors (Table 1). A smaller panel of inflammatory mediators and growth factors produced by THP-1 cells and primary monocytes was assessed quantitatively using multiplexed anti-cytokine bead arrays (Figure 1A & B). Though differences in the baseline levels of many cytokines were observed between preparations of primary monocytes, likely the result of the use of primary cells derived from different donors in each experiment, the N. gonorrhoeae-induced cytokine secretion profile seen in THP-1 cells was very similar to isolated human circulating primary monocytes. Granulocyte-colony stimulating factor was produced by both THP-1 and monocytes. MIP1α and MIP1β were also both induced dramatically in both cell types while other chemotactic factors, including MCP-1 and RANTES were induced to a much lesser extent and inconsistently. TNF-α, IL-1 (α and β), IL-8, and IL-10 were all produced in response to N. gonorrhoeae in THP-1 and primary monocytes. THP-1 cells also generated significant levels of IL-6 secretion in response to N. gonorrhoeae infection, which was not observed in preparations of primary monocytes. As expected, T-cell cytokines, including interferon-γ, IL-2, IL-4, IL-5, and IL-17, were not produced in significant quantities by these cells in response to gonococcus. These data indicate that N. gonorrhoeae similarly activates the production of chemotactic and proinflammatory responses in primary monocytes and monocyte derived cell lines.
Table I.
Inflammatory mediator production by THP-1 cells induced by N. gonorrhoeae exposure
| Cytokine | fold induction* |
|---|---|
| MIP-3α | 115.3 |
| MCP-2 | 54.0 |
| MIP-1β | 46.0 |
| MMP-9 | 38.6 |
| MIP-1α | 21.2 |
| I-309 | 20.4 |
| IL-1β | 11.7 |
| ACTIVIN A | 10.9 |
| MMP-13 | 9.4 |
| IP-10 | 9.3 |
| MMP-1 | 9.2 |
| GM-CSF | 9.1 |
| STNFR2 | 8.2 |
| GCP-2 | 7.9 |
| MIG | 7.5 |
| MCP-3 | 7.4 |
| IL-10 | 7.2 |
| IL-15 | 7.0 |
| GRO | 6.1 |
| CD14 | 5.6 |
| ONCOSTATIN M | 5.4 |
| IL-7 | 5.3 |
| IL-5 | 5.0 |
cytokines with ≥5 fold induction over untreated cells are reported.
Figure 1. Infection with N. gonorrhoeae induces production of chemotactic and inflammatory cytokines from primary monocytes and monocyte derived cell lines.
A) THP-1 cells at 1×106 cell/ml were incubated with N. gonorrhoeae at the indicated MOI as noted in the experimental methods. At 4 hours, extracellular bacteria were killed by addition of gentamicin. Supernatants were collected and cytokine production quantitated using multiplexed cytokine bead arrays. Each of two bars at each dose of infection represents an independent experiment. B) Primary monocytes were isolated from human blood as described in the Materials and Methods. The cells were seeded in plates at a density of 1.0 ×106 cells/ ml and were infected with gonococcus at an MOI of 0.2. The samples were processed and cytokines measured as described in A. The * indicates cytokine levels below 1 pg/ml and “H” indicates cytokine levels were greater than the highest standard measured, 150,000 pg/ml.
N. gonorrhoeae induced IL-1β and IL-18 production requires inflammasome components ASC and NLRP3
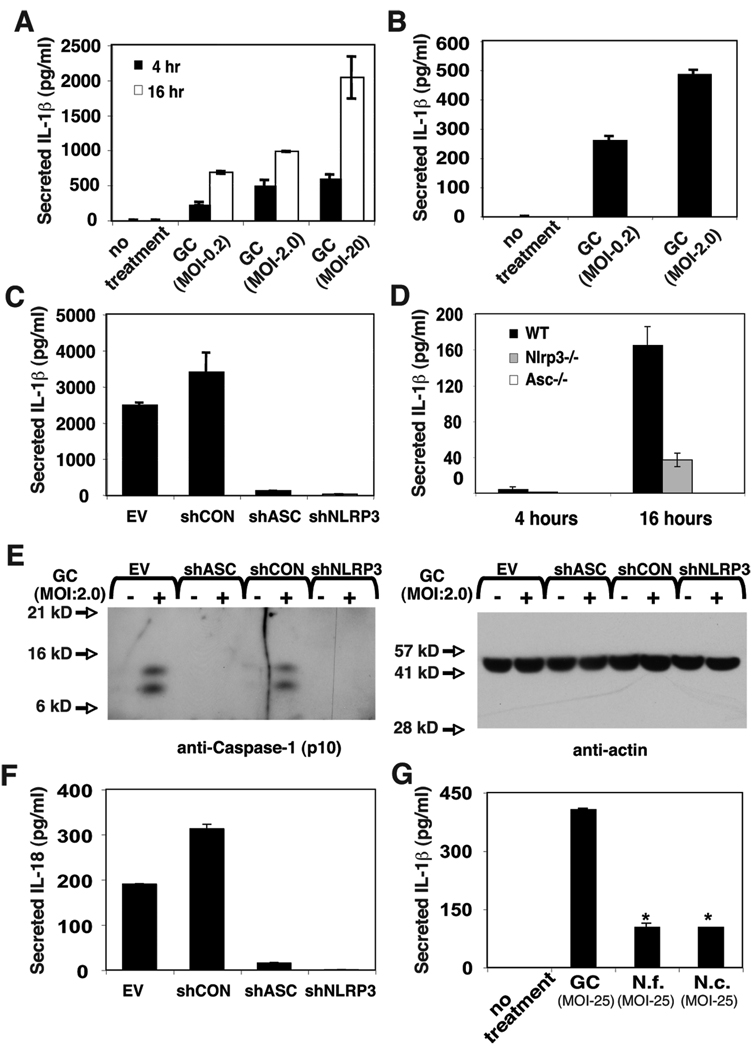
Urine and cervical fluid from N. gonorrhoeae infected individuals contain significant IL-1β (5, 6). The cellular source of IL-1β in these samples is not known, but resident macrophages derived from circulating monocytes are known to mediate significant IL-1β production. Using ELISA, we confirmed that N. gonorrhoeae exposure potently provoked IL-1β secretion from THP-1 cells and primary monocytes (Figure 2A and B). The NLR-containing inflammasomes have recently been shown to play an important role in the activation of the IL-1β converting enzyme caspase-1 in response to numerous proinflammatory stimuli. We sought to determine whether NLRP3 and ASC, two components of the NLRP3 inflammasome, also play a role in the production of IL-1β in response to whole N. gonorrhoeae. We have previously generated stable THP-1-derived cell lines with expression of the inflammasome components NLRP3 or ASC “knocked-down” by expression of small hairpin RNA’s targeting the mRNA encoding those proteins (28, 33). The levels of NLRP3 and ASC-encoding mRNA and proteins are reduced by greater than 90% in these cells (28, 33). These cells demonstrated dramatic reductions in IL-1β secretion in response to gonococci when compared to control cell lines with intact expression of NLRP3 and ASC (Figure 2C). Additionally, bone-marrow-derived macrophages (BMDM) derived from Asc −/− and Nlrp3 −/− mice showed insignificant IL-1β secretion when compared to BMDM from wild-type mice (Figure 2D). Immunoblot analysis for the p10 subunit of mature, active caspase-1 demonstrated that both NLRP3 and ASC were required N. gonorrhoeae-induced activation of caspase-1 in THP-1 cells, indicating that IL-1β secretion under these conditions was being regulated at the level of cytokine maturation caspase-1 (Figure 2D). Because secretion of mature IL-18 has also been shown to require activation of caspase-1, we tested whether the secretion of IL-18 also required NLRP3 and ASC. THP-1 derived cells secreted significant levels of IL-18 in response to N. gonorrhoeae and this secretion required intact expression of NLRP3 and ASC (Figure 2E). These results indicate that production of IL-1β and other caspase-1-processed cytokines by gonococcus-exposed macrophages relies primarily on activation of NLRP3/ASC dependent signaling. Because many Neisseria species are commensal organisms that colonize mucosal surfaces but do not induce robust inflammatory responses, we also examined whether non-pathogenic Neisseria species would activate NLRP3/ASC-mediated IL-1β secretion. Both N. cinerea and N. flavescens caused significantly less IL-1β secretion than did N. gonorrhoeae even at MOI as high as 25 (Figure 2F). The IL-1β secretion elicited by these commensal Neisseria species was also eliminated in THP-1 cells with “knocked-down” expression of NLRP3 or ASC (Supplemental Figure 1). These data suggest that the ability of N. gonorrhoeae to activate the NLRP3-dependent inflammasome and elicit IL-1β secretion may be associated with the virulence or pathogenesis of infections caused by this organism.
