Abstract
Introduction
Intensity-modulated radiation therapy (IMRT) is an advanced treatment delivery technique that can improve the therapeutic dose ratio. Its use in the treatment of inoperable non-small cell lung cancer (NSCLC) has not been well studied. This report reviews our experience with IMRT for patients with inoperable NSCLC.
Methods and Materials
We performed a retrospective review of fifty-five patients with stage I–IIIB inoperable NSCLC treated with IMRT at our institution between 2001–2005. The study endpoints were toxicity, local control, and overall survival.
Results
With a median follow-up of 26 months, the 2-year local control and overall survival rates for stage I/II patients were 50% and 55% respectively. For the stage III patients, 2-year local control and overall survival rates were 58% and 58% respectively with median survival time of 25 months. Six patients (11%) experienced grade 3 acute pulmonary toxicity. There were no acute treatment-related deaths. Two patients (4%) had grade 3 or worse late treatment-related pulmonary toxicity.
Conclusions
IMRT treatment resulted in promising outcomes for inoperable NSCLC patients.
Keywords: Non-small cell lung cancer, Intensity-Modulated Radiotherapy, Local Control
INTRODUCTION
In the United States, lung cancer is the leading cause of cancer death in both men and women. Radiotherapy, often in conjunction with chemotherapy, is the primary treatment option for medically inoperable patients or for patients who have locally advanced disease. 1 A recent prospective trial of chemoradiation demonstrated a 2-year overall survival of 22%–33% in patients with stage III disease. 2 The treatment of lung cancer with radiotherapy is technically challenging and local tumor control with standard radiation doses and techniques is often difficult to achieve.3 Given that local recurrence is a leading cause of death in this patient population, techniques for improving local control may have an impact on survival rates.
One such method is treatment to higher doses and more accurate and precise delivery of the radiation. Several studies from the University of Michigan, the Radiation Oncology Therapy Group (RTOG), and Memorial Sloan-Kettering Cancer Center (MSKCC) have shown the value of dose escalation to doses as high as 102.9 Gy in controlling locoregional recurrences in NSCLC.4–7 However, even in these studies it was difficult to meaningfully escalate dose in patients with large tumors. The value of dose escalation was demonstrated in a study from MSKCC where Rengan et al. reviewed the treatment of stage III tumors with large (≥ 100cc) gross tumor volumes (GTV) using 3D-CRT and found that a 10 Gy increase in dose correlated to a 36.4% decrease in local failure rates.8
However, dose escalation is often difficult even with 3D-CRT. To avoid treatment related complications such as severe pneumonitis, it is necessary to keep the mean lung dose (MLD) below approximately 20 Gy.9,10 The use of inverse treatment planning (ITP) and IMRT may improve the ability to deliver tumorcidal doses to the planning target volume (PTV) while preserving the integrity of surrounding normal tissues. A planning study by Liu et al. compared IMRT and 3D-CRT plans of ten NSCLC patients to see if IMRT could reduce the irradiated volumes of normal lung and other critical structures while maintaining adequate dose to the PTV. Results showed statistically significant differences in V20, V30, and MLD with the values in the IMRT plans being lower. Further, with IMRT they were able to maintain or decrease the V45 of the esophagus and heart as compared with 3D- CRT. The benefits seemed most pronounced in medium to large tumors. 11 Murshed et al. used a class solution of 9 coplanar and equispaced beams to retrospectively design IMRT plans for 41 patients who had been treated with 3D-CRT and compared dosimetric parameters. Results showed that with IMRT there was a 10% reduction in the predicted rate of radiation pneumonitis and an overall decrease in the MLD of >2 Gy.12 Christian et al, in a study of 10 patients with NSCLC, retrospectively compared 5 different IMRT plans using different fields (3,5,7, and 9 coplanar field and a 6 noncoplanar field ) to a six-field noncoplanar 3D-CRT plan for each patient. They found that using IMRT reduced the V20 when more than 3 fields were used as compared to the 3D-CRT plan. There also was a relationship between number of coplanar beams and reduction in dose to the normal lung with 9 beams being superior to 3,5 and 7.13
Further advantages of IMRT and ITP are highlighted in other planning studies. Grills et al. found that even in node-positive patients, the use of IMRT allowed for a reduction in the lung V20 and the MLD by 15% and the lung normal tissue complication probability (NTCP) by 30% as compared to 3D-CRT.14 Schwarz et al. showed that the use of IMRT in large concave tumors allows for dose escalation of up to 17% when compared to 3D-CRT, with both methods having a homogeneous dose distribution in the PTV. This study indicated that IMRT has potential to allow dose escalation in patients with stage III disease.15
Despite numerous reports on the dosimetric advantages of IMRT16,17, there is limited information on the clinical use of IMRT in NSCLC. In 2001, MSKCC began treating selected patients with large tumors or tumors near critical locations with IMRT. This report reviews those patients and assesses their survival outcomes and treatment-related toxicities.
