Abstract
Objective and rationale
Alcohol and nicotine are commonly co-abused; one possible explanation for co-abuse is that each drug ameliorates the aversive effects of the other. Both drugs have dose-dependent effects on learning and memory. Thus, this study examined the interactive effects of acute ethanol and acute, chronic, or withdrawal from chronic nicotine on fear conditioning in C57BL/6J mice.
Materials and methods
Conditioning consisted of auditory conditioned stimulus-foot-shock unconditioned stimulus pairings. For acute studies, saline or ethanol, then saline or nicotine was administered before training, and saline or nicotine was also administered before testing. For chronic and withdrawal studies, saline or nicotine was administered chronically, and ethanol or saline was administered before training.
Results
Acute nicotine (0.09 mg/kg) reversed ethanol-induced deficits (1.0 and 1.5 g/kg) in contextual and cued fear conditioning, whereas a low dose of ethanol (0.25 g/kg) reversed nicotine (6.3 mg kg−1 day−1) withdrawal-induced deficits in contextual conditioning. Tolerance developed for the effects of nicotine on ethanol-induced deficits in conditioning and cross-tolerance between chronic nicotine and acute ethanol was seen for the enhancing effects of ethanol on conditioning.
Conclusions
The complex and sometimes polar actions of ethanol and nicotine on behavior may contribute to co-abuse of these drugs. Specifically, smoking may initially reduce the aversive effects of ethanol, but tolerance develops for this effect. In addition, low doses of alcohol may lessen nicotine withdrawal symptoms.
Keywords: Addiction, Learning, Withdrawal, Tolerance, Acetylcholine
Introduction
Ethanol and nicotine are the two most commonly co-abused drugs, and many studies report a positive correlation between high-risk heavy use of each (Dawson 2000; John et al. 2003; Larsson and Engel 2004). For example, smoking rates have decreased in the general population, yet alcoholics are less successful at quitting smoking than the general population (Dawson 2000). In addition, smokers are at higher risk for alcohol dependence than non-smokers (John et al. 2003), and decreased smoking is associated with better outcomes in alcohol abstinence (Friend and Pagiano 2005). Although studies have shown that nicotine interacts with other drugs such as cocaine in modulating reward pathways (Kelley and Rowan 2004) and that cocaine can reverse locomotor deficits in nicotine-withdrawn mice (Vihavainen et al. 2006), quitting cocaine use is not associated with increased smoking (Patkar et al. 2006). Furthermore, the nicotine patch does not produce tolerance to the positive effects of cocaine or caffeine (Sobel et al. 2004). This suggests that there is some specificity in the molecular and behavioral interactions of ethanol and nicotine.
Whereas the legality of both nicotine and ethanol is undoubtedly a factor in the co-abuse, research suggests that other factors may also be involved. Interactions between the drugs may contribute to co-abuse. For example, co-use may enhance reward; co-administration of ethanol and nicotine produces additive effects on dopamine release in the nucleus accumbens, a key component in the reward pathway (Tizabi et al. 2007). In addition, the amelioration of the aversive properties of one drug by the other may also contribute to co-abuse. Furthermore, the development of cross-tolerance between nicotine and ethanol (Collins et al. 1988, 1993, 1996) could lead to increased drug use.
Changes in learning and synaptic plasticity have been strongly implicated in addiction (Hyman et al. 2006; Kalivas and O’Brien 2007; Kelley 2004), but the effects of coadministration of ethanol and nicotine on learning are only beginning to be elucidated. Many studies have demonstrated that impairments in learning and memory are associated with high doses of ethanol; this is seen for tasks that include passive avoidance, delay matching-toposition, free recall, radial maze, and both delay and trace fear conditioning (Bammer and Chesher 1982; Gibson 1985; Gould 2003a; Higgins et al. 1992; Weitemier and Ryabinin 2003). Research has also demonstrated that acute nicotine enhances learning in many of the same tasks (Davis et al. 2005b; Gould and Lommock 2003; Hahn et al. 2002; Rezvani and Levin 2003). Although few studies have examined the interactive effects of ethanol and nicotine on cognitive tasks, research suggests that nicotine is able to reverse ethanol-induced impairments in fear conditioning, cued and free recall, and conditioned taste aversion (Gould and Lommock 2003; Kunin et al. 1999; Meyerhoff et al. 2006), whereas ethanol blocks the enhancing effects of nicotine on other measures (Rezvani and Levin 2003). If nicotine can reverse ethanol-induced cognitive deficits, this may be one factor in the co-abuse of these drugs.
Chronic drug effects may also contribute to the co-abuse of ethanol and nicotine. In support, smokers report feeling less intoxicated compared to non-smokers after administration of an identical dose of alcohol (Madden et al. 1995), suggesting that chronic administration of nicotine may produce tolerance to some of the effects of ethanol. In fact, in mice, the pharmacodynamic effects of ethanol and nicotine result in asymmetrical cross-tolerance between the drugs. Chronic nicotine treatment decreases sensitivity to some of the impairing effects of acute ethanol, but chronic ethanol treatment does not alter the effects of acute nicotine (Collins et al. 1988, 1993, 1996; Lopez et al. 2001). Although it has been demonstrated that chronic nicotine treatment results in tolerance to the effects of nicotine on fear conditioning (Davis et al. 2005a), no studies have yet examined whether chronic nicotine treatment produces cross-tolerance to the effects of ethanol on fear conditioning.
Just as ethanol may interact with the chronic effects of nicotine, ethanol may alter nicotine withdrawal symptoms. Whereas acute nicotine enhances contextual fear conditioning, withdrawal from nicotine disrupts contextual conditioning (Davis et al. 2005a). Thus, fear conditioning is impaired by both high doses of ethanol and withdrawal from chronic nicotine, yet it is unclear whether there are additive effects of ethanol treatment and nicotine withdrawal. If such additive effects exist, they may contribute to the difficulty alcoholics experience in abstaining from nicotine. Moreover, it has been demonstrated that low doses of ethanol enhance learning (Gulick and Gould 2007; Hernandez and Powell 1986; Hernandez and Valentine 1990); thus, a dose of ethanol that enhances learning could reverse nicotine withdrawal deficits.
The present study used contextual and cued fear conditioning to examine the interactive effects of ethanol and nicotine on both types of learning. A goal of the current study was to examine how acute, chronic, and withdrawal from chronic nicotine interact with a range of acute doses of ethanol that includes both impairing and enhancing doses. Finally, we also examined whether nicotine administration altered blood alcohol concentration to determine whether the interactive effects of ethanol and nicotine are due to changes in the metabolism of ethanol.
Materials and methods
Subjects
Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) housed in groups of four mice per cage with ad libitum access to food and water were tested at 8–12 weeks of age (20–30 g; 8–14 mice per condition). A 12-h light–dark cycle (lights on at 7:00 a.m.) was maintained, with all testing done between 9:00 a.m. and 5:00 p.m. Procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus
Training and context testing took place in identical conditioning chambers housed in sound-attenuating boxes (MED Associates, St. Albans, VT, USA) as described in Gulick and Gould (2007). A computer running MED Associates software controlled presentation of stimuli. Chambers were cleaned with 70% ethanol before each session. Testing for freezing to the conditioned stimulus (CS) occurred in a separate room in altered context chambers that were housed in sound-attenuating boxes. In addition to the difference in location of the chambers, visual cues, chamber dimensions, floor construction, and a vanilla extract olfactory cue (no olfactory cue was present in the training chambers) further distinguished the altered context chambers from the original training chambers.
Procedure
Two CS–US pairings
On training day, mice were placed in conditioning chambers. After a 120-s baseline period, they received two co-terminating CS (30-s, 85-dB white noise)-unconditioned stimulus (US; 2-s, 0.57-mA foot shock) pairings. At 120 and 270 s, the CS sounded for 30 s; the US occurred during the last 2 s of the CS. The mice remained in the chamber for 30 s after the second CS–US presentation for a total of 5.5 min. Baseline freezing behavior was recorded during the first 120 s, and immediate freezing was recorded between the first and second CS presentations (methods based on Gould and Higgins 2003). Mice were tested 24 h after training. Freezing to the context was assessed and then freezing to the CS was assessed 1 h later. To evaluate freezing to the context, mice were placed in the training chamber for 5 min, and freezing was recorded. To evaluate freezing to the CS, mice were placed in the altered context. During the first 3 min of the altered context test, the CS was not presented (pre-CS test). During the last 3 min, the CS was continuously presented (CS test). Freezing was assessed during the entire 6-min period.