Figure 2. Infection with N. gonorrhoeae induces caspase-1-dependent cytokine production by THP-1 cells and PBMC-derived monocytes.
A) THP-1 cells at 1×106 cell/ml were incubated with gonococci (GC) at the indicated MOI as noted in the experimental methods. At 4 hours, extracellular bacteria were killed by addition of gentamicin. Supernatants were collected after 4 hours (black bars) and 20 hours (white bars) and secreted IL-1β was measured by ELISA. B) Primary monocytes were isolated from PBMC by adherence. The cells were seeded at 1×106 cell/ml and incubated with GC at the indicated MOI. At 4 hours, extracellular bacteria were killed by addition of gentamicin and supernatants were collected. C) THP-1-derived cell lines stabily transduced with shRNA expressing retrovirus were infected with GC at MOI of 2.0 and IL-1β production determined at 4 hours as described in A. The shRNA’s are directed to knock down expression as follows: shCON - negative control (scrambled sequence with base content equal to shASC); shASC – shRNA directed against Apoptotic Speck Containing-protein; shNLRP3 – shRNA directed against NLRP3. D) Bone marrow derived macrophages were isolated from C57/B6 mice which were either wild type (WT) or bearing genetic knock out of the genes encoding NLRP3 (Nlrp3−/−) or ASC (Asc −/−), cultured and infected with GC (MOI-0.2) as described in the materials and methods. At the indicated time points, culture supernatant was removed and assayed for the presence of IL-1β using ELISA. E) Immunoblot analysis for activated caspase-1 (P10 subunit) and control protein, actin, was performed on protein extracts from cellular infections described in (C) as described in the materials and methods. F) shRNA-expressing THP-1 cells were infected with GC (MOI-2.0) as described in (C) and secreted IL-18 was measured using ELISA. G) THP-1 cells were infected with pathogenic and commensal Neisseria species at the indicated MOI as described in A; N. gonorrhoeae (GC), Neisseria flavescens (N.f.), or Neisseria cinerea (N.c.). Secreted IL-1β was measured using ELISA. Experiments were performed in triplicate and results from representative experiments are shown. Error bars represent the standard error of the mean for duplicate or triplicate measurements of IL-1β.
N. gonorrhoeae induces NLRP3-dependent cell death
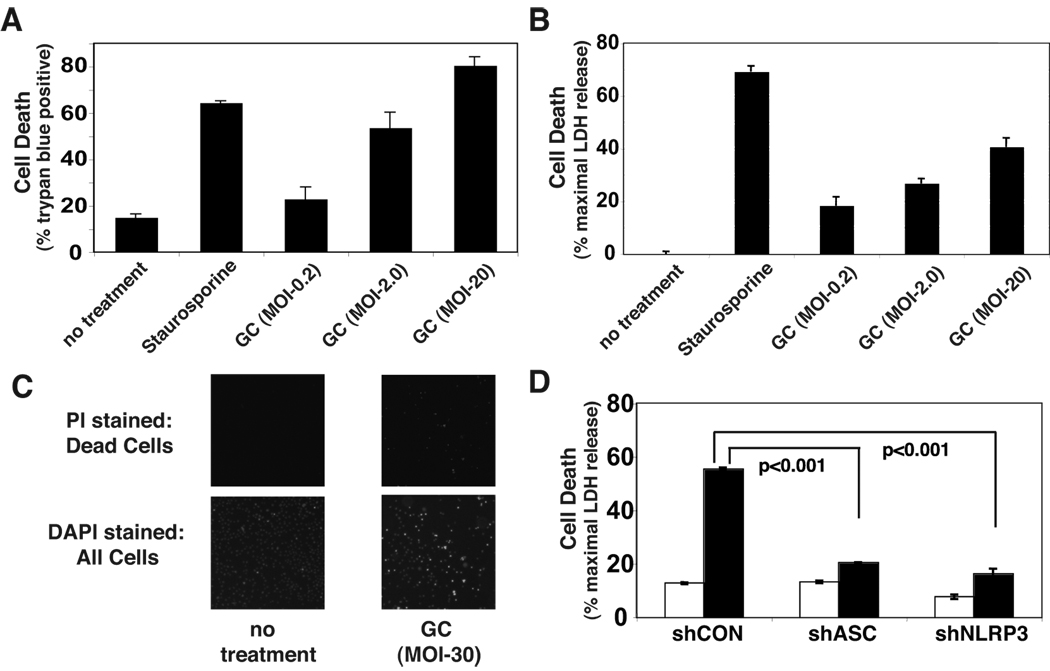
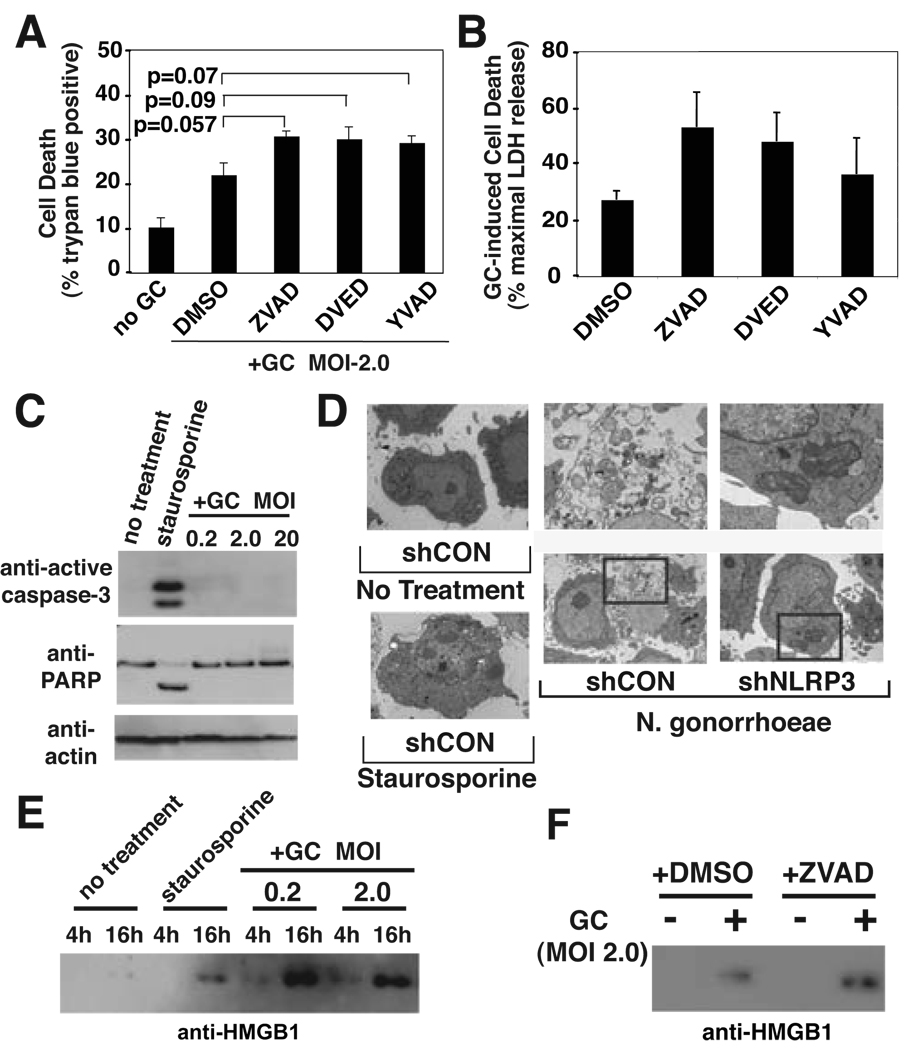
In addition to provoking cytokine secretion, N. gonorrhoeae has been found to induce apoptosis in B-lymphocytes (3). We found that N. gonorrhoeae also induced cell death in THP-1 cells as measured by release of cytoplasmic lactate dehydrogenase (LDH) into the culture media (Figure 3A) and loss of membrane impermeability to propridium iodide (Figure 3B) or Trypan Blue (Figure 3C). Similar to IL-1β secretion, gonococcal-induced cell death required expression of NLRP3 and ASC (Figure 3D). Activation of members of the cysteine protease family known as caspases is an apical event in the induction of apoptotic cell death. Under some circumstances, caspase-1 activity is thought to be capable of causing apoptotic cell death (37). We tested whether N. gonorrhoeae-induced cell death depends on the activity of caspase-1 or other apoptotic caspases by incubating the cells with cell permeable inhibitors of caspases prior to infection. The inhibitory action of these agents was confirmed by showing that lysates from treated cells inhibited activity of exogenously added caspase-1 (YVAD) or caspase-1 and caspase-3 (DEVD and ZVAD) while lysate from control cells had no inhibitory effects, consistent with the reported inhibitory activity of these agents at the concentrations used in the experiment (Supplemental Figure 2) (38). A general caspase inhibitor (Z-VAD) and specific inhibitors of either caspase-1 (YVAD) or caspase-3 (DEVD, which actually inhibited both caspase-1 and caspase-3 at the concentration utilized) failed to reduced N. gonorrhoeae-induced cell death (Figure 4A & B).. In fact, inhibitors of caspase activity showed a trend towards increased cell death, though none of these increases were statistically significant.