METHODS/MATERIALS
A total of 55 patients with biopsy-proven inoperable NSCLC stages I–IIIB were treated with IMRT between 2001 and 2005 at MSKCC to doses of 60 Gy or more. The MSKCC Institutional Review Board approved a retrospective review of these patient’s outcomes. Pathological diagnosis was made by CT-guided fine needle aspiration in 56% of the patients (31 patients). Seven patients (13%) underwent mediastinoscopy, 3 patients (5%) underwent thoracotomy, and 14 patients (25%) underwent bronchoscopy. Specific patient characteristics are presented in Table 1. Fifty-three patients (96%) had a FDG-PET for staging and GTV delineation before initiating radiation treatment. For those patients who received FDG-PET both before and after chemotherapy, the GTV was derived from the pre-chemotherapy scan. All patients had a complete metastatic work-up including CT or MRI of the head, FDG-PET, and/or bone scans.
Table 1.
Patient Characteristics
| n | |
|---|---|
| Age (years) | |
| Median | 67 |
| Range | 32–88 |
|
| |
| Gender | |
| Male | 23 (42) |
| Female | 32 (58) |
|
| |
| Histological subtype | |
| Adenocarcinoma | 20 (36) |
| Squamous Cell Cancer | 20 (36) |
| NSCLC, NOS | 15 (27) |
|
| |
| Stage | |
| I/II | 15 (27) |
| IIIA | 6 (11) |
| IIIB | 23 (42) |
| Recurrent | 6 (11) |
| Re-staged IIIA/IIIB | 5 (83) |
|
| |
| KPS (%) | |
| Median | 80 |
| Range | 60–100 |
|
| |
| GTV (cm3) | |
| Median | 136 |
| Range | 4–1060 |
|
| |
| PTV (cm3) | |
| Median | 459 |
| Range | 63–1890 |
Abbreviations: NSCLC= non-small cell lung cancer; NOS= not otherwise specified; KPS= Karnofsky Performance Status; GTV= gross tumor volume; PTV= planning target volume.
Data is presented as number of patients (n), with percentages in parentheses, unless otherwise specified.
All patients received a planning CT scan (Model PQ 5000 AcQSim, Marconi/Philips Medical Systems, Cleveland OH) and for treatment were immobilized in a supine position with their arms raised in a customized alpha-cradle mold (Alpha Cradle Molds, Akron, OH). The gross tumor volume (GTV) consisted of all known sites of disease with no elective nodal targets. The GTV was considered large if it was ≥ 100cc.8,18 The PTV was determined from the GTV by a radiation oncologist who used an automatic margining tool which added a standard margin of 10–18mm and critical normal structures were contoured by the physician or by the treatment planner which incorporated presumed microscopic extension and organ motion. Treatment planning was performed with the MSKCC treatment-planning system, which has previously been described 19–22. Tissue inhomogeneity corrections were applied to all dose calculations.23 Treatments were delivered with 6 MV photons utilizing the sliding window IMRT method on Varian linear accelerators with dynamic multi-leaf collimator (600C, 2100C or 2100 EX Linacs with Mark I, Mark II or Millenium DMLC, depending on the patient and machine availability).23 Beam directions were manually chosen by the planner to satisfy the clinical dosimetric objectives for the tumor and surrounding normal structures. The planners generally attempted to keep dose to the contralateral lung low, although formal dosimetric criteria were not applied to this structure. The planning goal was to deliver the prescription dose to at least 95% of the PTV while meeting normal tissue constraints described below. All patients were treated with conventional fractionation (1.8 –2.0 Gy) with no planned treatment breaks. The MU’s per beam (i.e. for each 2 Gy treatment) ranged from 150–250 for IMRT, which is similar to those for 30–60 degree wedged fields when using 3D-CRT.
The prescription doses for this group of patients ranged between 60 Gy to 90 Gy with a mean prescription dose of 6950 cGy. The spinal cord and total lung were the dose-limiting tissues. For the lung, we required that the NTCP not exceed 25%24 as calculated with the Lyman model25 using the dose-volume histogram (DVH) reduction scheme of Kutcher and Burman and the model parameters of Burman et al. for severe radiation pneumonitis.26,27 A parallel architecture model was also used in the evaluation process.28 This model views an organ in terms of functional subunits working in parallel and provides a scheme for calculating the fdam from the DVH; the destruction of a critical fraction of subunits is required to cause a complication.29 If the NTCP exceeded 25%, treatment to the initially intended dose was still permitted if fdam did not exceed 28%. The maximum spinal cord dose was kept below 50 Gy. A volumetric dose display was used to detect hot spots exceeding 110%–115% of prescription outside the PTV which were reduced or eliminated by re-planning with additional constraints. A sample case has been presented in Figures 1&2.
