Abstract
Waldenstrom macroglobulinemia (WM) is associated with a precursor condition, monoclonal gammopathy of undetermined significance (MGUS) of immunoglobulin-M (IgM) type. The etiology of these conditions is unknown. Recent studies at the population level have provided new data regarding familial aggregation of these disorders and other B-cell malignancies. Studies of familial clusters of WM have demonstrated an increased frequency of IgM MGUS compared to the general population and have provided new data suggesting that the phenotypic spectrum may also include polyclonal gammopathy and hypoglobulinemia. While the preponderance of immunoglobulin abnormalities in relatives of WM cases involves IgM, other immunoglobulin types (IgG and IgA) may also be affected. Large collaborative studies are needed to confirm these findings, which present an opportunity to define the earliest lesion(s) in the WM oncogenic pathway.
Keywords: MGUS, monoclonal gammopathy of undetermined significance, Waldenstrom macroglobulinemia, IgM, precursor disease, genetic susceptibility, polyclonal gammopathy, hypergammaglobulinemia, hypoglobulinemia
Introduction
Waldenstrom macroglobulinemia (WM) is a chronic lymphoproliferative disorder of unknown etiology characterized by aberrant production of monoclonal immunoglobulin (Ig)-M in the setting of histological evidence of lymphoplasmacytic lymphoma (LPL).1 Based on long-term follow-up data from the Mayo Clinic, a personal history of the precursor condition, monoclonal gammopathy of undetermined significance (MGUS) of IgM class is associated with an excess risk of developing WM.2 Indeed, MGUS is one of the most common premalignant disorders in western countries with a prevalence of 3.2% in the Caucasian general population 50 years of age or older.3 It is an asymptomatic condition characterized by the presence of a monoclonal immunoglobulin (M-protein) in the absence of any clinical signs or symptoms of WM, multiple myeloma, or other lymphoproliferative malignancies.4 Based on data from the Mayo Clinic, IgM MGUS accounts for about 14% of all MGUS.3
The average annual overall risk for MGUS patients to progress to a hematologic or lymphoid malignancy is about 1%5 while the average annual risk of progression for IgM MGUS cases is higher (around 1.5%),2 and the pattern of malignant outcomes is quite different. Whereas the risk of progression to chronic lymphocytic leukemia (CLL) or non-Hodgkin lymphoma (NHL) is essentially no greater for IgG and IgA MGUS than the expected incidence in the general population, the risks of progression to those endpoints for IgM MGUS are significantly elevated (5.7 for CLL and SIR=14.8 for NHL).2
Descriptive patterns
Data from the US Surveillance, Epidemiology and End Results (SEER) Registry estimate the US incidence of WM to be 0.36 per 100 000 person-years.6 WM incidence is about two-fold higher in men compared to women and increases in an age-dependent fashion. Moreover, WM exhibits a distinct pattern reflecting racial disparity; age-adjusted incidence rates are at least two-fold greater in whites compared to blacks or Asians. Our data in Ghanaian men suggest that IgM MGUS exhibits a similar racial pattern, with a very low prevalence of IgM MGUS among African blacks.7 The proportion of MGUS due to IgM also appears to be very low in Asian populations,8 which have lower rates of MGUS overall in comparison to other races.
While little is known about geographic patterns for WM, there appear to be variations in the geographic distribution of IgM MGUS, with notably high (24%) and low (6–8%) proportions reported in western France9 vs. the Mediterranean basin10 and Sweden,11 respectively. The highest absolute frequencies of IgM MGUS have been reported in western France (0.64%)9 and in an elderly community-based population in rural southern U.S. (0.61%).12 However, because of limited comparability across studies and changes in methodology over time, it is unknown whether these observed differences are real or reflect some unrecognized selection or other bias. Nevertheless, these findings could be compatible with either genetic and/or environmental influences and merit further study.