Figure 3. N. gonorrhoeae-induces NLRP3-dependent cell death in THP-1 cells.
A) THP-1 cells were infected with GC at the indicated MOI as described in Figure 2 or treated with staurosporine as a positive control for cell death. After 4 hours the cells were stained with trypan blue and the percentage of viable cells counted using the Nexcelom Cellometer Auto T4. B) Culture supernatants from infected cells were assayed for LDH released from injured or dead cells using a fluorometric assay. Levels of LDH above the background of LDH present in untreated cell culture supernatants are reported as a percent of the maximal LDH activity detected after detergent lysis. C) THP-1 cells were uninfected or infected with GC at an MOI of 0.2 for 20 hours. The cells with compromised membrane integrity were stained with the membrane impermeant dye, propidium iodide (left panels). All cells were subsequently stained with Hoechst 33342 to indicate total cell population (right panels). D) LDH released from cell lines expressing shRNA targeting the inflammasome components ASC or NLRP3 after a 4 hour exposure to GC at MOI of 2.0 was assayed. Results shown are representative of at least 3 independent experiments. Error bars are standard error of the mean for duplicate or triplicate measurements of cell death.
Figure 4. N. gonorrhoeae-induced cell death uses a necrotic mechanism.
A) and B) THP-1 cells were pretreated with vehicle (DMSO), 20 µM pan-caspase inhibitor (AC-VAD-CHO), 20 µM caspase-3 inhibitor (AC-DVED-CHO), or 20 µM caspase-1 inhibitor (AC-YVAD-CHO) for 30 min, then infected with GC at MOI of 2.0 for 4 hours. Cell death was assayed by Trypan Blue exclusion (A) or LDH release (B). Error bars represent standard deviation of triplicate measurements. Representative experiments of at least three independent infections are shown. C) THP-1 cells were treated for 4 hours with either staurosporine or GC at the indicated MOI. Lysates from these cells were analyzed by SDS-PAGE and immunoblot directed against active caspase-3, PARP, or actin. D) THP-1 derived cell-lines expressing either a control hairpin RNA (shCON) or a hairpin RNA directed towards NLRP3 (shNLRP3) were untreated, treated with staurosporine, or infected with GC at an MOI of 2.0 as indicated. The cells were processed and examined by transmission electron microscopy as described in the Materials and Methods. Normal cellular morphology is demonstrated in the untreated cells (upper left panel), apoptotic morphology is demonstrated by a staurosporine-treated cell (lower left panel), a representative intact dying control cell with associated bacteria is shown in the lower middle panel and a NLRP3 knockdown cell with a large burden of internalized N. gonorrhoeae and no morphologic features of cell death is shown in the lower right panel. The upper middle and upper right panels show bacteria associated with these cells. In panel E and F, N. gonorrhoeae-induced HMGB1 release by THP-1 cells was analyzed by SDS-PAGE of the cell free culture supernatants and immunoblot against HMGB1. In E, Cells were treated for the indicated time period with either staurosporine or the indicated MOI of GC. In F, cells were treated with vehicle (DMSO) or pan caspase inhibitor as described in A and B, followed by exposure to GC for 4 hours at an MOI of 2.