Figure 1.
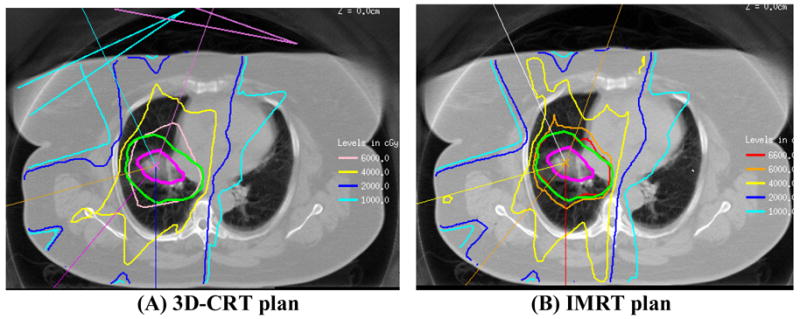
This is a comparison of a three-dimensional conformal radiation therapy (3D-CRT) plan (A) versus an intensity-modulated radiation therapy (IMRT) plan (B) for a 79 year old female with a T2N0M0 (stage IB) non-small cell lung cancer of the right lower lobe. The tumor was 4.7 × 3.1 cm in the right middle lobe. The patient was deemed medically inoperable was referred for definitive radiation treatment. Due to her medical co-morbidities she was also not a candidate for systemic chemotherapy. This case was ideal for IMRT due to the patient’s small lung volumes. Here are the transverse planes through the isocenter for the two plans. With 3D-CRT plan (A), the PTV D95 was approximately 58 Gy with a NTCP of 21%. With the IMRT plan (B), the PTV D95 was 64.5 Gy (the prescription dose was 66 Gy) and NTCP was 25%. In the images, the green heavy contour is the PTV and the magenta heavy contour is the GTV. The patient was treated to 6600 cGy with no radiation treatment-related complications. The patient has continued to have no evidence of disease for 2 years since the completion of radiation treatment.
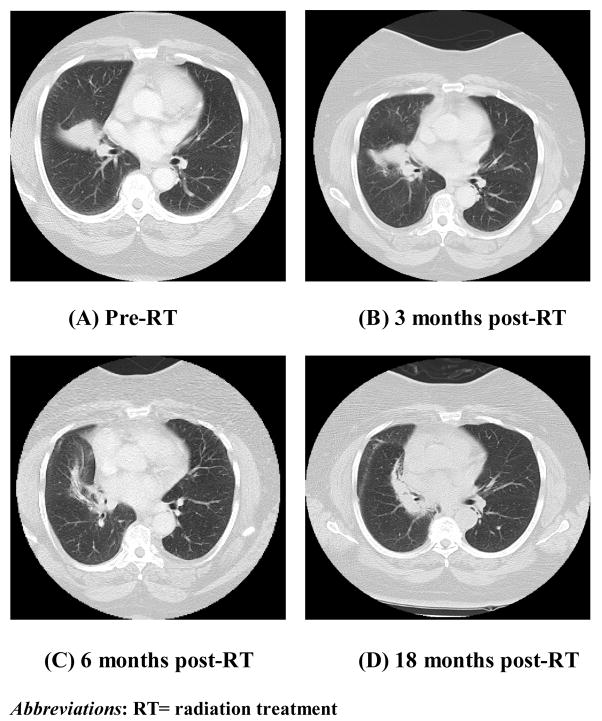
Figure 2.
These four images (A–D) are the pre-treatment and follow-up CT scans for the patient presented in Figure 1. The CT scan at 18 months after completion of radiation treatment shows dense fibrotic change without evidence of tumor recurrence.
Acute toxicities and late treatment-related complications were recorded based on a modified version of the RTOG toxicity scoring system.30 Acute reactions included those experienced during or within the first 4 months after the start of radiation treatment and were graded according to the most severe reaction. Any complications that began or persisted after 4 months were considered to be late reactions. Those patients who required oxygen or corticosteroid therapy were classified as having grade 3 radiation pneumonitis.
The first follow-up visit was one month after completion of radiation treatment and then every 3–4 months for the first 2 years. For the next 3 years patients were seen biannually. After 5 years, patients were usually seen once a year. Each follow-up visit consisted of a comprehensive physical examination and a CT scan of the chest.