Genetic susceptibility
Familial aggregation is an important indicator of the potential influence of genetic factors, and identification of multiple-case families has contributed to the elucidation of the genetic basis for many conditions.13 Our recent large population-based study demonstrated that first-degree relatives of patients diagnosed with WM or LPL have increased risk to develop LPL/WM and MGUS, as well as CLL and NHL.14 These findings were consistent with prior population-based studies showing evidence for co-aggregation of various types of lymphoproliferative disorders.15,16
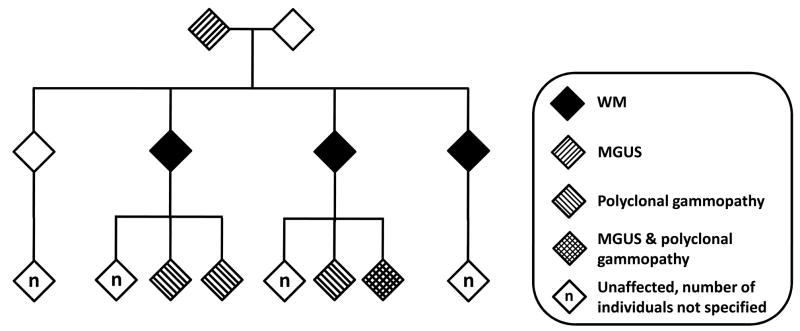
IgM MGUS appears to be a component in the spectrum of familial WM, with an estimated prevalence of about 10% in first-degree relatives of WM patients in multiple-case WM families (Figure 1).17 IgM MGUS is more common in first-degree relatives compared to more distant family members and is most commonly observed in siblings of cases. It must be noted, however, that parents or older relatives of WM patients are rarely available for screening, given the age-dependence of both IgM MGUS and WM. Our recent finding that the evidence for genetic linkage was increased when we considered patients with IgM MGUS to be affected provides further support for this relationship.18 Modifier genes and/or environmental exposures may further modulate genetic risk. For example, in our linkage study, we found evidence for linkage for WM in regions of chromosomes 1, 3, 4 and 6. We have also recently shown that, in two large studies of US veterans, elevated risk of WM is associated with hepatitis C infection19 and history of hepatitis, immunodeficiency virus, and rickettsiosis.20
Figure 1. Example of pedigree exhibiting co-aggregation of Waldenstrom macroglobulinemia (WM), monoclonal gammopathy of undetermined significance (MGUS), and polyclonal gammopathy.
Gender is not specified. Polyclonal gammopathies include both hyper- and hypoglobulinemia. The individual with both monoclonal and polyclonal gammopathy was originally found to have an IgM polyclonal elevation and subsequently developed an IgM monoclonal gammopathy.
The natural history of IgM MGUS in a familial context remains subject to investigation, since an understanding of whether the age of onset or rate and characteristics of progression of IgM MGUS differ between the familial and sporadic settings may have substantial clinical implications for risk assessment, screening, and prevention. We have found that serum immunofixation electrophoresis (IFE) is a sensitive technique for identifying at-risk individuals within families.21 While serum IFE detected small-volume IgM monoclonal bands, that were below the detection limit with immunoelectrophoresis, in both relatives and unrelated individuals, serial serum IFE demonstrated that the monoclonal bands persisted only in relatives of WM patients. We then followed three multiple-case families over a period of 20–30 years, and found that about half of the relatives with IgM MGUS ultimately progressed to WM, some within 15 years.22 Thus, the rate of progression in the familial setting is likely to be substantially greater than that observed in the general population.2,23 The extent to which familial patients subsequently develop symptomatic disease necessitating therapy remains to be clarified.