We tested whether acute nicotine shifts the dose-response curve for the acute effects of ethanol on fear conditioning. Following protocols from prior research (Gould 2003a), saline or ethanol (0.5, 1.0, 1.5 g/kg) was administered 15 min before training, via intraperitoneal (i.p.) injection, with saline or 0.09 mg/kg nicotine administered i.p. 5 min before training and testing both for contextual and for cued conditioning. Due to the partial reversal of ethanol-induced deficits in fear conditioning by 0.09 mg/kg nicotine, a second experiment examined the effects of a lower and a higher dose of nicotine (0.045 or 0.18 mg/kg) on the disruption of fear conditioning by ethanol. To test whether chronic nicotine alters the learning deficits associated with ethanol treatment, mice were chronically treated with saline or 6.3 mg kg−1 day−1 nicotine for 14 days. This dose of nicotine was chosen based on previous research indicating that 6.3 mg kg−1 day−1 nicotine produces plasma nicotine levels that are similar to those produced by acute administration of 0.09 mg/kg of nicotine (Davis et al. 2005a). Training occurred on day 13; either saline or ethanol (0.5, 1.0, 1.5 g/kg) was administered i.p. 15 min before the training session, and testing occurred on day 14.
We also examined whether the deficits in contextual fear conditioning produced by acute ethanol and withdrawal from chronic nicotine were additive. Mice were chronically treated with saline or 6.3 mg kg−1 day−1 nicotine for 12 days, after which, the osmotic mini-pumps were removed. Training occurred 24 h later on day 13, and either saline or ethanol (0.5, 1.0, 1.5 g/kg) was administered i.p. 15 min before the session. Testing occurred on day 14.
One CS–US pairing
To test if the interactive effects of nicotine and ethanol on cued fear conditioning were dependent on the level of conditioning and to rule out potential ceiling effects, we conditioned mice with a single CS–US pairing and a shorter duration CS. On training day, mice were placed in conditioning chambers in which they received a single coterminating CS (15-s, 85-dB white noise)–US (2-s, 0.57-mA foot shock) pairing 135 s after the baseline period. The mice remained in the chamber for 30 s after the end of the CS–US presentation for a total of 3 min (based on Gould et al. 2004). Mice were tested 24 h after training as described for the two CS–US pairing protocol. This reduced conditioning paradigm was used to examine the interactive effects of an acute dose of ethanol that enhanced learning (0.25 g/kg i.p. 15 min before training) with acute (0.09 mg/kg i.p. 5 min before training and each testing session), chronic (6.3 mg kg−1 day−1 for 14 days), and withdrawal from chronic nicotine on fear conditioning.
Cued test first
To test whether there is an order effect on testing day, we examined whether the effects of ethanol and nicotine would differ if testing for cued conditioning occurred before testing for contextual conditioning. Following the two CS–US pairing protocol discussed previously, with the exception that cued testing occurred 1 h before context testing, saline or ethanol (0.5, 1.0, 1.5 g/kg) was administered i.p. 15 min before training and saline or 0.09 mg/kg nicotine administered i.p. 5 min before training and both testing sessions.
Scoring
Freezing behavior was observed using a time-sampling procedure. At 10-s intervals, mice were assessed for 1 s for freezing as defined as the absence of visible movement with the exception of respiration (based on Gould and Wehner 1999).
Blood alcohol concentration
The effects of nicotine on blood alcohol concentrations (BACs) were assessed. Mice (four to five per condition) received injections of ethanol (0.25, 0.5, 1.0, 1.5) 15 min before blood collection and injections of saline or nicotine (0.09 mg/kg) 5 min before blood collection. These time points were chosen to approximate the blood alcohol levels during training. Blood was collected in a capillary tube (Analox Instruments, Lunenberg, MA, USA) and spun down in a centrifuge for 2 min at 14,000 rpm. The plasma was then analyzed by an AM1 Analyzer (Analox Instruments) to obtain BAC (mg/dl). Each sample was analyzed twice, and a mean from the two readings was used for data analysis (Gould and Lommock 2003).
Drugs
Acute nicotine (nicotine hydrogen tartrate salt dissolved in physiological saline, 0.045, 0.09, or 0.18 mg/kg i.p., doses reported as freebase weight; Sigma, St. Louis, MO, USA) was administered 5 min before training and before both testing sessions. Because nicotine is present at both training and testing for the chronic treatment condition, acute nicotine was also administered before training and before each testing session. Chronic nicotine (6.3 mg kg−1 day−1 dissolved in physiological saline) was administered via osmotic mini-pump (model 1002; Alzet, Cupertino, CA, USA), for 12–14 days. Ethanol (0.25, 0.5, 1.0, 1.5 g/kg dissolved in saline, Fisher Scientific, Pittsburgh, PA, USA) was given i.p. 15 min before each training session. Injection volume for nicotine was 0.01 ml/g body weight and ethanol was 20% v/v in saline. Controls received physiological saline. Dose, injection route, and administration time were based on prior research (Gould 2003a; Gould and Lommock 2003; Gould and Wehner 1999).
Surgeries
Mini-osmotic pumps that administered 0.25 µl/h of solution were filled with 100 µl of nicotine or saline solution and inserted subcutaneously via an incision in the middle back of the mouse. For withdrawal experiments, pumps were removed 12 days after the initial surgery. Surgeries were performed under sterile conditions with 5% isoflurane used as an anesthetic.
Statistical testing