A combination of clinical assessment, CT, and pathology results were used to assess local control. FDG-PET may have been used to aid in follow-up for some patients, but was not considered as a definitive measure of recurrence. Local recurrence was defined as an increase in radiographic abnormality within the irradiated volume that was not believed to be radiation induced scarring or pneumonitis. Estimates of local control, local failure, and overall survival were calculated using the Kaplan-Meier method31 from the initiation of treatment, whether it was chemotherapy or radiotherapy.
RESULTS
Fifty-five patients with inoperable NSCLC were treated to 60 Gy or more using IMRT between 2001 and 2005 at MSKCC. IMRT was used over 3D-CRT for most of these patients due to large tumor volumes (GTV ≥ 100cc)8,18, unfavorable tumor geometry, proximity of the tumor to critical organs, and/or small lung volumes. Among the patients 29% (16 patients) were stage I/II and 71% (39 patients) were stage IIIA/IIIB. Patients who presented with recurrent disease were re-assigned to the stage they would have been grouped with based on tumor characteristics. Six patients (11%) had recurrent disease 5 of whom were re-staged as stage IIIA/IIIB. For the purpose of this analysis, patients with stages IIIA and IIIB disease were collectively categorized as stage III.
Thirty- four patients (62%) had tumors that were inoperable based on site and stage, 19 patients (35%) were medically unfit for surgery due to their co-morbidities, and 2 patients (4%) chose radiation therapy over surgery. Thirteen patients (24%) were treated with radiotherapy alone, 29 patients (53%) were treated with induction chemotherapy followed by radiotherapy, and the remaining 13 patients (24%) were treated with concurrent chemoradiation therapy. Among the 39 stage III patients, 23 (59%) received neoadjuvant platinum-based chemotherapy followed by radiation, 13 (33%) received platinum-based chemotherapy and radiotherapy concurrently, and 3 patients (8%) received radiotherapy alone. Mean radiation prescription dose was 6950 cGy (6000–9000 cGy). The median GTV was 136cc.
Outcome
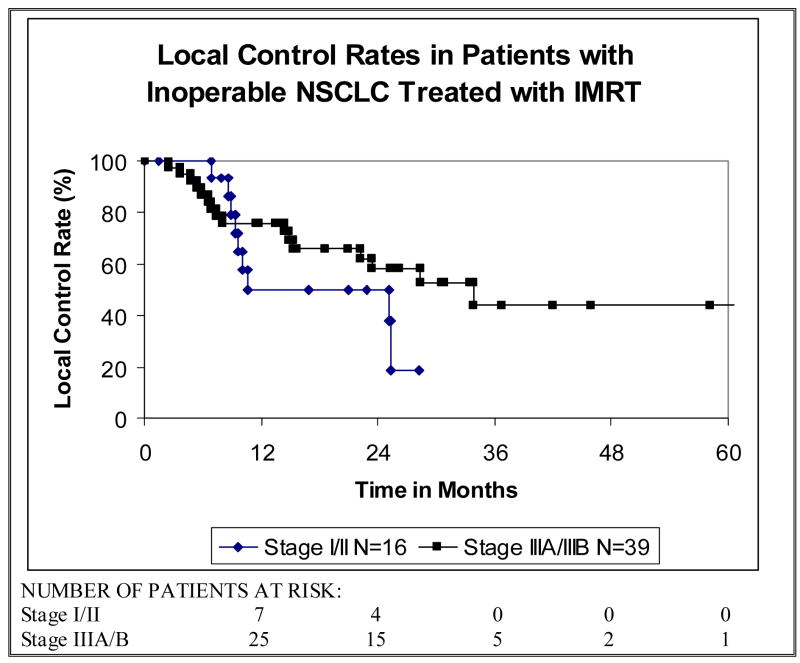
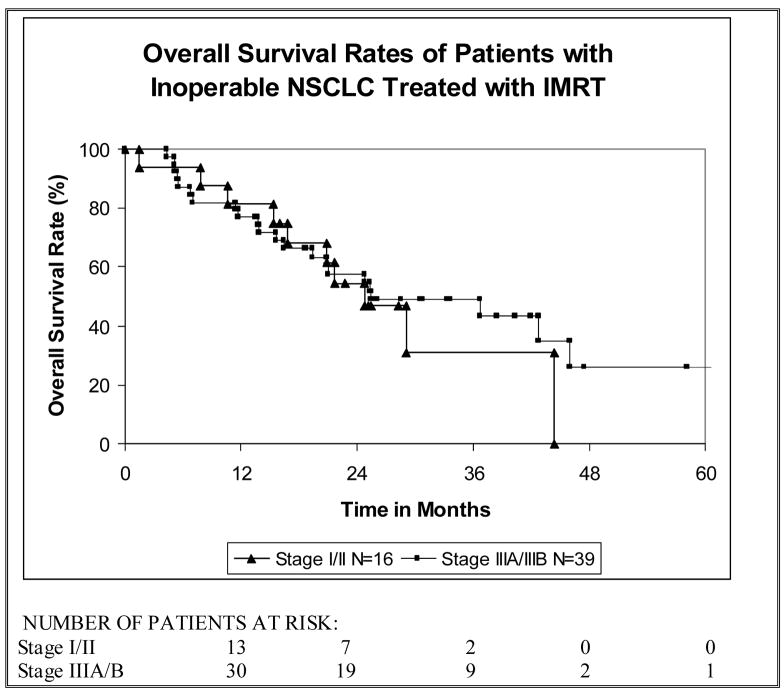
In the 16 patients with stage I/II tumors 2-year local control (LC) and overall survival (OS) rates were 50% (Figure 3) and 55% (Figure 4) respectively. In patients with stage III (IIIA/IIIB) disease, 2-year LC was 58% (Figure 3) and 2-year OS was 58% (Figure 4) with a median survival time of 25 months. The disease-free survival (DFS) rate in all patients at two years was 41% with a median DFS time of 12 months and the cancer-specific survival (CSS) rate at two years was 63%. As shown in Figure 3, the OS rate among all patients at two years was 57% with a median survival time of 25 months. Median follow-up time among all patients was 21 months (range, 0–61 months) and among survivors was 26 months (range, 14–61).
Figure 3.
The local control rates of patients with inoperable non-small cell lung cancer (NSCLC) treated with intensity-modulated radiation therapy (IMRT).
Figure 4.