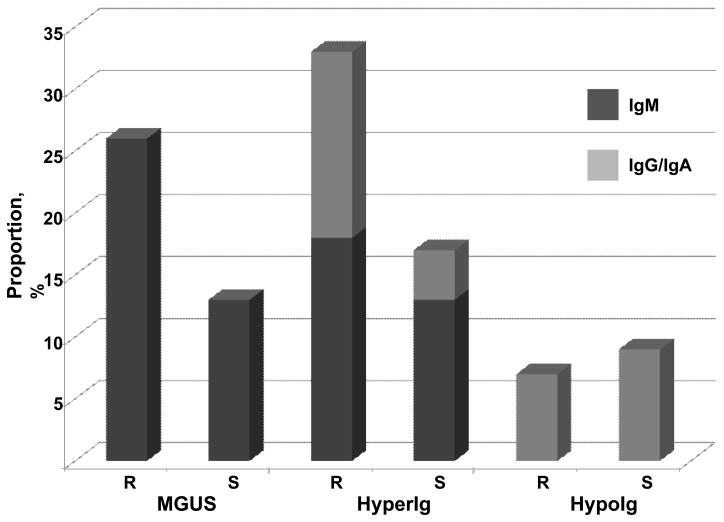
Another question raised by the longitudinal study is the significance of diverse polyclonal gammopathies, including polyclonal IgM, as well as hypoglobulinemia, in relatives of WM patients. We observed persistent polyclonal gammopathy and hypoglobulinemia that affected IgA and IgG as well as IgM in 41% of first-degree relatives (Figure 2).22 Many of these abnormalities were persistent and could not be readily explained clinically. While the population prevalence of quantitative immunoglobulin abnormalities is not defined, the high frequency of any immunoglobulin abnormality – together with the IgM predominance – was striking in this group. Especially noteworthy were patients with polyclonal IgM gammopathy that progressed to IgM MGUS. These observations suggest that other immunoglobulin abnormalities may also fall within the phenotypic spectrum of familial WM.
Figure 2. Distribution of monoclonal gammopathy of undetermined significance (MGUS) and polyclonal hyper- and hypoimmunoglobulinemia in first-degree relatives and spouses of familial Waldenstrom macroglobulinemia (WM) patients.
We screened 27 first-degree relatives from three multiple-case WM families. Seven relatives (26%) had MGUS, all of IgM type; 11 relatives (41%) had quantitative polyclonal abnormalities: 9 relatives had polyclonal immunoglobulin elevations (5 IgM, 1 IgG, 3 IgA); and 2 relatives had hypoglobulinemia (1 IgG, 1 IgA). Among spouses, 2 (9%) had MGUS, both of IgM type; 4 (17%) had polyclonal immunoglobulin elevations (3 IgM, 1 IgG); and 2 (9%) had hypoglobulinemia (1 IgG, 1 IgA). Abbreviations: R, blood-relatives; S, spouses; hyperIg, hypergammaglobulinemia; hypoIg, hypoglobulinemia.
Future directions
While there have been exciting developments in our understanding of disease susceptibility, we clearly need additional progress in therapeutic interventions. Based on SEER data, approximately 45–50% of WM patients diagnosed during 1988 – 2004 did not survive beyond 5 years from diagnosis.24 Until effective prevention strategies are identified, routine screening for MGUS has limited clinical utility and cannot be recommended outside research studies.
Though rare, WM occupies a critical biological niche in the continuum among hematologic lymphoid neoplasms. At present, our understanding of the initiation and determinants of progression remains limited. Large population-based studies are needed to define risk factors and to characterize the nature of progression to WM. Important questions to be addressed include whether all patients progress through an IgM MGUS phase or may develop WM de novo. Among individuals found to have IgM MGUS, it is unknown whether progression is inevitable or requires a quantitative threshold, below which progression may not occur. For example, small monoclonal bands found in a hospital setting have been shown to be transient in some cases.25 Longitudinal studies, with repeated evaluation by serum protein electrophoresis, will be needed to address these questions. Family studies provide an important context in which to explore these issues, since the prevalence of IgM MGUS appears to be higher in the familial setting than in the general population. In addition, the role of polyclonal gammopathy and hypoglobulinemia, particularly affecting IgM, should be further studied to determine whether they represent independent specific phenotypic indicators of risk. Our preliminary observations of hypoglobulinemia and polyclonal gammopathy with subsequent development of IgM MGUS in relatives of WM patients should be validated as a first step in determining whether these conditions represent yet another identifiable stage in the development of WM. Alternatively, these abnormalities may represent a spectrum of phenotypes that are indicative of a more generalized underlying immune dysregulation, which has been shown to be a risk factor for lymphomagenesis.