Data were analyzed using a two-way analysis of variance (ANOVA) for most behavioral studies. Tukey’s post hoc analysis was used to identify significant individual differences at the p<0.05 level. Blood alcohol concentration data were analyzed with a one-way ANOVA, as were the cued test first data. Statistics were calculated with SPSS (Version 13; SPSS, Chicago, IL, USA).
Results
Coadministration of acute ethanol with acute nicotine
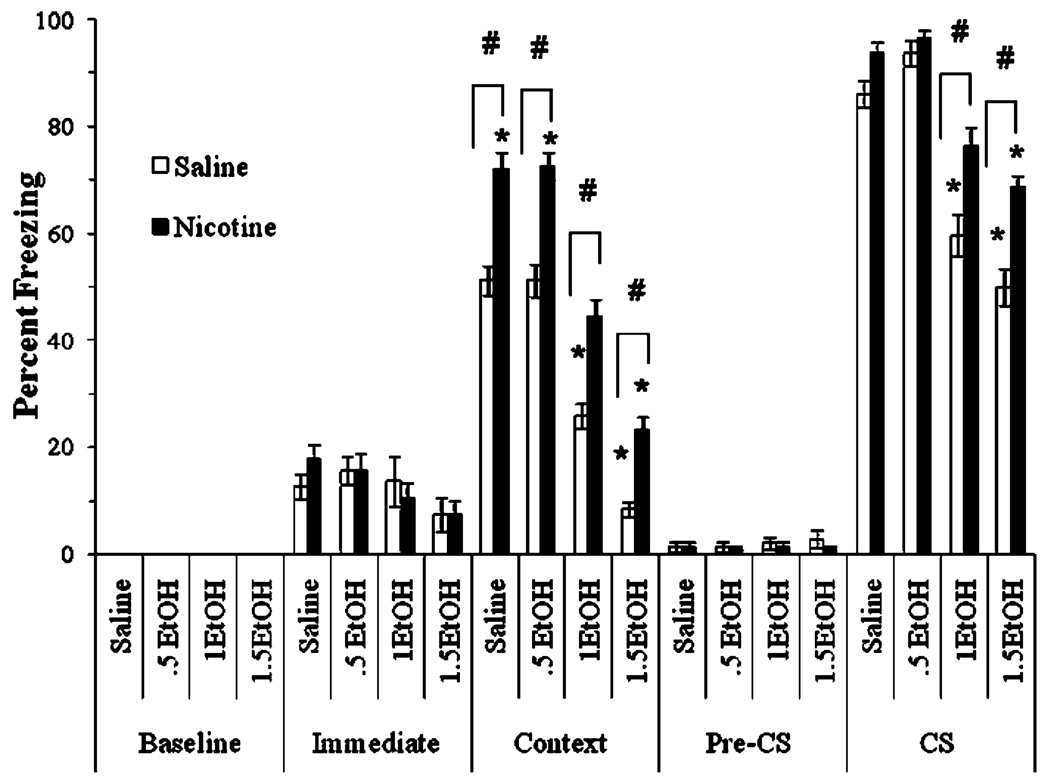
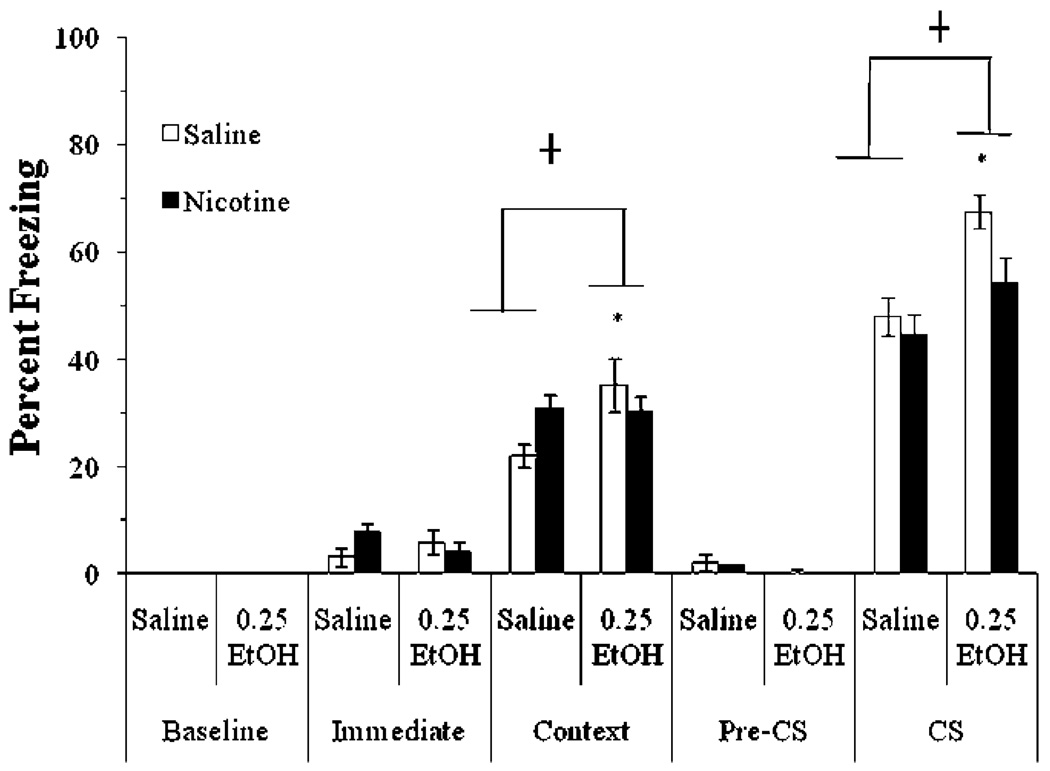
We examined whether acute nicotine (0.09 mg/kg) shifts the dose-response curve for ethanol (0.5, 1.0, or 1.5 g/kg)-induced disruption of contextual and cued fear conditioning. The levels of baseline freezing, measured before the first CS presentation, and of immediate freezing, measured between the first and second CS presentation, were similar across all groups. Significant main effects of ethanol, F (3,60)=167.85, p<0.001, and of nicotine, F(1,62)=120.51, p<0.001, on freezing to the context on testing day were found. There was no significant interaction of ethanol and nicotine on freezing to the context. There was a significant main effect of ethanol on freezing to the CS on testing day, F(7,56)=45.37, p<0.001, and a significant interaction of ethanol and nicotine on freezing to the CS on testing day, F (3, 60)=4.29, p<0.01. There was no significant difference in freezing during the pre-CS period (Fig. 1).
Fig. 1.
Acute nicotine reversed ethanol-induced impairments in contextual and cued conditioning (mean±SEM; asterisk indicates significant difference from controls, p<0.05; number sign indicates significant difference between nicotine and saline treatment within each ethanol condition, p<0.05)
Post hoc analysis revealed that for context testing, groups administered 1.0 and 1.5 g/kg ethanol froze significantly less than the saline and 0.5 g/kg ethanol groups (p<0.001). The 0.5 g/kg ethanol group was not significantly different from the saline group. Nicotine enhanced learning in the saline and 0.5 g/kg ethanol groups compared to controls (p<0.001). Nicotine also partially reversed the deficits seen with higher doses of ethanol such that nicotine-treated groups froze significantly more than the saline-treated groups administered 1.0 or 1.5 g/kg ethanol (p<0.001). The group administered 1.0 g/kg ethanol and nicotine was not significantly different from saline controls; the group administered 1.5 g/kg ethanol and nicotine froze significantly less than groups administered saline or 0.5 g/kg ethanol (p<0.001), but more than the group administered 1.5 g/kg ethanol alone (p<0.001).
Post hoc analysis of cued fear conditioning revealed that groups administered 1.0 or 1.5 g/kg ethanol froze significantly less than groups administered saline or 0.5 g/kg ethanol (p<0.001). Nicotine partially reversed this deficit such that groups administered nicotine with 1.0 or 1.5 g/kg ethanol froze significantly more than groups administered saline and ethanol (p<0.001). The group administered 1.0 g/kg ethanol and nicotine was not significantly different from saline controls; the group administered 1.5 g/kg ethanol and nicotine froze significantly less than controls (p<0.001) but more than the group administered 1.5 g/kg ethanol alone (p<0.001). Nicotine had no significant effect on the groups administered saline or 0.5 g/kg ethanol. Thus, acute nicotine shifted the dose response curve for the disruption of contextual and cued conditioning by ethanol to the right.
The next experiment examined whether a higher dose of nicotine (0.18 mg/kg) could fully reverse ethanol (1.0 g/kg)-induced deficits in conditioning. The levels of baseline freezing and immediate freezing were similar across all groups. A significant effect of ethanol on freezing to the context and to the CS on testing day was found, F(1,26)= 88.05, p<0.001 and F(1,26)=4.71, p<0.05, respectively, but no other significant effects were found.
Post hoc analysis revealed that during both context and cued testing, the groups administered ethanol [context ethanol alone, mean (mean percent freezing)=29.17; cued ethanol alone, mean=73.61; context ethanol and nicotine, mean=26.67; cued ethanol and nicotine, mean=77.08] froze significantly less than control groups (context, mean=52.97; Cued, mean=85.42) regardless of nicotine administration (p<0.05).
Similarly, we also examined whether a lower dose of nicotine (0.045 mg/kg) could fully reverse ethanol (1.0 g/kg)-induced deficits in conditioning. The levels of baseline freezing and immediate freezing were similar across all groups. Significant main effects of ethanol, F(3,23)=120.59, p<0.001, and of nicotine, F(1,25)=11.63, p<0.01, on freezing to the context on testing day were found; a significant main effect of ethanol on freezing to the CS on testing day was also found, F(3,23)=11.63, p<0.001. There were significant interactions of ethanol and nicotine on freezing to the context and the cue on testing day, F(3,23)= 17.31, p<0.001 and F(3,23)=4.33, p<0.05, respectively. There was no significant difference in freezing during the pre-CS period.
Post hoc analysis revealed that during context testing, the groups administered ethanol (saline and ethanol, mean= 10.48; nicotine and ethanol, mean=16.19) froze significantly less than the other groups (saline alone, mean=45.71; saline and nicotine, mean=28.09; p<0.05). In cued testing, the group administered nicotine and ethanol (mean=57.94) froze significantly more than the group administered ethanol alone (mean=46.83) but significantly less than the group administered saline alone (mean=88.10; p<0.05). The nicotine alone group (mean=76.19) was not significantly different from the control group. These results indicate that the 0.09 mg/kg dose of nicotine is most effective for reversing ethanol-associated deficits in fear conditioning.