The overall survival rates of patients with inoperable non-small cell lung cancer (NSCLC) treated with intensity-modulated radiation therapy (IMRT).
Acute Toxicity
The frequency of acute treatment-related toxicities including fatigue, nausea, esophagitis, skin reactions, and acute pulmonary toxicities are presented in Table 2. Of the 55 patients, six (11%) experienced grade 3 acute pulmonary toxicity and two (4%) experienced grade 3 acute esophagitis. There were no acute treatment-related deaths.
TABLE 2.
Rate of Acute Reactions in all Patients by Grade
| ACUTE REACTION | FREQUENCY OF REACTION (%) n=55 | |||||
|---|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Fatigue | 10 (18%) | 27 (49%) | 17 (31%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Nausea | 40 (73%) | 12 (22%) | 3 (6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Esophagitis | 15 (27%) | 26(47%) | 12 (22%) | 2 (4%) | 0 (0%) | 0 (0%) |
| Lung | 2 (4%) | 37 (67%) | 10 (18%) | 6 (11%) | 0 (0%) | 0 (0%) |
| Heart | 54 (98%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Skin | 25 (45%) | 24 (44%) | 4 (7%) | 2 (4%) | 0 (0%) | 0 (0%) |
Late Toxicity
The two treatment-related late complications that were assessed were late esophagitis and late pulmonary toxicity. Frequency of these late toxicities by grade is presented in Table 3. Forty-nine patients (89%) were alive 4 months after beginning treatment. Among these patients, one (2%) experienced grade 3 late pulmonary toxicity and one patient (2%) who had acute pulmonary toxicity died from radiation-induced pneumonitis. The crude rate of overall acute and late pulmonary complication rate was 13%.
TABLE 3.
Rate of Late Reactions by Grade in Survivors ≥ 4 months after Starting Treatment
| LATE REACTION | FREQUENCY OF LATE REACTION (%) n=49 | |||||
|---|---|---|---|---|---|---|
| Grade 0 | Grade I | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Late Pulmonary | 11 (23%) | 28 (57%) | 8 (16%) | 1 (2%) | 0 (0%) | 1 (2%) |
| Late Esophageal | 38 (78%) | 8 (16%) | 3 (6%) | 0 (0%) | 0 (0%) | 0 (0%) |
Seventy-eight percent (38 patients) of the patients alive at 4 months (n=49) had no evidence of late esophagitis (grade 0) and no patients were observed having > grade 2 late esophageal toxicity.
Discussion
This study is among the first reports of treatment outcome with significant follow-up on the use of IMRT for inoperable NSCLC. The results from our study are encouraging, especially for the stage III patients where the 2-year OS rate was 58%. Recently, two prospective trials were conducted using chemoradiation for patients with inoperable stage III disease. Results from the LAMP trial, which was a phase II prospective study of patients randomized to three different platinum-based chemoradiation regimens, showed 2-year OS rates ranging from 22–33%.2 In SWOG 9504, a phase II trial of patients with unresectable stage IIIB NSCLC treated with concurrent platinum-based chemoradiation followed by consolidation with docetaxel, the 2-year OS was 54%.32 When compared to these prospective trials, our results using IMRT for a patient population with large tumor volumes, are promising.