Our group has recently shown that activation of NLRP3 by mutation or exposure to the bacteria Shigella flexneri causes pyronecrosis, a program of cell death with necrotic features (28). Because gonococcus-induced cell death depends on NLRP3 and is independent of caspase activities, we sought to further evaluate the process of N. gonorrhoeae-induced cell death. During apoptosis both caspase-3 and Poly-ADP Ribose Polymerase (PARP) are cleaved proteolytically. We examined these proteins by immunoblot analysis after exposure to gonococci or the known apoptotic stimulus staurosporine. N. gonorrhoeae-induced cell death was not associated with proteolytic activation of caspase-3 or proteolytic cleavage of PARP (Figure 4C). Necrotic cell death and apoptosis can also be differentiated by ultrastructural changes observed with transmission electron microscopy. A control THP-1-derived cell line treated with gonococcus demonstrated necrotic morphology including extensive vacuolization, break down of the plasma membrane, and intact nuclei. Intracellular bacteria were often observed in association with these dying cells (Figure 4D). This morphology is clearly different from nuclear condensation and membrane blebbing observed in cells treated with staurosporine, a known inducer of apoptosis (Figure 4D). In marked contrast, THP-1 cells lacking NLRP3 expression did not exhibit significant cell death, even though they could be found containing abundant intracellular bacteria (Figure 4D). Necrotic cell death is associated with the early release of intracellular contents, which can further trigger proinflammatory signaling pathways. HMGB1 is a chromatin-associated protein that can activate proinflammatory signaling through the RAGE cell surface receptor following release from necrotic cells. We found that exposure of THP-1 cells to N. gonorrhoeae resulted in release of HMGB1 by 4 hours, while induction of apoptosis by staurosporine did not (Figure 4E). At later time points, HMGB1 is found in the supernatants of apoptotic cells but the quantity is much less than that seen with exposure to gonococcus. As expected, the HMGB1 release induced by N. gonorrhoe exposure is not blocked by addition of a pan-caspase inhibitor. In sum, these data indicate that N. gonorrhoeae-induced cell death occurs primarily through NLRP3-mediated pyronecrosis.
Activation of the cysteine-proteinase Cathepsin B is required for NLRP3-dependent signaling in response to gonococcus
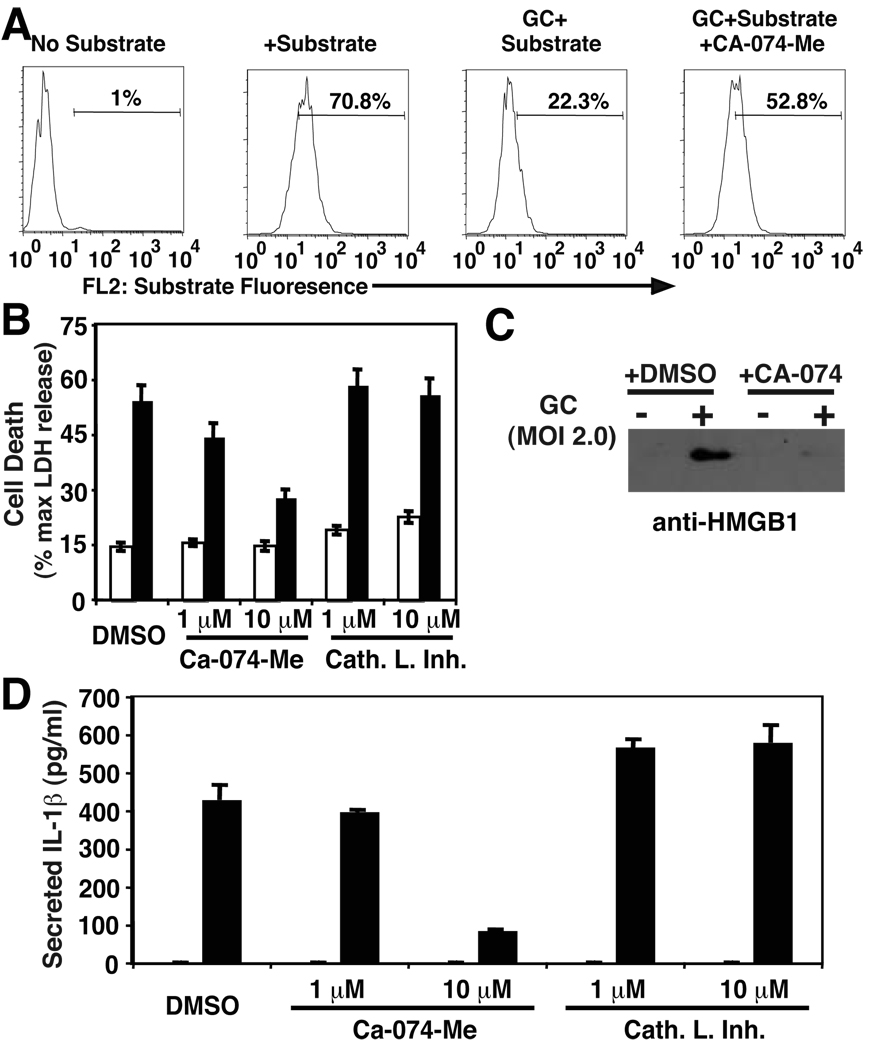
Both IL-1β secretion and cell death in response to the pore-forming microbial toxin, nigericin are blocked by inhibitors of the cysteine proteinase, Cathepsin B (39). Similarly, NLRP3-dependent pyronecrosis, which does not require caspase-1 activity, can also be abrogated by Cathepsin B inhibitors (28). The enzymatic activity of this protease has been shown to be activated by exposure to Salmonella typhimurium (40). Additionally, the break down of lysosomal integrity in response to inflammation-inducing crystals, including silica and aluminum, has recently been implicated in inflammasome activation in response to Danger Associated Molecular Patterns (23, 41). It is not known whether activation of this enzyme is a common feature of bacterial infections and Pathogen Associated Molecular Pattern-mediated activation of this pathway. We sought to determine whether Cathepsin B was playing a role in N. gonorrhoeae-induced inflammatory signaling. First, we found that treatment of THP-1 cells with N. gonorrhoeae induced the cleavage of a cell-permeable fluorescent Cathepsin B substrate, Magic Red™-RR, and reduction of intracellular fluorescence associated with exposure of cells to this substrate. The cleavage of this substrate could be partially reversed by addition of the specific cell-permeable Cathepsin B inhibitor, CA-074-me (Figure 5A). We suspect that Magic Red™-RR can also be cleaved by other non-specific intracellular proteases, which are not susceptible to inhibition by CA-074-me, thus explaining the partial reversal of substrate cleavage we have observed. Incubation of the cells with this inhibitor also blocked N. gonorrhoeae-induced cell death and release of IL-1β and HMGB1 (Figures 5B to D). As previously demonstrated for LPS-induced TNF α secretion, we observed that inhibition of Cathepsin B caused a mild reduction in gonococcus-induced TNFα secretion (supplemental Figure 3), suggesting Cathepsin B may play a role in multiple cytokine secretion pathways in response to bacterial pathogens (42). Thus, Cathepsin B activation appears to be an apical step in the downstream activities of NLRP3, ASC, and the inflammasome.