Conclusion
Given the fact that WM is a rare disease, collaborative approaches are essential to the careful definition and study of the role of IgM MGUS and polyclonal hyper- and hypogammopathy, which present an opportunity to identify the earliest lesions in the oncogenic pathway(s) to WM and other B-cell malignancies. Ultimately, the goal of these investigations will be to delineate the precise pathogenetic mechanisms underlying the development of IgM MGUS and to identify a cohort of patients who are at high risk for progression to malignancy.
Acknowledgments
This research was supported by grants from the Swedish Cancer Society, Stockholm County Council, the Karolinska Institutet Foundations, and the Intramural Research Program of the National Cancer Institute, NIH.
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Reference List
- 1.Berger F, Isaacson PG, Piris MA, Harris NL, Muller-Hermelink HK, Nathwani BN, Swerdlow SH. Lymphoplasmacytic lymphoma/Waldenström macroglobulinemia. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2001. pp. 132–4. [Google Scholar]
- 2.Kyle RA, Therneau TM, Rajkumar SV, Remstein ED, Offord JR, Larson DR, Plevak MF, Melton LJ. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102:3759–64. doi: 10.1182/blood-2003-03-0801. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, Dispenzieri A, Katzmann JA, Melton LJ. Prevalence of monoclonal gammopathy of undetermined significance. New England Journal of Medicine. 2006;354:1362–9. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 4.Anon Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. British Journal of Haematology. 2003;121:749–57. [PubMed] [Google Scholar]
- 5.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, Melton L. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. New England Journal of Medicine. 2002;346:564–9. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 6.Groves FD, Travis LB, Devesa SS, Ries LAG, Fraumeni JF., Jr Waldenström’s macroglobulinemia: incidence patterns in the United States, 1988 – 1994. Cancer. 1998;82:1078–81. [PubMed] [Google Scholar]
- 7.Landgren O, Katzmann JA, Hsing AW, Pfeiffer RM, Kyle RA, Yeboah ED, Biritwum RB, Tettey Y, Adjei AA, Larson DR, Dispenzieri A, Melton LJ, Goldin LR, McMaster ML, Caporaso NE, Rajkumar SV. Prevalence of monoclonal Gammopathy of undetermined significance among men in Ghana. Mayo Clinic Proceedings. 2007;82:1468–73. doi: 10.1016/S0025-6196(11)61089-6. [DOI] [PubMed] [Google Scholar]
- 8.Iwanaga M, Tagawa M, Tsukasaki K, Kamihira S, Tomonaga M. Prevalence of monoclonal gammopathy of undetermined significance: Study of 52,802 persons in Nagasaki city, Japan. Mayo Clinic Proceedings. 2007;82:1474–9. doi: 10.1016/S0025-6196(11)61090-2. [DOI] [PubMed] [Google Scholar]
- 9.Saleun JP, Vicariot M, Deroff P, Morin JF. Monoclonal gammopathies in the adult population of Finistere, France. J Clin Pathol. 1982;35:63–8. doi: 10.1136/jcp.35.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anagnostopoulos A, Evangelopoulou A, Sotou D, Gika D, Mitsibounas D, Dimopoulos MA. Incidence and evolution of monoclonal gammopathy of undetermined significance (MGUS) in Greece. Ann Hematol. 2002;81:357–61. doi: 10.1007/s00277-002-0493-0. [DOI] [PubMed] [Google Scholar]
- 11.Axelsson U, Bachmann R, Hällén J. Frequency of pathological proteins (M-components) in 6,995 sera from an adult population. Acta Medica Scandinavica. 1966;179:235–47. doi: 10.1111/j.0954-6820.1966.tb05453.x. [DOI] [PubMed] [Google Scholar]
- 12.Cohen HJ, Crawford J, Rao MK, Pieper CF, Currie MS. Racial differences in the prevalence of monoclonal gammopathy in a community-based sample of the elderly. Am J Med. 1998;104:439–44. doi: 10.1016/s0002-9343(98)00080-1. [DOI] [PubMed] [Google Scholar]