Coadministration of acute ethanol with chronic nicotine
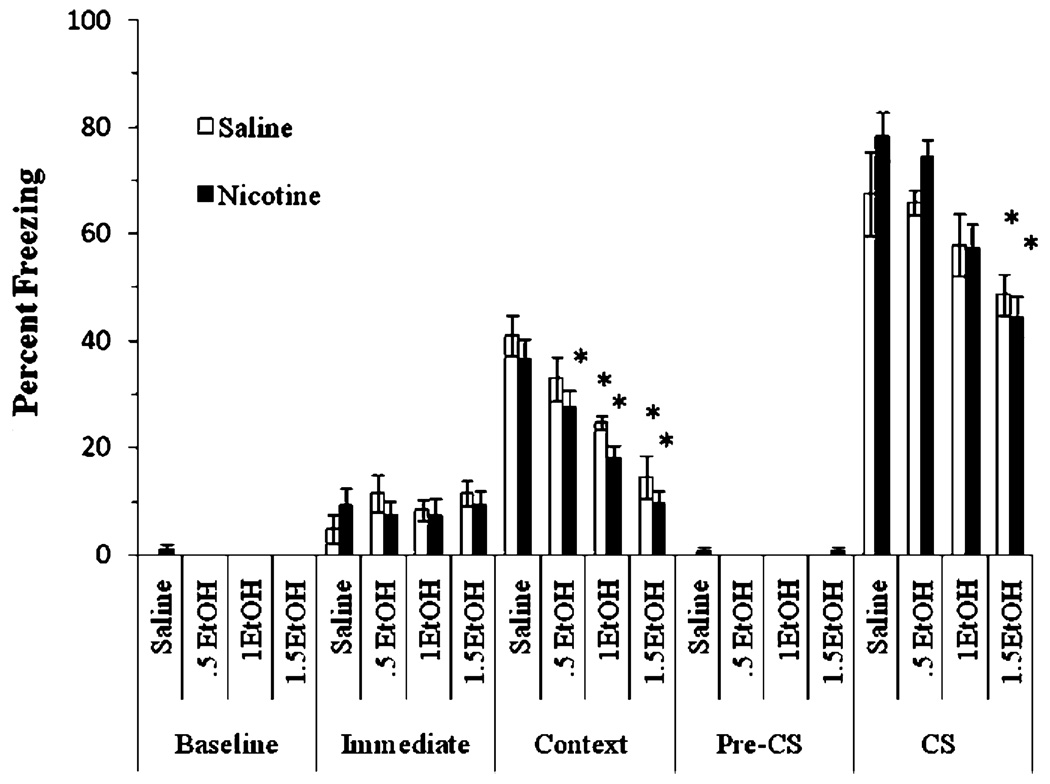
The next experiment examined whether chronic nicotine (6.3 mg kg−1 day−1 nicotine for 14 days) altered the effects of acute ethanol (0.5, 1.0, or 1.5 g/kg) on fear conditioning. If tolerance develops for the effects of nicotine on ethanolinduced deficits in fear conditioning, then a chronic dose of nicotine that has been shown to produce the same plasma nicotine levels as the 0.09 mg /kg dose of acute nicotine (Davis et al. 2005a) should not reverse ethanol-induced deficits in fear conditioning. The levels of baseline freezing and immediate freezing were similar across all groups. Significant main effects of ethanol, F(3,60)=26.96, p< 0.001, and nicotine, F(1,62)=8.18, p<0.01, on freezing to the context at testing were found, as well as a significant main effect of ethanol on freezing to the CS at testing, F(3,60)=14.84, p<0.001. There was no significant difference in freezing during the pre-CS period and no significant interaction of ethanol and nicotine on freezing in either test (Fig. 2).
Fig. 2.
Acute ethanol impaired contextual and cued conditioning, but chronic nicotine did not reverse these deficits and actually contributed to deficits in contextual fear conditioning with the 0.5 g/kg dose of ethanol (mean±SEM; asterisk indicates significant difference from controls, p<0.05)
Post hoc analysis revealed that during the context testing, all groups treated with 1.0 g/kg or 1.5 g/kg ethanol, as well as the group treated with both 0.5 g/kg ethanol and chronic nicotine, froze significantly less than the salinetreated controls (p<0.05). Post-hoc analysis also revealed that groups in the 1.5 g/kg ethanol conditions froze significantly less to the cue than groups administered saline or 0.5 g/kg ethanol (p<0.05) regardless of nicotine treatment. This shows that administration of 6.3 mg kg−1 day−1 of chronic nicotine produced tolerance for the ability of nicotine to reverse the ethanol-induced deficits in both contextual and cued conditioning, and slightly sensitized mice to the disruptive effects of ethanol, as deficits were seen in contextual fear conditioning in the group administered chronic nicotine and 0.5 g/kg ethanol. There were no significant differences between saline- and nicotine-treated groups that did not receive ethanol. Thus, tolerance was also seen for the ability of nicotine to enhance contextual fear conditioning, as reported previously (Davis et al. 2005a).
Administration of acute ethanol after withdrawal from chronic nicotine treatment
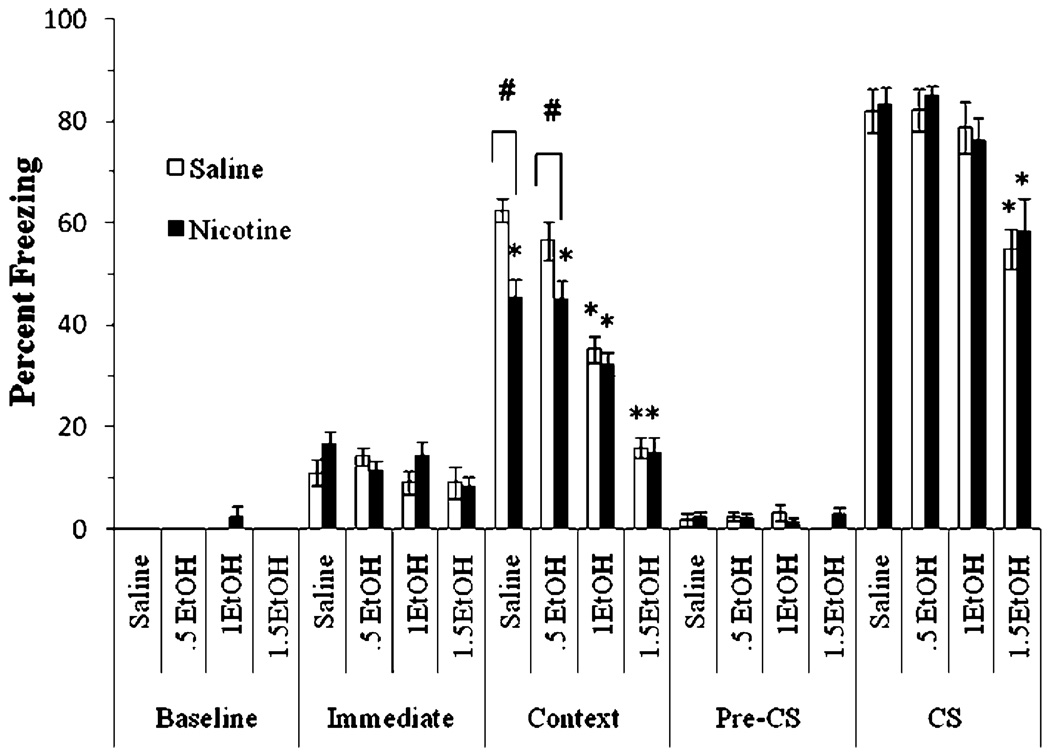
The interactive effects of withdrawal from chronic nicotine and acute ethanol on fear conditioning were also examined. The levels of baseline freezing and immediate freezing were similar across all groups. Significant main effects of ethanol, F(3,80)=72.64, p<0.001, and nicotine, F(1,82)=15.15, p<0.001, on freezing to the context at testing were found, as well as a significant main effect of ethanol on freezing to the CS, F(3,80)=18.78, p<0.001. There was also a significant interaction of ethanol and nicotine on contextual freezing at testing, F(3, 80)=3.15, p<0.05 (Fig. 3). No significant difference in freezing during the pre-CS period existed.
Fig. 3.
Both acute ethanol and withdrawal from chronic nicotine independently impaired conditioning (mean±SEM; asterisk indicates significant difference from controls, p<0.05; number sign indicates significant difference between nicotine and saline treatment within each ethanol condition, p<0.05)
Post hoc analysis revealed that for the context testing, the groups administered 1.0 and 1.5 g/kg ethanol froze significantly less than the saline-treated group (p<0.05). Additionally, nicotine-withdrawn groups administered saline or 0.5 g/kg ethanol froze significantly less than salinewithdrawn groups administered saline or 0.5 g/kg ethanol (p<0.05), but nicotine-withdrawn groups administered 1.0 or 1.5 g/kg ethanol were not significantly different from saline-withdrawn groups administered 1.0 or 1.5 g/kg ethanol. Post hoc analysis also revealed that groups administered 1.5 g/kg ethanol froze significantly less to the cue than all other groups (p<0.05). There were no other significant differences. Thus, withdrawal from chronic nicotine only altered contextual fear conditioning and did not interact with the ethanol doses tested.