Figure 5. N. gonorrhoeae-mediated Cathepsin B activation is required to mediate NLRP3-dependent IL-1β secretion and cell death.
Cathepsin B activity was measured via degradation of a fluorescent substrate, Magic Red™ Cathepsin B substrate (Immunochemistry Technologie). Magic Red™ Cathepsin B substrate was added to THP-1 cells treated or not treated with GC. A cohort of GC-treated cells were also treated with 10 µM Ca-074-me, a specific inhibitor of Cathepsin B. Degradation of the Magic Red substrate was measured via Flow Cyometry on a BD FACSCalibur. Analysis of the data was accomplished with FloJo software with detection of the optimal Magic Red Substrate fluorescence emission in FL-2 on a logarithmic scale. A) Representative FACS plot of THP-1 cells with various treatment conditions. The percentage of Magic Red+ cells is indicated for each plot. A number in the third panel indicates increased Cathepsin B activity upon GC infection. B) THP-1 cells were treated with inhibitors of Cathepsin B, Cathepsin L, or DMSO vehicle at the indicated concentration for 15 min prior to infection with GC at MOI of 2.0. Cell death was assessed after 4 hours using release of LDH into the culture media as noted in the materials and methods.. C) THP-1 cells were pretreated with vehicle (DMSO) or 10 µM Cathepsin B inhibitor prior to infection with N. gonorrhoeae at MOI of 2.0. The cell culture supernatant from each condition was analyzed by SDS-PAGE and immunoblot with antibody directed to HMGB1. D) Cell culture supernatants from cells described in (B) were assayed for IL-1β using ELISA. In B and D, open bars indicate cells not treated with N. gonorrhoeae and closed bars indicate cells treated with N. gonorrhoeae. Error bars represent standard deviation. Experiments were performed in triplicate.
N. gonorrhoeae lipooligosaccharide activates NLRP3-mediated signaling
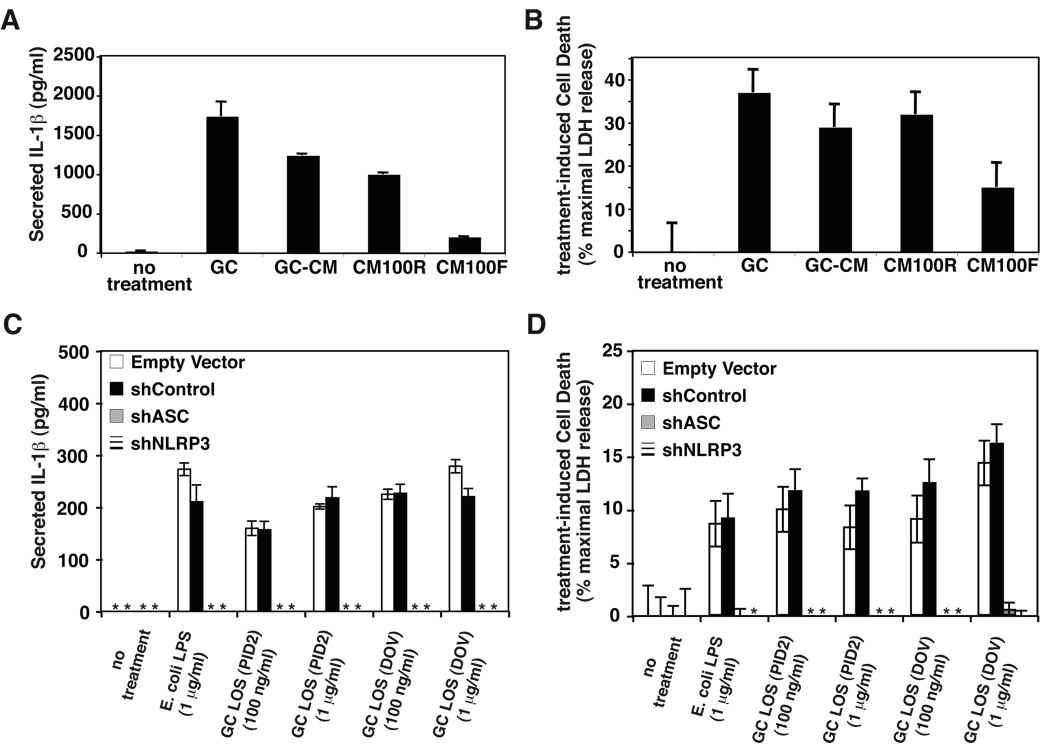
We sought to characterize the mechanism by which N. gonorrhoeae induced NLRP3 activation. We found that N. gonorrhoeae-conditioned media could activate IL-1β secretion and cell death in THP-1 cells (Figure 6A and 6B). N. gonorrhoeae and other pathogenic Neisseria species are known to produce membrane blebs containing large amounts of lipooligosaccharide (LOS) as well as gonococcal outer membrane proteins. Much of the IL-1β and cell death-stimulating activity in N. gonorrhoeae-conditioned media was retained by a 100kD ultrafiltration device, suggesting that the bulk of the activity was found in outer membrane blebs. However, a small portion of the activity also passed through the filter (Figure 6A and 6B). To assess whether N. gonorrhoeae LOS was one of the active components in N. gonorrhoeae-conditioned media, purified LOS from two different N. gonorrhoeae strains were used to stimulate THP-1 derived cell lines. These isolated LOS induced both IL-1β secretion and cell death in THP-1 cells (Figure 6C and 6D). As with stimulation with whole, live gonococci, these LOS-induced effects required intact expression of both NLRP3 and ASC (Figure 6C and 6D). E. coli lipopolysaccharide (LPS) can activate NLRP3-dependent IL-1β secretion in mouse macrophages (18–22). However, in mouse macrophages, E. coli LPS-induced IL-1β secretion is dramatically enhanced by the addition of extracellular ATP, which activates the P2×7 potassium channel and opens a large pore formed by the protein pannexin, presumably providing cytoplasmic access to the LPS (43). This additional stimulation is not absolutely required in human macrophages, monocytes, or the monocyte-derived THP-1 cell line. We found no difference in the ability of isolated E. coli LPS and gonococcal LOS to stimulate these NLRP-3 mediated activities (Figure 6C and 6D). This suggests that it is the physiologic shedding of LOS in outermembrane blebs by N. gonorrhoeae is one mechanism by which these bacteria activate NLRP3-mediated signaling.