- 13.Risch NJ, Whittemore AS. Genetic concepts and methods in epidemiologic research. In: Schottenfeld D, Fraumeni JFJ, editors. Cancer Epidemiology and Prevention. 3. Oxford University Press; New York: 2006. pp. 89–98. [Google Scholar]
- 14.Kristinsson SY, Bjorkholm M, Goldin LR, McMaster ML, Turesson I, Landgren O. Risk of lymphoproliferative disorders among first-degree relatives of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia patients: a population-based study in Sweden. Blood. 2008;112:3052–6. doi: 10.1182/blood-2008-06-162768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldin LR, Pfeiffer RM, Li XJ, Hemminki K. Familial risk of lymphoproliferative tumors in families of patients with chronic lymphocytic leukemia: results from the Swedish Family-Cancer Database. Blood. 2004;104:1850–4. doi: 10.1182/blood-2004-01-0341. [DOI] [PubMed] [Google Scholar]
- 16.Goldin LR, Landgren O, McMaster ML, Gridley G, Hemminki K, Li X, Mellemkjaer L, Olsen JH, Linet MS. Familial aggregation and heterogeneity of non-Hodgkin lymphoma in population-based samples. Cancer Epidemiol Biomarkers Prev. 2005;14:2402–6. doi: 10.1158/1055-9965.EPI-05-0346. [DOI] [PubMed] [Google Scholar]
- 17.McMaster ML. Familial Waldenstrom’s macroglobulinemia. Semin Oncol. 2003;30:146–52. doi: 10.1053/sonc.2003.50063. [DOI] [PubMed] [Google Scholar]
- 18.McMaster ML, Goldin LR, Bai Y, Ter-Minassian M, Boehringer S, Giambarresi TR, Vasquez LG, Tucker MA. Genomewide linkage screen for Waldenstrom macroglobulinemia susceptibility loci in high-risk families. Am J Hum Genet. 2006;79:695–701. doi: 10.1086/507687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, Engels EA. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. Jama-Journal of the American Medical Association. 2007;297:2010–7. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 20.Koshiol J, Gridley G, Engels EA, McMaster ML, Landgren O. Chronic immune stimulation and subsequent Waldenstrom macroglobulinemia. Archives of internal medicine. 2008;168:1903–9. doi: 10.1001/archinternmed.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMaster ML, Csako G. Protein electrophoresis, immunoelectrophoresis and immunofixation electrophoresis as predictors for high-risk phenotype in familial Waldenstrom macroglobulinemia. International Journal of Cancer. 2008;122:1183–8. doi: 10.1002/ijc.23229. [DOI] [PubMed] [Google Scholar]
- 22.McMaster ML, Csako G, Giambarresi TR, Vascluez L, Berg M, Saddlemire S, Hulleyl B, Tucker MA. Long-term evaluation of three multiple-case Waldenstrom macroglobulinemia families. Clinical Cancer Research. 2007;13:5063–9. doi: 10.1158/1078-0432.CCR-07-0299. [DOI] [PubMed] [Google Scholar]
- 23.Baldini L, Goldaniga M, Guffanti A, Broglia C, Cortelazzo S, Rossi A, Morra E, Colombi M, Callea V, Pogliani E, Ilariucci F, Luminari S, Morel P, Merlini G, Gobbi P. Immunoglobulin M monoclonal gammopathies of undetermined significance and indolent Waldenstrom’s macroglobulinemia recognize the same determinants of evolution into symptomatic lymphoid disorders: proposal for a common prognostic scoring system. J Clin Oncol. 2005;20(23):4662–8. doi: 10.1200/JCO.2005.06.147. [DOI] [PubMed] [Google Scholar]
- 24.SEER*Stat Database. Incidence - SEER 13 Regs Limited-Use, Nov 2004 Sub (1973–2002 varying) National Cancer Institute, DCCPS Surveillance Research Program Cancer Statistics Branch; 2005. [Google Scholar]
- 25.Strobel SL. Transient paraproteinemia: An intriguing immunological anomaly. Annals of Clinical and Laboratory Science. 2003;33:265–70. [PubMed] [Google Scholar]