Coadministration of a lower dose of ethanol with nicotine
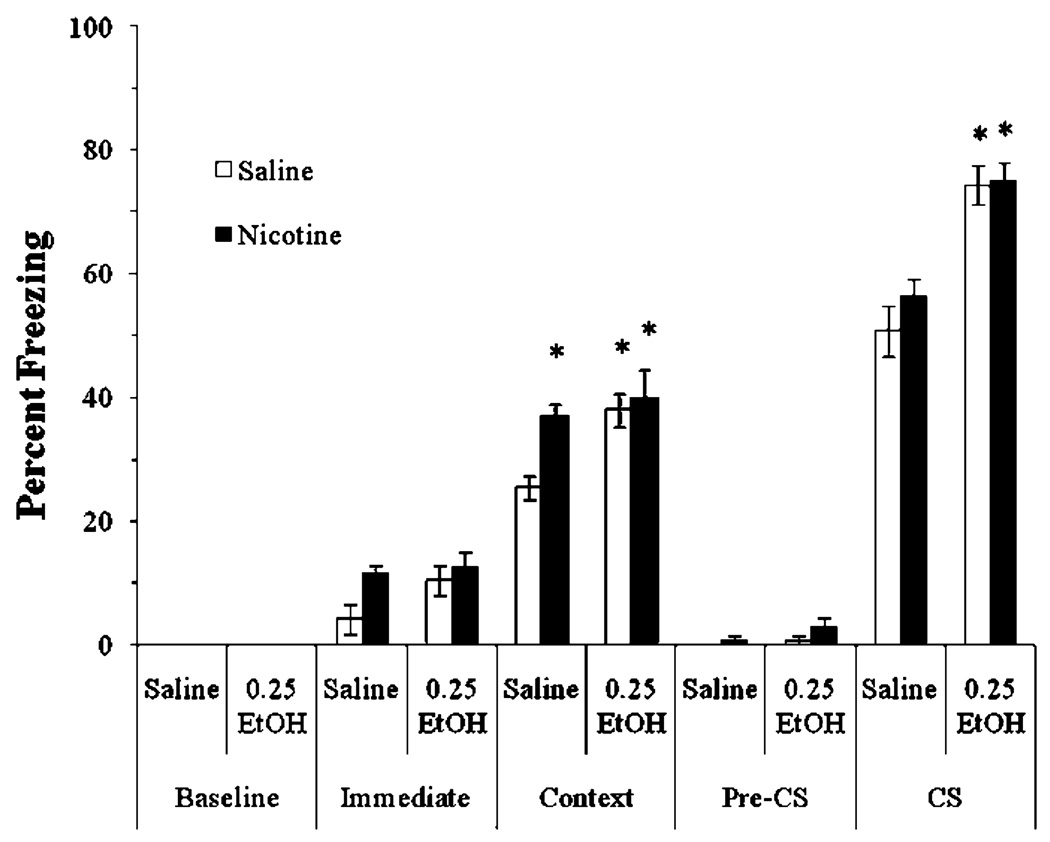
We examined if 0.25 g/kg ethanol, a dose shown to enhance fear conditioning in mice (Gulick and Gould 2007), would alter the effects of acute nicotine on fear conditioning. To avoid potential ceiling effects in the enhancement of fear conditioning, we reduced the strength of conditioning by using a single 15-s CS. The levels of baseline freezing and immediate freezing were similar across all groups. Significant main effects of ethanol, F(3,28)=8.17, p<0.01, and nicotine, F(1,31)=6.50, p<0.05, on freezing to the context at testing were found, as well as a significant main effect of ethanol on freezing to the CS, F(3,28)=46.76, p<0.001. There was no significant interaction of ethanol and nicotine in either test, nor was there a significant difference in freezing during the pre-CS period (Fig. 4).
Fig. 4.
Acute nicotine and a low dose of ethanol enhanced contextual conditioning in a nonadditive manner. The low dose of ethanol also enhanced cued conditioning (mean±SEM; asterisk indicates significant difference from controls, p<0.05)
Post hoc analysis revealed that groups in all drug conditions froze to the context significantly more than controls (p<0.05). There were no significant differences in freezing between groups administered nicotine, ethanol, or nicotine and ethanol. Post hoc analysis also revealed that the groups administered ethanol alone or ethanol and nicotine froze to the cue significantly more than controls (p<0.001), but the group administered nicotine alone froze at levels similar to controls, showing that acute ethanol enhances both contextual and cued conditioning at low doses, whereas acute nicotine enhances only contextual conditioning.
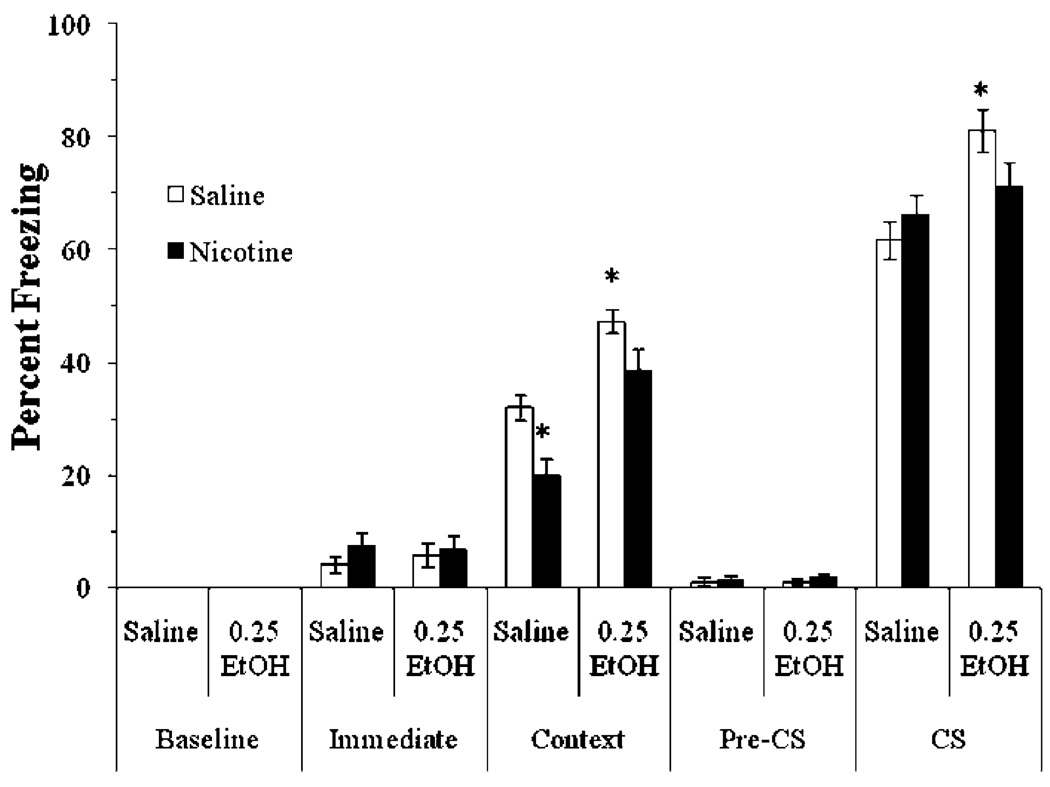
We next examined if chronic nicotine treatment altered the effects of 0.25 g/kg ethanol on fear conditioning. The levels of baseline freezing and immediate freezing were similar across all groups. A significant main effect of ethanol on contextual freezing was found, F(3,27)=11.67, p<0.01; there were also significant main effects of ethanol, F(3,27)=19.96, p<0.001, and of nicotine, F(1,30)=7.40, p<0.05, on CS freezing, and a significant interaction of ethanol and nicotine on freezing during context testing, F(3,28)=4.66, p<0.05, but not during cued testing. There was no significant difference in freezing during the pre-CS period (Fig. 5).
Fig. 5.