Figure 6. N. gonorrhoeae LOS induces NLRP3-dependent IL-1β secretion and pyronecrosis in THP-1 cells.
A) and B) N. gonorrhoeae -conditioned media (GC-CM), N. gonorrhoeae-conditioned media 100kD retentate (CM100R) and filtrate (CM100F) were prepared as described in the materials and methods. THP-1 cells were incubated with live N. gonorrhoeae (GC) at an MOI of 1 or conditioned media preparations from and equivalent amount of bacteria for 4 hours and secreted IL-1β and cell death were assessed as previousl described. C) and D) THP-1-derived cell lines stably transduced with shRNA expressing retrovirus (described in Figure 2) were incubated with the indicated concentration of E. coli derived LPS or purified gonococcal LOS from strains PID2 or DOV (15, 35). After 4 hours, cell death was determined by measurement of LDH release into the media and secreted IL-1β was determined by ELISA. The shRNA’s are directed to knock down expression as follows: shCON - negative control (scrambled sequence with base content equal to shASC); shASC – shRNA directed against Apoptotic Speck Containing-protein; shNLRP3 – shRNA directed against NLRP3. LDH release and IL-1β secretion into the media that was not detectable is indicated by an asterisk (*). Representative experiments (of three) are shown, the bars indicate mean values of triplicate measurements with error bars representing the SEM.
DISCUSSION
Monocyte-derived cells, including macrophages and dendritic cells, play critical roles in the innate immune response to pathogens. Macrophages are sentinels which serve to provide chemotactic factors for polymorphonuclear (PMN) cells migrating to the site of an infection. Dendritic cells, and macrophages to a lesser extent, present pathogen derived antigens to lymphocytes in order to stimulate protective adaptive immune responses. NLRP3 and its associated inflammasome complex have been implicated in inflammatory responses by monocyte-derived cells to numerous pathogens. We have now shown that infection with N. gonorrhoeae can activate the NLRP3-mediated signaling. This activation is critical for the elaboration of IL-1β and IL-18 by the infected cells.
In murine macrophages, many extracellular IL-1β-inducing stimuli require activation of the purogenic P2×7 receptor and subsequent cytoplasmic potassium depletion in order to activate caspase-1 and elicit IL-1β secretion (28). Recently, lysosomal disruption has been implicated as another mechanism of NLRP3 activation that occurs during crystal-induced IL-1β secretion (41). S. flexneri requires the Shigella virulence plasmid to induce NLRP3-mediated caspase-1 activation and cell death in murine macrophage, implicating that an intact type III secretion system and/or bacterial invasion is required to activate this process. N gonorrhoeae may activate NLRP3 -mediated signaling through multiple mechanisms. Of note, the gonococcus lacks well characterized secreted protein exotoxins and a type III secretion system. N. gonorrhoeae does express a secreted proteinase, IgA-protease, that actually cleaves and inactivates the lysosome-associated membrane protein-1 (LAMP-1) in phagosomes. This cleavage results in blockade of the antimicrobial activity of these phagosomes and may promote bacterial survival and cytoplasmic penetration by the gonococcus (44, 45). Although it is possible that this cytoplasmic penetration by N. gonorrhoeae and the phagosomal disruption that must precede it can lead to NLRP3 activation, we have found that N. gonorrhoeae produces soluble factors that can activate this pathway. Gonococcal LOS appears to be one such factor. N. gonorrhoeae has a heterogeneous LOS (46). The LOS structures expressed by strains of N .gonorrhoeae vary as a result of phase-variable expression of a number of genes involved in core polysaccharide biosynthesis(47–49). The expression of several larger molecular weight LOS appears to be critical in the infectivity of this bacterium in experimental human infection models (50). Recently the potency of LOS derived from different gonococcal strains in activating IL-1β secretion by THP-1 cells has been correlated to the proportion of LOS-derived lipid A that is decorated by phosphotidyl ethanolamine (51). Studies into the virulence of and ability to activate NLRP3-signaling by gonococcal strains with defined variation in LOS stuctures, both oligosaccharide and lipid A, may shed light on the role of this host inflammatory signaling pathway in the pathogenesis of gonococcal infection.
Pathogen induced cell death is an increasingly recognized phenomena. Gonococcal surface proteins known as opacity proteins have been reported to induce apoptosis in B-cells through activation of the B-cell surface receptor CEACAM-1 (3). N. gonorrhoeae has also been shown to induce apoptosis in epithelial cell lines through translocation of the N. gonorrhoeae membrane protein PorB to the mitochondrial membrane (52, 53). However, exposure to N. gonorrhoeae and gonococcal PorB in epithelial and PMN cells actually induces expression of antiapoptotic genes (54–57). It is unclear what the role of these opposing activities on apoptotic cell death play in gonococcal infection. It has been suggested that they may preserve the host cell as a nutrient rich environment until these cells have been depleted of nucleotide energy sources (56). Prior to this study, N. gonorrhoeae-induced cell death in immune cells was not thoroughly characterized with regard to the hallmarks of apoptosis, such as dependence on apoptotic caspase activity. While epithelial cell lines exert very little antimicrobial activity towards the gonococcus, cells of the immune system may pose a significant threat to the replicating bacteria. Thus, it is not surprising that multiple host cell death pathways may be differentially affected by the gonococcus, particularly depending on the host cell type in question. We have now demonstrated that N. gonorrhoeae-induced cell death in monocytic cells lacks apoptotic hallmarks. Instead, the process appears morphologically consistent with necrosis. Furthermore, N. gonorrhoeae-induced cell death depends on the NLRP3/ASC signaling network in these cells.
Gonococcal infection is associated with localized inflammation with extensive PMN recruitment to the site of infection. Unlike apoptosis, necrosis causes the release of many proinflammatory intracellular substances, which lead to PMN infiltration of the necrotic areas. Additionally, IL-1β is known to promote infiltration of leukocytes through upregulation of adhesion molecule and chemotactic factor expression. Measurable levels of IL-1β have been found in the urine of human subjects with experimental gonococcal infection at the time of they develop active infection. Given the findings in this report, N. gonorrhoeae-induced NLRP3 activation and the resulting IL-1β production and pyronecrosis in resident tissue macrophages is likely to be a major contributor to the host inflammatory response during gonococcal urethritis. The full compliment of gonococcus-derived factors that can activate NLRP3 remains to be determined.