Inverse treatment planning (ITP) with IMRT appears to be a good treatment planning option with favorable outcomes for patients who have larger tumors with difficult geometry in critical locations. In a preliminary retrospective study of 59 NSCLC patients treated with IMRT, Yom et al. reported crude OS and LC rates at 6 months of 87% and 47%. IMRT was used in these cases because of the larger tumor volumes, tumor location near critical structures, and the necessity of normal lung sparing due to prior thoracic irradiation.33 In such cases, high indicators of lung toxicity such as MLD, V20, NTCP, or fdam as well as doses to the spinal cord or esophagus often limit dose escalation with 3D-CRT.6,9,10,24–29 As highlighted in the treatment-planning studies cited above, IMRT plans can often maintain these dosimetric predictors of toxicity at acceptable levels at higher target doses.11,12,14,15 Clinically this increase in maximum deliverable dose with IMRT may lead to favorable outcomes for patients with stage III disease.
Concerns have been expressed regarding the clinical use of IMRT for NSCLC.34 Recently, there have been indications that the irradiation of large fractions of the lung to doses well below 20 Gy may be correlated with both acute and late pulmonary toxicity.35 Although it has been shown that IMRT can reduce the MLD, V>10, and the dose to the esophagus and spinal cord36 there is conflicting data on whether IMRT also increases the volume of lung receiving smaller yet potentially toxic doses of radiation. In the studies by Liu et al. and Murshed et al. there was minimal increase in the percent volume of lung receiving at least 5 Gy (V5) for plans with 9 equispaced beams, which was attributed to leakage dose.37,12 Both studies commented that the lung volume receiving low doses may be reduced by more efficient delivery methods (reducing the number of monitor units, hence leakage) and by reducing the number of beams. Schwarz et al. noted that when IMRT plans are derived to deliver more homogeneous dose distributions the V5 and the integral dose could be maintained or reduced when compared to 3D-CRT plans.15 Additional clinical data are needed to better understand the relation between VD<10 and early or late lung complications and further planning studies are needed to determine how best to limit the low dose part of the DVH if this is desirable.
Respiratory motion is another concern with using IMRT for lung cancer. Unlike static 3D-CRT, in IMRT the dose from each beam is not delivered all at once, but instead it is delivered to by smaller subfields that move as a function of time. Therefore the use of IMRT in lung cancer has been questioned because with “the dynamic delivery of IMRT, it is not clear how the (planned) doses will add (up to the delivered doses) when the target is also moving”34. However, using software simulations and statistical analysis, Bortfeld et al. reported that respiratory motion has no significant effect (<1%) on the planned versus the delivered dose if treatment is delivered in 30 or more fractions. They also found that the variation in dose due to organ motion was the same in both IMRT and 3D-CRT techniques.38 Chui et al. had similar observations, based on a study of the clinical IMRT plans for 3 breast cancer and 4 lung cancer patients. They also concluded that for treatments of approximately 30 or more fractions, dose variance to the PTV due to respiratory motion was similar for both IMRT delivered with a dynamic multileaf collimator as it was for static 3D-CRT fields.39
The pencil-beam tissue inhomogeneity correction implemented in the MSKCC planning system is known to have limitations in dealing with situations of electronic disequilibrium such as exist at lung-soft tissue interfaces. In part because of this limitation, we do not reduce GTV to PTV margins for IMRT plans (compared to 3D-CRT plans) and we use 6 MV photons rather than higher energies. We have previously compared the planning system’s dose distributions and dose-volume histograms (DVH) with Monte-Carlo calculations of the same plans for 3D-CRT and IMRT lung plans and have observed similar effects for both techniques.40–42
The outcomes reported in our study with using IMRT are comparable to results from other studies that have used 3D-CRT for NSCLC. In a prospective dose escalation study, Hayman et al. reported 2-year OS of 40% with median survival time of 18 months and 2-year progression-free survival of 17% in patients with stage I-IIIB disease.4,5 RTOG 9311 reported 2-year LC rates ranging from 50%–78% and OS rates between 20%–50% for patients with stage I–IIIB disease.6 This is especially encouraging considering that our patient population most likely represented a less favorable group of patients given the large median gross tumor volume.
We have observed neither excessive rates of lung complications nor inferior local control rates that might indicate underdosing due to the “interplay” between respiratory motion and intensity-modulated beam delivery. Because patients did not have 3D-CRT plans to compare with the IMRT plans, this study cannot reach definite conclusions as to the conditions under which one technique is superior to the other. However, our results do support the consideration of IMRT as a useful tool for treating larger tumors (GTV ≥ 100cc)8,18 with unfavorable locations to doses that may be difficult to achieve with 3D-CRT.