Acute ethanol enhanced contextual and cued conditioning, but chronic nicotine blocked enhancement of conditioning by ethanol, suggesting cross-tolerance (mean±SEM; asterisk indicates significant difference from controls and cross indicates significant differences between groups collapsed by ethanol treatment, p<0.05)
Post hoc analysis revealed that the group administered ethanol alone froze to the context and to the cue significantly more than saline controls (p<0.001). There were no significant differences in freezing to the context or to the cue between the chronic nicotine groups and the saline controls. However, nicotine blocked the enhancing effect of ethanol on freezing to the context; the group administered nicotine and ethanol was not significantly different from the saline controls. In addition, post hoc analysis revealed that the group administered ethanol alone froze to the cue significantly more than the group administered chronic nicotine and ethanol, whereas the latter group was not significantly different from saline controls (p<0.005). These results suggest that chronic nicotine treatment produces cross-tolerance to the enhancing effects of ethanol on fear conditioning.
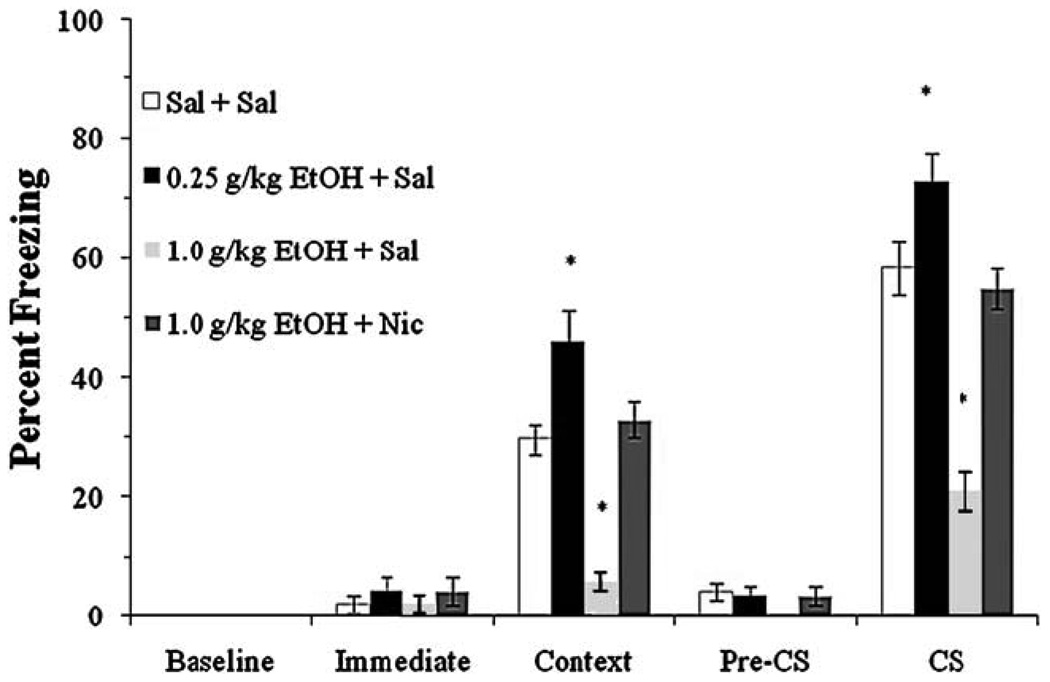
Finally, we examined whether a low dose of ethanol would reverse nicotine-withdrawal-associated deficits in contextual fear conditioning. The levels of baseline freezing and immediate freezing were similar across all groups. Significant main effects of ethanol, F(3,36)=40.29, p<0.001, and nicotine, F(1,38)=14.89, p<0.001, on freezing to the context at testing were found, as well as a significant main effect of ethanol on freezing to the CS at testing, F (3,36)=11.48, p<0.01, and a significant interaction of ethanol and nicotine on freezing to the CS, F(3,36)=4.18, p<0.05. There was no significant difference in freezing during the pre-CS period (Fig. 6).
Fig. 6.
Withdrawal from chronic nicotine impaired contextual conditioning, whereas a low dose of ethanol enhanced contextual and cued conditioning and reversed nicotine withdrawal-associated deficits in contextual fear conditioning (mean±SEM; asterisk indicates significant difference from controls, p<0.05)
Post hoc analysis revealed that the group administered ethanol alone froze to the context and cue significantly more than saline controls (p<0.01), whereas the nicotine withdrawal alone group froze significantly less to the context (p<0.05). There were no significant differences in freezing between the ethanol-treated nicotine withdrawal group and the saline controls. These results indicate that a low dose of ethanol reverses nicotine-withdrawal-associated deficits in contextual conditioning. Interestingly, ethanol did not enhance cued fear conditioning in mice withdrawn from chronic nicotine, suggesting that the cross-tolerance that developed with chronic nicotine treatment lasts beyond cessation of nicotine treatment.
Effect of cued testing first
To determine whether the effects of ethanol and nicotine interact with the order of testing, we examined the effects of acute ethanol (0.25 or 1.0 g/kg) and nicotine (0.09 mg/kg) on fear conditioning when cued testing occurred 1 h before context testing. The levels of baseline freezing and immediate freezing were similar across all groups. A significant difference in freezing to the context at testing was found, F(3,28)=27.14, p<0.001, as well as a significant difference in freezing to the CS, F(3,28)=34.14, p<0.001, but there was no significant difference in freezing during the pre-CS period (Fig. 7).
Fig. 7.
Testing cued conditioning first did not alter the effects of either ethanol or nicotine on conditioning (mean±SEM; asterisk indicates significant difference from controls, p<0.05)
Post hoc analysis revealed that the group administered saline and 0.25 g/kg ethanol froze to the context and to the cue significantly more than the saline alone group (p<0.05).
The group administered saline and 1.0 g/kg ethanol froze significantly less to the context and the cue than the saline alone group (p<0.05). There was no difference in freezing to the context and the cue between the group administered 1.0 g/kg ethanol and nicotine and the group administered saline. Thus, the order of testing does not alter the drug effects.
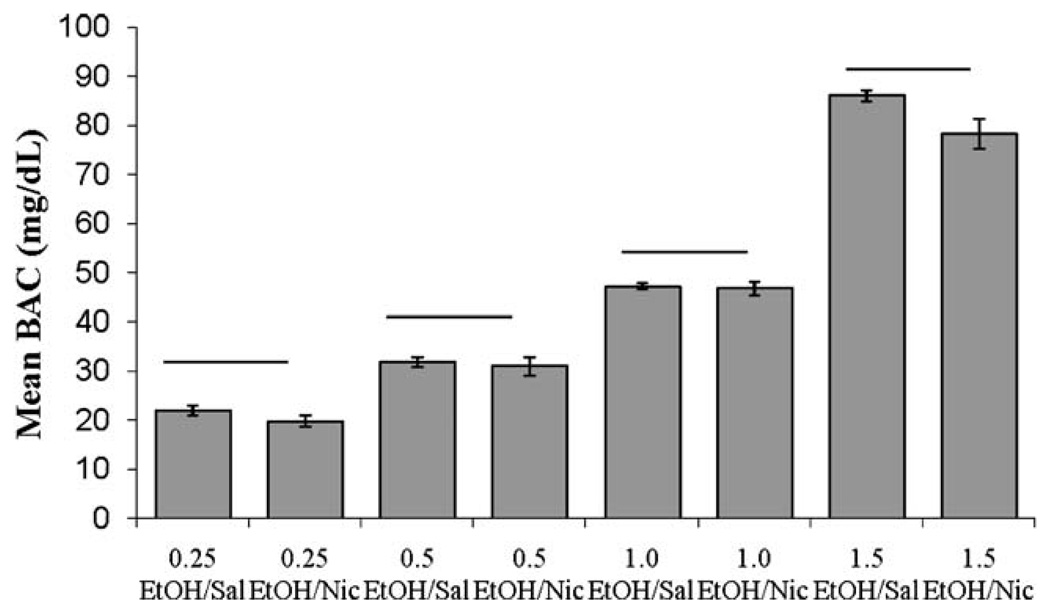
Blood alcohol concentration
The effects of nicotine on BAC were examined. There were significant differences in the mean blood alcohol levels between groups, F(9,30)=236.03, p<0.001. Tukey post hoc analyses revealed significant differences in BAC between all doses of ethanol (p<0.001), but no significant differences within ethanol dose between groups administered saline or nicotine, suggesting that nicotine treatment did not significantly alter ethanol metabolism (Fig. 8).
Fig. 8.