Other N. gonorrhoeae-associated molecular patterns such as N. gonorrhoeae-derived nucleic acids, peptidoglycan, and lipoproteins may also be able to directly or indirectly activate NLRP3 and contribute to gonococcus-induced NLRP3 activation. Our studies have focused on a population of N. gonorrhoeae, which is predominately piliated and express Opa proteins with opaque phenotypes. In future studies we will attempt to determine whether adherence or cytoplasmic penetration mediated by these adherence factors enhance N. gonorrhoeae’s ability to activate this inflammatory signaling pathway.
N. gonorrhoeae-induced inflammation is associated with many of the major complications of clinical diseases caused by this organism, including pelvic inflammatory disease associated infertility. Inflammation is generally considered an important component of the host immune response to pathogens. The gonococcus is an exclusive and highly adapted human pathogen, which has not evolved significant anti-inflammatory mechanisms despite many other immunosuppressive properties. This suggests the host inflammatory response to N. gonorrhoeae provides the organism with some survival or transmission advantage and plays a role in the virulence of the pathogen. The data presented in this work demonstrate that N. gonorrhoeae is innately detected by the NLRP3-inflammasome signaling platform to elicit inflammatory signals, including IL-1β secretion and pyronecrosis. The NLRP3 signaling pathway is present in many phagocytic cells including macrophages and other antigen presenting cells. Activation of this pathway could potentially lead to a robust inflammatory response and at the same time cause death of the antigen presenting cells, thereby explaining the paradoxical host immune responses seen in natural gonococcal infection.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Deborah Taxman for providing shRNA-expressing THP-1-cell lines and Victoria Madden of the UNC Microscopy Services laboratory for her assistance with transmission electron microscopy.
Footnotes
This work was supported by the UNC Multidisciplinary Clinical Research Career Development Award (NIH RR023248) and the Developmental Awards Program of the National Institutes of Health NIAID Sexually Transmitted Infections and Topical Microbicide Cooperative Research Centers (STI-TM CRC) grants to the University of Washington (AI 31448) and the University of North Carolina (AI031496) to J.A.D.; Additional support for this project was provided by the NIH: AI63031 to J.P-Y.T.; AI031496 P.F.S. and C.E.T.; AI063927 to G.A.J.; and the Lineberger Postdoctoral Training Programs supported D.T.B. B.P.O’C. is supported by the Irvington Institute Fellowship.
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org
REFERENCES
- 1.Tapsall JW. Antibiotic resistance in Neisseria gonorrhoeae. Clin Infect Dis. 2005;41 Suppl 4:S263–S268. doi: 10.1086/430787. [DOI] [PubMed] [Google Scholar]
- 2.Fox KK, Thomas JC, Weiner DH, Davis RH, Sparling PF, Cohen MS. Longitudinal evaluation of serovar-specific immunity to Neisseria gonorrhoeae. Am J Epidemiol. 1999;149:353–358. doi: 10.1093/oxfordjournals.aje.a009820. [DOI] [PubMed] [Google Scholar]
- 3.Pantelic M, Kim YJ, Bolland S, Chen I, Shively J, Chen T. Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect Immun. 2005;73:4171–4179. doi: 10.1128/IAI.73.7.4171-4179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey KH, Schneider H, Kuschner RA, Trofa AF, Cross AS, Deal CD. Inflammatory cytokine response to experimental human infection with Neisseria gonorrhoeae. Ann N Y Acad Sci. 1994;730:322–325. doi: 10.1111/j.1749-6632.1994.tb44280.x. [DOI] [PubMed] [Google Scholar]
- 6.Ramsey KH, Schneider H, Cross AS, Boslego JW, Hoover DL, Staley TL, Kuschner RA, Deal CD. Inflammatory cytokines produced in response to experimental human gonorrhea. J Infect Dis. 1995;172:186–191. doi: 10.1093/infdis/172.1.186. [DOI] [PubMed] [Google Scholar]
- 7.Hedges SR, Mayo MS, Mestecky J, Hook EW, 3rd, Russell MW. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect Immun. 1999;67:3937–3946. doi: 10.1128/iai.67.8.3937-3946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fichorova RN, Desai PJ, Gibson FC, 3rd, Genco CA. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun. 2001;69:5840–5848. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey HA, Post DM, Apicella MA. Immortalization of human urethral epithelial cells: a model for the study of the pathogenesis of and the inflammatory cytokine response to Neisseria gonorrhoeae infection. Infect Immun. 2002;70:5808–5815. doi: 10.1128/IAI.70.10.5808-5815.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rarick M, McPheeters C, Bright S, Navis A, Skefos J, Sebastiani P, Montano M. Evidence for cross-regulated cytokine response in human peripheral blood mononuclear cells exposed to whole gonococcal bacteria in vitro. Microb Pathog. 2006;40:261–270. doi: 10.1016/j.micpath.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 13.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 14.Zhang P, Schwartz O, Pantelic M, Li G, Knazze Q, Nobile C, Radovich M, He J, Hong SC, Klena J, Chen T. DC-SIGN (CD209) recognition of Neisseria gonorrhoeae is circumvented by lipooligosaccharide variation. J Leukoc Biol. 2006;79:731–738. doi: 10.1189/jlb.0405184. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Mosoian A, Li-Yun Chang T, Zerhouni-Layachi B, Snyder A, Jarvis GA, Klotman ME. Gonococcal lipooligosaccharide suppresses HIV infection in human primary macrophages through induction of innate immunity. J Infect Dis. 2006:751–759. doi: 10.1086/506360. [DOI] [PubMed] [Google Scholar]
- 16.Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 17.Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J Biol Chem. 2003;278:46252–46260. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- 18.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 19.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 20.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. Critical Role for Cryopyrin/Nalp3 in Activation of Caspase-1 in Response to Viral Infection and Double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 21.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 22.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- 26.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 27.Fujisawa A, Kambe N, Saito M, Nishikomori R, Tanizaki H, Kanazawa N, Adachi S, Heike T, Sagara J, Suda T, Nakahata T, Miyachi Y. Disease-associated mutations in CIAS1 induce cathepsin B-dependent rapid cell death of human THP-1 monocytic cells. Blood. 2006 doi: 10.1182/blood-2006-07-033597. [DOI] [PubMed] [Google Scholar]