Footnotes
Conflicts of Interest Notification
Actual or potential conflicts of interest do not exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curran W, Scott CB, Langer CJ, et al. Long-term benefit is observed in a Phase III comparison of sequential vs. concurrent chemo-radiation for patients with unresected stage III NSCLC: RTOG 9410. Proc Am Soc Clin Oncol. 2003;22:621. [Google Scholar]
- 2.Belani CP, Choy H, Bonomi P, et al. Combined Chemoradiotherapy Regimens of Paclitaxel and Carboplatin for Locally Advanced Non–Small-Cell Lung Cancer: A Randomized Phase II Locally Advanced Multi-Modality Protocol. J Clin Oncol. 2005;23(25):5883–5891. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 3.Qiao X, Tullgren O, Lax I, et al. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer. 2003;41:1–11. doi: 10.1016/s0169-5002(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 4.Hayman JA, Martel MK, Ten Haken RK, et al. Dose escalation in non-small cell lung cancer using three-dimensional conformal radiation therapy: Update of a phase I trial. J Clin Oncol. 2001;19:127–136. doi: 10.1200/JCO.2001.19.1.127. [DOI] [PubMed] [Google Scholar]
- 5.Narayan S, Henning GT, Randall TK, et al. Results following treatment to doses of 92.4 or 102.9 Gy on a phase I dose escalation study for non-small cell lung cancer. Lung Cancer. 2004;44:79–88. doi: 10.1016/j.lungcan.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 7.Rosenzweig KE, Fox JL, Leibel SA, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer. 2005;103:2118–2127. doi: 10.1002/cncr.21007. [DOI] [PubMed] [Google Scholar]
- 8.Rengan R, Rosenzweig KE, Venkatraman E. Improved local control with higher doses of radiation in large-volume stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;60(3):741–747. doi: 10.1016/j.ijrobp.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 10.Kwa SL, Lebesque JV, Theuws JC, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data in 504 patients. Int J Radiat Oncol Biol Phys. 1998;42:1–9. doi: 10.1016/s0360-3016(98)00196-5. [DOI] [PubMed] [Google Scholar]
- 11.Liu HH, Wang X, Dong L, et al. Feasibility of sparing lung and other thoracic structures with intensity-modulated radiotherapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1268–1279. doi: 10.1016/j.ijrobp.2003.09.085. [DOI] [PubMed] [Google Scholar]
- 12.Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58(4):1258–1267. doi: 10.1016/j.ijrobp.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 13.Christian JA, Bedford JL, Webb S, et al. Comparison of inverse-planned three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;67(3):735–741. doi: 10.1016/j.ijrobp.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 14.Grills IS, Yan D, Martinez AA, et al. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation therapy, and elective nodal irradiation. Int J Radiat Oncol Biol Phys. 2003;57:875–890. doi: 10.1016/s0360-3016(03)00743-0. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz M, Alber M, Lebesque LV, et al. Dose heterogeneity in the target volume and intensity-modulated radiotherapy to escalate the dose in the treatment of non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2005;62(2):561–70. doi: 10.1016/j.ijrobp.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Yorke E. Advantages of IMRT for dose escalation in radiation therapy for lung cancer.[Abstract] Med Phys. 2001;28:1291. [Google Scholar]
- 17.Sidhu K, Ford EC, Spirou S, Yorke E, et al. Optimization with both dose-volume and biological constraints for lung IMRT.[Abstract] Med Phys. 2001;28(6):1261. [Google Scholar]
- 18.Willner J, Baier K, Caragiani E, et al. Dose, volume, and tumor control predictions in primary radiotherapy of non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;52(2):382–389. doi: 10.1016/s0360-3016(01)01823-5. [DOI] [PubMed] [Google Scholar]
- 19.Mohan R, Barest G, Brewster LJ, et al. A comprehensive three-dimensional radiation treatment planning system. Int J Radiat Oncol Biol Phys. 1988;15:481–495. doi: 10.1016/s0360-3016(98)90033-5. [DOI] [PubMed] [Google Scholar]
- 20.Burman C, Chui CS, Kutcher G, et al. Planning, delivery and quality assurance of intensity-modulated radiotherapy using dynamic multileaf collimator: A strategy for large-scale implementation for the treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1997;39:863–873. doi: 10.1016/s0360-3016(97)00458-6. [DOI] [PubMed] [Google Scholar]