Average blood alcohol concentration (mg/dl) 15 min after ethanol injection and 5 min after nicotine injection. Higher doses of ethanol produced significantly greater blood alcohol concentrations, but there was no effect of nicotine treatment on blood alcohol concentration (mean±SEM; bar indicates no difference in BAC)
Discussion
Despite the frequent co-abuse of ethanol and nicotine, relatively few studies have examined the interactive effects of these drugs on cognitive function. The current study extends prior ethanol and nicotine research by demonstrating that the interactive effects of acute ethanol and nicotine shift as nicotine administration is changed from acute to chronic and into withdrawal from chronic nicotine. Acute nicotine interacted with only impairing doses of acute ethanol to reverse ethanol-induced deficits in contextual and cued fear conditioning. Withdrawal from chronic nicotine interacted with only an enhancing dose of acute ethanol such that acute ethanol reversed nicotine-withdrawal-associated deficits in contextual fear conditioning. In addition, chronic nicotine treatment resulted in cross-tolerance to the enhancing but not the disruptive effects of ethanol on contextual and cued fear conditioning.
Reversal of ethanol-induced impairments in learning by nicotine may be one factor underlying co-abuse of these drugs. As expected, acute nicotine enhanced contextual conditioning and reversed ethanol-induced impairments in fear conditioning, replicating and extending previous research (Davis et al. 2005b; Gould and Lommock 2003) by examining the effects of a full range of ethanol doses. Thus, smokers and even non-smokers may use tobacco in settings where alcohol is consumed to reduce the aversive effects of alcohol consumption. It should be noted that the current study does not provide experimental evidence that the interactions of nicotine and ethanol on cognition directly increase addiction or co-abuse. Whereas self-administration paradigms are essential to examining the craving and consumption of these drugs, our study focuses only on the cognitive effects of the drugs and proposes that one aspect of addiction and abuse may include cognitive changes induced by the drugs.
Reversal of the cognitive deficits associated with nicotine withdrawal by a low dose of ethanol may be another factor underlying co-abuse. In the current study, we found, as have other researchers (Gulick and Gould 2007; Hernandez and Powell 1986; Hernandez and Valentine 1990), that low doses of ethanol enhance learning. Although there were no additive effects of nicotine withdrawal and high doses of ethanol on disruption of contextual fear conditioning, nicotine-withdrawal-associated deficits in contextual fear conditioning were reversed by a low dose of ethanol. Nicotine withdrawal may lead to increased ethanol consumption in an attempt to reverse withdrawal-associated cognitive deficits, but increased consumption of ethanol also induces cognitive deficits, which could lead to smoking relapse in an attempt to ameliorate ethanol-associated deficits, producing a downward spiral into co-abuse. This hypothesis is supported by findings that nicotine withdrawal is associated with increases in both alcohol craving and consumption (Palfai et al. 2000), that the impairments associated with ethanol are reversed by nicotine (Gould and Lommock 2003; Lyon et al. 1975), and, finally, that ethanol decreases the ability to limit nicotine consumption (McKee et al. 2006). Despite these findings, it is important to remember that the cognitive effects of these drugs comprise only one aspect of co-abuse; alcohol and nicotine also act on the brain’s reward system, and interactive effects on this system could increase consumption as well.
Chronic drug use is often characterized by the development of tolerance to the acute effects of the drug. Replicating previous work in our laboratory, we found that chronic treatment with a dose of nicotine that produced the same plasma nicotine levels as an acute dose of nicotine that enhanced contextual fear conditioning had no effect on contextual or cued fear conditioning (Davis et al. 2005a). Interestingly, chronic nicotine also produced cross-tolerance to the enhancing effects of ethanol. Although studies have demonstrated that cross-tolerance can exist between nicotine and ethanol (Collins et al. 1988, 1993), it has not been shown for the cognitive enhancing effects of ethanol until now. Considering that chronic nicotine treatment produces tolerance to the effects of nicotine on contextual conditioning, it is not surprising that in the current study, chronic nicotine failed to reverse ethanol-induced impairments in conditioning. This suggests that smokers may increase their chronic nicotine consumption in an attempt to reverse ethanol-induced impairments in learning.
Whereas the neural mechanisms underlying the interactive effects of ethanol and nicotine on learning are unknown, several potential molecular and cellular mechanisms exist. Nicotine acts primarily at nACh receptors, whereas ethanol acts on a wide variety of targets that include nAChRs (Bowers et al. 2005; Escher and Mittleman 2004; Larsson et al. 2002; Melia et al. 1996; Meyerhoff et al. 2006; Wehner et al. 2004; White et al. 2000). Thus, ethanol may directly interact with nicotine by altering nAChR function; in support, ethanol stabilizes the open state of nAChRs, producing a twofold leftward shift in the acetylcholine response curve (Forman and Zhou 1999). Ethanol also acts on many other receptors, including GABAA and glutamatergic NMDA receptors (for review see White et al. 2000), and nAChRs have been shown to modulate both GABAergic and glutamatergic function (Alkondon and Albuquerque 2001; Gray et al. 1996). Thus, ethanol and nicotine may interact by modulating GABAergic and glutamatergic function. In support, ethanol enhances NMDA-receptor-mediated activity at low doses and inhibits it at high doses (Lima-Landman and Albuquerque 1989); the ability of ethanol to dose-dependently potentiate or depress NMDA-receptor-mediated activity suggests that the action of ethanol on these receptors may underlie both the enhancing and impairing effects of ethanol on learning. Furthermore, research suggests that the cognitive enhancing effects of nicotine may involve interactions with NMDA receptors (Lewis and Gould 2005). Thus, if higher doses of ethanol disrupt learning by inhibiting NMDA receptor function, nicotine could reverse this by facilitating NMDA receptor function.
The effects of nicotine alone, ethanol alone, or coadministration of these drugs on learning may also be mediated by different brain regions. Whereas nicotine alone altered only contextual fear conditioning (Gould 2003b; Gould and Wehner 1999; and present results), which is hippocampus-dependent (Logue et al. 1997), ethanol altered both contextual and cued fear conditioning (Celerier et al. 2000; Gould 2003a; Melia et al. 1996; and present results), both of which depend on the amygdala (Phillips and LeDoux 1992). Enhancement of contextual fear conditioning by nicotine is due to actions in the hippocampus (Davis et al. 2007), whereas ethanol may act on the amygdala to alter both types of learning. Furthermore, nicotine reversed ethanol-induced deficits in both contextual and cued conditioning, but only enhanced contextual fear conditioning, suggesting that these two effects of nicotine may involve different brain regions. Clearly, better understanding of the interactive effects of nicotine and ethanol will be gained once the underlying neural substrates are identified.
Finally, some studies have suggested that nicotine can increase the metabolism and elimination of ethanol, thus, decreasing peak blood alcohol levels (Chen et al. 2001), but we found no effect of nicotine on blood alcohol concentration, replicating previous research (Gould and Lommock 2003; Parnell et al. 2006). This apparent conflict can be explained by the differences in drug administration protocols. A recent study demonstrated that nicotine alters blood alcohol concentration after intragastric intubation, but not i.p. injection of nicotine, and only at higher doses of nicotine than those used in the current study (Parnell et al. 2006). These results suggest that a shift in ethanol metabolism is not responsible for the behavioral effects seen in this study.
In summary, the interactive effects seen in the current study suggest a cycle of drug co-use that could lead to increased consumption of both drugs. Ethanol and nicotine may initially be consumed for their rewarding properties, but more nicotine may be consumed to counteract the impairing effects of ethanol. Withdrawal from nicotine may increase ethanol consumption in an attempt to reverse nicotine-withdrawal-associated impairments; increased ethanol consumption could lead to increased nicotine consumption as ethanol-induced impairments recur. Finally, chronic nicotine produces tolerance to the effects of both nicotine and ethanol; thus, consumption of both drugs may increase to achieve the same effects that were initially achieved with lower levels of consumption.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant AA015515 (T.J.G.). All research complies with current US laws for the care and use of laboratory animals.
Contributor Information
Danielle Gulick, Department of Psychology, Neuroscience Program, Temple University, Philadelphia, PA 19122, USA.
Thomas J. Gould, Email: tgould@temple.edu, Department of Psychology, Neuroscience Program, Temple University, Philadelphia, PA 19122, USA; Department of Psychology, Center for Substance Abuse Research, Temple University, Weiss Hall, Philadelphia, PA 19122, USA.