- 28.Willingham SB, Bergstralh DT, O'Connor W, Morrison AC, Taxman DJ, Duncan JA, Barnoy S, Venkatesan MM, Flavell RA, Deshmukh M, Hoffman HM, Ting JP. Microbial Pathogen-Induced Necrotic Cell Death Mediated by the Inflammasome Components CIAS1/Cryopyrin/NLRP3 and ASC. Cell Host and Microbe. 2007;2:147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, Kurtz S, Coffield VM, Accavitti-Loper MA, Su L, Vogel SN, Braunstein M, Ting JP. The CATERPILLER protein Monarch-1 is an antagonist of TLR, TNFalpha , and M. tuberculosis-induced pro-inflammatory signals. J Biol Chem. 2005;3:3. doi: 10.1074/jbc.M502820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nachamkin I, Cannon JG, Mittler RS. Monoclonal antibodies against Neisseria gonorrhoeae: production of antibodies directed against a strain-specific cell surface antigen. Infect Immun. 1981;32:641–648. doi: 10.1128/iai.32.2.641-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knapp JS, Totten PA, Mulks MH, Minshew BH. Characterization of Neisseria cinerea, a nonpathogenic species isolated on Martin-Lewis medium selective for pathogenic Neisseria spp. J Clin Microbiol. 1984;19:63–67. doi: 10.1128/jcm.19.1.63-67.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerse AE, Cohen MS, Drown PM, Whicker LG, Isbey SF, Seifert HS, Cannon JG. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994;179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taxman DJ, Zhang J, Champagne C, Bergstralh DT, Iocca HA, Lich JD, Ting JP. Cutting edge: ASC mediates the induction of multiple cytokines by Porphyromonas gingivalis via caspase-1-dependent and -independent pathways. J Immunol. 2006;177:4252–4256. doi: 10.4049/jimmunol.177.7.4252. [DOI] [PubMed] [Google Scholar]
- 34.Haskill S, Johnson C, Eierman D, Becker S, Warren K. Adherence induces selective mRNA expression of monocyte mediators and proto-oncogenes. J Immunol. 1988;140:1690–1694. [PubMed] [Google Scholar]
- 35.Pridmore AC, Jarvis GA, John CM, Jack DL, Dower SK, Read RC. Activation of toll-like receptor 2 (TLR2) and TLR4/MD2 by Neisseria is independent of capsule and lipooligosaccharide (LOS) sialylation but varies widely among LOS from different strains. Infect Immun. 2003;71:3901–3908. doi: 10.1128/IAI.71.7.3901-3908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 37.Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 38.Margolin N, Raybuck SA, Wilson KP, Chen W, Fox T, Gu Y, Livingston DJ. Substrate and inhibitor specificity of interleukin-1 beta-converting enzyme and related caspases. J Biol Chem. 1997;272:7223–7228. doi: 10.1074/jbc.272.11.7223. [DOI] [PubMed] [Google Scholar]
- 39.Hentze H, Lin XY, Choi MS, Porter AG. Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ. 2003;10:956–968. doi: 10.1038/sj.cdd.4401264. [DOI] [PubMed] [Google Scholar]
- 40.Hang HC, Loureiro J, Spooner E, van der Velden AW, Kim YM, Pollington AM, Maehr R, Starnbach MN, Ploegh HL. Mechanism-based probe for the analysis of cathepsin cysteine proteases in living cells. ACS Chem Biol. 2006;1:713-–723. doi: 10.1021/cb600431a. [DOI] [PubMed] [Google Scholar]
- 41.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ha SD, Martins A, Khazaie K, Han J, Chan BM, Kim SO. Cathepsin B is involved in the trafficking of TNF-alpha-containing vesicles to the plasma membrane in macrophages. J Immunol. 2008;181:690–697. doi: 10.4049/jimmunol.181.1.690. [DOI] [PubMed] [Google Scholar]
- 43.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Lin L, Ayala P, Larson J, Mulks M, Fukuda M, Carlsson SR, Enns C, So M. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol Microbiol. 1997;24:1083–1094. doi: 10.1046/j.1365-2958.1997.4191776.x. [DOI] [PubMed] [Google Scholar]
- 45.Hopper S, Vasquez B, Merz A, Clary S, Wilbur JS, So M. Effects of the immunoglobulin A1 protease on Neisseria gonorrhoeae trafficking across polarized T84 epithelial monolayers. Infect Immun. 2000;68:906–911. doi: 10.1128/iai.68.2.906-911.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Apicella MA, Shero M, Jarvis GA, Griffiss JM, Mandrell RE, Schneider H. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect Immun. 1987;55:1755–1761. doi: 10.1128/iai.55.8.1755-1761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang QL, Gotschlich EC. Variation of gonococcal lipooligosaccharide structure is due to alterations in poly-G tracts in lgt genes encoding glycosyl transferases. J Exp Med. 1996;183:323–327. doi: 10.1084/jem.183.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shafer WM, Datta A, Kolli VS, Rahman MM, Balthazar JT, Martin LE, Veal WL, Stephens DS, Carlson R. Phase variable changes in genes lgtA and lgtC within the lgtABCDE operon of Neisseria gonorrhoeae can modulate gonococcal susceptibility to normal human serum. J Endotoxin Res. 2002;8:47–58. [PubMed] [Google Scholar]
- 49.Gotschlich EC. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J Exp Med. 1994;180:2181–2190. doi: 10.1084/jem.180.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider H, Cross AS, Kuschner RA, Taylor DN, Sadoff JC, Boslego JW, Deal CD. Experimental human gonococcal urethritis: 250 Neisseria gonorrhoeae MS11mkC are infective. J Infect Dis. 1995;172:180–185. doi: 10.1093/infdis/172.1.180. [DOI] [PubMed] [Google Scholar]
- 51.John CM, Liu M, Jarvis GA. Profiles of structural heterogeneity in native lipooligosaccharides of neisseria and cytokine induction. J Lipid Res. 2008 doi: 10.1194/jlr.M800184-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muller A, Gunther D, Brinkmann V, Hurwitz R, Meyer TF, Rudel T. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. Embo J. 2000;19:5332–5343. doi: 10.1093/emboj/19.20.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muller A, Gunther D, Dux F, Naumann M, Meyer TF, Rudel T. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. Embo J. 1999;18:339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simons MP, Nauseef WM, Griffith TS, Apicella MA. Neisseria gonorrhoeae delays the onset of apoptosis in polymorphonuclear leukocytes. Cell Microbiol. 2006;8:1780–1790. doi: 10.1111/j.1462-5822.2006.00748.x. [DOI] [PubMed] [Google Scholar]
- 55.Morales P, Reyes P, Vargas M, Rios M, Imarai M, Cardenas H, Croxatto H, Orihuela P, Vargas R, Fuhrer J, Heckels JE, Christodoulides M, Velasquez L. Infection of human fallopian tube epithelial cells with Neisseria gonorrhoeae protects cells from tumor necrosis factor alpha-induced apoptosis. Infect Immun. 2006;74:3643–3650. doi: 10.1128/IAI.00012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Binnicker MJ, Williams RD, Apicella MA. Gonococcal porin IB activates NF-kappaB in human urethral epithelium and increases the expression of host antiapoptotic factors. Infect Immun. 2004;72:6408–6417. doi: 10.1128/IAI.72.11.6408-6417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Binnicker MJ, Williams RD, Apicella MA. Infection of human urethral epithelium with Neisseria gonorrhoeae elicits an upregulation of host anti-apoptotic factors and protects cells from staurosporine-induced apoptosis. Cell Microbiol. 2003;5:549–560. doi: 10.1046/j.1462-5822.2003.00300.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.