- 21.Spirou SV, Chui CS. A gradient inverse planning algorithm with dose-volume constraints. Med Phys. 1998;25:321–333. doi: 10.1118/1.598202. [DOI] [PubMed] [Google Scholar]
- 22.Chui CS, LoSasso T, Spirou S, et al. Dose calculation for photon beams with intensity modulation generated by dynamic jaw or multileaf collimations. Med Phys. 1994;21:1237–1244. doi: 10.1118/1.597206. [DOI] [PubMed] [Google Scholar]
- 23.Spirou SV, Chui CS. Generation of arbitrary intensity profiles by dynamic jaws or multileaf collimators. Med Phys. 1994;21:1031–1047. doi: 10.1118/1.597345. [DOI] [PubMed] [Google Scholar]
- 24.Rosenzweig KE, Mychalzhak B, Fuks Z, et al. Final report of the 70.2-Gy and 75.6-Gy dose levels of a phase I dose escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable non-small cell lung cancer. Cancer. 2000;6:82–87. [PubMed] [Google Scholar]
- 25.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res. 1985;104(Suppl):S13–S19. [PubMed] [Google Scholar]
- 26.Kutcher Gj, Burman C, Brewster L, et al. Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys. 1991;21:137–146. doi: 10.1016/0360-3016(91)90173-2. [DOI] [PubMed] [Google Scholar]
- 27.Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21:123–135. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- 28.Withers HR, Taylor JMG, Maciejewski B. Treatment volume and tissue tolerance. Int J Radiat Oncol Biol Phys. 1988;14:751–759. doi: 10.1016/0360-3016(88)90098-3. [DOI] [PubMed] [Google Scholar]
- 29.Yorke Ed, Jackson A, Rosenzweig KE, et al. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2002;54 (2 SU):329–339. doi: 10.1016/s0360-3016(02)02929-2. [DOI] [PubMed] [Google Scholar]
- 30.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 32.Gandara DR, Chansky K, Albain KS, et al. Consolidation Docetaxel After Concurrent Chemoradiotherapy in Stage IIIB Non–Small-Cell Lung Cancer: Phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21(10):2004–2010. doi: 10.1200/JCO.2003.04.197. [DOI] [PubMed] [Google Scholar]
- 33.Yom S, Liao Z, Liu HH, et al. Analysis of acute toxicity results of intensity-modulated radiation therapy (IMRT) in the treatment of non-small cell lung cancer. Lung Cancer. 2005;49(2):S52. Abstract: [Google Scholar]
- 34.Stevens C, Guerrero T, Forster K. Lung Cancer Radiotherapy. In: Palta Jatinder, Mackie T Rockwell., editors. Intensity-Modulated Radiation Therapy: the State of the Art. Madison, Wisconsin: Medical Physics Publishing; 2003. pp. 645–662. [Google Scholar]
- 35.Seppenwoolde Y, Lebesque JV, De Jaeger KD, et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2003;55(3):724–735. doi: 10.1016/s0360-3016(02)03986-x. [DOI] [PubMed] [Google Scholar]
- 36.Graham MV, Purdy JA, Emani B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3-D treatment for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1999;45(2):323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 37.Liu HH, Wang X, Dong L, et al. Feasibility of sparing lung and other thoracic structures with intensity-modulated radiotherapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58(4):1268–1279. doi: 10.1016/j.ijrobp.2003.09.085. [DOI] [PubMed] [Google Scholar]
- 38.Bortfeld T, Jokivarsi K, Goitein M, et al. Effects of intra-fraction motion on IMRT dose delivery: statistical analysis and simulation. Phys Med Biol. 2002;47(13):2209–2220. doi: 10.1088/0031-9155/47/13/302. [DOI] [PubMed] [Google Scholar]
- 39.Chui CS, Yorke E, Hong L. The effects of intra-fraction organ motion on the delivery of intensity-modulated field with multilead collimator. Med Phys. 2003;30(7):1736–1746. doi: 10.1118/1.1578771. [DOI] [PubMed] [Google Scholar]
- 40.Yorke ED, Wang L, Rosenzweig KE, et al. Evaluation of deep inspiration breath hold lung treatment plans with Monte Carlo dose calculation. Int Jnl Radat Oncol Biol Phys. 2002;53:1058–1070. doi: 10.1016/s0360-3016(02)02778-5. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Yorke E, Chui CS, et al. Monte Carlo evaluation of 6 MV intensity modulated radiotherapy plans for head and neck and lung treatments. Med Phys. 2002;29:2705–2717. doi: 10.1118/1.1517291. [DOI] [PubMed] [Google Scholar]
- 42.Della Biancia C, Yorke E, Chui CS, et al. Comparison of end normal inspiration and expiration for gated intensity modulated radiation therapy (IMRT) of lung cancer. Radiother and Oncol. 2005;75:149–156. doi: 10.1016/j.radonc.2005.01.008. [DOI] [PubMed] [Google Scholar]