References
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor a7 and a4b2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophys. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Bammer G, Chesher GB. An analysis of some effects of ethanol on performance in a passive avoidance task. Psychopharmacology. 1982;77:66–73. doi: 10.1007/BF00436101. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM. Deletion of the α7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcohol Clin Exp Res. 2005;29(3):295–302. doi: 10.1097/01.alc.0000156116.40817.a2. [DOI] [PubMed] [Google Scholar]
- Celerier A, Ognard R, Decorte L, Beracochea D. Deficits of spatial and non-spatial memory and of auditory fear conditioning following anterior thalamic lesions in mice: comparison with chronic alcohol consumption. Eur J Neurosci. 2000;12:2575–2584. doi: 10.1046/j.1460-9568.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Parnell SE, West JR. Nicotine decreases blood alcohol concentration in neonatal rats. Alcohol Clin Exp Res. 2001;23(1):18–25. [PubMed] [Google Scholar]
- Collins AC, Burch JB, DeFiebre CM, Marks MJ. Tolerance to and cross-tolerance between ethanol and nicotine. Pharmacol Biochem Behav. 1988;29:365–373. doi: 10.1016/0091-3057(88)90170-0. [DOI] [PubMed] [Google Scholar]
- Collins AC, Romm E, Selvaag S, Turne S, Marks MJ. A comparison of the effects of chronic nicotine infusion on tolerance to nicotine and cross-tolerance to ethanol in long- and short-sleep mice. J Pharmacol Exp Ther. 1993;266(3):1390–1397. [PubMed] [Google Scholar]
- Collins AC, Wilkins LH, Slobe BS, Cao JZ, Bullock AE. Long-term ethanol and nicotine treatment elicit tolerance to ethanol. Alcohol Clin Exp Res. 1996;20(6):990–999. doi: 10.1111/j.1530-0277.1996.tb01936.x. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005a;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett. 2005b;394(3):202–205. doi: 10.1016/j.neulet.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 2000;59:235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Escher T, Mittleman G. Effects of ethanol and GABAb drugs on working memory. Psychopharmacology. 2004;176:166–174. doi: 10.1007/s00213-004-1875-x. [DOI] [PubMed] [Google Scholar]
- Forman SA, Zhou Q. Novel modulation of a nicotinic receptor channel mutant reveals that the open state is stabilized by ethanol. Mol Pharmacol. 1999;55:102–108. doi: 10.1124/mol.55.1.102. [DOI] [PubMed] [Google Scholar]
- Friend KB, Pagano ME. Changes in cigarette consumption and drinking outcomes: findings from Project MATCH. J Subst Abuse Treat. 2005;29(3):221–229. doi: 10.1016/j.jsat.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson WE. Effects of alcohol on radial maze performance in rats. Physiol Behav. 1985;35:1003–1005. doi: 10.1016/0031-9384(85)90273-2. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Ethanol disrupts fear conditioning in C57BL/6J mice. J Psychopharmacol. 2003a;17(1):77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine produces a within-subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integr Physiol Behav Sci. 2003b;38(2):124–132. doi: 10.1007/BF02688830. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci. 2003;117(6):1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102(1–2):31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning. Behav Brain Res. 2004;155(1):167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Acute ethanol has biphasic effects on short-and long-term memory in both foreground and background contextual feat conditioning in C57BL/6 mice. Alcohol Clin Exp Res. 2007;31(9):1528–1537. doi: 10.1111/j.1530-0277.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman I. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology. 2002;162(2):129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Hernandez LL, Powell DA. Ethanol enhancement of Pavlovian conditioning: comparison with instrumental conditioning. Psychopharmacology. 1986;88:75–81. doi: 10.1007/BF00310516. [DOI] [PubMed] [Google Scholar]
- Hernandez LL, Valentine JD. Mild ethanol intoxication may enhance Pavlovian conditioning. Drug Dev Res. 1990;20:155–167. [Google Scholar]
- Higgins ST, Rush CR, Hughes JR, Bickel WK, Lynn M, Capeless MA. Effects of cocaine and alcohol, alone and in combination, on human learning and performance. J Exp Anal Behav. 1992;58(1):87–105. doi: 10.1901/jeab.1992.58-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman EH, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hake U. Probabilities of alcohol high-risk drinking, abuse, or dependence estimated on grounds of tobacco smoking and nicotine dependence. Addiction. 2003;98:805–814. doi: 10.1046/j.1360-0443.2003.00381.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301564. in press. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kelley BM, Rowan JD. Long-term, low-level adolescent nicotine exposure produces dose-dependent changes in cocaine sensitivity and reward in adult mice. Int J Dev Neurosci. 2004;22(5–6):339–348. doi: 10.1016/j.ijdevneu.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kunin D, Smith BR, Amit Z. Nicotine and ethanol interaction on conditioned taste aversions induced by both drugs. Pharmacol Biochem Behav. 1999;62(2):215–221. doi: 10.1016/s0091-3057(98)00155-5. [DOI] [PubMed] [Google Scholar]
- Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurol Biobehav Rev. 2004;27:713–720. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Larsson A, Svensson L, Soderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Gould TJ. Latent inhibition of cued fear conditioning: an NMDA receptor-dependent process that can be established in the presence of anisomycin. Eur J Neurosci. 2005;20(3):818–826. doi: 10.1111/j.1460-9568.2004.03531.x. [DOI] [PubMed] [Google Scholar]
- Lima-Landman MT, Albuquerque EX. Ethanol potentiates and blocks NMDA-activated single-channel currents in rat hippocampal pyramidal cells. FEBS Lett. 1989;247(1):61–67. doi: 10.1016/0014-5793(89)81241-4. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111(1):104–133. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Lopez MF, White N, Randall CL. Alcohol tolerance and nicotine cross-tolerance in adolescent mice. Add Biol. 2001;6:119–127. doi: 10.1080/13556210020040190. [DOI] [PubMed] [Google Scholar]
- Lyon RJ, Tong JE, Leigh G, Clare G. The influence of alcohol and tobacco on the components of choice reaction time. J Stud Alcohol. 1975;36(5):587–596. doi: 10.15288/jsa.1975.36.587. [DOI] [PubMed] [Google Scholar]
- Madden PAF, Heath AC, Starner GA, Whitfield JB, Martin NG. Alcohol sensitivity and smoking history in men and women. Alcohol Clin Exp Res. 1995;19(5):1111–1120. doi: 10.1111/j.1530-0277.1995.tb01588.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Kishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006;189(2):201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, LeDoux JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74(2):313–322. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Tizabi Y, Staley JK, Durazzo TC, Glass JM, Nixon SJ. Smoking comorbidity in alcoholism: neurobiological and neurocognitive consequences. Symposium conducted for Alcohol Clin Exp Res. 2006;30(2):253–264. doi: 10.1111/j.1530-0277.2006.00034.x. Chair. [DOI] [PubMed] [Google Scholar]
- Palfai TP, Monti PM, Ostafin B, Hutchison K. Effects of nicotine deprivation on alcohol-related information processing and drinking behavior. J Abnorm Psychology. 2000;109(1):96–105. doi: 10.1037//0021-843x.109.1.96. [DOI] [PubMed] [Google Scholar]
- Parnell SE, West JR, Chen WJ. Nicotine decreases blood alcohol concentrations in adult rats: a phenomenon potentially related to gastric function. Alcohol Clin Exp Res. 2006;30(8):1408–1413. doi: 10.1111/j.1530-0277.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- Patkar A, Mannelli P, Peindl K, Murray HW, Meier B, Leone FT. Changes in tobacco smoking following treatment for cocaine dependence. Am J Drug Alcohol Abuse. 2006;32(2):135–148. doi: 10.1080/00952990500479209. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotine-alcohol interactions and attentional performance on an operant visual signal detection task in female rats. Pharmacol Biochem Behav. 2003;76:75–83. doi: 10.1016/s0091-3057(03)00193-x. [DOI] [PubMed] [Google Scholar]
- Sobel BF, Sigmon SC, Griffiths RR. Transdermal nicotine maintenance attenuates the subjective and reinforcing effects of intravenous nicotine, but not cocaine or caffeine, in cigarette-smoking stimulant abusers. Neuropsychopharmacology. 2004;29(5):991–1003. doi: 10.1038/sj.npp.1300415. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42:413–416. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- Vihavainen T, Mijatovic J, Piepponen TP, Tuominen RK, Ahtee L. Effect of morphine on locomotor activity and striatal monoamine metabolism in nicotine-withdrawn mice. Behav Brain Res. 2006;173(1):85–93. doi: 10.1016/j.bbr.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- White AM, Matthews DB, Best PJ. Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus. 2000;10:88–93. doi: 10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]